Preprint
Article
Assessing Functional Capacity in ME/CFS: A Patient Informed Questionnaire
Altmetrics
Downloads
4548
Views
6332
Comments
1
supplementary.pdf (1.16MB )
This version is not peer-reviewed
Submitted:
28 September 2023
Posted:
29 September 2023
You are already at the latest version
Alerts
Abstract
Myalgic Encephalomyelitis / Chronic Fatigue Syndrome (ME/CFS) is an acquired disease with significant morbidity that affects both children and adults. Effective tools to assess functional capacity (FC) are severely lacking which has significant consequences for timely diagnosis, assessments for patient disability benefits and assessing the impact and effectiveness of interventions. In interventional research the inability to assess FC can result in an incomplete assessment of the potential effect of the intervention. Specifically of concern is that if an intervention is effective in reducing symptom load, patients may increase their activity level to reach a pre-intervention symptom load. Thus, if FC is not accurately assessed, beneficial treatment outcomes may be missed. To address this issue, using extensive, repeated patient feedback we have developed a new questionnaire, FUNCAP, to achieve optimal FC assessment in ME/CFS patients. The questionnaire covers eight domains and activity types: A. Personal hygiene / basic functions, B. Walking / movement, C. Being upright, D. Activities in the home, E. Communication, F. Activities outside the home, G. Reactions to light and sound, and H. Concentration. Through five rounds of anonymous web-based surveys and a further test - retest validation round, two versions of the questionnaire were developed; a longer version comprising 55 questions (FUNCAP55) to improve diagnostic and disability benefit/ insurance FC assessments and a shorter version (FUNCAP27) for interventional research and less extensive FC assessments. FUNCAP may also be useful in other conditions where fatigue and PEM is present, such as Long Covid.
Keywords:
Subject: Medicine and Pharmacology - Neuroscience and Neurology
1. Introduction
Myalgic Encephalomyelitis / Chronic Fatigue Syndrome (ME/CFS) is an acquired disease affecting children and adults with a probable prevalence in the U.S., when post exertional malaise (PEM) is present, of approximately 0.8% [1]. Central features are typically sudden onset of severe reduction of pre-illness functional capacity (FC) for all activities, extensive fatigue, PEM, cognitive symptoms such as “brainfog”, non-restorative sleep, pain, and sensory hypersensitivity [2]. After extensive reviews by committees in the U.S and U.K it was concluded that ME/CFS is a serious, systemic disease with multiple symptoms and no known cause or cure. There is an urgent need for research to further understand the disease (REF: National Academy of Medicine- IOM, NICE).
The Canadian Consensus Criteria (CCC) [3] and Fukuda [4] diagnostic criteria for adults and the Jason criteria [5] for children have been recommended for diagnosing ME/CFS in the Norwegian National Guidelines for CFS/ME since 2014. The presence of PEM is mandatory in these except for the Fukuda criteria, where it is an optional symptom [4]. The Norwegian National Guidelines state PEM is a cardinal symptom. We therefore think that PEM is most likely present in most Norwegian patients diagnosed with ME/CFS by specialists. Although symptoms of ME/CFS have been extensively described and reviewed [6,7] the lack of effective tools to assess FC has significant consequences. First, a substantial reduction in FC to less than 50% after ME/CFS onset is required for ME/CFS diagnosis in the International Consensus Criteria (ICC) [8]. This magnitude of FC reduction is also recommended in Norway regardless of which diagnostic criteria are used. The lack of precise tools targeting FC therefore hampers ME/CFS diagnostics. Second, broadly assessing FC reduction in ME/CFS is paramount when health authorities assess the right to, and need for, disability benefits and in health insurance evaluations [9]. Third, in ME/CFS interventional research accurate assessments of FC changes after an intervention is essential. In this setting, assessing severity of symptoms such as fatigue, pain, nausea, and sleep disturbance, whilst still important, is inadequate. If an intervention has a positive effect and reduces symptoms, patients may, consciously or unconsciously, increase their total level of activity and reach a pre-intervention symptom burden, but with increased FC as a benefit. If researchers then only assess symptom severity they may miss a positive effect of the intervention. Additionally, when evaluating potential biomarkers for ME/CFS it is important to assess whether the candidate biomarker correlates with ME/CFS disease severity, highlighting the importance of FC assessment.
The aim of the present study was to develop a new questionnaire to assess FC accurately and precisely in ME/CFS patients using extensive ME/CFS patient feedback for diagnostic and disability/ insurance FC assessments, and a shorter version for research and less extensive FC assessments.
2. Materials and Methods
The study surveys were anonymous, internet based and limited to one response per IP address. In all, six survey rounds were undertaken with the initial three Norwegian rounds targeting the development of survey content. The fourth was an English language, international round. The fifth was the main Norwegian survey, and the sixth a Norwegian retest round. Although there were time restrictions for each survey, they remained open to submissions throughout the allocated time periods. Carers could answer on behalf of patients who were too ill to answer themselves.
Invitations were issued through social media accounts belonging to the Norwegian ME Association (NMEF). NMEF offers a range of moderated support groups on Facebook, both for patients and carers, with approximately 11000 participants, with some overlap between groups. Roughly half of these participants are non-members of NMEF. NMEF also has followers on Facebook, Twitter (X) and Instagram, supporting a reach far beyond NMEF membership.
The Norwegian National Guidelines for CFS/ME of 2014 are generally adhered to when diagnosing ME/CFS in Norway. The CCC [3] are usually employed for adults, with Fukuda criteria [4] being used for some cases. Only the CCC specifically requires PEM to be present. However, the Norwegian National Guidelines for CFS/ME states PEM is an essential symptom making its presence highly likely in the study’s Norwegian participants. For individuals less than 18 years of age the Jason criteria [5], which also requires PEM, are recommended in these guidelines. We based the classification of disease severity on the classification in Norwegian National Guidelines for CFS/ME which is based on the ICC Criteria´s definitions of severity [8].
Individual items of the questionnaire were separated into eight domains according to activity types: A. Personal hygiene / basic functions, B. Walking / movement, C. Being upright, D. Activities in the home, E. Communication, F. Activities outside the home, G. Reactions to light and sound, and H. Concentration.
The SurveyMonkey platform was used in all survey rounds. Only one response per IP address was allowed, except when we additionally asked for the respondent to, if possible, recruit a healthy control, such as a family member, carer, or friend to answer the questionnaire. Open-ended criticisms/ responses/ ideas were actively encouraged in the first three rounds.
SPSS version 27 was used for all statistical analyses. 2- tailed p-values were adhered to in all analyses.
3. Results
3.1. Initial survey rounds developing the FUNCAP questionnaire
Round 1. Undertaken 11th to 15th March 2022 yielded 290 respondents. The initial set of items (Round 1) were chosen by KS and TS based on their extensive clinical experience as a doctor with extensive ME/CFS clinical experience, and patient support experience, respectively. Items asked respondents to record the frequency of undertaking specific activities during the previous month in domains similar to those in the final FUNCAP questionnaire. In addition, background data, such as age, year of disease onset and sex were included.
Major respondent feedback in Round 1 was that items regarding frequency of undertaking various activities did not address the consequences and impact of doing them. Furthermore, capability regarding activity frequency did not consider other activities the respondent undertook. Also, respondents described having chosen not to undertake certain activities because doing so, repeatedly, resulted in negative consequences including extended decreased capacity for all activities and increased symptoms (PEM). Many noted that frequency of undertaking specific activities depended on choice, preferences, and access to support from others. Instead, respondents suggested we ask questions about the consequences of undertaking a given activity. Also, more precise descriptions of items were requested, including replacing terms such as “a short” or “long walk” with “Walk 100m to 1 km”. Other comments noted that too few of the items were appropriate for the most and least severely affected, too few adequately covered cognitive problems, and sensitivity to light and sound was inadequately addressed. Respondents requested considering the effect of undertaking an activity on the capacity to undertake other activities.
Round 2. Undertaken 22nd April – 19th May 2022 yielded 435 respondents. Revisions were made to the Round 2 questionnaire based on Round 1 respondents´ input. A major change was asking about the consequences of undertaking the various activities as opposed to the frequency. An ordinal six-point scale describing consequences was introduced including targeting consequences of undertaking an activity on the capacity to carry out other activities, and the duration of such consequences. We also specified that responses be given for an average day in the previous month. Inadequate items targeting sensory exposure (light and sound) were corrected. Specifying time criteria to perform a specific item was included where appropriate.
Major respondent feedback in Round 2 stated that there were activities they were unable to perform with inadequate items regarding cognitive/ social endurance, and items regarding the least affected.
Round 3. Undertaken 30th May – 21st June 2022. Changes were made according to Round 2 feedback as deemed appropriate including adding an option stating that the respondent was unable to undertake an activity at all, increasing the response scale to seven points. Items better assessing cognitive/ social endurance capacity and the least affected were also added in addition to further changes in wording. Respondents were asked to, if possible, invite responses from healthy family members, or other relatives or friends (healthy controls, HC). This round yielded 636 ME/CFS, and 366 HC respondents. Some non-substantial suggestions for changes were made in this round. Most ME/CFS respondents (75%) reported that they felt the questionnaire was highly suitable for describing their FC.
The FUNCAP questionnaire version used in Round 3 was used extensively in clinical practice by KS and by several other doctors with extensive knowledge and experience in diagnosing and managing ME/CFS patients of all ages and severities of disease. Feedback from these clinicians resulted in minor adjustments to the wording of the questionnaire. Feedback was unanimously positive from patients and clinicians regarding the usefulness of the questionnaire in assessing FC in ME/CFS patients. It was most useful and effective when given/ e-mailed to a patient several days prior to a consultation. The final FUNCAP questionnaire consisted of 55 items spanning eight domains and is herein referred to as FUNCAP55.
3.2. Round 4 and main Round 5 using finalized FUNCAP55 questionnaire
Round 4. English version. Undertaken 9th Sep – 30th Nov 2022. The FUNCAP55 questionnaire from Round 3 was revised with minor changes and translated into English and back translated to Norwegian by an experienced psychologist with extensive knowledge of questionnaire development and extensive knowledge of ME/CFS. Only minor adjustments in wording of items were made to FUNCAP55 after Round 3.
There were 2,186 initial respondents of whom 1,999 had been diagnosed with ME/CFS. 1,413 of these were below 60 years of age and included in the present study. Respondents’ characteristics are presented in the right column in Table 1. There were 341 (24%) from the UK, 294 (21%) from USA, 140 (10%) from Australia, 111 (8%) from Canada, 73 (5%) from Northern Ireland, 68 from Norway (5%), 67 (5%) from Sweden, and 60 (4%) from Germany. Of the remaining respondents all, except 52, were from Europe. Thirty-seven had mild ME/CFS, 1,030 moderate, 301 severe, and 45 very severe.
Round 5. The results from the Norwegian Round 5 survey yielded the main data used in the presented study. It included ME/CFS and HC respondents. It was open from 6th March 2023 to 6th April 2023. Round 5 FUNCAP55 questionnaire was very similar to Round 4, with two items (visiting, and receiving a visit from a friend) substituted by the item “Participating in a conversation with three people for approximately ½ hour”. There were initially 1,463 ME/CFS and 223 HC respondents. Only those less than 60 years of age were included in the data analyses in the present paper to reduce the potential confounder of increased frequency of unrelated fatiguing conditions with increasing age. This left 1,263 ME/CFS respondents in this study. Of 223 HC respondents, 188 were less than 60 years of age. Among these, 10 HC respondents were excluded as they were outliers based on their scores on various items indicating they were incompatible with being HC leaving 178 HC included respondents.
Among 1,263 ME/CFS respondents, 878 (69%) were diagnosed in a hospital by a consultant and 313 (25%) by a general practitioner. For 72 (6%) the questionnaire was completed by another person due to the patients being too ill to complete it themselves. In this last group we did not ask whether a consultant or general practitioner had diagnosed ME/CFS. The question regarding ME/CFS severity was structured according to ICC 2011: Very severe: Totally bedridden and in need of help with basic functions. Severe: Mostly bedridden. Moderate: Mostly housebound. Mild: At least a 50% reduction in activity level compared to before disease onset. Better than mild: Symptomatic, but less than 50% reduction in activity level. Among ME/CFS respondents 19 (2%) were very severe, 136 (11%) severe, 733 (58%) moderate, 360 (29%) mild, and 15 (1%) better than mild. Background data are described in Table 1.
The individual questionnaire items were scored: 0: I cannot do this. 1: My capacity will be severely reduced for at least three days. 3: I can do little else on the same day. 4: I must limit other activities on the same day. 5: This rarely affects other activities. 6: Unproblematic- does not affect other activities.
3.3. Validating the FUNCAP55 questionnaire
For Round 5 ME/CFS respondents (HC excluded) we evaluated the relationships between all items in the questionnaire and how they correlated and the total variance. For this purpose, we used principal components analyses (PCA) with Varimax rotation and Kaiser normalization of the final set of 55 items in the A to H sub-scores in the questionnaire. Initially, default Eigen values larger than one were used as criteria for component identification resulting in five components with initial Eigen values of 29.2, 3.8, 2.4, 1.3 and 1.1, respectively. The last two components were not meaningful based on the high component loadings as opposed to the first three (see interpretation below). Based on this, a forced three component model was chosen. The three resulting components yielded interpretable component loadings. Component 1 was associated with items demanding little FC in a normal population, pointing to their presumed importance for FC assessment in the most severely affected ME/CFS patients. Component 2 was associated with items demanding much more FC pointing to importance for the less severe ME/CFS severity degrees. Component 3 was associated with items regarding communication and concentration. We chose an arbitrary high cutoff component loading greater than or equal to 0.6 for each item for evaluating interpretability of the individual items. These interpretations were supported by high (>0.6) component loadings of different sets of the individual 55 items on these three components. Component one, two and three explained 25, 24 and 16%, respectively, of the cumulative 64% variance explained by the three-component model. Component 1 had high component loadings on the following FUNCAP55 item numbers (FUNCAP55 full questionnaire, Supplemental Material: Table S1): 1 to 9, 15, 16, 18, 23, 24, 33 and 37. These items assess activities requiring very little FC in a healthy population. Component 2 had high component loadings on FUNCAP55 item numbers 11 to 14, 19 to 21, 25, 31, 32, 34 to 36, 38, 39, 44, 45, 54, and 55. These items assess activities requiring substantially more FC in a healthy population compared to those regarding Component 1. Component 3 had high component loadings on FUNCAP55 item numbers 26, 27, 29, 46, 48 to 51 and 53 which assess communication and concentration. There was no overlap between items with high loadings (>0.6) on the three components.
An identical, exploratory PCA, as used for Round 5 ME/CFS respondents, was repeated with the English Round 4 FUNCAP55 ME/CFS data using the same strategies described above. Using Eigen values larger than one identified six components with only a three-component solution being interpretable. Component interpretation was the same as in the corresponding Norwegian component analysis. A similar component analysis on FUNCAP27 Round 4 data resulted in a three-component solution with default Eigen value above one for component identification using component interpretation as described above.
3.4. Creating a shortened version of FUNCAP55
Corresponding to our stated aim we created a shorter version of FUNCAP55 by identifying items to retain or remove. Using the Round 5 data we first calculated mean sub-scores for each of the eight domains A to H as the average of all individual item scores (0 to 6) within each domain. We then calculated the correlations between each individual item score (0 to 6) within a domain and the corresponding mean sub-score for that domain. The Pearson correlations were within the ranges: A: 0.7 to 0.91. B: 0.68 to 0.93. C: 0.78 to 0.91. D: 0.82 to 0.90. E: 0.82 to 0.90. F: 0.70 to 0.90. G: 0.68 to 0.85. H: 0.56 to 0.81. These high correlations supported redundancy in the number of items required for objective FC assessment. We retained those items for the short FUNCAP version deemed most likely to identify variation in FC across the entire range of ME/CFS disease severity, from very severe to mild. Based on this strategy, 27 items were retained across the eight A to H domains resulting in the FUNCAP27 version (Table 2). The Pearson correlation coefficients between the FUNCAP55 vs FUNCAP27 A to H sub-scores, including the Total Scores (mean of A to H sub-scores), for the ME/CFS respondents were A: 0.96. B: 0.98. C: 0.98. D: 0.93. E: 0.97. F: 0.98. G: 0.96. H: 0.96. Total Score: 0.99. All p values were < 0.009 (with Bonferroni correction).
An identical, exploratory PCA, as described above, was repeated with the set of 27 items in the A to H domains in the Round 5 ME/CFS group. Default Eigen values larger than one was used as the initial criteria for component identification, resulting in three components. The corresponding Eigen values of these were 15.2, 2.1 and 1.4. Component one, two and three explained 26, 26 and 14%, respectively, of the cumulative 65% variance explained by the model. We used the same high score cutoff component loading of greater than or equal to 0.6 for each item for evaluating interpretability of individual items. Component 1 had high component loadings on FUNCAP27 items (retained numberings from FUNCAP55 with corresponding FUNCAP27 numberings in parenthesis): 1 (1), 5 (2), 7 (3), 8 (4), 15 (7), 18 (9), 33 (15), and 41 (19). Component 2 had high component loadings on the following FUNCAP27 items numbers: 10 (21), 13 (6), 21 (10), 25 (11), 32 (14), 35 (16), 38 (17), 39 (18), 44 (21) and 55 (27). Component 3 had high component loadings on the FUNCAP27 items 46 (22), 48 (23), 51 (24) and 53 (25). The two item numbers in bold typeface were the only items with high component loadings in FUNCAP27 that did not have high component loadings on the same components in FUNCAP55. Item number 54 (26) although present in both questionnaires had high component loading on FUNCAP55 but not on FUNCAP27 (the last loading being 0.58). Using the same strategy as described for the FUNCAP55 model, the three components identified in FUNCAP27 yielded the same three interpretations as for FUNCAP55.
Pearson correlations between Round 4 FUNCAP55 and FUNCAP27 sub-scores, including the Total Score, for ME/CFS respondents were very high: A: 0.97. B: 0.97. C: 0.98. D: 0.92. E: 0.97. F: 0.98. G: 0.97. H: 0.97. Total Score: 0.99. All were statistically significant with 2-tailed p-values < 0.006 (with Bonferroni correction). Correlations among FUNCAP 27 sub-scores were very similar to those in Round 5.
To assess internal consistency (reliability) of the FUNCAP55 and FUNCAP27 questionnaire A to H domain sub-scores and Total Scores, Cronbach´s alpha was assessed. For FUNCAP55 / FUNCAP27 this was: A: 0.92 / 0.77; B: 0.93 / 0.78; C: 0.91 / 0.83; D: 0.93 / 0.84; E: 0.91 / 0.80; F: 0.93 / 0.85; G: 0.87 / 0.78; H: 0.89 / 0.84; Total Score: 0.96 / 0.95. This corresponds to good (0.8 to < 0.9) or excellent (> = 0.9) for all FUNCAP55 scores and for all but three FUNCAP27 scores which were acceptable (0.7 – 0.79).
3.5. Round 5, FUNCAP results: ME/CFS vs HC
ME/CFS respondents had significantly lower mean scores (0 to 6) on all individual item scores compared to HC in both FUNCAP55 (Supplementary material: Table S1) and FUNCAP27 (Table 2). The same was true for all mean A to H sub-scores and Total Score for FUNCAP55 (Supplementary material: Table S3) and FUNCAP27 (Table 4, Figure 1).
The difference in mean scores (0 to 6) between ME/CFS and HC respondents ranged from 0.6 vs 5.5 for item H27 “Managing a full working day (non-physical work such as office work, classes or lectures)” to 5.4 vs 6.0 for item H22 “Reading a short text, such as a mobile phone text message” (Table 2).
There were high correlations between individual A to H sub-scores for the FUNCAP27 for ME/CFS respondents. The highest (0.82) being between sub-score B: “Walking/ moving around” and F: “Activities outside your home” with the lowest (0.61) being between sub-score A: “Personal hygiene / basic functions” and H: “Concentration” (Table 3). Correlations were much lower for the HC respondents (Table 3). The corresponding correlations for FUNCAP55 were also high (Supplementary material: Table S2)
Table 3.
Round 5. Correlations among the eight A to H FUNCAP27 sub-scores (in addition to Total Score, i.e., the mean of the eight A to H sub-scores) for the ME/CFS respondents (n=1263) and HC (healthy controls, n = 178). All correlations were statistically significant (p < 0.006 with Bonferroni correction) except the correlation between sub-scores A and C among HC respondents. Column letter titles (A to H) correspond to identical domain names in row titles.
Table 3.
Round 5. Correlations among the eight A to H FUNCAP27 sub-scores (in addition to Total Score, i.e., the mean of the eight A to H sub-scores) for the ME/CFS respondents (n=1263) and HC (healthy controls, n = 178). All correlations were statistically significant (p < 0.006 with Bonferroni correction) except the correlation between sub-scores A and C among HC respondents. Column letter titles (A to H) correspond to identical domain names in row titles.
| Domains, ME/CFS respondents | A | B | C | D | E | F | G | H | TS |
| A. Personal hygiene / basic functions | 1 | 0.77 | 0.79 | 0.70 | 0.69 | 0.74 | 0.66 | 0.61 | 0.86 |
| B. Walking / moving around | 1 | 0.76 | 0.77 | 0.71 | 0.82 | 0.66 | 0.65 | 0.88 | |
| C. Being upright | 1 | 0.74 | 0.75 | 0.76 | 0.71 | 0.66 | 0.89 | ||
| D. Activities in home | 1 | 0.73 | 0.81 | 0.66 | 0.68 | 0.88 | |||
| E. Communication | 1 | 0.78 | 0.73 | 0.77 | 0.88 | ||||
| F. Activities outside your home | 1 | 0.73 | 0.71 | 0.92 | |||||
| G. Reactions to light and sound | 1 | 0.67 | 0.84 | ||||||
| H. Concentration | 1 | 0.81 | |||||||
| TS. Total Score (mean of A-H sub-scores) | 1 | ||||||||
| Domains, HC Respondents | A | B | C | D | E | F | G | H | TS |
| A. Personal hygiene / basic functions | 1 | 0.38 | 0.19 | 0.40 | 0.30 | 0.35 | 0.20 | 0.29 | 0.43 |
| B. Walking / moving around | 1 | 0.24 | 0.61 | 0.51 | 0.55 | 0.33 | 0.49 | 0.69 | |
| C. Being upright | 1 | 0.40 | 0.35 | 0.28 | 0.31 | 0.37 | 0.48 | ||
| D. Activities in home | 1 | 0.75 | 0.74 | 0.52 | 0.66 | 0.89 | |||
| E. Communication | 1 | 0.68 | 0.65 | 0.73 | 0.87 | ||||
| F. Activities outside your home | 1 | 0.53 | 0.66 | 0.83 | |||||
| G. Reactions to light and sound | 1 | 0.69 | 0.74 | ||||||
| H. Concentration | 1 | 0.85 | |||||||
| TS. Total Score (mean of A-H sub-scores) | 1 |
All Pearson Correlation p-values were < 0.009 (with Bonferroni correction).
Table 4.
Round 5. FUNCAP27: Mean sub-scores (SD) for the eight A to H domains and Total Score (mean of A to H sub-scores) for the ME/CFS vs HC respondents. All mean differences were statistically significant with t-test p-values < 0.009 with Bonferroni correction.
Table 4.
Round 5. FUNCAP27: Mean sub-scores (SD) for the eight A to H domains and Total Score (mean of A to H sub-scores) for the ME/CFS vs HC respondents. All mean differences were statistically significant with t-test p-values < 0.009 with Bonferroni correction.
| ME/CFS (n = 1263) |
HC (n = 178) |
Mean difference 95% CI |
|
|---|---|---|---|
| A Personal hygiene / Basic functions | 4.5 (1.1) | 6.0 (0.1) | -1.5 (-1.6 to -1.3) |
| B Walking / Moving around | 3.1 (1.2) | 5.9 (0.3) | -2.8 (-2.9 to -2.6) |
| C Being upright | 4.1 (1.4) | 5.9 (0.2) | -1.8 (-2.0 to -1.6) |
| D Activities in the home | 2.2 (1.4) | 5.7 (0.5) | -3.5 (-3.7 to -3.3) |
| E Communication | 3.3 (1.0) | 5.8 (0.4) | -2.5 (-2.6 to -2.3) |
| F Activities outside your home | 2.7 (1.3) | 5.8 (0.4) | -3.1 (-3.3 to -2.9) |
| G Reactions to light and sound | 3.4 (1.3) | 5.8 (0.4) | -2.3 (-2.5 to -2.1) |
| H Concentration | 3.2 (1.0) | 5.8 (0.4) | -2.5 (-2.7 to -2.4) |
| Total score | 3.3 (1.1) | 5.8 (0.3) | -2.5 (-2.7 to -2.3) |
The sub-scores for the eight A to H domains for the ME/CFS respondents were close to normally distributed over the range of possible scores (0 to 6) except for domain D (Activities in the home) which had a high number of respondents with a score of 0 (Supplementary material: Figure S2). This was explained by 320 (23%) ME/CFS respondents being unable to undertake the task in item D10: “Heavier housework (washing floors, vacuuming etc.) for approx. ½ hour continuously” and 227 (18%) ME/CFS respondents being unable to undertake item D11 “Cooking a complicated meal from scratch, approx. 1 hour of preparation”.
Next, we sought to further validate FUNCAP27 by determining how accurately FUNCAP27 could distinguish between the different groups of ME/CFS severity. Severity degree was based on patient reported disease severity degree. The mean sub-scores for the eight A to H domains and Total Score were compared between very severe, severe, moderate, mild, and better than mild ME/CFS subgroups and HCs. The mean A to H sub-scores and Total Score were lowest for the most severely ill ME/CFS patient group, rising consecutively with less severe degrees of disease for all scales to the highest mean sub-scores in the HC group (Figure 1). We ran nine separate ANOVA analyses with Tukey Post Hoc tests with the dependent variables being the A to H sub-scores and Total Score and the independent variables being the ME/CFS and HC severity groups. All ANOVA analyses were statistically significant with p-values < 0.009 with Bonferroni correction. Post Hoc analyses were statistically significant corresponding to the decreasing degree of severity except that they were not statistically significant between the better than mild vs mild and between the HC and mild and/ or better than mild groups for most analyses. Almost identical results were found for corresponding FUNCAP55 analyses. In summary, this supported that FUNCAP27 and FUNCAP55 sub- scores, and Total Score reflected ME/CFS severity degree.
3.3. Round 6: FUNCAP27 test – retest reliability
Round 6: A new round was undertaken to assess test-retest reliability of FUNCAP27. Since FUNCAP27 may be relevant in research and in many clinical settings, the shorter version was chosen to assess test–retest reliability. It was undertaken from 31st July until 16th August 2023. ME/CFS and HC respondents were asked to complete FUNCAP27 twice, two weeks apart. Respondents were asked to write a code of their own choice in the test round and re-enter the same code in the retest round two weeks later to enable pairing of the responses. Only respondents younger than 60 years of age were included leaving 301 with a diagnosis of ME/CFS (234 from a hospital consultant and 67 from a general practitioner) and 32 HC. Of these, five ME/CFS respondents had very severe ME/CFS, 43 severe, 167 moderate, 80 mild and one better than mild. Mean age was 43 (SD 0.6) for the ME/CFS and 45 (SD 1.8) for HC. Among the ME/CFS respondents the test – retest Pearson correlations for the various sub-scores were A: 0.93, B: 0.85, C: 0.90, D: 0.89, E: 0.84, F: 0.94, G: 0.89, H: 85 and 0.96 for the Total Score with all p-values < 0.009 with Bonferroni correction).
The survey asked ME/CFS respondents for changes in their health between the test and the retest timepoint on a scale of 1: I am much worse, 2: I am a little worse, 3: No change, 4: I am a little better, 5: I am much better. This scale was positively correlated with a variable calculated as Total Score at Retest minus Total score at Test time points (Pearson Correlation 0.23, p < 0.001). The ME/CFS respondents were asked how well they felt the questionnaire assessed their functional capacity in various domains on a scale 1: Very bad, 2: Bad, 3: Good, 4: Very good. The domains were Sensitivity to light and sound, with the distribution among the responses being 1: 1%, 2: 9%, 3: 69%, 4: 22%; Being upright: 1: 0.3%, 2: 11%, 3: 63%, 4: 26%, Physical function: 1: 1%, 2: 14%, 3: 64%, 4: 22% and Cognitive function: 1: 1%, 2: 10%, 3: 63%, 4: 26%. They were further asked, on a scale from 0-100, where 0 was Very bad and 100 was Very good, how they experienced answering the survey. The ME/CFS respondents score (n= 298) had a mean value of 78 (SD 18).
Sex, age and ME/CFS disease severity distributions were similar in all rounds.
4. Discussion
The present study demonstrates the development of a patient informed, novel questionnaire, FUNCAP, for assessment of functional capacity (FC) in ME/CFS patients. Its development was based on repeated rounds of anonymous web-based surveys including large numbers of ME/CFS respondents and HC, providing repeated feedback for successively optimizing questionnaire content. The number of initial items was reliably halved from 55 to 27 for a substantially shortened questionnaire version. Mean scores for all individual items and eight sub-scores were significantly lower among ME/CFS respondents compared to HC. Mean sub-scores were consistently lower with increasing disease severity. Internal consistency was high and test-retest evaluation supported reliable questionnaire responses. A large international English language survey (Round 4) of FUNCAP55 showed similar background data regarding age, sex distribution, age at disease onset and disease duration, compared to the main Norwegian Round 5 findings with mean domain sub-scores and Total Score being very similar between the two.
Extensive and successive feedback from ME/CFS respondents in refining questionnaire content was central in supporting content validity. Examples include responses relevant to assessing variability in FC across the ME/CFS disease spectrum, from targeting basic personal hygiene to managing a full working day. High internal consistency, as shown by high Cronbach alpha levels, supports reliability of the questionnaire content. The very high Cronbach alpha level for FUNCAP55, although reassuring, indicated redundancy in questionnaire items, supporting the strategy to develop a version with reduced number of items - FUNCAP27. It was not possible to assess concurrent validity since there are no external “gold standard” methods to assess FC in ME/CFS patients. Given the generally high correlations between the eight sub-scores it is not surprising that the PCA did not identify the eight domains as separate components (except for the Concentration subscale). This is consistent with ME/CFS affecting all FC domains assessed by FUNCAP and is inherent in many of the disease criteria for ME/CFS [6]. This was, as expected, entirely different and much more individually variable among the HC respondents with much lower correlations reflecting that the healthy controls had variable FC across domains. High test–retest reliability was supported by the high correlations between repeated responses to FUNCAP27 taken two weeks apart.
An important strength of the present study is that it was undertaken in a country, Norway, with a well-developed, universal healthcare system with national guidelines for the diagnosis and management of ME/CFS. This makes an accurate diagnosis of ME/CFS more likely, although it is still probably underdiagnosed in Norway. The main strength of the development of FUNCAP is its reliance on input, in the form of open-ended responses and critique, from ME/CFS patients themselves who have the most relevant information for the questionnaire. Using anonymous, web-based surveys facilitated broad participation since this required minimal respondent activity and maximum flexibility in completing the questionnaire. This was particularly important for the most severely affected patients. We also believe respondents were motivated to participate in consecutive survey rounds as they could directly observe how their feedback resulted in the changes made to the questionnaire. The existence of a large, and well- organized ME/CFS patient organization and extensive pre-existing social media groups facilitated study recruitment. The large number of participants and inclusion of HC further supports the generation of reliable results. The PCA on Round 5 data supports FUNCAP sensitivity to FC both among the most, and the least severely affected ME/CFS patients.
The low number of components, their high component loadings and the high correlations between the eight sub-scores in the separate correlation analyses supported the basis for item reduction. Two opposite concerns governed the final choice of the extent of item retention in the shortened FUNCAP version of 27 vs 55 items: As few items as possible while retaining accurate FC estimation. In a research situation assessing potential improvement in FC after an intervention, these two concerns are adequate. However, if the goal is an assessment and documentation of the range of general human activities attainable given a certain FC, a much broader range of items are mandatory. A good example is evaluation of capacity for holding a job vs need of disability benefits. We believe that FUNCAP27 is most appropriate for the first, and FUNCAP55 the latter scenario. In other situations, the choice may vary. When evaluating whether the criteria for ME/CFS diagnosis is fulfilled, FUNCAP55 is probably most appropriate, whereas FUNCAP27 may be preferable in follow up of ME/CFS patients.
The similarity in mean sub-scores and standard deviations in the Norwegian Round 5 survey compared to the same English, international Round 4 survey is striking and supports the generalizability of our findings. This is further supported by very similar findings on the PCA in Round 4 compared to Round 5 data.
A limitation of the study is that we could not independently confirm that respondents had ME/CFS, relying instead on self-reporting as patient medical records were not available. We do, however, find it unlikely that people would use time and energy to participate as ME/CFS respondents if they had not received a ME/CFS diagnosis. Of note, there were relevant response options in Round 3 for being under evaluation for possible ME/CFS or believing that they had ME/CFS without being under evaluation for this- to exclude respondents from the ME/CFS group. It was not the aim of the present study to undertake a large population-based study and we do not know therefore how generalizable our findings are to the entire population of ME/CFS patients in Norway and elsewhere. This, however, is not a major limitation as our aim was to develop a questionnaire and not, for example, test interventions or investigate epidemiological data such as disease prevalence.
The severity classification of respondents was not verifiable and could be inaccurate. It was probably based on the respondent’s own assessment as, in our clinical experience, severity degree is often lacking or inaccurate when ME/CFS diagnoses are given. The small proportion of our respondents with very severe ME/CFS is similar to that found in another recent survey using the same definitions of degree of severity with 10% having very severe disease compared to 14% in our study [10]. The few classified as better than mild in the present study are underrepresented since many people with this severity degree would probably not have responded to our survey. Most of these may previously have satisfied ME/CFS criteria but improved over time.
PEM is a cardinal symptom of ME/CFS. It has recently been extensively discussed and targeted with the DePaul Post-Exertional Malaise Questionnaire (DPEMQ) developed with feedback from ME/CFS patients (Jason 2021). Of special interest for the present study, Jason states that “There needs to be items on questionnaires that assess items such as what would happen if a patient were to engage in exertion producing activities, as well as if they are pacing to reduce symptom exacerbation” [11]. This echoes feedback from respondents in Round 1 of the present study.
The Total Score in the present study may be imprecise as a reliable indicator of “total FC” in ME/CFS patients. Some FUNCAP subscales may be more important than others in this regard. Also, the concept of “total FC” is dependent on personal and societal settings of individuals and what aspects of FC are most important to them. For example, patients with relatively extensive orthostatic intolerance, but relatively high FC regarding concentration/ cognitive could conceivably fill a position where it is possible to work from home, or work lying down, e.g., an IT job. Further research regarding such aspects is warranted. On the other hand, if FUNCAP is used in research settings to assess the effect of an intervention in ME/CFS patients on FC, the Total Score could be an important outcome measure, reducing the number of statistical analyses and spurious findings, thereby increasing interpretability of results.
No objective or validated method exists to accurately assess FC in ME/CFS patients. There is support for cardiopulmonary testing, use of an activity monitor, and the SF36 Physical Functioning subscale to corroborate the ICC classification of ME/CFS severity [12]. However, this does not imply that these tools accurately assess FC in ME/CFS patients as the four-level ICC severity classification is a course FC scale. The FC assessment accuracy sought in the present study is much higher. In a systematic review of questionnaires used to assess activity limitations and participation restrictions in individuals with CFS, SF36 was described as the most frequently used tool [13]. PEM was not a prerequisite in many of the included studies in this systematic review. SF36 scales were found to have unknown internal consistency, content validity, and interpretability when used in this setting. Still, the SF36 subscales, especially the Role Physical and Social Function subscales, but also Physical Functioning indicated distinct disability in ME/CFS patients compared to patients with multiple sclerosis and HC [14]. Not incorporating consequences of PEM and general lack of items central to FC in ME/CFS, such as being upright, being exposed to light and sound, and cognitive activities are major shortcomings of SF36. Also, SF36 does not include the necessity of choosing between activities emphasized as being central by respondents in the present study. The widespread use of SF36 in many other diseases and several previous ME/CFS studies, in our opinion, does not overcome these disadvantages. It is important to note that SF36 is a questionnaire targeting patient quality of life, not FC specifically. The aforementioned review [13] found the CFS-Activities and Participation Questionnaire (CFS-APQ) [15] was the best available tool as of 2015 for FC assessment in CFS and ME/CFS patients [13]. Its content and construct validity were judged to be moderate, based on study populations using the Fukuda CFS inclusion criteria [4]. This is problematic since these criteria do not require PEM to be present. Furthermore, consequences of PEM are not adequately incorporated into the items of the questionnaire. We maintain that the CFS-APQ target many important restrictive activities for ME/CFS patients, but to a much lesser extent than FUNCAP.
FUNCAP does not attempt to assess the full range of ME/CFS symptoms per se, which include fatigue, pain, nausea, and unrefreshing sleep. Assessment of such symptoms would be important additions to FC assessment, for example in research targeting effectiveness of interventions or treatments. The same is true if FUNCAP is used in clinical settings or when assessing a patient with ME/CFS for disability benefits or insurance evaluations. Additionally, FUNCAP may not only be useful in FC assessment in ME/CFS, but also in other conditions with fatigue and PEM such as Long Covid [16].
5. Conclusions
Our findings support the utility of FUNCAP55 as an effective tool for assessing FC in ME/CFS patients. It may prove valuable in clinical diagnostic or follow-up consultations and for assessing liability for disability benefits. The shorter version, FUNCAP27, may prove valuable in research on effectivity of treatments and interventions in ME/CFS patients. FUNCAP may prove important in exposing the often hidden, but drastically reduced FC in ME/CFS and emphasize the need for more research funding and general societal recognition of the severe nature of ME/CFS.
Supplementary Materials
See separate file.
Author Contributions
Conceptualization, K.S., T.S.; Data Curation, K.S., T.S.; Formal Analysis, K.S., T.S.; Investigation, K.S., T.S.; Methodology, K.S., T.S.; Software, T.S. and K.S.; Visualization, T.S., K.S.; Writing—Original Draft, K.S., T.S.; Writing—Review and Editing, K.S., T.S., K.A.S and S.R.C. All authors have read and agreed to the published version of the manuscript.
Funding
KAS and SRC’s ME/CFS research is supported by the UK charity Invest in ME Research.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki. After consultation with the Regional Committees for Medical and Health Research Ethics, ethical review and approval were waived for this study due to it being an anonymous, online survey.
Informed Consent Statement
Patient consent was waived due to the survey being an anonymous, online survey.
Data Availability Statement
Selected data presented in this study are available on reasonable request from the corresponding author.
Acknowledgments
Our main gratitude goes to the ME/CFS patients and their carers for providing extensive and time-consuming responses, forming the basis of this paper. We also thank Anne Bolstad for translation and back-translation to / from English of the questionnaire.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Valdez, A.R.; Hancock, E.E.; Adebayo, S.; Kiernicki, D.J.; Proskauer, D.; Attewell, J.R.; Bateman, L.; DeMaria, A., Jr.; Lapp, C.W.; Rowe, P.C.; et al. Estimating Prevalence, Demographics, and Costs of ME/CFS Using Large Scale Medical Claims Data and Machine Learning. Front Pediatr 2018, 6, 412. [Google Scholar] [CrossRef] [PubMed]
- Nacul, L.C.; Lacerda, E.M.; Campion, P.; Pheby, D.; Drachler Mde, L.; Leite, J.C.; Poland, F.; Howe, A.; Fayyaz, S.; Molokhia, M. The functional status and well being of people with myalgic encephalomyelitis/chronic fatigue syndrome and their carers. BMC Public Health 2011, 11, 402. [Google Scholar] [CrossRef] [PubMed]
- Carruthers, B.M.; Jain, A.K.; De Meirleir, K.L.; Peterson, D.L.; Klimas, N.G.; Lerner, A.M.; Bested, A.C.; Flor-Henry, P.; Joshi, P.; Powles, A.C.P.; et al. Myalgic Encephalomyelitis/Chronic Fatigue Syndrome. Journal of Chronic Fatigue Syndrome 2003, 11, 7–115. [Google Scholar] [CrossRef]
- Fukuda, K.; Straus, S.E.; Hickie, I.; Sharpe, M.C.; Dobbins, J.G.; Komaroff, A. The chronic fatigue syndrome: a comprehensive approach to its definition and study. International Chronic Fatigue Syndrome Study Group. Ann Intern Med 1994, 121, 953–959. [Google Scholar] [CrossRef] [PubMed]
- Jason, L.A.; Jordan, K.; Miike, T.; Bell, D.S.; Lapp, C.; Torres-Harding, S.; Rowe, K.; Gurwitt, A.; De Meirleir, K.; Van Hoof, E.L.S. A Pediatric Case Definition for Myalgic Encephalomyelitis and Chronic Fatigue Syndrome. Journal of Chronic Fatigue Syndrome 2006, 13, 1–44. [Google Scholar] [CrossRef]
- Committee on the Diagnostic Criteria for Myalgic Encephalomyelitis/Chronic Fatigue, S.; Board on the Health of Select, P.; Institute of, M. The National Academies Collection: Reports funded by National Institutes of Health. In Beyond Myalgic Encephalomyelitis/Chronic Fatigue Syndrome: Redefining an Illnes; National Academies Press (US) Copyright 2015 by the National Academy of Sciences. All rights reserved.: Washington (DC), 2015.
- National Institute for Health and Care Excellence: Guidelines. In Myalgic encephalomyelitis (or encephalopathy)/chronic fatigue syndrome: diagnosis and management; National Institute for Health and Care Excellence (NICE) Copyright © NICE 2021.: London, 2021.
- Carruthers, B.M.; van de Sande, M.I.; De Meirleir, K.L.; Klimas, N.G.; Broderick, G.; Mitchell, T.; Staines, D.; Powles, A.C.; Speight, N.; Vallings, R.; et al. Myalgic encephalomyelitis: International Consensus Criteria. J Intern Med 2011, 270, 327–338. [Google Scholar] [CrossRef] [PubMed]
- Podell, R.; Dimmock, M.E.; Comerford, B.B. Documenting disability in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS). Work 2020, 66, 339–352. [Google Scholar] [CrossRef] [PubMed]
- Sommerfelt, K.; Schei, T.; Angelsen, A. Severe and Very Severe Myalgic Encephalopathy/Chronic Fatigue Syndrome ME/CFS in Norway: Symptom Burden and Access to Care. J Clin Med 2023, 12. [Google Scholar] [CrossRef] [PubMed]
- Jason, L.A.; Holtzman, C.S.; Sunnquist, M.; Cotler, J. The development of an instrument to assess post-exertional malaise in patients with myalgic encephalomyelitis and chronic fatigue syndrome. J Health Psychol 2021, 26, 238–248. [Google Scholar] [CrossRef] [PubMed]
- van Campen, C.; Rowe, P.C.; Visser, F.C. Validation of the Severity of Myalgic Encephalomyelitis/Chronic Fatigue Syndrome by Other Measures than History: Activity Bracelet, Cardiopulmonary Exercise Testing and a Validated Activity Questionnaire: SF-36. Healthcare (Basel) 2020, 8. [Google Scholar] [CrossRef] [PubMed]
- Vergauwen, K.; Huijnen, I.P.; Kos, D.; Van de Velde, D.; van Eupen, I.; Meeus, M. Assessment of activity limitations and participation restrictions with persons with chronic fatigue syndrome: a systematic review. Disabil Rehabil 2015, 37, 1706–1716. [Google Scholar] [CrossRef] [PubMed]
- Kingdon, C.C.; Bowman, E.W.; Curran, H.; Nacul, L.; Lacerda, E.M. Functional Status and Well-Being in People with Myalgic Encephalomyelitis/Chronic Fatigue Syndrome Compared with People with Multiple Sclerosis and Healthy Controls. Pharmacoecon Open 2018, 2, 381–392. [Google Scholar] [CrossRef]
- Nijs, J.E.A. <ptj0444.pdf>. Physical Therapy 2003, 83, 444–454. [Google Scholar] [PubMed]
- Bonilla, H.; Quach, T.C.; Tiwari, A.; Bonilla, A.E.; Miglis, M.; Yang, P.C.; Eggert, L.E.; Sharifi, H.; Horomanski, A.; Subramanian, A.; et al. Myalgic Encephalomyelitis/Chronic Fatigue Syndrome is common in post-acute sequelae of SARS-CoV-2 infection (PASC): Results from a post-COVID-19 multidisciplinary clinic. Front Neurol 2023, 14, 1090747. [Google Scholar] [CrossRef] [PubMed]
Figure 1.
Round 5. Mean (+ - 1 SD) A to H sub-scores and Total Score on the FUNCAP27 questionnaire for the ME/CFS (n=1263) and healthy control (HC, n=178) respondents according to the respondent’s classification of ME/CFS severity as follows: Very severe: Totally bedbound and in need of care for basic functions (n=19). Severe: Mostly bedridden (n=136). Moderate: Mostly housebound (n=733). Mild: At least 50% reduction in pre-illness activity level (n=360). Better than mild: Less than 50% reduction in pre-illness activity level (n=15).
Figure 1.
Round 5. Mean (+ - 1 SD) A to H sub-scores and Total Score on the FUNCAP27 questionnaire for the ME/CFS (n=1263) and healthy control (HC, n=178) respondents according to the respondent’s classification of ME/CFS severity as follows: Very severe: Totally bedbound and in need of care for basic functions (n=19). Severe: Mostly bedridden (n=136). Moderate: Mostly housebound (n=733). Mild: At least 50% reduction in pre-illness activity level (n=360). Better than mild: Less than 50% reduction in pre-illness activity level (n=15).
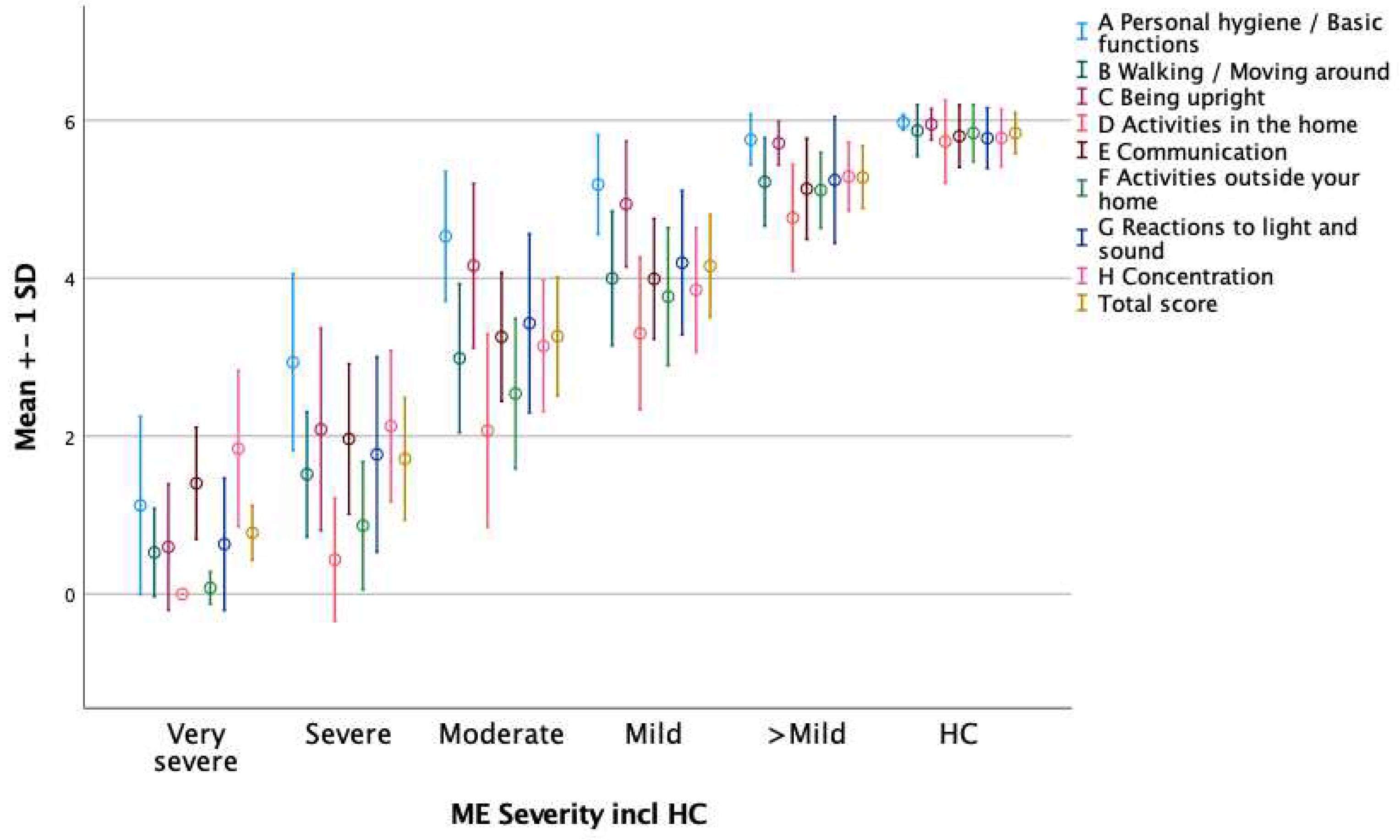
Table 1.
Round 5 (Norwegian) ME/CFS and healthy control (HC) respondent and Round 4 (International – English) ME/CFS respondents characteristics. Numbers of respondents (per cent). Not all respondents answered all questions; the number of responses may not therefore add up to the total number within the group.
Table 1.
Round 5 (Norwegian) ME/CFS and healthy control (HC) respondent and Round 4 (International – English) ME/CFS respondents characteristics. Numbers of respondents (per cent). Not all respondents answered all questions; the number of responses may not therefore add up to the total number within the group.
| ME/CFS Round 5 (n = 1263) |
HC Round 5 (n = 178) |
ME/CFS Round 4 (n = 1413) |
|
|---|---|---|---|
| Female / Male* | 1102 / 149 (88) | 118 / 56 (68) | 1175 / 216 (85) |
| Age < 19 years | 48 (4) | 3 (2) | 15 (1) |
| Age 20–39 years | 420 (33) | 67 (38) | 446 (32) |
| Age 40+ years | 795 (63) | 108 (61) | 952 (67) |
| Onset 0–15 years | 194 (15) | 174 (12) | |
| Onset 16–29 years | 424 (29) | 539 (38) | |
| Onset 30+ years | 646 (45) | 700 (50) | |
| Duration 0–5 years | 143 (12) | 290 (21) | |
| Duration 6–15 years | 666 (56) | 595 (42) | |
| Duration 16+ years | 372 (31) | 528 (37) | |
| Disability benefits | 1038 (82) | 5 (3) | |
| Parttime < 50% work/ education | 120 (10) | 4 (2) | |
| Parttime 50 – 100% work/ education | 33 (3) | 11 (6) | |
| Full time work/ education | 30 (2) | 149 (84) | |
| Stay at home | - | 6 (3) | |
| None of these | 42 (3) |
* 12 ME/CFS and 4 HC Round 5 respondents and 22 Round 4 respondents answered that they preferred not to state their sex.
Table 2.
Round 5 and 4. Mean (SD) scores and mean difference in score for the 27 items in the main Norwegian Round 5 FUNCAP27 for ME/CFS (ME, n = 1236) and healthy control (HC, n = 178) respondents. The right two columns show corresponding mean scores (SD) from Round 4 respondents (international round in English language, n = 1413). The numbers in parenthesis after each item number indicates the number the same item had in FUNCAP55. All t-test p-values for the differences in mean scores on each item between ME/CFS respondents and controls were statistically significant (p < 0.003 with Bonferroni correction).
Table 2.
Round 5 and 4. Mean (SD) scores and mean difference in score for the 27 items in the main Norwegian Round 5 FUNCAP27 for ME/CFS (ME, n = 1236) and healthy control (HC, n = 178) respondents. The right two columns show corresponding mean scores (SD) from Round 4 respondents (international round in English language, n = 1413). The numbers in parenthesis after each item number indicates the number the same item had in FUNCAP55. All t-test p-values for the differences in mean scores on each item between ME/CFS respondents and controls were statistically significant (p < 0.003 with Bonferroni correction).
| Round 5 | Round 4 | ||||||
|---|---|---|---|---|---|---|---|
| ME | HC | HC-ME | ME | ||||
| Items | Mean | SD | Mean | SD | Mean diff. | Mean | SD |
| A1 (1) Using toilet (not bedpan or bedside commode) | 5.3 | 0.9 | 6.0 | 0.1 | 0.7 | 5.1 | 1.1 |
| A2 (5) Showering standing up | 3.4 | 1.8 | 6.0 | 0.2 | 2.6 | 3.3 | 2.0 |
| A3 (7) Getting dressed in regular clothes | 4.8 | 1.2 | 6.0 | 0.1 | 1.2 | 4.7 | 1.2 |
| B4 (8) Walking a short distance indoors, from one room to another | 4.9 | 1.1 | 6.0 | 0.1 | 1.1 | 4.6 | 1.3 |
| B5 (10) Walking between approx. 100 m and 1 km on level ground (length of 1 to 10 football fields) | 3.0 | 1.7 | 6.0 | 0.2 | 2.9 | 2.6 | 1.9 |
| B6 (13) Physical activity with increased heart rate, for approx. 15 min | 1.4 | 1.4 | 5.7 | 0.8 | 4.3 | 1.5 | 1.6 |
| C7 (15) Sitting in bed for approx. 30 minutes | 5.2 | 1.2 | 6.0 | 0.1 | 0.8 | 5.0 | 1.4 |
| C8 (17) Sitting in an upright chair (dining chair) with feet on floor for approx. 2 hours | 3.2 | 1.9 | 5.9 | 0.5 | 2.7 | 2.8 | 2.1 |
| C9 (18) Standing up for approx.5 minutes, e.g., while queuing or while cooking | 3.9 | 1.6 | 6.0 | 0.1 | 2.0 | 3.9 | 1.8 |
| D10 (21) Heavier housework (washing floors, vacuuming etc.) for approx. ½ hour continuously | 1.9 | 1.5 | 5.7 | 0.6 | 3.8 | 1.8 | 1.7 |
| D11 (25) Cooking a complicated meal from scratch, approx. 1 hour of preparation | 2.6 | 1.6 | 5.8 | 0.5 | 3.2 | 1.9 | 1.7 |
| E12 (27) Having a conversation for approx. 5 minutes | 5.2 | 1.0 | 6.0 | 0.1 | 0.8 | 5.0 | 1.1 |
| E13 (30) Participating in a conversation with three people for approx.x.½2 hour | 3.1 | 1.5 | 5.9 | 0.4 | 2.7 | 3.1 | 1.6 |
| E14 (32) Participating in a dinner party, party or family event | 1.7 | 1.2 | 5.6 | 0.8 | 3.9 | 1.8 | 1.4 |
| F15 (33) Stepping right outside your home | 4.5 | 1.5 | 6.0 | 0.1 | 1.5 | 4.2 | 1.8 |
| F16 (35) Going to a shop for groceries | 2.9 | 1.5 | 5.9 | 0.4 | 3.0 | 2.2 | 1.7 |
| F17 (38) Using public transport (bus or train) | 2.4 | 1.8 | 5.9 | 0.4 | 3.5 | 2.1 | 1.9 |
| F18 (39) Participating in organized leisure activities such as classes, sports etc. | 1.0 | 1.4 | 5.6 | 0.8 | 4.6 | 1.1 | 1.5 |
| G19 (41) Staying in a room with normal lighting, without sunglasses, for approx. 1 hour | 5.0 | 1.4 | 6.0 | 0.1 | 1.0 | 4.9 | 1.5 |
| G20 (42) Staying outdoors in daylight without sunglasses for approx. 2 hours | 3.2 | 1.9 | 5.8 | 0.5 | 2.6 | 3.4 | 2.1 |
| G21 (44) Staying in a noisy environment, (shopping mall, café or open plan office) for approx. 1 hour | 2.2 | 1.4 | 5.5 | 0.7 | 3.4 | 2.6 | 1.8 |
| H22 (46) Reading a short text, such as a mobile phone text message | 5.4 | 0.9 | 6.0 | 0.1 | 0.6 | 5.4 | 0.9 |
| H23 (48) Reading and understanding a non-fiction text, such as an official document one A4 page long | 3.1 | 1.7 | 5.8 | 0.5 | 2.7 | 4.0 | 1.6 |
| H24 (51) Using social media to stay in touch with others | 4.6 | 1.2 | 5.9 | 0.3 | 1.4 | 4.6 | 1.3 |
| H25 (53) Focusing on a task for approx. 10 minutes continuously | 3.8 | 1.5 | 5.9 | 0.3 | 2.1 | 4.3 | 1.3 |
| H26 (54) Focusing on a task for approx. 2 hours continuously | 1.9 | 1.6 | 5.6 | 0.8 | 3.7 | 2.4 | 1.8 |
| H27 (55) Managing a full working day (non-physical work such as office work, classes or lectures) | 0.6 | 1.1 | 5.5 | 0.9 | 4.8 | 0.8 | 1.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Copyright: This open access article is published under a Creative Commons CC BY 4.0 license, which permit the free download, distribution, and reuse, provided that the author and preprint are cited in any reuse.
MDPI Initiatives
Important Links
© 2024 MDPI (Basel, Switzerland) unless otherwise stated






