Submitted:
29 September 2023
Posted:
29 September 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction: Maize's Crucial Role in Global Nutrition
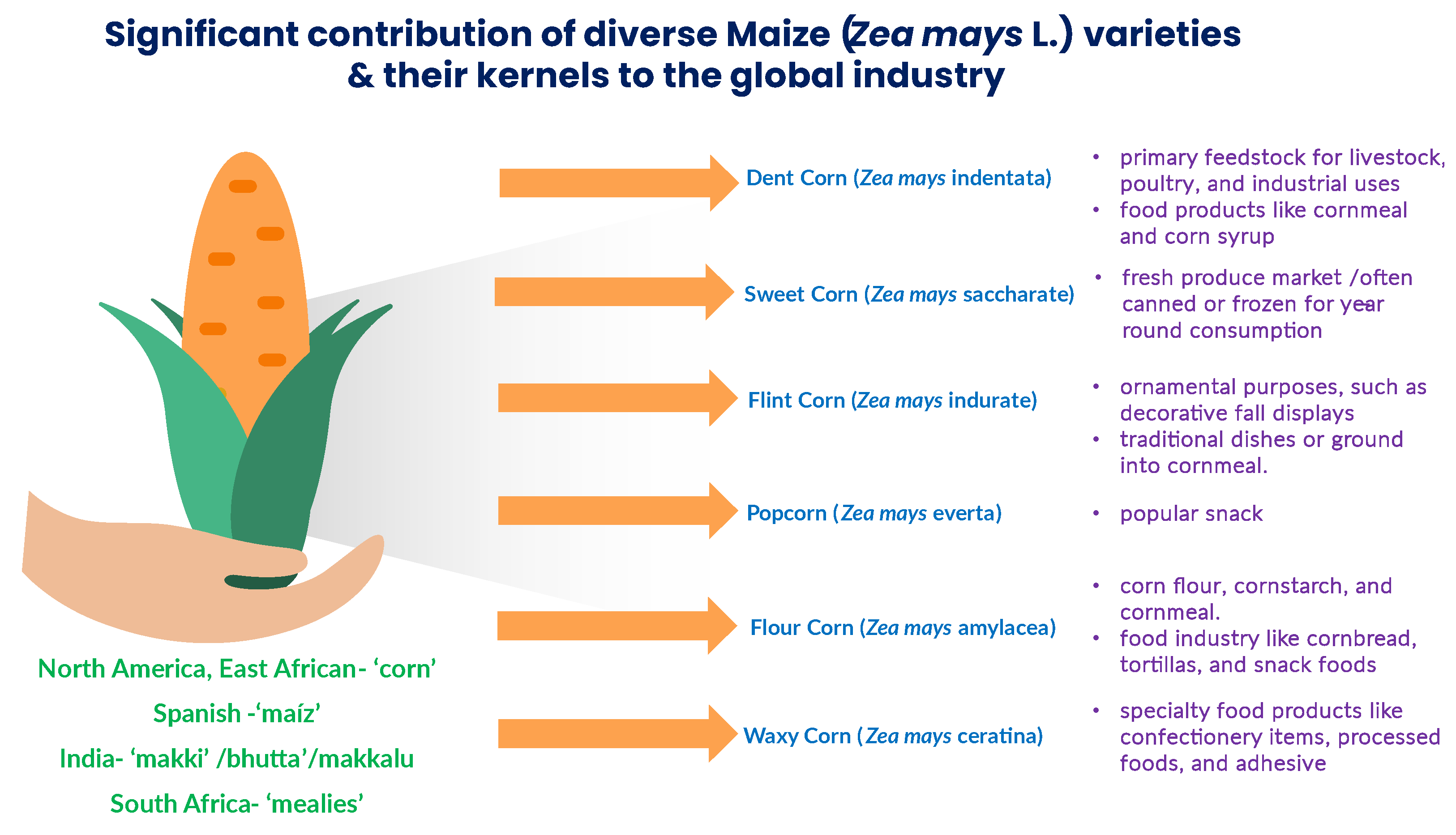
2. Maize's Nutritional Profile: Beyond Basics
2.1. Unveiling the intricate nutrient composition of Maize
2.2. Implications of macronutrient and micronutrient content
2.3. Exploring the value of dietary fiber in Maize
3. Maize's Nutritional Profile: Beyond Basics
4. Biofortification: Empowering Maize with Essential Nutrients
4.1. Pioneering provitamin A enrichment in Maize
4.2. Unravelling zinc and iron biofortification efforts
4.3. Revolutionary breeding techniques for nutrient elevation
5. Genetic Mastery: Transforming Maize's Nutritional Landscape
5.1. A profound exploration of genetic modification in Maize
5.2. Case studies showcasing nutrient-enriched transgenic Maize strains
6. Cultivating Nutrient Excellence: Agronomic Strategies
7. Preserving Nutrient Integrity: Maize Processing Traditions
7.1. Whole-Grain Maize
7.2. Nixtamalization
7.3. Hydrothermal treatment
7.4. Elevating nutrient retention during culinary and industrial processing
8. Bridging Nutritional Gaps: The Role of Maize Varieties
8.1. Unearthing the spectrum of nutrient content variation within Maize strains
8.2. Championing nutrient-rich Maize cultivars for nutritional empowerment
9. Safeguarding Nutrients Beyond Harvest: Post-Harvest Strategies
9.1. Unveiling storeroom techniques to counter nutrient depletion
9.2. Vigilance against mold proliferation and mycotoxin contamination
10. Enlightened Choices: Empowering Consumers Through Education
11. Championing Change: Success Stories and Global Impact
12. Tomorrow's Promise: Pioneering Nutritional Frontiers
12.1. Identifying Roadblocks and Potential Solutions for Elevating Maize's Nutritional Potency
12.1.1. Biotic and Abiotic Stress Factors
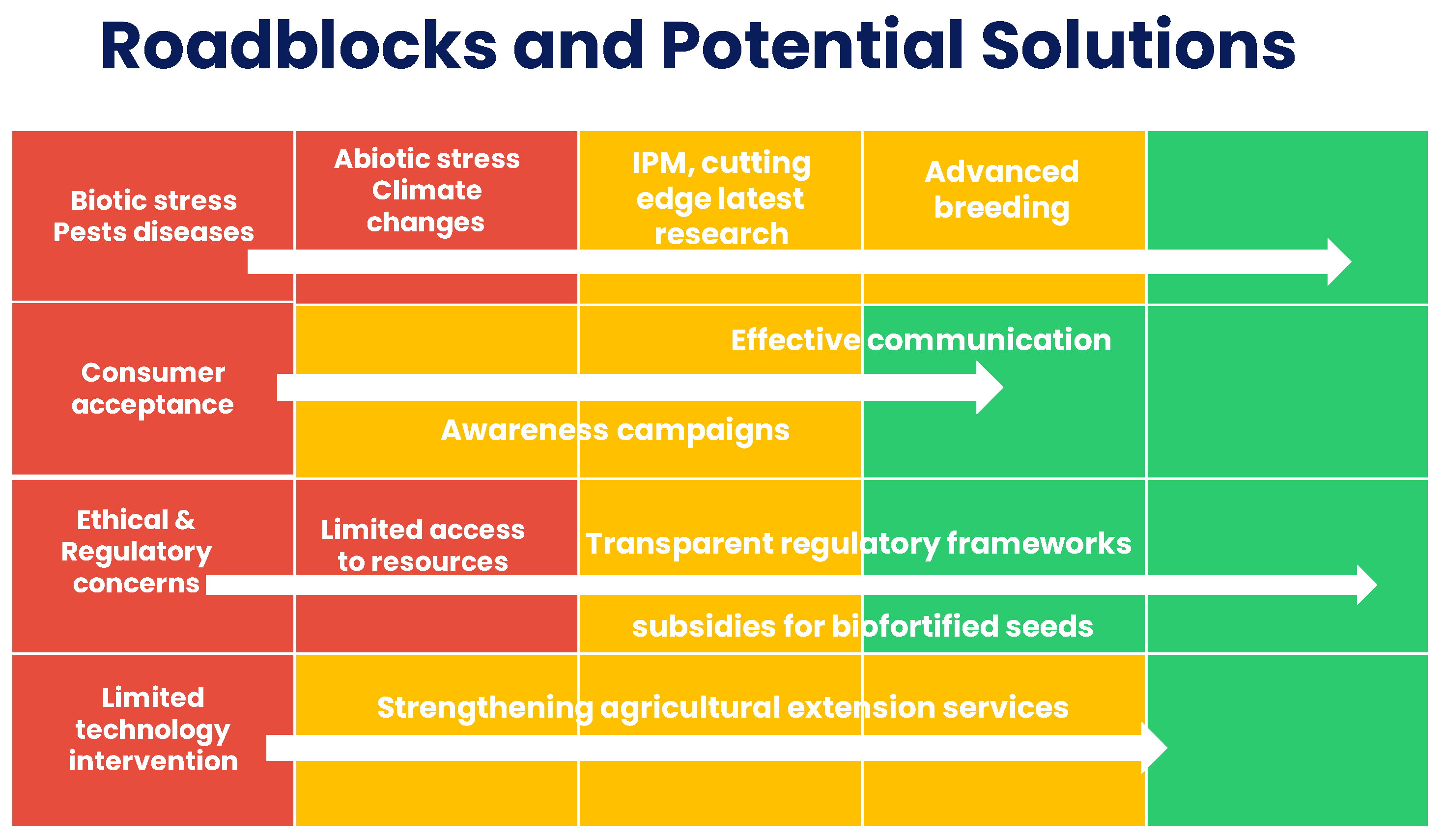
12.1.2. Socioeconomic Barriers
12.1.3. Consumer Acceptance
12.1.4. Regulatory and Ethical Considerations
13. In Conclusion: Forging a Nourished Future with Maize
References
- Sah RP, KUMAR A, Rana M, Kumar U. Maize (Corn). Forage Crops of the World, 2-volume set: Volume I: Major Forage Crops; Volume II: Minor Forage Crops. 2022 May 29:15.
- Onuwa, G. Fostering Sustainable Productivity through Maize Technology Intensification: Participant responses. In Handbook of Research on Green Technologies for Sustainable Management of Agricultural Resources; IGI Global, 2022; pp. 427–436. [Google Scholar]
- Revilla, P.; Alves, M.L.; Andelković, V.; Balconi, C.; Dinis, I.; Mendes-Moreira, P.; Malvar, R.A. Traditional Foods From Maize (Zea mays L.) in Europe. Front. Nutr. 2022, 8, 683399. [Google Scholar] [CrossRef] [PubMed]
- Rouf Shah, T.; Prasad, K.; Kumar, P. Maize—A potential source of human nutrition and health: A review. Cogent Food Agric. 2016, 2, 1166995. [Google Scholar] [CrossRef]
- USDA. United States Department of Agriculture (USDA). National nutrient database for standard reference. https://fdc.nal.usda.gov/. 2020. [Google Scholar]
- Nuss, E.T.; Tanumihardjo, S.A. Maize: A paramount staple crop in the context of global nutrition. Comprehensive Reviews in Food Science and Food Safety 2010, 9, 417–436. [Google Scholar] [CrossRef] [PubMed]
- Sands, A.L.; Leidy, H.J.; Hamaker, B.R.; Maguire, P.; Campbell, W.W. Consumption of the slow-digesting waxy maize starch leads to blunted plasma glucose and insulin response but does not influence energy expenditure or appetite in humans. Nutr. Res. 2009, 29, 383–390. [Google Scholar] [CrossRef] [PubMed]
- Galani, Y.J.H.; Orfila, C.; Gong, Y.Y. A review of micronutrient deficiencies and analysis of maize contribution to nutrient requirements of women and children in Eastern and Southern Africa. Crit. Rev. Food Sci. Nutr. 2020, 62, 1568–1591. [Google Scholar] [CrossRef]
- Gibbon, B.C.; Larkins, B.A. Molecular genetic approaches to developing quality protein maize. Trends Genet. 2005, 21, 227–233. [Google Scholar] [CrossRef]
- Orthoefer, F.; Eastman, J.; List, G. Corn oil: Composition, processing, and utilization. In Corn: Chemistry and Technology, 2nd ed.; 2003; pp. 671–693. [Google Scholar]
- CRA. Corn oil, 5th ed.; Corn Refiners Association: Washington, DC, 2006. [Google Scholar]
- Kumar, D.; Jhariya, A.N. Nutritional, medicinal and economical importance of corn: A mini review. Res J Pharm Sci 2013, 2319, 555X. [Google Scholar]
- Sen, C.K.; Khanna, S.; Roy, S. Tocotrienols: Vitamin E beyond tocopherols. Life Sci. 2006, 78, 2088–2098. [Google Scholar] [CrossRef]
- Kopsell, D.A.; Armel, G.R.; Mueller, T.C.; Sams, C.E.; Deyton, D.E.; McElroy, J.S.; Kopsell, D.E. Increase in nutritionally important sweet corn kernel carotenoids following mesotrione and atrazine applications. Journal of Agricultural and Food Chemistry 2009, 57, 6362–6368. [Google Scholar] [CrossRef]
- Demeke, K.H. Nutritional Quality Evaluation of Seven Maize Varieties Grown in Ethiopia. Biochem. Mol. Biol. 2018, 3, 45. [Google Scholar] [CrossRef]
- Harrabi, S.; St-Amand, A.; Sakouhi, F.; Sebei, K.; Kallel, H.; Mayer, P.M.; Boukhchina, S. Phytostanols and phytosterols distributions in corn kernel. Food Chemistry 2008, 111, 115–120. [Google Scholar] [CrossRef]
- Murdia, L.K.; Wadhwani, R.; Wadhawan, N.; Bajpai, P.; Shekhawat, S. Maize utilization in India: An overview. American Journal of Food and Nutrition 2016, 4, 169–176. [Google Scholar]
- Eckhoff, S. R.; Paulsen, M. R. Maize. In Cereal grain quality; Springer: Dordrecht, The Netherlands, 1996; pp. 77–112. [Google Scholar]
- Aliyu, K.T.; Huising, J.; Kamara, A.Y.; Jibrin, J.M.; Mohammed, I.B.; Nziguheba, G.; Adam, A.M. Understanding nutrient imbalances in maize (Zea mays L.) using the diagnosis and recommendation integrated system (DRIS) approach in the Maize belt of Nigeria. Scientific Reports 2021, 11, 16018. [Google Scholar] [CrossRef] [PubMed]
- Gwirtz, J.A.; Garcia-Casal, M.N. Processing maize flour and corn meal food products. Ann. New York Acad. Sci. 2013, 1312, 66–75. [Google Scholar] [CrossRef]
- Suri, D.J.; Tanumihardjo, S.A. Effects of different processing methods on the micronutrient and phytochemical contents of maize: From A to Z. Comprehensive Reviews in Food Science and Food Safety 2016, 15, 912–926. [Google Scholar] [CrossRef]
- Brodowska, M.S.; Wyszkowski, M.; Bujanowicz-Haraś, B. Mineral Fertilization and Maize Cultivation as Factors Which Determine the Content of Trace Elements in Soil. Agronomy 2022, 12, 286. [Google Scholar] [CrossRef]
- Abdel-Aal, E.-S.M.; Akhtar, H.; Zaheer, K.; Ali, R. Dietary Sources of Lutein and Zeaxanthin Carotenoids and Their Role in Eye Health. Nutrients 2013, 5, 1169–1185. [Google Scholar] [CrossRef]
- Gopalan, C.; Rama Sastri, B. V.; Balasubramanian, S. C. Nutritive value of Indian foods; National Institute of Nutrition, Indian Council of Medical Research, 1971. [Google Scholar]
- Gensberger, S.; Mittelmaier, S.; Glomb, M.A.; Pischetsrieder, M. Identification and quantification of six major α-dicarbonyl process contaminants in high-fructose corn syrup. Anal. Bioanal. Chem. 2012, 403, 2923–2931. [Google Scholar] [CrossRef]
- Tappy, L.; Lê, K.-A. Metabolic Effects of Fructose and the Worldwide Increase in Obesity. Physiol. Rev. 2010, 90, 23–46. [Google Scholar] [CrossRef]
- Parker, K.; Salas, M.; Nwosu, V.C. High fructose corn syrup: Production, uses and public health concerns. Biotechnol Mol Biol Rev 2010, 5, 71–78. [Google Scholar]
- Bawa, A.S.; Anilakumar, K.R. Genetically modified foods: Safety, risks, and public concerns—A review. Journal of Food Science and Technology 2013, 50, 1035–1046. [Google Scholar] [CrossRef] [PubMed]
- Norris, M. L. Will GMOs hurt my body? The public’s concerns and how scientists have addressed them. Science in the News, 2015. [Google Scholar]
- Li, Y.; Zhang, L.; Liu, H.; Yang, Y.; He, J.; Cao, M.; Zhong, W.; Lin, Y.; Zhuo, Y.; Fang, Z.; et al. Effects of the Ratio of Insoluble Fiber to Soluble Fiber in Gestation Diets on Sow Performance and Offspring Intestinal Development. Animals 2019, 9, 422. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.W.; E Jones, A.; Riddell-Mason, S. Ten Different Dietary Fibers Have Significantly Different Effects on Serum and Liver Lipids of Cholesterol-Fed Rats. J. Nutr. 1994, 124, 78–83. [Google Scholar] [CrossRef] [PubMed]
- Bouis, H.E.; Saltzman, A. Improving nutrition through biofortification: A review of evidence from Harvest Plus, 2003 through 2016. Global food security 2017, 12, 49–58. [Google Scholar] [CrossRef]
- Manjeru, P.; Van Biljon, A.; Labuschagne, M. The development and release of maize fortified with provitamin A carotenoids in developing countries. Crit. Rev. Food Sci. Nutr. 2017, 59, 1284–1293. [Google Scholar] [CrossRef]
- Gannon, B.; Kaliwile, C.; A Arscott, S.; Schmaelzle, S.; Chileshe, J.; Kalungwana, N.; Mosonda, M.; Pixley, K.; Masi, C.; A Tanumihardjo, S. Biofortified orange maize is as efficacious as a vitamin A supplement in Zambian children even in the presence of high liver reserves of vitamin A: A community-based, randomized placebo-controlled trial. Am. J. Clin. Nutr. 2014, 100, 1541–1550. [Google Scholar] [CrossRef]
- Palmer, A.C.; Healy, K.; Barffour, M.A.; Siamusantu, W.; Chileshe, J.; Schulze, K.J.; Labrique, A.B. Provitamin A carotenoid–biofortified maize consumption increases pupillary responsiveness among Zambian children in a randomized controlled trial. The Journal of nutrition 2016, 146, 2551–2558. [Google Scholar] [CrossRef]
- Zuma, M.K.; Kolanisi, U.; Modi, A.T. The Potential of Integrating Provitamin A-Biofortified Maize in Smallholder Farming Systems to Reduce Malnourishment in South Africa. Int. J. Environ. Res. Public Health 2018, 15, 805. [Google Scholar] [CrossRef]
- White, P.; Broadley, M. Biofortifying crops with essential mineral elements. Trends Plant Sci. 2005, 10, 586–593. [Google Scholar] [CrossRef]
- Zhu, C.; Naqvi, S.; Gomez-Galera, S.; Pelacho, A.M.; Capell, T.; Christou, P. Transgenic strategies for the nutritional enhancement of plants. Trends Plant Sci. 2007, 12, 548–555. [Google Scholar] [CrossRef]
- Grusak, M.A. Enhancing Mineral Content in Plant Food Products. In Proceedings of the 42nd Annual Meeting of the American-College-of-Nutrition; pp. 178S–183S.
- Roberts, L.A.; Pierson, A.J.; Panaviene, Z.; Walker, E.L. Yellow Stripe1. Expanded Roles for the Maize Iron-Phytosiderophore Transporter. Plant Physiol. 2004, 135, 112–120. [Google Scholar] [CrossRef] [PubMed]
- Drakakaki, G.; Marcel, S.; Glahn, R.P.; Lund, E.K.; Pariagh, S.; Fischer, R.; Stoger, E. Endosperm-specific co-expression of recombinant soybean ferritin and Aspergillus phytase in maize results in significant increases in the levels of bioavailable iron. Plant Molecular Biology 2005, 59, 869–880. [Google Scholar] [CrossRef] [PubMed]
- Davila-Hicks, P.; Theil, E.C.; Lönnerdal, B. Iron in ferritin or in salts (ferrous sulfate) is equally bioavailable in nonanemic women. Am. J. Clin. Nutr. 2004, 80, 936–940. [Google Scholar] [CrossRef] [PubMed]
- Lönnerdal, B.; Bryant, A.; Liu, X.; Theil, E.C. Iron absorption from soybean ferritin in nonanemic women. Am. J. Clin. Nutr. 2006, 83, 103–107. [Google Scholar] [CrossRef]
- Brown, K.H.; Rivera, J.A.; Bhutta, Z.; Gibson, R.S.; King, J.C.; Lonnerdal, B.; Ruel, B.; Sandtrom, B.; Wasantwisut, E.; Hotz, C. International Zinc Nutrition Consultative Group: Assessment of the risk of zinc deficiency in populations and options for its control. The Food and Nutrition Bulletin 2004, 25, S99–203. [Google Scholar]
- Hotz, C. The Potential to Improve Zinc Status through Biofortification of Staple Food Crops with Zinc. Food Nutr. Bull. 2009, 30, S172–S178. [Google Scholar] [CrossRef]
- Whitt, S.R.; Wilson, L.M.; Tenaillon, M.I.; Gaut, B.S.; Buckler IV, E.S. Genetic diversity and selection in the maize starch pathway. Proceedings of the National Academy of Sciences 2002, 99, 12959–12962. [Google Scholar] [CrossRef]
- Palaisa, K.; Morgante, M.; Tingey, S.; Rafalski, A. Long-range patterns of diversity and linkage disequilibrium surrounding the maize Y1 gene are indicative of an asymmetric selective sweep. Proceedings of the National Academy of Sciences 2004, 101, 9885–9890. [Google Scholar] [CrossRef]
- Karn, A.; Gillman, J.D.; A Flint-Garcia, S. Genetic Analysis of Teosinte Alleles for Kernel Composition Traits in Maize. G3 Genes|Genomes|Genetics 2017, 7, 1157–1164. [Google Scholar] [CrossRef]
- Fan, L.; Bao, J.; Wang, Y.; Yao, J.; Gui, Y.; Hu, W.; Zhu, J.; Zeng, M.; Li, Y.; Xu, Y. Post-Domestication Selection in the Maize Starch Pathway. PLoS ONE 2009, 4, e7612. [Google Scholar] [CrossRef]
- Huang, Y.; Wang, H.; Zhu, Y.; Huang, X.; Li, S.; Wu, X.; Zhao, Y.; Bao, Z.; Qin, L.; Jin, Y.; et al. THP9 enhances seed protein content and nitrogen-use efficiency in maize. Nature 2022, 612, 292–300. [Google Scholar] [CrossRef] [PubMed]
- Turnbull, C.; Lillemo, M.; Hvoslef-Eide, T.A. Global regulation of genetically modified crops amid the gene edited crop boom–a review. Frontiers in Plant Science 2021, 12, 630396. [Google Scholar] [CrossRef] [PubMed]
- Kettenburg, A.J.; Hanspach, J.; Abson, D.J.; Fischer, J. From disagreements to dialogue: Unpacking the Golden Rice debate. Sustain. Sci. 2018, 13, 1469–1482. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Yu, Y.; Li, G.; Xie, L.; Guo, X.; Li, J.; Li, Y.; Hu, J. Genome-wide association study of vitamin E in sweet corn kernels. Crop. J. 2019, 8, 341–350. [Google Scholar] [CrossRef]
- Waltz, E. CRISPR-edited crops free to enter market, skip regulation. Nat. Biotechnol. 2016, 34, 582. [Google Scholar] [CrossRef] [PubMed]
- Zilberman, D.; Holland, T.G.; Trilnick, I. Agricultural GMOs—What we know and where scientists disagree. Sustainability 2018, 10, 1514. [Google Scholar] [CrossRef]
- Chrenková, M.; Sommer, A.; Čerešňáková, Z.; Nitrayová, S.; Prostredná, M. Nutritional evaluation of genetically modified maize corn performed on rats. Archives of Animal Nutrition 2002, 56, 229–235. [Google Scholar] [CrossRef] [PubMed]
- Venneria, E.; Fanasca, S.; Monastra, G.; Finotti, E.; Ambra, R.; Azzini, E.; Durazzo, A.; Foddai, M.S.; Maiani, G. Assessment of the Nutritional Values of Genetically Modified Wheat, Corn, and Tomato Crops. J. Agric. Food Chem. 2008, 56, 9206–9214. [Google Scholar] [CrossRef] [PubMed]
- Baktavachalam, G.B.; Delaney, B.; Fisher, T.L.; Ladics, G.S.; Layton, R.J.; Locke, M.E.; Schmidt, J.; A Anderson, J.; Weber, N.N.; A Herman, R.; et al. Transgenic maize event TC1507: Global status of food, feed, and environmental safety. GM Crop. Food 2015, 6, 80–102. [Google Scholar] [CrossRef]
- EFSA Panel on Genetically Modified Organisms (EFSA GMO Panel); Naegeli, H.; Bresson, J.L.; Dalmay, T.; Dewhurst, I.C.; Epstein, M.M.; Koukoulanaki, M. Assessment of genetically modified maize Bt11× MIR162× MIR604× 1507× 5307× GA21 and subcombinations, for food and feed uses, under Regulation (EC) No 1829/2003 (application EFSA-GMO-DE-2011-103). EFSA Journal 2019, 17, e05635. [Google Scholar]
- Boyle, J.H.; Dalgleish, H.J.; Puzey, J.R. Monarch butterfly and milkweed declines substantially predate the use of genetically modified crops. Proc. Natl. Acad. Sci. 2019, 116, 3006–3011. [Google Scholar] [CrossRef] [PubMed]
- Pellegrino, E.; Bedini, S.; Nuti, M.; Ercoli, L. Impact of genetically engineered maize on agronomic, environmental, and toxicological traits: A meta-analysis of 21 years of field data. Scientific Reports 2018, 8, 3113. [Google Scholar] [CrossRef] [PubMed]
- Woźniak, E.; Tyczewska, A.; Twardowski, T. A Shift Towards Biotechnology: Social Opinion in the EU. Trends Biotechnol. 2020, 39, 214–218. [Google Scholar] [CrossRef]
- Hull, R.; Head, G.; Tzotzos, G. T. (Eds.) Evolution of regulatory systems and national biosafety frameworks. In Genetically Modified Plants, 2nd ed.; Elsevier: Cambridge, Massachusetts, 2021; pp. 127–155. [Google Scholar]
- Cakmak, I. Agronomic Biofortification; International Food Policy Research Institute: Washington, DC, USA, 2014; pp. 56–97. [Google Scholar]
- Dimkpa, C.O.; Bindraban, P.S. Fortification of micronutrients for efficient agronomic production: A review. Agron. Sustain. Dev. 2016, 36. [Google Scholar] [CrossRef]
- Vanlauwe, B.; Descheemaeker, K.; Giller, K.E.; Huising, J.; Merckx, R.; Nziguheba, G.; Zingore, S. Integrated soil fertility management in sub-Saharan Africa: Unravelling local adaptation. Soil 2015, 1, 491–508. [Google Scholar] [CrossRef]
- Teklu, D.; Gashu, D.; Joy, E.J.M.; Amede, T.; Broadley, M.R. Effectiveness of Agronomic Biofortification Strategy in Fighting against Hidden Hunger. Agronomy 2023, 13, 2173. [Google Scholar] [CrossRef]
- Ngigi, P.B.; Lachat, C.; Masinde, P.W.; Du Laing, G. Agronomic biofortification of maize and beans in Kenya through selenium fertilization. Environ. Geochem. Health 2019, 41, 2577–2591. [Google Scholar] [CrossRef]
- Botoman, L.; Chimungu, J.G.; Bailey, E.H.; Munthali, M.W.; Ander, E.L.; Mossa, A.; Young, S.D.; Broadley, M.R.; Lark, R.M.; Nalivata, P.C. Agronomic biofortification increases grain zinc concentration of maize grown under contrasting soil types in Malawi. Plant Direct 2022, 6, e458. [Google Scholar] [CrossRef]
- Umar, W.; Hameed, M.K.; Aziz, T.; Maqsood, M.A.; Bilal, H.M.; Rasheed, N. Synthesis, characterization and application of ZnO nanoparticles for improved growth and Zn biofortification in maize. Arch. Agron. Soil Sci. 2020, 67, 1164–1176. [Google Scholar] [CrossRef]
- Serna-Saldivar, S.O.; Carrillo, E.P. Food uses of whole corn and dry-milled fractions. In Corn; AACC International Press: St. Paul, MN, USA, 2019; pp. 435–467. [Google Scholar]
- Palacios-Rojas, N.; McCulley, L.; Kaeppler, M.; Titcomb, T.J.; Gunaratna, N.S.; Lopez-Ridaura, S.; Tanumihardjo, S.A. Mining maize diversity and improving its nutritional aspects within agro-food systems. Compr. Rev. Food Sci. Food Saf. 2020, 19, 1809–1834. [Google Scholar] [CrossRef]
- Gallego-Castillo, S.; Taleon, V.; Talsma, E.F.; Rosales-Nolasco, A.; Palacios-Rojas, N. Effect of maize processing methods on the retention of minerals, phytic acid and amino acids when using high kernel-zinc maize. Curr. Res. Food Sci. 2021, 4, 279–286. [Google Scholar] [CrossRef] [PubMed]
- Sahasrabudhe, S. N. Corn characterization and development of a convenient laboratory scale alkaline cooking process. 2015. [Google Scholar]
- Sefa-Dedeh, S.; Cornelius, B.; Sakyi-Dawson, E.; Afoakwa, E.O. Effect of nixtamalization on the chemical and functional properties of maize. Food Chem. 2004, 86, 317–324. [Google Scholar] [CrossRef]
- González, R.; Reguera, E.; Mendoza, L.; Figueroa, J.M.; Sánchez-Sinencio, F. Physicochemical Changes in the Hull of Corn Grains during Their Alkaline Cooking. J. Agric. Food Chem. 2004, 52, 3831–3837. [Google Scholar] [CrossRef]
- Mora-Avilés, A.; Lemus-Flores, B.; Miranda-López, R.; Hernández-López, D.; Pons-Hernández, J.L.; Acosta-Gallegos, J.A.; Guzmán-Maldonado, S.H. Effects of common bean enrichment on nutritional quality of tortillas produced from nixtamalized regular and quality protein maize flours. Journal of the Science of Food and Agriculture 2007, 87, 880–886. [Google Scholar] [CrossRef]
- Serna-Saldivar, S.O. Understanding the functionality and manufacturing of nixtamalized maize products. J. Cereal Sci. 2021, 99, 103205. [Google Scholar] [CrossRef]
- Serna-Saldivar SO, Chuck-Hernandez C. Food uses of lime-cooked corn with emphasis in tortillas and snacks. In Corn; AACC International Press, 2019; pp. 469–500.
- Schaarschmidt, S.; Fauhl-Hassek, C. Mycotoxins during the Processes of Nixtamalization and Tortilla Production. Toxins 2019, 11, 227. [Google Scholar] [CrossRef] [PubMed]
- Rocha-Villarreal, V.; Hoffmann, J.F.; Vanier, N.L.; Serna-Saldivar, S.O.; García-Lara, S. Hydrothermal treatment of maize: Changes in physical, chemical, and functional properties. Food Chem. 2018, 263, 225–231. [Google Scholar] [CrossRef]
- Akinbolade, J.A.; Igbeka, J.C. Hydrothermal reactions on selected utilization qualities of parboiled Maize. Agricultural Engineering International: CIGR Journal 2012, 14, 238–245. [Google Scholar]
- Oladiran, D.A.; Emmambux, N.M. Locally Available African Complementary Foods: Nutritional Limitations and Processing Technologies to Improve Nutritional Quality—A Review. Food Rev. Int. 2020, 38, 1033–1063. [Google Scholar] [CrossRef]
- Goredema-Matongera, N.; Ndhlela, T.; Magorokosho, C.; Kamutando, C. N.; van Biljon, A.; Labuschagne, M. Multinutrient biofortification of maize (Zea mays L.) in Africa: Current status, opportunities and limitations. Nutrients 2021, 13, 1039. [Google Scholar] [CrossRef]
- Akinsola, O.T.; Alamu, E.O.; Otegbayo, B.O.; Menkir, A.; Maziya-Dixon, B. Evaluation of Quality and Acceptability of Snack (Kokoro) Produced From Synthetic Provitamin A Maize (Zea mays) Genotypes. Front. Sustain. Food Syst. 2020, 4. [Google Scholar] [CrossRef]
- Batool, M. Nutrient Management of Maize. 2023. [Google Scholar]
- Langyan, S.; Bhardwaj, R.; Kumari, J.; Jacob, S.R.; Bisht, I.S.; Pandravada, S.R.; Singh, A.; Singh, P.B.; Dar, Z.A.; Kumar, A.; et al. Nutritional Diversity in Native Germplasm of Maize Collected From Three Different Fragile Ecosystems of India. Front. Nutr. 2022, 9, 812599. [Google Scholar] [CrossRef] [PubMed]
- Qu, J.-H.; Liu, D.; Cheng, J.-H.; Sun, D.-W.; Ma, J.; Pu, H.; Zeng, X.-A. Applications of Near-infrared Spectroscopy in Food Safety Evaluation and Control: A Review of Recent Research Advances. Crit. Rev. Food Sci. Nutr. 2013, 55, 1939–1954. [Google Scholar] [CrossRef] [PubMed]
- Payne, W.Z.; Kurouski, D. Raman spectroscopy enables phenotyping and assessment of nutrition values of plants: A review. Plant Methods 2021, 17, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Abeshu, M.A.; Lelisa, A.; Geleta, B. Complementary feeding: Review of recommendations, feeding practices, and adequacy of homemade complementary food preparations in developing countries–lessons from Ethiopia. Frontiers in nutrition 2016, 3, 41. [Google Scholar] [CrossRef]
- Maqbool, M.A.; Issa, A.B.; Khokhar, E.S. Quality protein maize (QPM): Importance, genetics, timeline of different events, breeding strategies and varietal adoption. Plant Breed. 2021, 140, 375–399. [Google Scholar] [CrossRef]
- Kumar, P.; Choudhary, M.; Hossain, F.; Singh, N.K.; Choudhary, P.; Gupta, M.; Singh, V.; Chikappa, G.K.; Kumar, R.; Kumar, B.; et al. Nutritional quality improvement in maize (Zea mays): Progress and challenges. Indian J. Agric. Sci. 2019, 89, 895–911. [Google Scholar] [CrossRef]
- Yadava, D. K.; Choudhury, P. R.; Hossain, F.; Kumar, D. Biofortified varieties: Sustainable way to alleviate malnutrition; Indian Council of Agricultural Research: New Delhi, 2017. [Google Scholar]
- Otitodun, G.; Ala, A.; Nwaubani, S.; Omobowale, M.; Ajao, S.; Ogundare, M.; Olenloa, A.; Busari, G.; Abel, G.; Braimah, J.; et al. Assessing efficacies of insect pest management methods to preserve nutritional composition of bagged maize in storehouses located in markets in Nigeria. Afr. J. Food Agric. Nutr. Dev. 2021, 21, 17972–17988. [Google Scholar] [CrossRef]
- Hell, K.; Mutegi, C.; Fandohan, P. Aflatoxin control and prevention strategies in maize for Sub-Saharan Africa. Julius-Kühn-Archiv 2010, 425, 534. [Google Scholar]
- Hodges, R.; Bennett, B.; Bernard, M.; Rembold, F. Tackling post-harvest cereal losses in sub-Saharan Africa. Rural 21: The International Journal for Rural Development 2013, 47, 16–18. [Google Scholar]
- Suleiman, R. A.; Kurt, R. A. Current maize production, postharvest losses, and the risk of mycotoxins contamination in Tanzania. In 2015 ASABE Annual International Meeting; American Society of Agricultural and Biological Engineers, 2015; p. 1. [Google Scholar]
- Schnable, P.S.; Ware, D.; Fulton, R.S.; Stein, J.C.; Wei, F.; Pasternak, S.; Liang, C.; Zhang, J.; Fulton, L.; Graves, T.A.; Minx, P. The B73 maize genome: Complexity, diversity, and dynamics. Science 2009, 20, 1112–1115. [Google Scholar] [CrossRef] [PubMed]
- Bukowski, R.; Guo, X.; Lu, Y.; Zou, C.; He, B.; Rong, Z.; Wang, B.; Xu, D.; Yang, B.; Xie, C.; et al. Construction of the third-generation Zea mays haplotype map. GigaScience 2017, 7, gix134. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Xu, X.; Jin, W.; Xu, M.; Zhao, H.; Xiang, Z.; Song, W.; Ying, K.; Zhang, M.; Jiao, Y.; et al. Genome-wide patterns of genetic variation among elite maize inbred lines. Nat. Genet. 2010, 42, 1027–1030. [Google Scholar] [CrossRef] [PubMed]
- Jin, M.; Liu, H.; He, C.; Fu, J.; Xiao, Y.; Wang, Y.; Xie, W.; Wang, G.; Yan, J. Maize pan-transcriptome provides novel insights into genome complexity and quantitative trait variation. Sci. Rep. 2016, 6, 18936. [Google Scholar] [CrossRef]
- Springer, N.M.; Ying, K.; Fu, Y.; Ji, T.; Yeh, C.-T.; Jia, Y.; Wu, W.; Richmond, T.; Kitzman, J.; Rosenbaum, H.; et al. Maize Inbreds Exhibit High Levels of Copy Number Variation (CNV) and Presence/Absence Variation (PAV) in Genome Content. PLOS Genet. 2009, 5, e1000734. [Google Scholar] [CrossRef]
- Fu, J.; Cheng, Y.; Linghu, J.; Yang, X.; Kang, L.; Zhang, Z.; Zhang, J.; He, C.; Du, X.; Peng, Z.; et al. RNA sequencing reveals the complex regulatory network in the maize kernel. Nat. Commun. 2013, 4, 2832. [Google Scholar] [CrossRef]
- Hirsch, C.N.; Foerster, J.M.; Johnson, J.M.; Sekhon, R.S.; Muttoni, G.; Vaillancourt, B.; Peñagaricano, F.; Lindquist, E.; Pedraza, M.A.; Barry, K.; et al. Insights into the Maize Pan-Genome and Pan-Transcriptome. Plant Cell 2014, 26, 121–135. [Google Scholar] [CrossRef]
- Jiao, Y.; Zhao, H.; Ren, L.; Song, W.; Zeng, B.; Guo, J.; Wang, B.; Liu, Z.; Chen, J.; Li, W.; et al. Genome-wide genetic changes during modern breeding of maize. Nat. Genet. 2012, 44, 812–815. [Google Scholar] [CrossRef]
- Yang, N.; Liu, J.; Gao, Q.; Gui, S.; Chen, L.; Yang, L.; Huang, J.; Deng, T.; Luo, J.; He, L.; et al. Genome assembly of a tropical maize inbred line provides insights into structural variation and crop improvement. Nat. Genet. 2019, 51, 1052–1059. [Google Scholar] [CrossRef]
- Liu, J.; Fernie, A.R.; Yan, J. The Past, Present, and Future of Maize Improvement: Domestication, Genomics, and Functional Genomic Routes toward Crop Enhancement. Plant Commun. 2019, 1, 100010. [Google Scholar] [CrossRef]
- Doebley, J.; Stec, A.; Hubbard, L. The evolution of apical dominance in maize. Nature 1997, 386, 485–488. [Google Scholar] [CrossRef]
- Brutnell, T.P. Transposon tagging in maize. Functional & integrative genomics 2002, 2, 4–12. [Google Scholar]
- Mccarty, D.R.; Mark Settles, A.; Suzuki, M.; Tan, B.C.; Latshaw, S.; Porch, T.; Robin, K.; Baier, J.; Avigne, W.; Lai, J.; et al. Steady-state transposon mutagenesis in inbred maize. Plant J. 2005, 44, 52–61. [Google Scholar] [CrossRef] [PubMed]
- Till, B.J.; Reynolds, S.H.; Weil, C.; Springer, N.; Burtner, C.; Young, K.; Bowers, E.; Codomo, C.A.; Enns, L.C.; Odden, A.R.; Greene, E.A.; Comai, L. Discovery of induced point mutations in maize genes by TILLING. BMC Plant Biology 2004, 4, 12. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.-Y.; Li, Y.; Zhang, C.-Q. QTL mapping for stay-green in maize (Zea mays). Can. J. Plant Sci. 2012, 92, 249–256. [Google Scholar] [CrossRef]
- Zhao, X.; Peng, Y.; Zhang, J.; Fang, P.; Wu, B. Identification of QTLs and Meta-QTLs for Seven Agronomic Traits in Multiple Maize Populations under Well-Watered and Water-Stressed Conditions. Crop. Sci. 2018, 58, 507–520. [Google Scholar] [CrossRef]
- Wang, G.; Zhao, Y.; Mao, W.; Ma, X.; Su, C. QTL Analysis and Fine Mapping of a Major QTL Conferring Kernel Size in Maize (Zea mays). Front. Genet. 2020, 11. [Google Scholar] [CrossRef]
- Badu-Apraku, B.; Adewale, S.; Paterne, A.A.; Gedil, M.; Toyinbo, J.; Asiedu, R. Identification of QTLs for grain yield and other traits in tropical maize under Striga infestation. PLoS ONE 2020, 15, e0239205. [Google Scholar] [CrossRef]
- Yang, J.; Liu, Z.; Chen, Q.; Qu, Y.; Tang, J.; Lübberstedt, T.; Li, H. Mapping of QTL for grain yield components based on a DH population in maize. Scientific Reports 2020, 10, 7086. [Google Scholar] [CrossRef]
- Wang, L.; Zhou, Z.; Li, R.; Weng, J.; Zhang, Q.; Li, X.; Wang, B.; Zhang, W.; Song, W.; Li, X. Mapping QTL for flowering time-related traits under three plant densities in maize. Crop. J. 2020, 9, 372–379. [Google Scholar] [CrossRef]
- Fei, J.; Lu, J.; Jiang, Q.; Liu, Z.; Yao, D.; Qu, J.; Liu, S.; Guan, S.; Ma, Y. Maize plant architecture trait QTL mapping and candidate gene identification based on multiple environments and double populations. BMC Plant Biol. 2022, 22, 110. [Google Scholar] [CrossRef]
- Wang, M.; Zhang, R.; Zhao, Y.; Yao, J.; Li, W.; Yang, Z.; Sun, F.; Yang, X. Identifying QTL and candidate genes for prolificacy in maize. Crop. J. 2023, 11, 531–539. [Google Scholar] [CrossRef]
- Shikha, K.; Shahi, J.P.; Vinayan, M.T.; Zaidi, P.H.; Singh, A.K.; Sinha, B. Genome-wide association mapping in maize: Status and prospects. 3 Biotech 2021, 11, 244. [Google Scholar] [CrossRef] [PubMed]
- Wills, D.M.; Whipple, C.J.; Takuno, S.; Kursel, L.E.; Shannon, L.M.; Ross-Ibarra, J.; Doebley, J.F. From many, one: Genetic control of prolificacy during maize domestication. PLoS Genetics 2013, 9, e1003604. [Google Scholar] [CrossRef] [PubMed]
- Whipple, C.J.; Kebrom, T.H.; Weber, A.L.; Yang, F.; Hall, D.; Meeley, R.; Schmidt, R.; Doebley, J.; Brutnell, T.P.; Jackson, D.P. grassy tillers1 promotes apical dominance in maize and responds to shade signals in the grasses. Proceedings of the National Academy of Sciences 2011, 108, E506–E512. [Google Scholar] [CrossRef]
- Wang, J.; Lin, Z.; Zhang, X.; Liu, H.; Zhou, L.; Zhong, S.; Li, Y.; Zhu, C.; Lin, Z. krn1, a major quantitative trait locus for kernel row number in maize. New Phytol. 2019, 223, 1634–1646. [Google Scholar] [CrossRef]
- Sigmon, B.; Vollbrecht, E. Evidence of selection at the ramosa1 locus during maize domestication. Mol. Ecol. 2010, 19, 1296–1311. [Google Scholar] [CrossRef]
- Studer, A.; Zhao, Q.; Ross-Ibarra, J.; Doebley, J. Identification of a functional transposon insertion in the maize domestication gene tb1. Nature Genetics 2011, 43, 1160–1163. [Google Scholar] [CrossRef]
- Wang, H.; Nussbaum-Wagler, T.; Li, B.; Zhao, Q.; Vigouroux, Y.; Faller, M.; Bomblies, K.; Lukens, L.; Doebley, J.F. The origin of the naked grains of maize. Nature 2005, 436, 714–719. [Google Scholar] [CrossRef]
- Dong, Z.; Li, W.; Unger-Wallace, E.; Yang, J.; Vollbrecht, E.; Chuck, G. Ideal crop plant architecture is mediated by tassels replace upper ears1, a BTB/POZ ankyrin repeat gene directly targeted by TEOSINTE BRANCHED1. Proceedings of the National Academy of Sciences 2017, 114, E8656–E8664. [Google Scholar] [CrossRef]
- Lin, Z.; Li, X.; Shannon, L.M.; Yeh, C.-T.; Wang, M.L.; Bai, G.; Peng, Z.; Li, J.; Trick, H.N.; E Clemente, T.; et al. Parallel domestication of the Shattering1 genes in cereals. Nat. Genet. 2012, 44, 720–724. [Google Scholar] [CrossRef] [PubMed]
- Sosso, D.; Luo, D.; Li, Q.-B.; Sasse, J.; Yang, J.; Gendrot, G.; Suzuki, M.; E Koch, K.; McCarty, D.R.; Chourey, P.S.; et al. Seed filling in domesticated maize and rice depends on SWEET-mediated hexose transport. Nat. Genet. 2015, 47, 1489–1493. [Google Scholar] [CrossRef] [PubMed]
- Cobb, J.N.; Juma, R.U.; Biswas, P.S.; Arbelaez, J.D.; Rutkoski, J.; Atlin, G.; Hagen, T.; Quinn, M.; Ng, E.H. Enhancing the rate of genetic gain in public-sector plant breeding programs: Lessons from the breeder’s equation. Theor. Appl. Genet. 2019, 132, 627–645. [Google Scholar] [CrossRef] [PubMed]
- Prasanna, B.M.; Palacios-Rojas, N.; Hossain, F.; Muthusamy, V.; Menkir, A.; Dhliwayo, T.; Ndhlela, T.; Vicente, F.S.; Nair, S.K.; Vivek, B.S.; et al. Molecular Breeding for Nutritionally Enriched Maize: Status and Prospects. Front. Genet. 2020, 10, 1392. [Google Scholar] [CrossRef]
- Hossain, F.; Zunjare, R.U.; Muthusamy, V.; Bhat, J.S.; Mehta, B.K.; Sharma, D.; Talukder, Z.A.; Chhabra, R.; Katral, A.; Dutta, S.; Chand, G. Biofortification of maize for nutritional security. In Biofortification of Staple Crops; Singapore: Singapore, 2022; pp. 147–174. [Google Scholar]
| Nutritional content | Amount | Nutritional content | Amount |
|---|---|---|---|
| Phosphorus | 348 | Calcium | 10 |
| Calories | 342 | Fat | 3.6 |
| Potassium | 286 | Fibre | 2.7 |
| Magnesium | 139 | Iron | 2.3 |
| Sulphur | 114 | Amino Acids | 1.78 |
| Carotene | 90 | Minerals | 1.5 |
| Carbohydrates (g) | 66.2 | Thiamine | 0.42 |
| Sodium | 15.9 | Copper | 0.14 |
| Moisture (g) | 14.9 | Vitamin c | 0.12 |
| Protein | 11.1 | Riboflavin | 0.1 |
| Transgenic lines | Nutrient evaluation | Findings | References |
|---|---|---|---|
| Roundup Ready (RR) contain gene for glyphosate resistance | Crude protein, crude fibre, fatty acid, starch, ash, sugar, fat, amino acids, and macroelement | RR is equivalent in nutrition to isogenic corn | [56] |
| PR33P67 and Pegaso Bt: containing “Cry toxin” gene | fatty acids, vitamin C, antioxidants, total phenols, carotenoids, polyphenols, and mineral composition | Transgenic varieties are nutritionally equivalent to conventional varieties | [57] |
| TC1507: Insect-resistant and herbicide-tolerant | Fatty acid, vitamins, Minerals, amino acids, secondary metabolites, and anti-nutrients | Transgenic variety nutritionally comparable to conventional variety | [58] |
| Genetically modified six-event stack maize: Bt11 x MIR162 x MIR604 x 1507 x 5307 x GA21 | Folic acid, Potassium, zinc, arachidic acid, b-carotene, ferulic acid, and methionine | Nutritionally comparable to its non-GM corn | [59] |
| Nutrient | Method of Application | Rate of Application | % Increase in Grain | Yield increase (%) | References |
|---|---|---|---|---|---|
| Se | Basal | 5-20g ha-1 Na2SeO4 | 25–227 | - | [68] |
| Foliar | 423–819 | - | |||
| Zn | Basal | Elemental Zn (30 kg ha−1) | 15 | 11 | [69] |
| ZnO (8 kg Zn ha−1) | 59 | 44 | |||
| 28 | 11 | ||||
| Foliar | ZnO nanoparticle (2% solution) | 82 | 33 | [70] | |
| ZnO (2% solution) | 38 | 11 |
| Variety | Nutrition improved | Developed by | Year of Release |
|---|---|---|---|
| Vivek QPM 9 | Lys content: 4.19% of protein. Trp content: 0.83% of protein | ICAR-Vivekananda Parvatiya Krishi Anusandhan Sansthan, Almora | 2008 |
| Pusa HM4 Improved | Lys content: 3.62 % of protein. Trp content: 0.91% of protein | ICAR-IARI, New Delhi | 2017 |
| Pusa HM8 | Lys content: 4.18 % of protein. Trp content: 1.06 % of protein | ICAR-IARI, New Delhi | 2017 |
| Pusa HM9 Improved | Lys content: 2.97 % of protein. Trp content: 0.68 % of protein | ICAR-IARI, New Delhi | 2017 |
| Pusa Vivek QPM9 Improved (India’s first PrVit-A rich corn) | PrVit-A: 8.15 ppm. Lys content: 2.67 % of protein. Trp content: 0.74 % of protein | ICAR-IARI, New Delhi | 2017 |
| Pusa VH 27 Improved | PrVit-A: 5.49 ppm | ICAR-IARI, New Delhi | 2020 |
| Pusa HQPM 5 Improved | PrVit-A: 6.77 ppm. Lys content: 4.25 % of protein. Trp content: 0.94 % of protein | ICAR-IARI, New Delhi | 2020 |
| Pusa HQPM 7 Improved | PrVit-A: 7.10 ppm. Lys content: 4.19 % of protein. Trp content: 0.93 % of protein | ICAR-IARI, New Delhi | 2020 |
| IQMH 201 (LQMH 1) | Lys content: 3.03 % of protein. Trp content: 0.73 % of protein | ICAR-Indian Institute of Maize Research, Ludhiana | 2020 |
| IQMH 202 (LQMH 2) | Lys content: 3.04 % of protein. Trp content: 0.66 % of protein | ICAR-Indian Institute of Maize Research, Ludhiana | 2020 |
| IQMH 203 (LQMH 3) | Lys content: 3.48 % of protein. Trp content: 0.77 % of protein | ICAR-Indian Institute of Maize Research, Ludhiana | 2020 |
| Gene | Traits | References |
|---|---|---|
| gt1 | Plant architecture | [121,122] |
| ids1/Ts6 | Kernel row number | [123] |
| ra1 | Inflorescence architecture | [124] |
| tb1 | Plant architecture | [125,108] |
| tga1 | Hardened fruitcase | [126] |
| tru1 | Plant architecture | [127] |
| UB3 | Kernel row number | |
| ZmSh1-5.1+ZmSh1-5.2; ZmSh1-1 | Shattering | [128] |
| ZmSWEET4c | Seed filling | [129] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





