1. Introduction
Chemical control of plant pests, particularly soil-borne parasites, has generally been recognized as harmful to human health and contaminant for the environment, as well as ineffective in most cases; therefore, the use of biological tools to limit plant diseases and pests has been recommended [
1]. Plant parasitic nematodes (PPNs) are the most diffused and damaging soil-borne pests, although nematode damage has largely been underestimated because the symptoms of infection are often non-specific and unrecognizable [
2]. Among the many families of nematodes, the sole sedentary endoparasitic root-knot nematodes (RKNs),
Meloidogyne spp., produce yield and quality losses that can be economically estimated in more than €80 billion/year in worldwide agriculture [
3]. The invading motile worm-like juveniles (J2s) of RKNs penetrate the roots and move intercellularly through the cortex toward the central cylinder. They suck cell sap from few cells of the central cylinder and inject, through a protrusible stylet of their mouth apparatus, several digestive compounds secreted by pharyngeal glands. J2s soon become sedentary and, through two molts to J3 and J4, develop into adult gravid females, which lay hundreds of eggs in gelatinous masses outside the roots. Nematode secretions alter gene regulation, cell metabolism and structure as markedly as to transform the pierced cortical cells into giant nurse cells. Likewise, they produce hypertrophy and hyperplasia of the root tissue that result in the familiar visible galls or knots that are the only specific underground symptoms.
Biological control against RKNs implies the treatments of plants with specific rhizosphere microbes that can act as antagonists to nematodes in soil, through antibiosis and competition for food and space, and induce resistance by stimulating plant immune system [
4].
Trichoderma spp. and arbuscular mycorrhizal fungi (AMF) have extensively been reported to be effective as resistance inducers against nematodes [
5,
6,
7,
8]. AMF contained in commercial formulates of biocontrol agents (BCAs) have been proved to colonize roots of tomato and immunize plants against RKNs [
9].
Some non-parasitic plant growth promoting rhizobacteria (PGPR), such as
Pseudomonas fluorescens, have been used as low-cost and environmentally safe BCAs and adopted in the management programs of soil-borne pathogens, including PPNs [
10]. Competition for food and space, along with the production of metabolites effective in reducing egg hatching and juvenile vitality and induction of systemic resistance, are the main suppression mechanisms adopted by
P. fluorescens to limit RKN infections [
11,
12]. Also
Bacillus firmus, another PGPR, has been used commercially as a bionematicide since the early 2000s, as it induces paralysis and mortality to individuals of several nematode families, RKNs included [
13].
Such type of management of nematodes is in the frame of the recently proposed microbiome-mediated stress resistance in plants, or, more generally, microbiome-assisted agriculture [
14,
15]. We have long been studying the potential of presently commercially available formulates containing single or mixture of BCAs to control sedentary endoparasitic nematodes on vegetable crops in pots placed in a glasshouse [
16]. Paramount in this management strategy has been found the determination of the dosage expressed on a plant weight basis and also the period of time to allow endophytic fungi to colonize roots and immunize plants [
9].
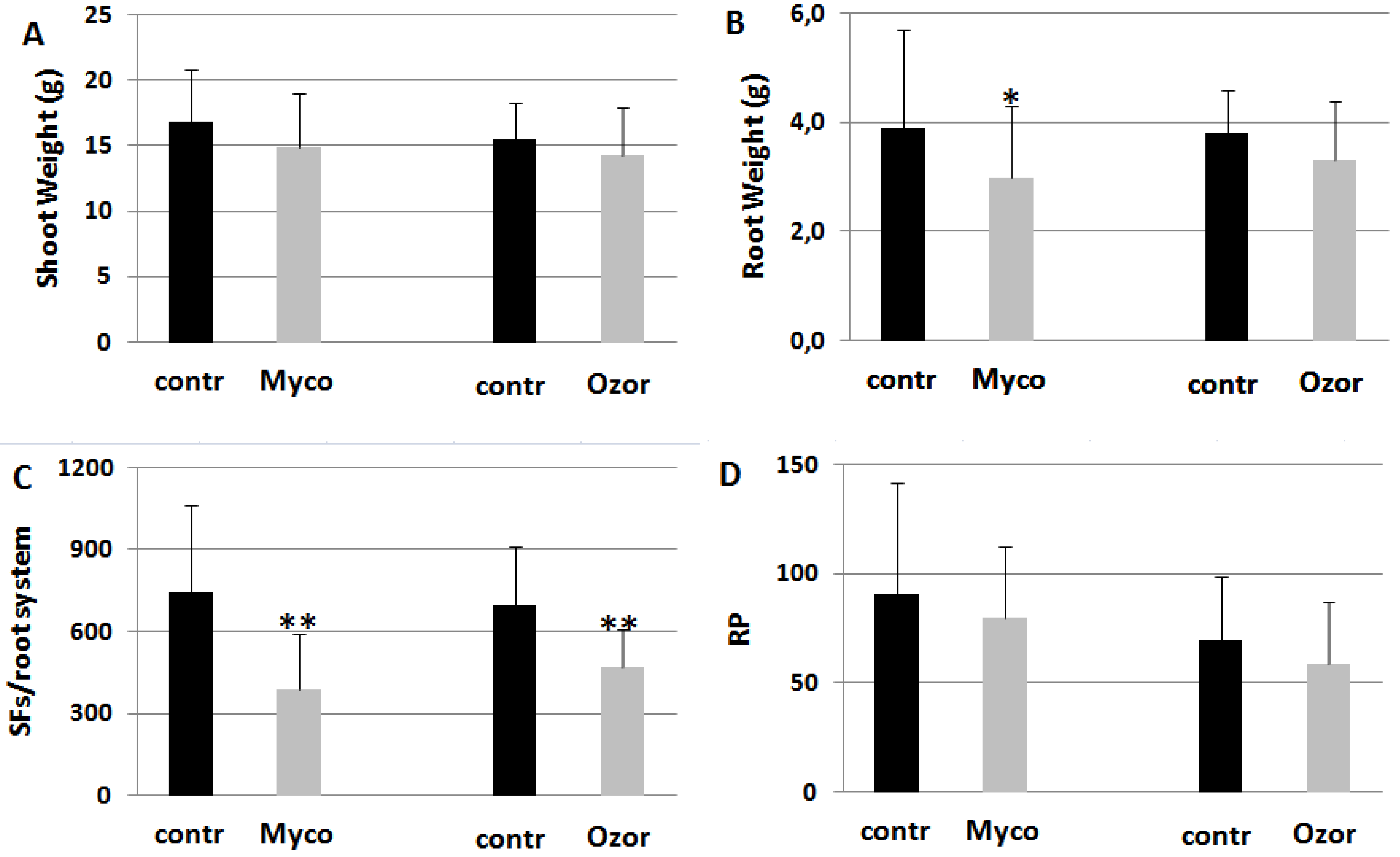
In this study, we have enriched the soil of tomato-potted plants with the previously used mixture of bacterial and fungal rhizosphere microbes (named as Myco in the text) or with an untested AMF commercial formulate (Ozor) and monitored the effects on plant growth, RKN infection, and on leaves attacked by the miner insect Tuta absoluta. As Myco was proved to be more effective than Ozor in limiting pest parasitism, we focused on the mixture to find the microbial component(s) that specifically elicited plant resistance to RKNs. Moreover, we mixed several chemicals with Myco, before providing microbial suspensions to plants, in the attempt to characterize the biochemical pathway that endophytic fungi use to colonize roots and immunize plants. Immunization and immune reaction to RKNs were monitored by means of the expression of two Pathogenesis Related-genes (PR-genes, such as PR-2 and PR-3) and the activities of the encoded cell-wall degrading enzymes endochitinases and β-1,3-glucanase.
3. Discussion
Enrichment for beneficial rhizosphere microbes can increase drought tolerance and stimulate plant immune system, thereby promoting plant growth and crop yield [
25,
26]. However, beneficial rhizosphere microbes are naturally selected by plants, or should be selected by farmers as an exogenously provided input to cropping systems to comply with the so-called microbiome-assisted agriculture [15J. In this study, we used a commercial mixture of beneficial rhizosphere microbes (named in the text as Myco) to test its effect on plant growth and protection against soil-borne parasites, such as RKNs, and foliar miner insects, such as T. absoluta. Actually, treatments with Myco have already been reported to activate plant immune response and prime tomato against RKNs [
6]. Moreover, it has been proven that, after Myco treatements to tomato, colonization of roots by AMF occurs although colonization capacity, that is, the amount of root colonized in a certain time or the time taken to colonize roots, is strictly dependent on determined doses of inoculum [
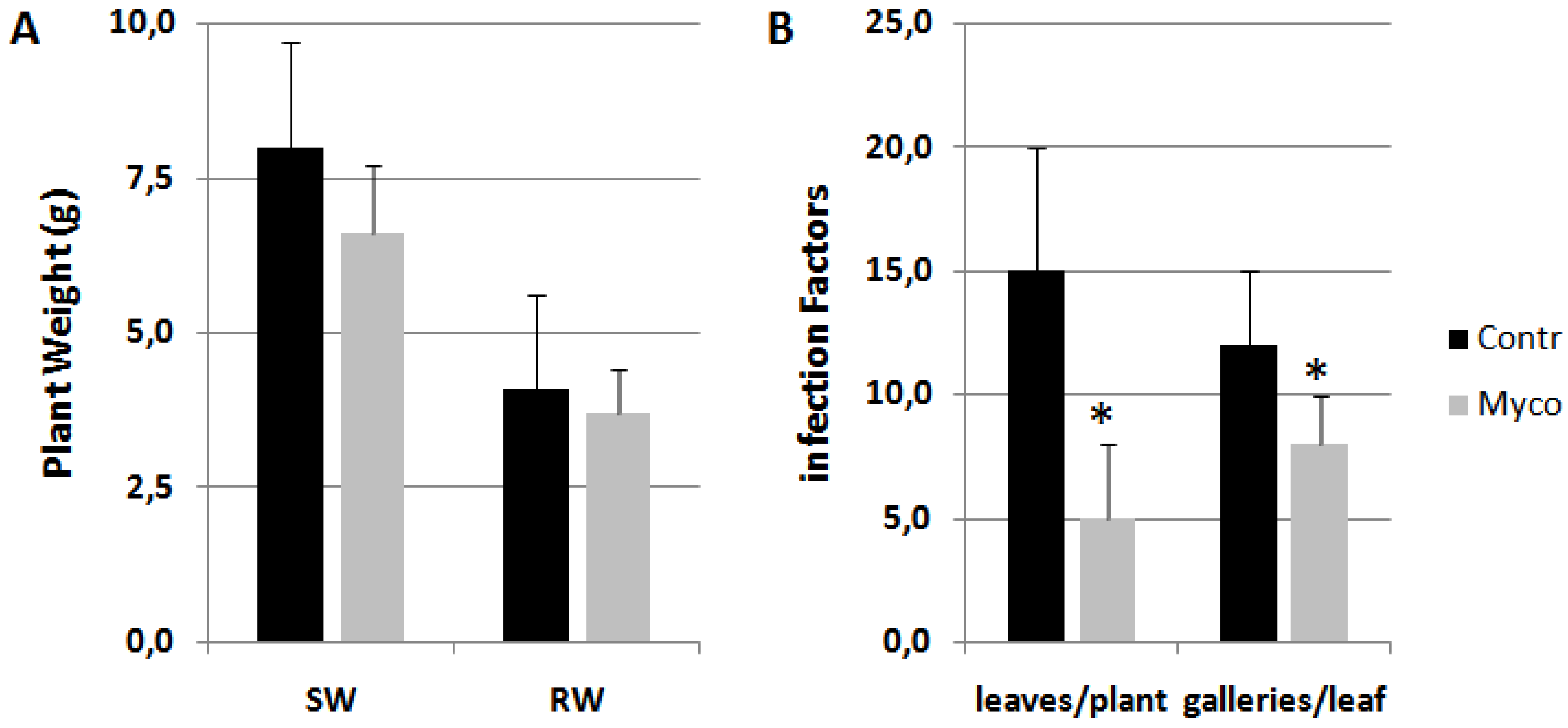
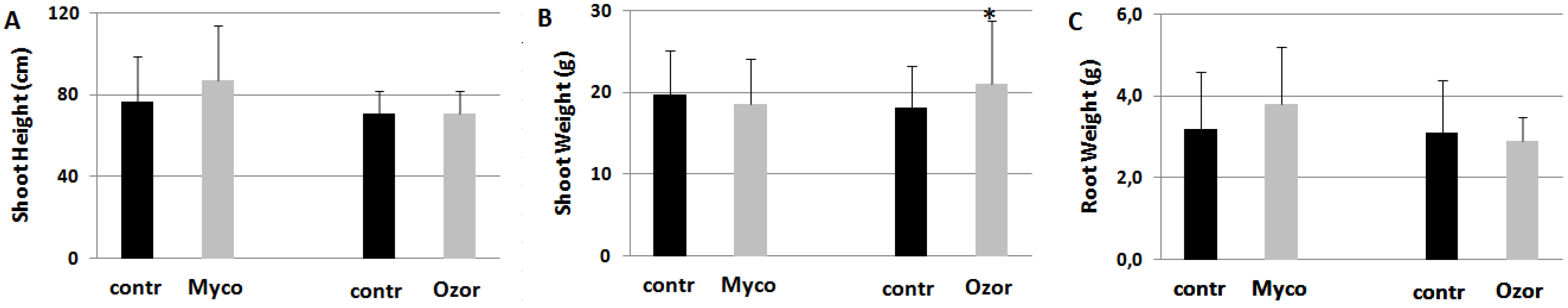
9]. Such doses were also important for the ability of treated roots to lessen RKN infection with respect to untreated roots. However, the role of each component of the mixture in defense activation of tomato has not been fully cleared thus far.
Besides AMF, that are the main components, Myco contains antagonistic fungi, such as Beauveria spp. with entomopathogenic properties, and Trichoderma harzianum. Trichoderma spp. cultivated in laboratory or contained in commercial formulates were successfully used for biocontrol of RKNs [
7,
16]. Moreover, Myco contains PGPR, such as Agrobacterium radiobacter, Bacillus subtilis, and Streptomyces spp., which are known agents of biocontrol. First, we used another commercial formulate, named as Ozor, which contains only AMF, to provide tomato plants as pre-treatements for immunization against RKNs. Ozor treatments were actually able to restrain nematode development in roots, compared with untreated roots, although not as much as Myco. The strong restriction of individuals developing in roots produced by these two commercial beneficial microbe formulates considerably lessens competition for food and enhances female fecundity, thereby allowing females to deposit a higher number of eggs in each mass than when under conditions of root high density. Therefore, reproduction rates sometimes are not significantly affected by beneficial microbe treatements. The higher attenuation of symptoms by Myco is also indicated by the healthier state of roots, compared with the highly infected control roots, indicated by lower weight. Difference between Myco and Ozor in the ability to control leaf mining by the miner insect T. absoluta was even larger. The mixture contained in Myco developed a root microbiome effective against aboveground insect pests as already reported for other microbiomes against foliar pests and pathogens [
27].
Although Myco was proven to be a good defense biostimulant, it did not show to act as a biofertilizer and growth promotor in not challenged plants, under the experimental conditions adopted in this study. In a previous study, plants were analyzed after much shorter periods of growth at which shoot weights were found to be enhanced in Myco-treated plants [
9]. On the other hand, in plants infected by pests, the consistent relief of symptoms shown by Myco-treated attacked plants was not sufficient to make a difference in terms of plant weight growth, probably because of the fitness costs spent in immunity response that counterbalanced the growth promoting effect exerted by Myco.
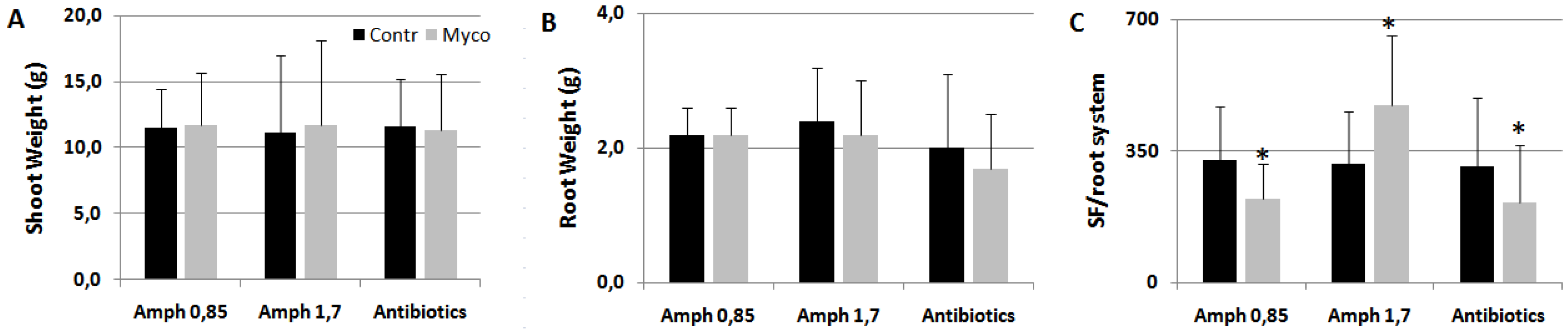
Once it was apparent that the mixture contained in Myco was more effective than Ozor, our task was to find which component of the mixture was specifically responsible of the effect. Amphotericin B, a potent antifungal agent, was added to Myco and the mixture incubated as usual before plants soil-drenching. When sufficient amounts of the agent were provided, Myco completely lost its property to lessen nematode infection, rather, its bacterial components increased root infection. On the contrary, antibiotics mixed with Myco did not alter its ability to restrain nematode development. Therefore, B. subtilis, a PGPR contained in Myco, was tested as a BCA against RKNs and compared with the PGPR P. fluorescens, which is known to control RKNs by the destruction of eggs and induction of plant defence mechanisms leading to systemic resistance [
28]. Treatements with B. subtilis increased infection indicators at any tested cell density; P. fluorescens was confirmed to effectively control nematode spread in the roots, at least at high suspension concentrations. Our findings seem to be in contrast to many studies in which, Bacillus spp., due to their nematicidal properties, have generally been recognized as effective BCAs for RKNs [
29]. However, such an inhibitory effect may be depending on the species, and within B. subtilis sp., on different strains, and most probably on methods and concentrations of the application [
30]. For comparison, we used the same low concentrations of B. subtilis suspensions per plant as those provided with Myco treatments and they did nor work. On the other hand, large amounts of studies have revealed that microbial-mediated modulation of host immune responses may facilitate nematode parasitism, and that native microbiota naturally associated with nematodes, such as the one present in the fresh soil used in this study, may protect nematodes against microbial antagonists [
31].
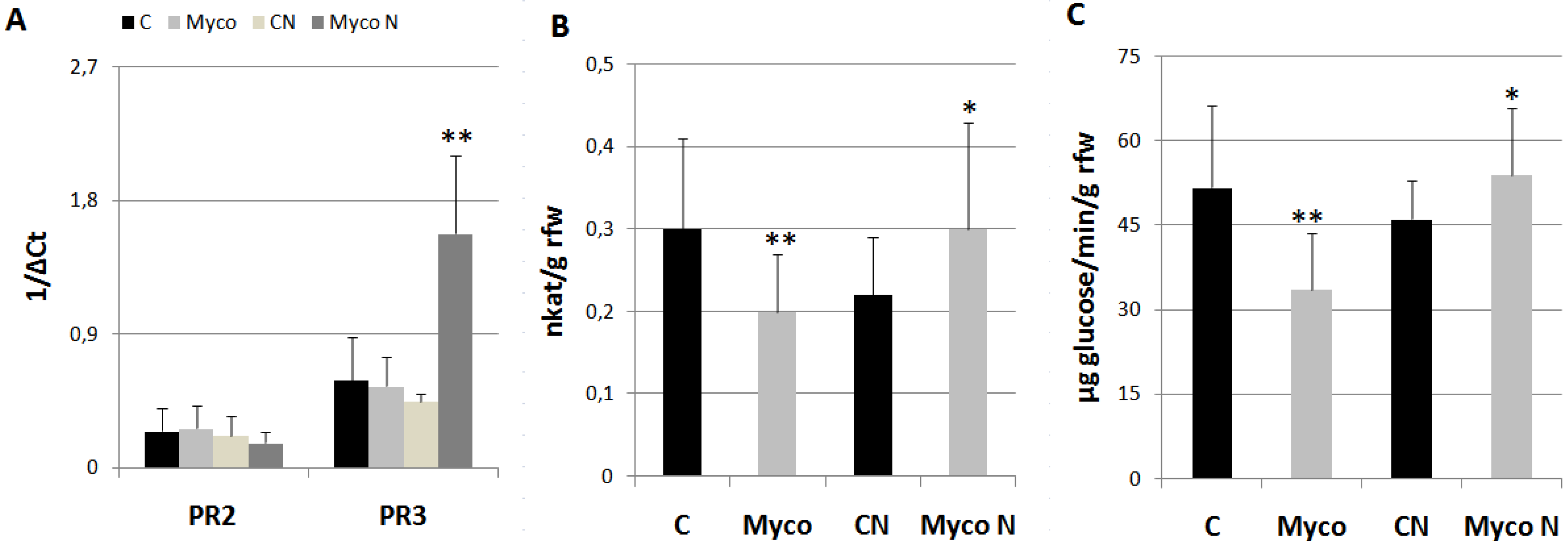
A series of experiments were undertaken to get insights on some possible molecular mechanisms underlying immune response to nematodes induced by Myco treatments. In this study, we focused on the SA-responsive gene PR-2, which encodes for the enzyme β-1,3-glucanase, and on the JA-responsive gene PR-3, which encodes for endochitinases; both genes have recently been reported to trigger defense mechanisms against Meloidogyne spp. [
32]. Both PR-2 and PR-3 were not responsive to Myco, at least 8 days after treatments. However, priming of plants was ascertained by the monitored over-expression of PR-3 in plants treated with Myco 3 days after nematode inoculation. Moreover, in roots of inoculated Myco-treated plants, both chitinase and glucanase activities were higher than in untreated roots. Cell-wall degrading enzymes, such as chitinase and β-1,3-glucanase, are generally recognized to have an important role in the control of Meloidogyne spp. by opportunistic fungi, such as Beauveria and Trichoderma spp., mainly because of their capacity in inhibiting eggs hatching and inducing J2s mortality [
33]. Roots of resistant soybean cultivars showed enhancement of chitinase activity when attacked by M. incognita [
34]. Augmented chitinase and β-1,3-glucanase activities observed in roots of Myco-treated plants 3 days after nematode inoculation can be considered as an early defense response induced by the interaction between roots and fungi present in the provided mixture. It was not a surprise the finding that, if not inoculated by nematodes, Myco-treated roots conversely showed an inhibition of the tested cell-wall degrading enzyme activities with respect to control roots. Generally, non-pathogenic members of the root microbiome actively interfere with plant immune signaling to permit root colonization by secreting immune-suppressive effector molecules, like plant pathogens [
35,
36]. In particular, it was reported that SA-mediated defense was deactivated by T. harzianum endophytes up to 15 days after inoculation to favor their colonization of roots [
7]. Secondary infections that may be carried out by soil-borne or above-ground plant parasites trigger immunity by overexpression of a series of genes involved in defense response. Immune response activation is responsible of the low developmental rate of nematodes in immunized plants; however, such an activation is preceded by root colonization of AMF and BCF present in the inoculum [
6,
9]. It is possible that AMF and BCF act together to contrast nematode attack, thus explaining the higher potential of Myco to limit the infection compared with Ozor.
To investigate on which type of metabolic pathway Myco exerts its action, a series of metabolites and enzyme inhibitors were added to the treatments. Such compounds were mixed with Myco suspensions before the 3-days incubation; controls were arranged with suspensions of the sole compounds. Exogenously added SA has since long been proved to be an active inducer of resistance against RKNs [
37]. The mixture of SA with Myco strenghtened the repressive effect of SA on the development of SFs in roots, although it generally seems that mixing SA with Myco poses greater difficulties to the beneficials contained in Myco in their root colonization and consequent protective action. On the other hand, as biotrophs, AMF colonization is negatively impacted by SA, which is why they attempt to impair SA-mediated defense response [
38]. However, when Myco was mixed with PCB that reduces SA levels, it completely lost its defense eliciting effect against RKNs, thus suggesting that, once roots have been colonized, AMF need suitable amounts of SA to trigger immune reaction. It is also likely that MIR needs ROS generation, since mixing Myco with DPI, which inhibits O
2●- generation, results in a loss of Myco protective effect against RKNs. On the contrary, DIECA is a O
2●- generator as it markedly inihibits SOD; DIECA limited plant growth and nematode development almost impeding reproduction because it creates an oxidative environment in cells hostile to both host and pest. It is possible that Myco mixed with DIECA may lower DIECA absorption by roots, thus relieving its toxicity to plants and nematodes but weakening its own potential in colonizing and immunizing plants. SHAM reduced symptoms of infection, but in contrast to DIECA, it promoted plant growth and healthy, since it inhibits the alternative cyanide-insensitive respiration that acts as a ROS scavanger [
39], thus favoring feeding and development of nematode juveniles. However, mixtures of Myco plus SHAM had effects on nematode infection similar to those obtained with the sole Myco, thus indicating that beneficials contained in Myco do not exploit plant cyanide-insensitive respiration and do not depend on copper enzyme activities to induce plant immune reaction. If it is MIR that Myco-treated plants use to constrast nematode attacks, it should mainly be mediated by SA-signaling, according to our findings.
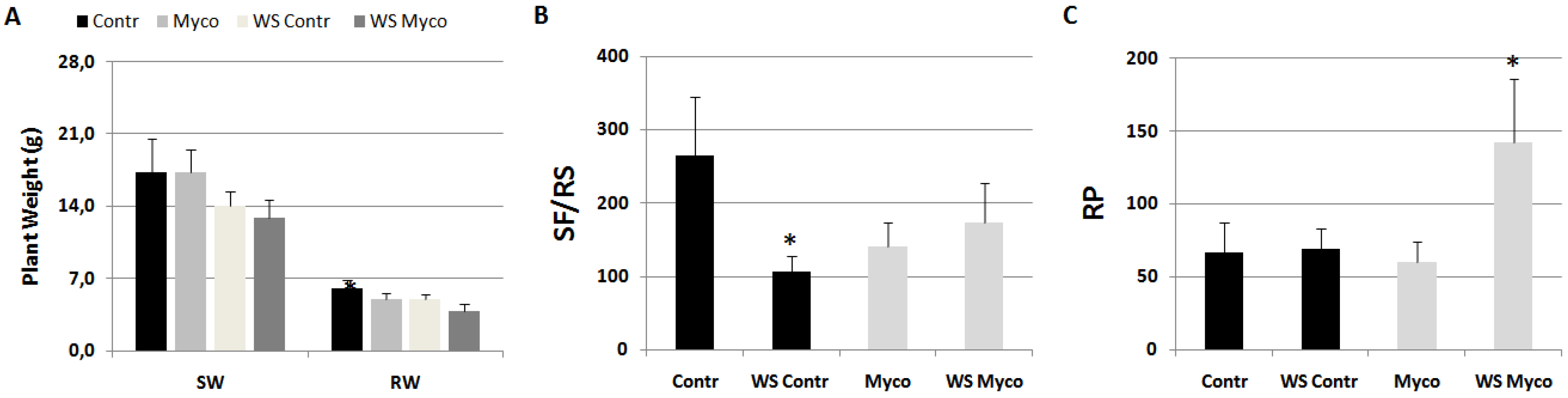
Among abiotic stresses, water stress is the most important growth-limiting factor, particularly in arid and semiarid regions. The role of beneficial PGPR and AMF in improving drought tolerance has recently been well described [
40,
41]. In our experimental conditions, though, Myco did not relieve the loss in plant weight caused by drought. Stressed roots hosted much fewer nematodes than unstressed roots because of food scarcity; this did not happen with Myco-treated roots, thus indicating that mycorrhizal roots save their capacity to feed a discrete number of parasites in spite of water stress. This finding indicates that mycorrhizal roots tolerate water stress and maintain their metabolic activities higher than not-mycorrhizal roots may do, although it does not result in weight gain.
In nature, rhizosphere augments the numbers of microbes normally present in bulk soil and makes a selection, through specific root exudates, for the most beneficial in terms of improved root architecture, nutrient uptake, abiotic stress tolerance and faster and stronger immunity reaction [
15]. Unfortunately, in most agricultural areas indigenous root microbiome has drastically been reduced by human agricultural practices or natural disturbances. Therefore, in the perspective of a microbiome-assisted agriculture, large exogenous applications of beneficial microbes should be predicted. Data shown in this paper indicate some of the conditions needed to make sure these treatments may be effective and anticipate the problems that farmers will have to face when commercial formulates are going to be used. Experiments are being done to ascertain the durability of microbiome benefits, in terms of protection against plant parasites, when several crop seasons are considered. Finally, it will be important to have information on the survival rates of the added microbe consortia in time and in competition with the indigenous ones present in the tested soils.
4. Materials and Methods
4.1. Treatment of Plants with a Mixture of Rhizosphere Microorganisms
Seeds of the tomato (Solanum lycopersicum L.) cultivars Roma VF, Regina, Fiaschetto, Principe Borghese, and Marmande, all susceptible to RKNs, were sown in special containers filled with sterilized topsoil at 23-25 °C in a glasshouse. Seedlings were transferred to 110-cm
3 clay pots filled with a freshly field-collected loamy soil and put in temperature-controlled benches (soil temperature 23-25°C). Seedlings were provided with a regular regime of 12 h light/day, and weekly fertilized with Hoagland’s solution, and allowed to grow to a weight of 3-5 g. A mixture of rhizosphere microorganisms was provided to plants by soil-drenching in pots a commercial formulate (Micosat F
®, named Myco in the text, C.C.S. Aosta, Italy) consisting in 25% of arbuscular mycorrhizal forming fungi (AMF) provided as root fragments containing intra-radical spores/vesicles and hyphae of Glomus spp. (Glomus spp. GB 67, G. mosseae GP11, G. viscosum GC 41). Myco contained also PGPR, such as Agrobacterium radiobacter AR 39, Bacillus subtilis BA 41, and Streptomyces spp. (6.2%, 4.0 x 10
8 C.F.U. g
-1 Myco). Amounts of formulate were dissolved in sterile distilled water added with peptone-glucose (0.7 g L
-1) and incubated in an orbital shaker at 25°C for 3 days in dark. The amount in gram of Myco provided per plant was fixed according to the fresh weight of plant at treatment (0.25 g Myco g
-1 pfw) since this dose had previously been proven to prime tomato plants against nematodes with minimal fitness costs [
6]. Since the used formulate contained both AMF and PGPR, some controls were made to investigate about which component were responsible for plant priming. Therefore, in some experiments, Myco and control suspensions were added with 0.85 and 1.7 mg amphotericin B g
-1 Myco, a potent antifungal compound, to exclude the fungal component from the soil-drenched formulate; conversely, minimal amounts (0.25 mg g
-1 Myco) of antibiotics, such as ampicillin and streptomycin, were added to incubation media to exclude rhizobacteria.
To have information on the metabolic pathways through which Myco induces priming of tomato against RKNs, a series of chemicals was added to Myco and control suspensions before incubation, as follows:
- salicylic acid (SA) as water solutions of potassium salicylate at 15-20 mg g-1 Myco
- salicylhydroxamic acid (SHAM) as water solutions at 0.8 mg g-1 Myco
- sodium diethyldithiocarbamate trihydrate (DIECA) as water solutions at 0.9 mg g-1 Myco
- diphenyliodonium chloride (DPI) as water solutions at 0.3 mg g-1 Myco
- paclobutrazol (PAC) as ethanol solutions at 3 mg g-1 Myco
In the cases of chemicals provided as ethanol solutions, the same amounts of ethanol were added to control suspensions.
The effect of a moderate water stress on Myco-induced priming of plants was tested, as well.
4.2. Treatment of Plants with AMF
Plants at the weight of 3-5 g were soil-drenched with the commercial formulate Ozor (Bioplanet, Italy), containing 500 propagules g-1 of Glomus intraradices CMCCROC7. Opportune amounts of the formulate were dissolved in sterile distilled water added with peptone-glucose (0.7 g L-1) and incubated in an orbital shaker at 25°C for 3 days in dark. The most effective dose was determined as 0.08 g Ozor g-1 pfw at treatment. Controls were provided with peptone-glucose suspensions incubated without Ozor.
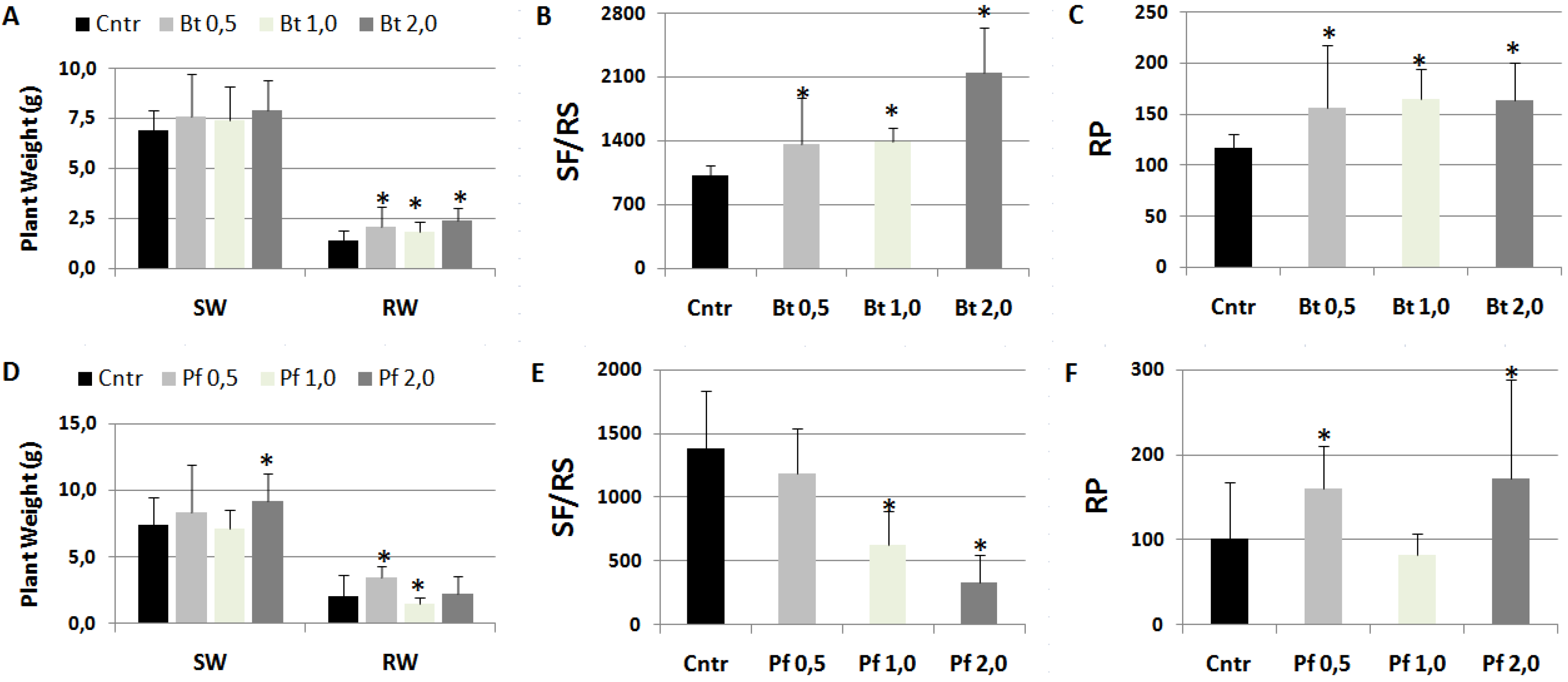
4.3. Treatment of Plants with PGPR
Pseudomonas fluorescens ATCC 13525 and Bacillus subtilis were used as potential elicitors of resistance of tomato against RKNs. P. fluorescens is not contained in the BCAs mixture provided as Myco, whilst B. subtilis is one of the component. P. fluorescens and B. subtilis were cultivated in shake cultures of King’s B medium and Agar Meat-Peptone, respectively, at 28°C for 24 h in the dark. Bacterial suspension density was brought to an absorbance of 1.0, which is equivalent to a bacterial concentration of 1.2 x 10
9 C.F.U. [
42]. Aliquots of this mother suspensions were diluted with sterile water to soil-drench tomato plants with 0.5, 1.0, 2.0 x 10
8 C.F.U./plant; control plants were provided with the same volumes of growing media.
4.4. Nematode Inoculation and Measurements of Infection Level
Field populations of the RKN Meloidogyne incognita (Kofi et White) Chitw. were reared on susceptible tomato in a glasshouse and used for inoculation of plants. Active second-stage juveniles (J2s) were obtained by incubation of egg masses in tap water at 25 °C in dark. At the second day of incubation, J2s were collected and concentrated by filtering through 500 mesh sieves. J2s were counted by means of a dissecting microscope at 25 × magnification. Aliquots of stirring suspensions, containing approx. 400 J2s, were poured into 2 holes made in the soil at the base of plants. Inoculations were made 5 days after Myco-treatment. The described inoculations of susceptible tomato plants with field nematode populations gave severe standard infections.
Plants were grown under the conditions described above and harvested 2 months after inoculation. Under the adopted experimental conditions, most nematodes completed their life cycle with the deposition of eggs outside the roots in gelatinous egg masses (EMs) in about one month. Afterwards, J2s start to hatch in the pot soil and re-infest roots as a second generation. The potential numbers of these second generation J2s are much higher than those of the first artificial inoculation. However, in our experiments, second generation J2s enter the roots and develop into sedentary forms (SF: J3, J4, swollen females), but adult females do not have enough time to produce EMs for reproduction. Therefore, reproduction rates are those determined by the J2s of the artificial inoculation, whereas the total reported numbers of SFs developed in the roots are mostly indicative of the secondary infection. Reproduction Potentials (RPs) indicate how many times the density of initial population has multiplied after plant harvest, whereas SF numbers reflect the galling state of the roots; higher the galling state higher the damage level of the plants.
At harvest, Myco-treated and control plants, inoculated or not with nematodes, were weighed and their length measured. Then, roots, cut from shoots, were washed free of soil debris. Each sample for nematode life-stage extractions was recovered from 2 root systems that were chopped into fragments; samples were weighed before being used for extraction of SFs and eggs. Other weighed samples were used to determine the numbers of EMs. EMs were red-colored by immersion of samples in 0.1 g L
−1 Eosin Yellow and stored in a refrigerator for at least 1 h. Root fragments were scored for red-colored EMs under a stereoscope (6 × magnification). Roots were softened by incubation in a mixture of the enzymes pectinase and cellulase at 37 °C in an orbital shaker before SF extraction to free sedentary nematodes from root tissue. Roots were then ground in physiological solution and SFs collected on a 90 µm sieve. Aliquots (2 mL) of stirring nematode suspensions were pipetted in small Petri dishes and the numbers of SFs counted under a stereoscope (12 × magnification). Eggs were extracted by stirring root samples in diluted bleach and counted under a stereoscope (25 × magnification) [
43].
If numbers of inoculated J2s are taken as the initial population in each pot (
Pi), and numbers of total eggs per root system as the final population (
Pf, the number of J2s free in soil is negligible in the small pots used in the experiments),
4.5. Determination of Tuta Absoluta Infestation
Pots containing Myco-treated and untreated tomato plants were randomly located in a glasshouse naturally infested by T. absoluta. Twenty-one days after, leaves of plants were examined for the amounts of insect galleries. Infested leaves of each plant were collected, separated from healthy leaves, and counted. The numbers of the insect galleries affecting each leaf were determined, as well.
4.6. Protein Extraction and Enzyme Activity Assays
Proteins were extracted from roots of plants harvested 8 days after Myco treatment and 3 days after nematode inoculation; as inoculation was carried out 5 days after Myco treatments, both plant groups, inoculated and not inoculated, had the same growth time. Root samples of all groups, were collected, dried, weighed and put on ice. Samples were ground in porcelain mortars by immersion in liquid nitrogen. Minced samples were further ground by a Polytron® PT–10–35 (Kinematica GmbH, Switzerland) in 0.1 M KPi buffer (1:5 w/v, pH 6.0) containing polyvinylpyrrolidone (4%, PVP) and the protease inhibitor phenylmethanesulfonyl fluoride (PMSF, 1 mM). Coarse homogenates were filtered through four layers of gauze and centrifuged at 12000 x g for 15 min. 10-ml syringes fixed with 0.45 µm nitrocellulose filters were used to further purify protein extracts. Proteins in the extracts were concentrated using ultra-filters (2-ml Vivaspin micro-concentrators, 10,000 molecular weight cut off, Sartorius Stedim, Biotech GmbH, Germany) by centrifugation (3000 x g) at 4°C. Retained protein suspensions were used for enzyme assays.
Chitinase activity (CHI) was detected as the amount of N-acetyl-D-glucosamine (NAG) produced from chitobiose by the β-glucuronidase introduced in the reaction mixture [
44]. Root extracts (100 µl) were diluted in 150 µl of 0.05 M sodium acetate buffer (pH 5.2) containing 0.5 M NaCl and mixed with suspended chitin (250 µl, 10 mg/ml) from shrimp shells (Sigma-Aldrich, Italy). Reactions were started by incubating the mixtures in 1.5 ml-Eppendorfs for 1 h at 37°C in an orbital incubator and stopped by boiling at 100°C for 5 min in a water bath. Then, reaction mixtures were centrifuged at 10,000 x
g for 5 min at room temperature, and 300 µl of supernatants collected and added with 5 µl β-glucuronidase (Sigma, type HP-2S, 9.8 units/ml). After start and end of reactions done as previously described, mixtures were cooled at room temperature, then again heated to 100°C for 3 min after having added 60 µl of 0.8 M potassium tetraborate (pH 9.1) and cooled. An incubation at 37°C for 20 min after addition of 1% 4-dimethylamino -benzaldehyde (1.2 ml, DMAB, Sigma) was the next step. Absorbance was read at 585 nm (DU-70, Bechman) and the amount of NAG produced was determined by means of a standard curve obtained with known concentrations (4.5-90 nmoles) of commercial NAG (Sigma). Blanks (negative controls) were mixtures in which tissue extracts were not added; positive controls were arranged by adding 10 µl chitinase from
Streptomyces griseus (Sigma, 200 units/g). Chitinase expressed as nanokatal g
-1 rfw, with 1 nkat defined as 1.0 nmol NAG produced per second at 37°C.
β-1,3-Endoglucanase (glucanase, GLU) activity was measured by its capacity to release glucose from laminarin (Sigma, Italy). Reaction mixtures, incubated at 37°C for 30 min, consisted in laminarin (0.4 mg) and 100 µl root extracts in 300 µl 0.1 M sodium acetate (pH 5.2). To test glucose production, Nelson alkaline copper reagent (300 µl) was added in the mixtures that were heated at 100°C for 10 min and then cooled at room temperature. Nelson chromogenic reagent (100 µl) was added and the absorbance of suspensions read at 500 nm [
45]. Negative and positive controls were arranged by substitution of root extracts with grinding buffer and addition of laminarinase (2 U/ml), respectively. Enzyme activity was expressed as µmol glucose equivalents released per minute per g rfw according to a standard curve created with known amounts (10-200 µg ml
-1) of commercial glucose (Sigma, Italy).
4.7. RNA Extraction, cDNA Synthesis, and Quantitative Real-Time Polymerase Chain Reaction
Roots from untreated and Myco-treated plants were collected and weighed at 8 dpt and 3 dpi by M. incognita. Root samples were ground to powder in a porcelain mortar in liquid nitrogen. Each total RNA extraction was carried out from 100 mg macerated tissue using an RNA-easy Plant Mini Kit (Qiagen, Germany) according to the instructions specified by the manufacturer. RNA quality was verified by electrophoresis runs on 1.0% agarose gel and quantified using a Nano-drop spectrophotometer. QuantiTect Reverse Transcripton Kit (Qiagen, Germany) with random hexamers was used for cDNA synthesis from 1 μg of total RNA, according to the manufacturer’s instructions. PCR mixtures (20-μl final volume) contained RNAse free water, 0.2 μM each of forward and reverse primers, 1.5 μl cDNA template and 10 μl SYBR® Select Master Mix (Applied Biosystems, Italy). PCR cycling consisted in an initial denaturation step at 95 °C (10 min); 40 cycles at 95 °C (30 s), at 58 °C (30 s), at 72 °C (30 s), with a final extension step at 60 °C (1 min). qRT-PCRs were performed in triplicate using an Applied Biosystems® StepOne™ instrument. The following tomato genes were tested: PR2 (NM001247229, encoding β-1,3-glucanase) and PR3 (Z151140, encoding endochitinase). For each oligonucleotide set, a no-template water control was used. Actin-7 (NM_001308447.1, ACT-7) was used as the reference gene for quantification, as it was experimented that its expression did not vary according to treatments. Gene transcript levels were expressed as 1/ΔCt, where ΔCt is the difference between the cycle thresholds of fluorescence signal of the tested gene and the signal of the reference gene (Actin 7).
4.8. Experimental Design and Statistical Analysis
Data from plants treated with different rhizosphere microorganisms were confronted with those from untreated control plants. Data shown in each figure and related supplementary table are the means of values coming from 3 different bioassays; in each bioassay, the same treatment was applied to groups of 8 tomato plants. Means of plant growth factors were then calculated from 24 replicates. Conversely, one value of infection factors was obtained from 2 plants, to have 4 replicates per experiment. Means of each factor, then, are calculated from 12 replicates. Conversely, in the experiments in which Myco is added with priming effectors, 2 bioassays were performed, then, means of growth factors had 16 whilst those of infection factors 8 replicates. Means ± standard deviations were separated by a paired t-test (Significance Levels: *P<0.05 or **P<0.01), using MS Excel Software.
For RNA extraction, plants coming from 2 independent bioassays were used; roots from 2 plants of the same group constituted one sample; RNA was extracted from 3 different samples of roots per treatment, harvested at 8 dpt and 3 dpi. qRT-PCR data are expressed as means (n=6) ± standard deviations of 1/ΔCt (Ct target gene - Ct actin). 1/ΔCt means ± standard deviations were differentiated by a paired t-test (Significance Levels: *P<0.05), using MS Excel Software.