Submitted:
29 September 2023
Posted:
29 September 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
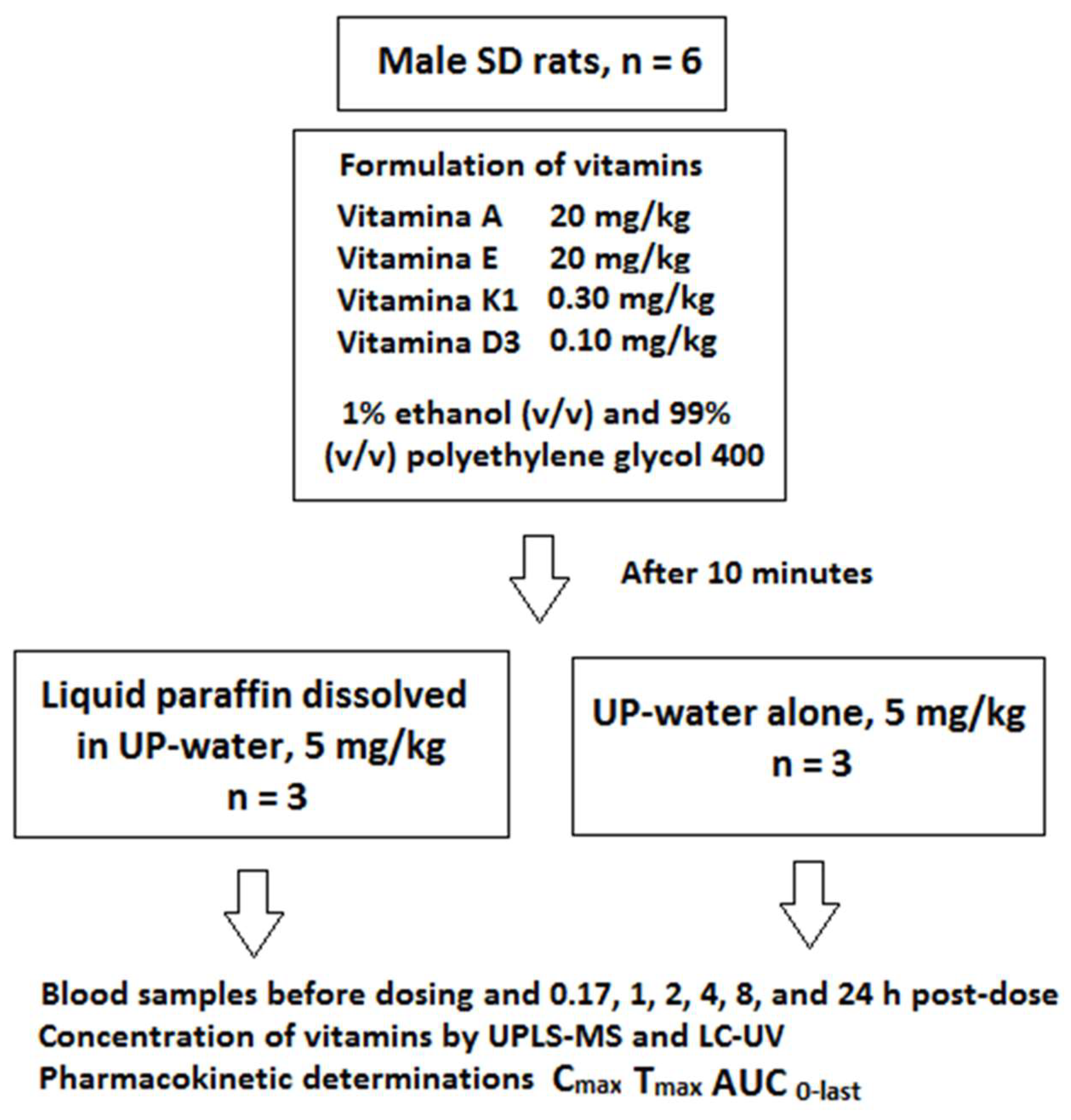
2.1. Study Design
2.2. Animal Experiment and Formulation Preparation
2.3. Analysis and Pharmacokinetic Parameters
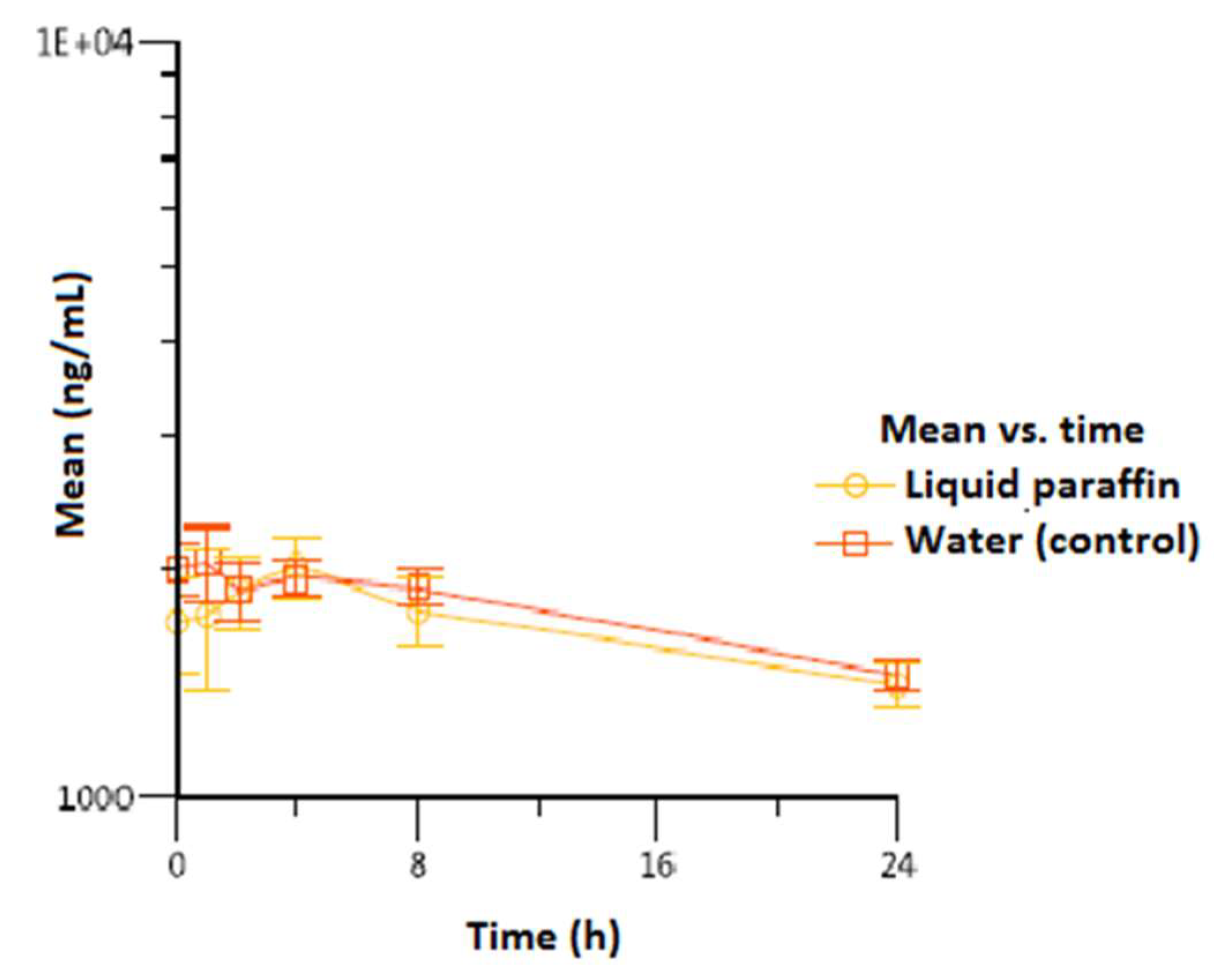
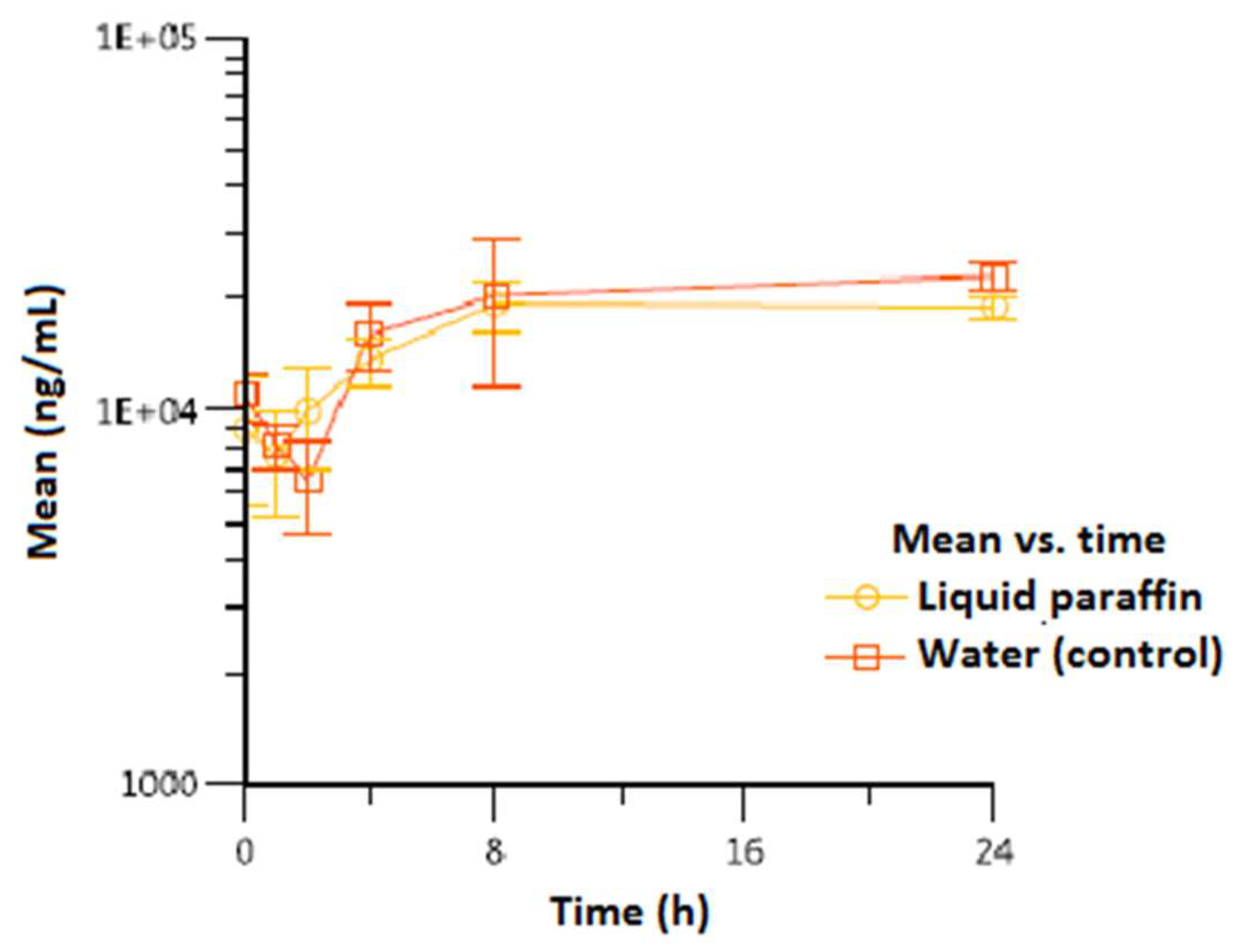
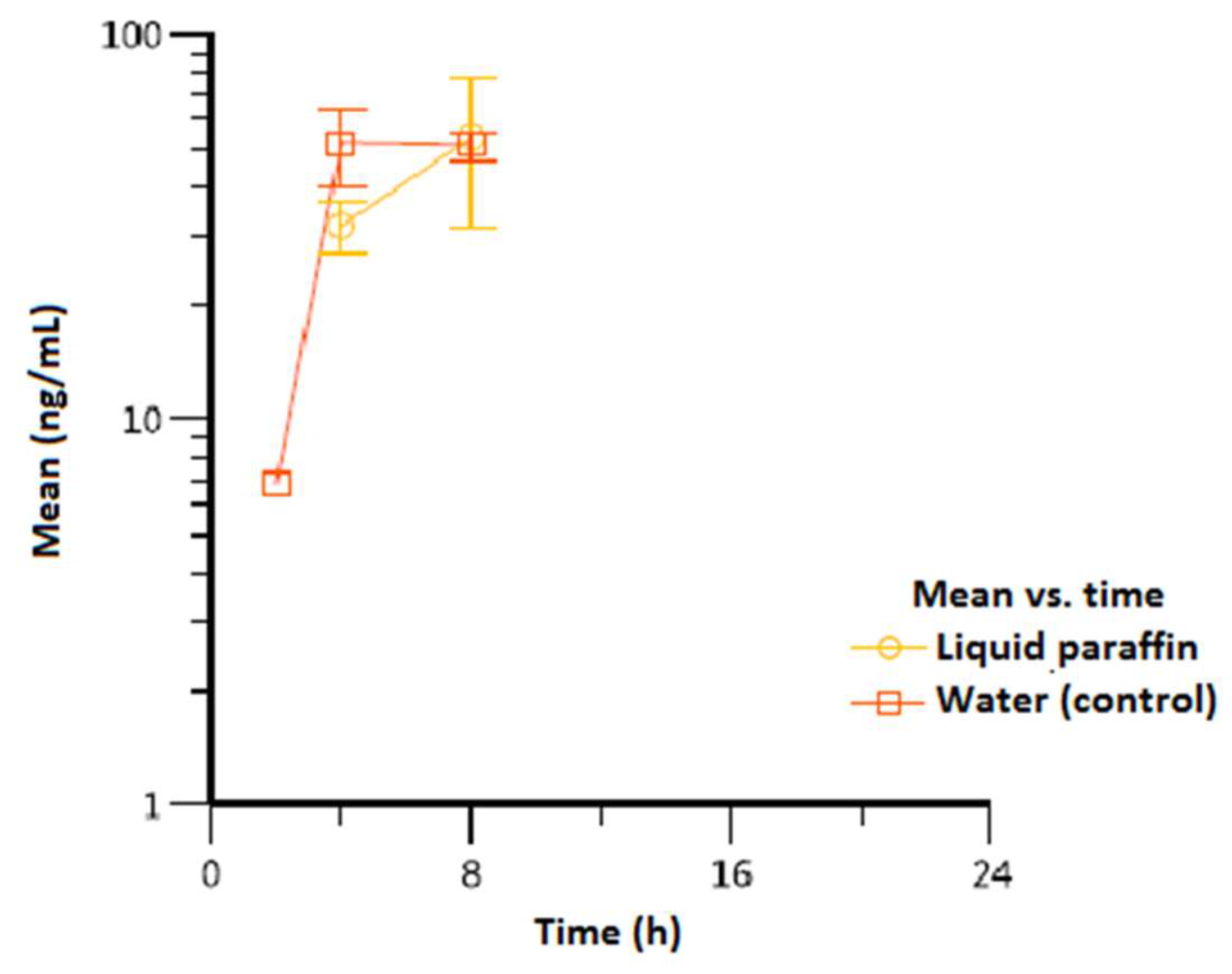
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Basilisco, G.; Coletta, M. Chronic constipation: a critical review. Dig Liver Dis 2013, 45, 886–893. [Google Scholar] [CrossRef] [PubMed]
- Rao, S.S. Constipation: evaluation and treatment of colonic and anorectal motility disorders. Gastroenterol Clin North Am 2007, 36, 687–711. [Google Scholar] [CrossRef] [PubMed]
- Cullen, G.; O'Donoghue, D. Constipation and pregnancy. Best Pract Res Clin Gastroenterol 2007, 21, 807–818. [Google Scholar] [CrossRef] [PubMed]
- Sobrao, C.W.; Corrêa Neto, I.J.P.; Ambar Pinto, R.; Sobrado, L.F.; Nahas, S.C.; Cecconello, I. Diagnosis and treatment of constipation: a clinical update based on the Rome IV criteria. J Coloproctol 2018, 32, 137–144. [Google Scholar]
- Pinto Sanchez, M.I.; Bercik, P. Epidemiology and burden of chronic constipation. Can J Gastroenterol 2011, 25 (Suppl B), B),11B–15B. [Google Scholar] [CrossRef]
- Higgins, P.D.; Johanson, J.F. Epidemiology of constipation in North America: a systematic review. Am J Gastroenterol 2004, 99, 750–759. [Google Scholar] [CrossRef]
- Peppas, G.; Alexiou, V.G.; Mourtzoukou, E.; Falagas, M.E. Epidemiology of constipation in Europe and Oceania: a systematic review. BMC Gastroentrol 2008, 8, 5. [Google Scholar] [CrossRef]
- Escudero Sanchís, A.; Bixquert Jiménez, M. Guía para prevenir y tratar el estreñimiento. Fundación Española de Aparato Digestivo (FEAD). Available online: https://www.saludigestivo.es/wp-content/uploads/2016/03/guia-estrenimiento-para-web-20120425180854.pdf. (accessed on 20 February 2021).
- Belsey, J.; Greenfield, S.; Candy, D.; Geraint, M. Systematic review: impact of constipation on quality of life in adults and children. Aliment Pharmacol Ther 2010, 31, 938–949. [Google Scholar] [CrossRef]
- Mearín, F. Impacto del estreñimiento crónico en la calidad de vida: mucho más importante de lo que parece. Prog Gastroenterol 2013, 36, 443–498. [Google Scholar] [CrossRef]
- Johanson, J.F.; Kralstein, J. Chronic constipation: a survey of the patient perspective. Aliment Pharmacol Ther 2007, 25, 599–608. [Google Scholar] [CrossRef]
- Sharif, F.; Crushell, E.; O’Driscoll, K.; Bourke, B. Liquid paraffin: a reappraisal of its role in the treatment of constipation. Arch Dis Child 2001, 85, 121–124. [Google Scholar] [CrossRef]
- Hadda, V.; Khilnani, G.C. Lipoid pneumonia: an overview. Expert Rev Respir Med 2010, 4, 799–807. [Google Scholar] [CrossRef] [PubMed]
- Alaminos García, P.; Colodro Ruiz, A.; Menduiña Guillén, M.J.; Báñez Sánchez, F.; Pérez Chica, G. Neumonía lipoidea exógena. Presentación de un nuevo caso. An Med Interna (Madrid) 2005, 22, 283–284. [Google Scholar] [CrossRef]
- Aliaga, F.; Chernilo, S.; Fernández, C.; Valenzuela, H.; Rodríguez, J.C. Neumonía lipoidea exógena: uma causa inabitual de nódulos pulmonares. Casos clínicos. Rev Med Chil 2017, 145, 1495–1499. [Google Scholar] [CrossRef] [PubMed]
- Osman, G.A.; Ricci, A.; Terzo, F.; Falasca, C.; Giovagnoli, M.R.; Bruno, P.; et al. Exogenous lipoid pneumonia induced by nasal decongestant. Clin Respir J 2018, 12, 524–531. [Google Scholar] [CrossRef]
- Bandla, H.P.; Davis, S.H.; Hopkins, N.E. Lipoid pneumonia: a silent complication of mineral oil aspiration. Pediatrics 1999, 103, E19. [Google Scholar] [CrossRef] [PubMed]
- Ohwada, A.; Yoshioka, Y.; Shimanuki, Y.; Mitani, K.; Kumasaka, T.; Dambara, T.; et al. Exogenous lipoid pneumonia following ingestion of liquid paraffin. Intern Med 2002, 41, 483–486. [Google Scholar] [CrossRef]
- Clark, J.H.; Russell, G.J.; Fitzgerald, J.F.; Nagamori, K.E. Serum beta-carotene, retinol, and alpha-tocopherol levels during mineral oil therapy for constipation. Am J Dis Child 1987, 141, 1210–1212. [Google Scholar]
- Ballantine, T.V.N.; Zeigler, D.; Greecher, C.P.; Smith, J.; Karl, S.R. The effect of mineral oil (MO) on fat-soluble vitamin levels. [Abstract]. JPEN J Parenter Enteral Nutr 1986, 10, S18. [Google Scholar]
- McClung, H.J.; Boyne, L.J.; Linsheid, T.; Heitlinger, L.A.; Murray, R.D.; Fyda, J.; et al. Is combination therapy for encopresis nutritionally safe? Pediatrics 1993, 91, 591–594. [Google Scholar] [CrossRef]
- Gal-Ezer, S.; Shaoul, R. The safety of mineral oil in the treatment of constipation--a lesson from prolonged overdose. Clin Pediatr (Phila) 2006, 45, 856–858. [Google Scholar] [CrossRef]
- Tulliez, J.; Bories, G.; Peleran, J.C. The effect of prolonged ingestion of paraffin oil in pigs: selective retention and interference with cholesterol metabolism. C R Acad Sci Hebd Seances Acad Sci D 1975, 280, 2261–2264. [Google Scholar]
- Abuasal, B.; Thomas, S.; Sylvester, P.W.; Kaddoumi, A. Development and validation of a reversed-phase HPLC method for the determination of γ-tocotrienol in rat and human plasma. Biomed Chromatogr 2011, 25, 621–627. [Google Scholar] [CrossRef]
- Craciun, A.M.; Groenen-van Dooren, M.M.; Vermeer, C. Nutritional vitamin K-intake and urinary gamma-carboxyglutamate excretion in the rat. Biochim Biophys Acta 1997, 1334, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Gershkovich, P.; Ibrahim, F.; Sivak, O.; Darlington, J.W.; Wasan, K.M. A simple and sensitive method for determination of vitamins D3 and K1 in rat plasma: application for an in vivo pharmacokinetic study. Drug Dev Ind Pharm 2014, 40, 338–344. [Google Scholar] [CrossRef] [PubMed]
- Kanazawa, H.; Miyata, C.; Nagata, Y.; Urano, S.; Matsushima, Y. Determination of alpha-tocopherol and alpha-tocopherylquinone in rat tissues and plasma by high-performance liquid chromatography with electrochemical detection. Chem Pharm Bull (Tokyo) 2000, 48, 1462–1466. [Google Scholar] [CrossRef]
- Tovar, A.; Ameho, C.K.; Blumberg, J.B.; Peterson, J.W.; Smith, D.; Booth, S.L. Extrahepatic tissue concentrations of vitamin K are lower in rats fed a high vitamin E diet. Nutr Metab (Lond) 2006, 3, 29. [Google Scholar] [CrossRef] [PubMed]
- Uchida, T.; Nomura, S.; Ichikawa, T.; Abe, C.; Ikeda, S. Tissue distribution of vitamin E metabolites in rats after oral administration of tocopherol or tocotrienol. J Nutr Sci Vitaminol (Tokyo) 2011, 57, 326–332. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Pan, X.; Chen, G.; Li, J.; Liu, L.; Liu, X.; Jin, S.; Xie, L.; Wang, G. Increased exposure of vitamin A by Chrysanthemum morifolium Ramat extract in rat was not via induction of CYP1A1, CYP1A2, and CYP2B1. J Food Sci 2012, 77, H121–127. [Google Scholar] [CrossRef]
- European Medicines Agency (EMA). Committee for Medical Products for Human Use (CMPH). Guideline on the investigation of bioequivalence. Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-investigation-bioequivalence-rev1_en.pdf (accessed on 20 February 2021).
- Bassotti, G.; Usai Satta, P.; Bellini, M. Chronic idiopathic constipation in adults: A review on current guidelines and emerging treatment options. Clin Exp Gastroenterol 2021, 14, 413–428. [Google Scholar] [CrossRef] [PubMed]
- Paré, P.; Fedorak, R.N. Systematic review of simulant and nonstimulant laxatives for the treatment of functional constipation. Can J Gastroenterol Hepatol 2014, 28, 549–557. [Google Scholar] [CrossRef] [PubMed]
- Gordon, M.; Naidoo, K.; Akobeng, A.K.; Thomas, A.G. Cochrane Review: Osmotic and stimulant laxatives for the management of childhood constipation (Review). Evid Based Child Health 2013, 8, 57–109. [Google Scholar] [CrossRef] [PubMed]
- Gordon, M.; MacDonald, J.K.; Parker, C.E.; Akobeng, A.K.; Thomas, A. Osmotic and stimulant laxatives for the management of childhood constipation. Cochrane Database Syst Rev 2016, Issue 8. Art. No.: CD009118. [CrossRef]
- Javert, C.T.; Macri, C. Prothrombin concentration and mineral oil. Am J Obstet Gynecol 1941, 42, 409–414. [Google Scholar] [CrossRef]
- Curtis, A.C.; Ballmer, R.S. The prevention of carotene absorption by liquid petrolatum. JAMA 1939, 113, 1785–1788. [Google Scholar] [CrossRef]
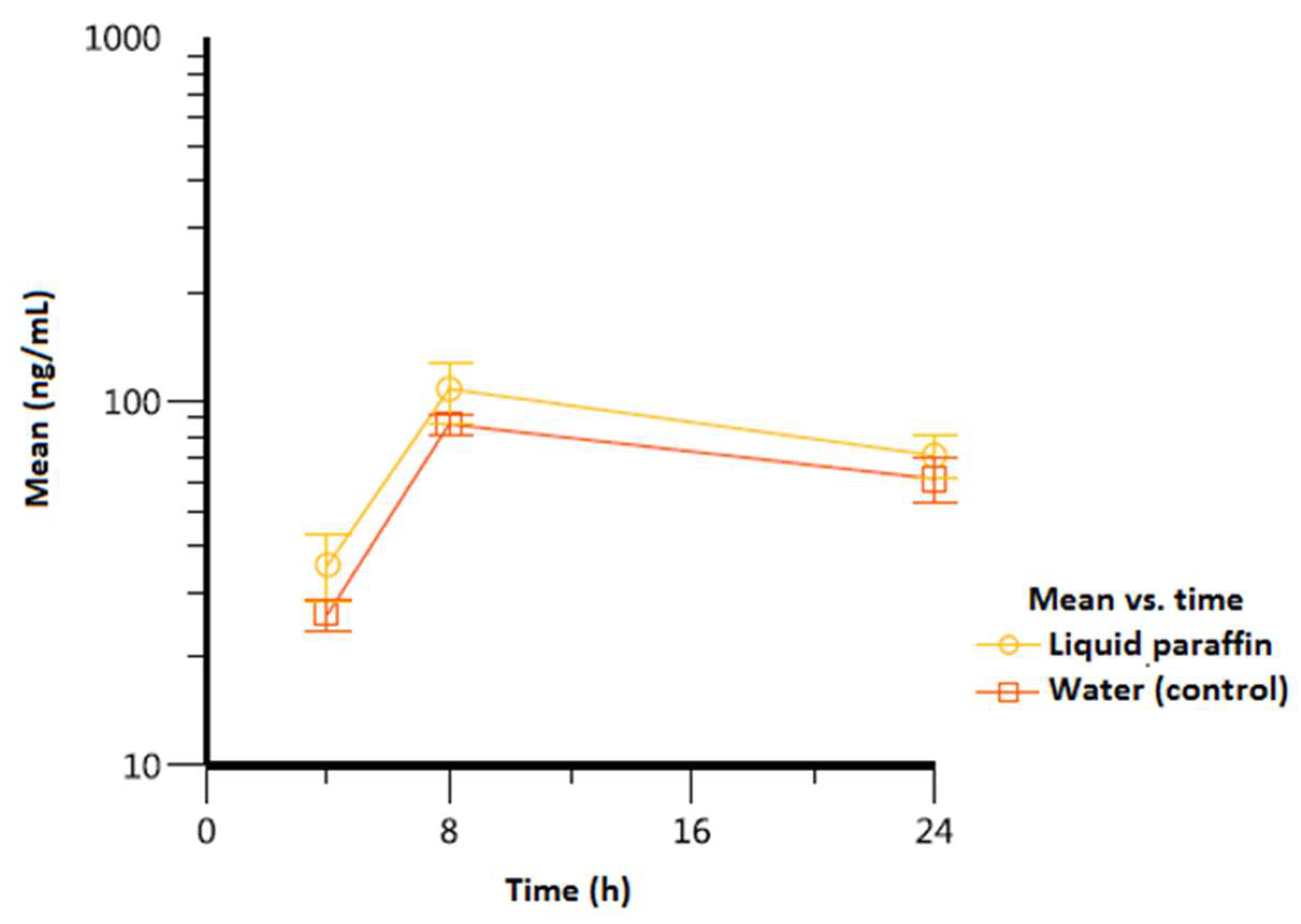
| Experimental condition |
Tmax, h per animal* |
Cmax, ng/mL mean (SD) |
Tlast, h per animal* |
Clast, ng/mL mean (SD) |
AUC0-last, h·ng/mL mean (SD) |
|---|---|---|---|---|---|
| Vitamin A | |||||
| Liquid paraffin | 4/4/4 | 1.023 (200) | 24/24/24 | 1.407 (96.1) | 40.358 (4.012) |
| Water (control) | 1/1/4 | 2.130 (130) | 24/24/24 | 1.453 (66.6) | 42.245 (1.717) |
| Vitamin E | |||||
| Liquid paraffin | 24/8/24 | 19.900 (2.784) | 24/24/24 | 18.967 (1.401) | 413.535 (48.526) |
| Water (control) | 24/8/24 | 25.367 (4.636) | 24/24/24 | 22.933 (1.801) | 458.453 (100.118) |
| Vitamin K1 | |||||
| Liquid paraffin | 8/8/8 | 54.1 (22.5) | 8/8/8 | 54.1 (22.5) | 637 (237) |
| Water (control) | 8/8/4 | 57.3 (7.70) | 8/8/8 | 51.8 (4.68) | 686 (57.0) |
| Vitamin D3 | |||||
| Liquid paraffin | 8/8/8 | 108 (20.6) | 24/24/24 | 71.2 (9.42) | 1.796 (136) |
| Water (control) | 8/8/8 | 86.7 (5.49) | 24/24/24 | 61.4 (8.47) | 1.463 (12.7) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




