1. Introduction
Chronic venous disease (CVD) is a frequently encountered disease and a common health care problem. The prevalence of this condition remains underestimated, early evidence of CVD being frequently overlooked by general practitioners. Clinical manifestations of CVD can range from mild to severe, such as telangiectasia, varicose veins (most notably), lipodermatosclerosis or venous ulceration [
1,
2]. Although the use of varicose veins as a graft remains controversial and often reserved for cases where there are no other healthy veins available, they can sometimes be a feasible option [
3]. In light ofthis data, we could assume that a large part of the collected samples could be used as a graft for bypass procedures. Most frequently, the harvesting procedure involves open surgery. This procedure, however, has some disadvantages, among which we mention the large incision that is prone to vicious scarring and a greater risk of complications such as hematoma, seroma or wound infection [
4]. However, interventional and endoscopic procedures are the current standards in the treatment of various venous diseases [
5], and recent data describing the possibility of endoscopic vein harvesting have been reported [
6]. Considering this aspect, as well as the fact that cryostripping is a minimally invasive procedure compared to classic surgery [
7], the question arises whether the veins harvested by cryosurgery retain their viability and can be used as grafts.
Cryosurgery for varicose veins removal is not a new procedure, and there are several articles in the literature that would support this procedure [
8,
9]. The saphenous vein freezing procedure and its use as a graft was originally described in 1975 [
10], when a single case treated with this procedure was reported. This case presentation has not been subsequently reconfirmed on a larger scale and to our knowledge, no other publications on this topic are available. Considering the period in which the articles cited above were published, the specimens were not investigated from a molecular point of view, which we consider essential for the viability of the normal or varicose vein specimen.
For these reasons, we will analyze in our study some of the components of the wall of varicose veins that cannot be highlighted by morphological and histochemical methods. We consider the immunohistochemical and molecular evaluation at the level of protein expression to be original, since we have not identified similar data in the literature. We believe that the molecular profile we are evaluating further complements the morphological and histochemical data in enabling graft viability from varicose veins.
The investigation of the molecular profile of the intima and the endothelium, respectively, was based on the application of two markers, namely CD34, a highly specific marker for the vascular endothelium, and Ki67, which signals cells during division and in the period immediately following it.
CD34 is predominantly considered a marker of hematopoietic stem and progenitor cells, but also of endothelial cell progenitors and mature endothelial cells. Although the molecule has been identified for half a century, its functional function is still only partially known [
11]. CD34 is a transmembrane phosphoglycoprotein identified in 1984 on hematopoietic cells. It has an extracellular site with numerous glycosylation sites. There is a single transmembrane helix and a cytoplasmic tail, the most common ligand being L-selectin. Although current studies have not elucidated the functions of this molecule, current data suggest the involvement of the molecule in cytoarchitectonics and the regulation of cell differentiation and proliferation. It is possible that the CD34 molecule is involved in the trafficking of hematopoietic progenitors. Immunohistochemically identified CD34 has the final reaction product localized to the endothelium of blood vessels and usually does not stain the endothelium of lymphatic vessels. Much of the current data supports that CD34+ endothelial cells are dormant, considered to have major implications in adhesion and migration. In this way, CD34 is directly involved in the phenomenon of endothelial sprouting, respectively in angiogenesis [
12]. Considering the brief data above, we consider CD34 evaluation to be a mandatory step in testing the viability of a possible vein graft.
Ki67 is a highly specific marker for cells in division and in the immediate postmitotic period. It is not a specific marker for endothelial cells at all, but it identifies dividing cells by nuclear reaction end product. Endothelial cells have a long lifespan, which under normal conditions exceeds 1000 days. When stimulated by different agents they can proliferate relatively rapidly, demonstrating viability as well as the ability to repair endothelial damage. Ki67 is a marker that is actively involved in defining whether a lesion is benign or malignant, as well as in the chemotherapy response of individual cases [
13]. The proliferation rate of endothelial cells in the tumor area has been investigated by few authors, possibly due to the absence of a standardized methodology. Double immunostaining for the endothelial marker-Ki67 was especially practiced in tumors, and cells with double cytoplasmic and nuclear signal were considered proliferative endothelial [
14,
15]. There are no known data on the proliferation rate of the endothelium of varicose veins, an aspect that we analyze in addition to the study of the endothelial reaction for CD34.
The aim of this study was to evaluate the viability of the venous fragments harvested by cryostripping, in order to determine if the technique lends itself to the harvesting of venous fragments which potentially can be used as grafts.
2. Materials and Methods
Patients. The study included 109 venous specimens, each one of them being taken from patients operated for varicose veins by cryostripping method. The principle of this procedure consists of venous catheterisation with a special probe which is cooled to −85 °C. Due to these very low temperatures, the venous intimal layer strongly adheres to the probe, thus enabling the stripping of the insufficient vein. The probe is made of smooth metal materials, and it can be easily inserted in the venous lumen even if the vein path is tortuous. By connecting the probe to the ERBOKRYO device (Erbe USA Incorporated 2225 Northwest Parkway Marietta, GA 30067, USA) using liquid nitrogen the cooling is subsequently carried out. When the insufficient vein adheres to the probe (this phenomenon usually occurs in about 5 seconds), it is removed by repeated traction [
6]. Each specimen was subsequently divided in 6 samples, one sample being directly fixed in formalin, and the others being maintained in serum for two, four, twelve, twenty-for and forty-eight hours and after that fixed. For each case, all the 6 samples were microsopically analyzed then in both immunohistochemical stains, CD34 and Ki67, in order to establish the viability of the specimen in time.
Immunohistochemistry. The simple immunohistochemical methods were performed in a fully automated and standardized procedure for all cases, with the Leica Bond-Max autostainer (Leica Biosystems, Newcastle upon Tyne, UK). Paraffin sections were treated to the Bond Epitope Retrieval Solution 2 for 20 minutes (Leica Biosystems, Newcastle Ltd.). Endogenous peroxidase was blocked with 3% hydrogen peroxide for 5 minutes. The sections were then incubated for 30 minutes with the primary antibodies-CD34 (Leica Bond, RTU, clone QBEnd 10) and Ki 67 (Leica Bond, RTU, clone MM1). For visualization the Bond Polymer Refine Detection System was used. It includes the secondary antibody (8 minutes) and the polymer with an incubation time of 8 minutes. The chromogen was 3, 3 diamino-benzidine dihydrochloride and hematoxylin was used as counterstain.
Microscopic evaluation and image analysis. The quantification methods took into account the following aspects. For CD34, the localization of the end product of the reaction (the three layers of the venous wall: intima, media and adventitia), the continuity of the endothelium and the associated positive tissue elements. For Ki67, only the positive cellular elements of the endothelium level were considered, and they were evaluated as microscopic field density at x400 magnification.The morphologically and immunohistochemically stained sections were analyzed on Zeiss Axiocam 506 micro-scopes (Jena, Germany) and Nikon AY260, both equipped with a real-time imaging system and software for digital microscopic image analysis.
Statistical analyses. Statistical analyses were performed using Statistical Package forSocial Sciences version 16 (SPSS Inc., Champaign, IL, USA). The results were statistically analyzed using Chi-squared test. The Kolmogorov-Smirnov test was used to analyze the normal distribution of variables. A resulting p-value of <0.05 was considered statistically significant and was assessed at a 95% confidence interval.
3. Results
The study included 109 venous specimens collected from patients undergoing varicose vein cryostentric surgery. Each specimen was divided into six groups: one was immediately fixed in formalin (zero-hourtime) and subsequently fixed, and the other was placed in physiological solution for 2, 4, 12, 24 and 48 hours before fixation. Immunohistochemical analysis was performed on each sample to assess the expression of CD34 and Ki67, two markers of endothelial cell viability and proliferation.
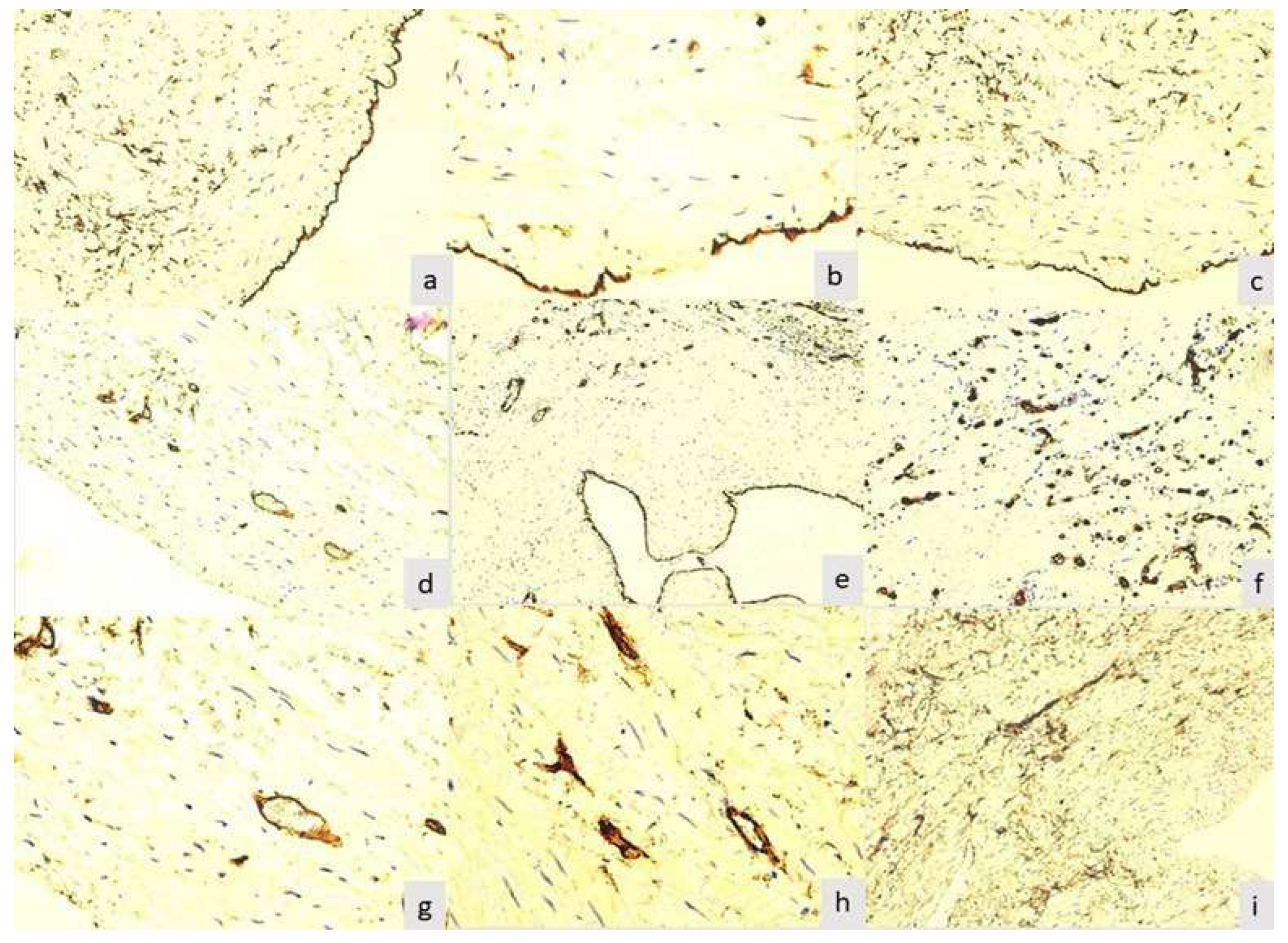
The final product of the immunoreaction for CD34 for zero-hourtimewas located at all three levels of the wall of varicose veins excised by cryostripping. At the level of the intima, the layer of endothelial cells was intensely and homogeneously positive (p=0.001). This aspect was found in 89(81.61%) of the 109 samples studied (
Figure 1a).
Between zero-hour time and 12 hours, no major changes were observed in the samples studied, which were also statistically significant (zero-hour time: p-value=0.0021; 2 hours: p-value=0.0136; 4 hours: p-value=0.0348). Changes in all layers appeared after 12 hours (12 hours: p-value=0.0499; 24 hours: p-value= 0.0582), but especially at the intima layer started to be seen after the samples had been in physiological solution for 12 hours, so that at the endothelium level lacunar expressions appeared in 32 (29.35%) cases and at 24 hours multiple lacunar expressions appeared in 53 (48.62%) cases.
After 48 hours in physiological solution, it was noticed a single (
Figure 1b) or multiple (
Figure 1c) lacunar expression.These aspects underlined damage to the endothelium induced by the surgical procedure used, associated with the duration of preservation of the specimens in physiological serum. Therefore, at the level of the endothelium, 37 (33.94%) specimens were negative for this immunoreaction (
Figure 1d). Small blood vessels in the media served as an internal positive control, with positive endothelium. In 97 (88.99%) of the samples studied, the subintimal space did not show positive cells for this reaction.
Three cases were found that showed typical aspects of recanalized thrombus, where some particular aspects were observed. The endothelium of all evaluated lumens was intensely positive for CD34. In its immediate vicinity in the subintimal space, was not observed any other positive cells or small neoformation vessels(
Figure 1e).Small vessels, with characteristic morphology for capillaries and postcapillary venules, were present at a relatively large distance from the neointimal space, being included in a tissue dominated by connective cellular elements, with rare fibers and without acute or chronic inflammatory infiltrate.The small vessels in the recanalized thrombi showed thick endothelium with a visible lumen or not (
Figure 1f), signaling the presence of neoformation vessels. The density of these vessels was very high per surface unit, coinciding with the proliferation phase of the endothelium. For this reason, these aspects recapitulate some stages of physiological angiogenesis, but we believe that the thrombosed venous vessel model is particularly useful not only for understanding some stages of angiogenesis, but also for testing inhibitory medication on this process.
After 48 hours, in 46 (42.2%) of the 109 samples studied, was observed the normal appearance of the venous media, respectively showing smooth muscle cells and small blood vessels in the middle area (
Figure 1g). Some cases showed average blood vessels with one or more buds, suggesting endothelial cell activation (
Figure 1h). The vessels in which were noticed aspects of budding did not present visible lumen in optical microscopy, being classified as immature. In the other cases, and especially in those with partial or no endothelium stained for CD34, was found obliteration of the border between media and adventitia. Adventitial cells diffusely and heterogeneously invaded the media, up to the vicinity of the subintimal space (
Figure 1i). The adventitial cells show long, elongated cytoplasmic extensions, which tend to be a dense network in the external portion of the media, being in direct continuity with the adventitia. Although the morphology in this technical form is a static investigation, we believe that these aspects suggest the mobilization and migration of adventitial cells towards the other layers of the venous wall, a phenomenon that obviously occurs during the evolution of the venous disease.
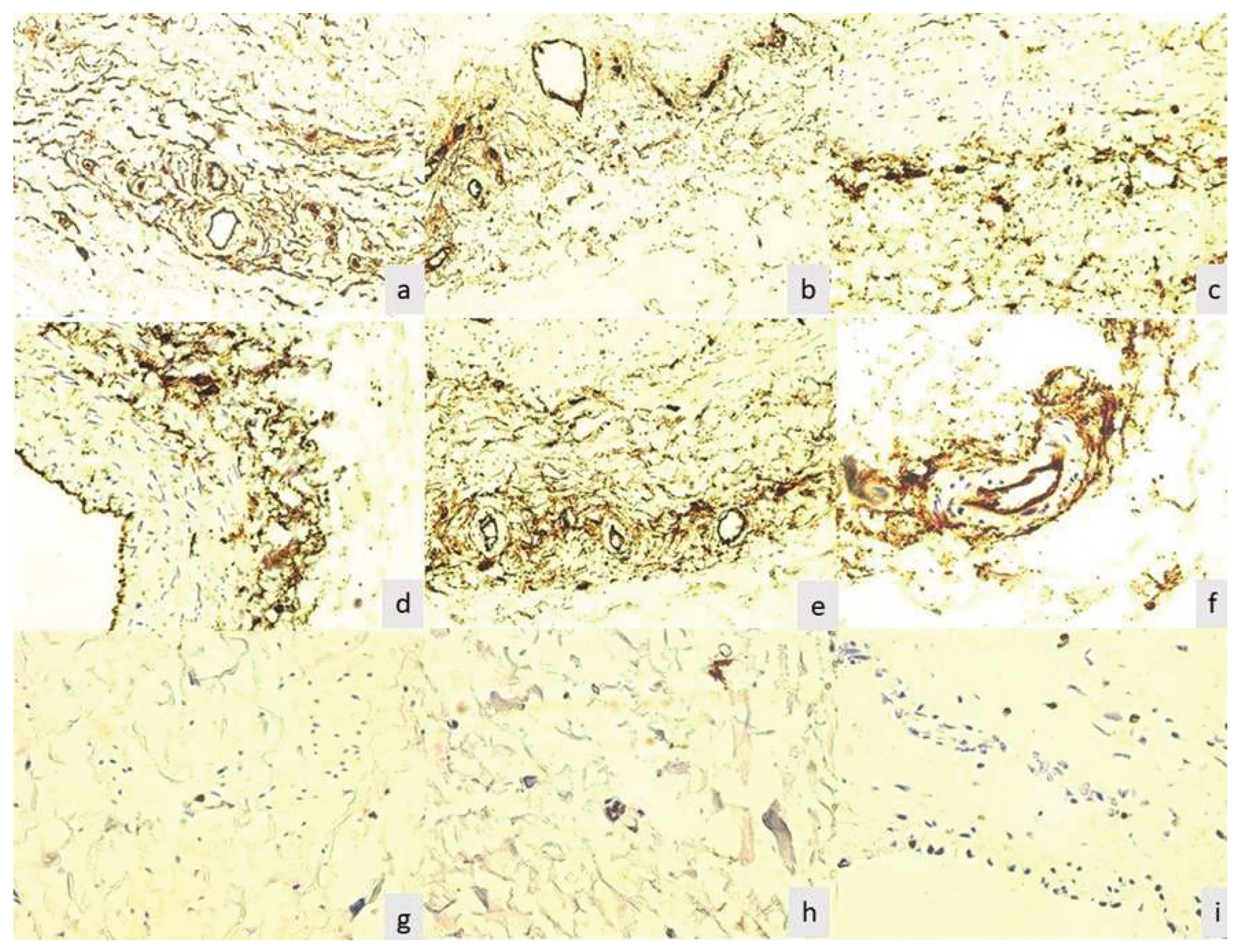
The adventitia was intensely stained in all the evaluated cases after 48 hours. In this immunoreaction, it appears in the form of a cellular network with higher density around the vasa vasorum (
Figure 2a). This aspect is independent of the nature of the arteriolar or venular vessel it surrounds. In cases where the lumen of the vessel is wider, the density of adventitial cells was reduced (
Figure 2b).In the cases with major morphological changes of the media and intima, was noted the mobilization and concentration of adventitial cells at the border of the media (
Figure 2c), as well as their presence in the middle of the media (
Figure 2d).In these cases, it was no longer observed vessels with a permeable lumen, previously described for the tunica media. In the adventitia, CD34-positive cells strictly adhered to the arteriolar media, as seen in
Figure 2e,f.
In
Table 1, the study presents a comprehensive analysis of CD34 expression across the three layers of venous wall tissue (intima, media, and adventitia) at various time points, ranging from zero-hour to 48 hours, following varicose vein cryostripping surgery. These results shed light on the dynamic changes in CD34 expression, reflecting endothelial cell activity and the impact of preservation duration in physiological solution. Notably, at the zero-hour time point, CD34 expression was intensely positive in the intima, demonstrating endothelial vitality. The study observed significant alterations in CD34 expression at the 12-hour time point, particularly in the intima, where lacunar expressions began to appear, indicating endothelial damage. These changes continued to evolve at the 24-hour and 48-hour marks, emphasizing the importance of preservation duration. The data also highlight variations in CD34 expression between the different layers of the venous wall. Overall, this comprehensive analysis provides valuable insights into the time-dependent alterations in CD34 expression within the context of varicose vein pathology and surgical intervention.
The final product of the immunohistochemical reaction for Ki67 was nuclear restricted. Only cells with a clear and intensely brown-stained nucleus were considered positive, regardless of the examined venous layer. Was evaluated the reaction from endothelium, the venous media and the vasa vasorum from adventitia. All specimens that were fixed after 48 hourswere negative for Ki67.
In zero-hour time, it was noted that the highest number of positive cells at the level of the small vessels in the adventitia of the extirpated varicose veins, on a cross section, between 1 and 3 positive nuclei can be observed on a cross and oblique section (
Figure 2 g, h). It was found that Ki67 positive nuclei in the adventitia in 37 (33.94%) cases. In comparison, none of the cellular elements of adventitia were positive for this reactionafter 12 hours.
On average, 11.92% of samples showing only rare smooth muscle cells were positive, a subjective percentage of less than 1% of the cells, well below the normal rate of multiplication in smooth muscle tissue of the vascular wall. The endothelium showed Ki67-positive cells only in isolated form, reflecting the long lifespan of these cells (
Figure 2i). It was reported positive endothelial reaction in 11 (10.09%) specimens. The reaction was negative for all cellular elements forming the valves captured by the histological section. In the case of recanalized thrombi, was not noticed a positive reaction to any of the cell types mentioned above.
Table 2 shows a detailed examination of Ki67 expression within the vein wall at different time points after varicose vein cryosurgery. The results provide valuable insight into cell proliferation and activity in the context of venous tissue. In particular, at time zero hours, the three layers of the venous wall show the highest number of Ki67 positive cells, especially in small vessels. However, after 12 and 24 hours, Ki67-positive cells were absent in the adventitia, highlighting the transitional nature of this proliferation. Interestingly, all specimens fixed after 12 hours showed a marked decrease in expression, and after 48 hours showed Ki67-negative expression, highlighting the end of proliferation at prolonged preservation periods.
4. Discussion
Due to the increased incidence of vascular diseases [
16,
17], vascular procedures are worldwide widely practiced. Along with stenting and endarterectomy [
18,
19], revascularization surgical procedures are frequently practiced today [
20]. For example, coronary artery bypass grafting is a key cardiac surgery procedure and is the main treatment for patients with multivessel coronary artery disease [
21]. Vascular grafts, as either interpositional conduits or bypass grafts, can be used for revascularization procedures in the upper extremity, too. Vein grafts are more readily available and can be easier to harvest [
22]. The most frequently used conduit for this procedure is the great saphenous vein. The most important determining factor for the surgery success is the graft patency rate. The technique of harvesting the vein has evolved over the last 30 years from total open harvesting to endoscopic with minimal access technique [
23]. While the total open harvesting technique presents some disadvantages, such as a longer healing period, a visible scar and even the risk of a keloid scar, the risk of a seroma or hematoma, and even infection, minimally invasive techniques became methods of choice [
24,
25]. Also, endovenous procedures are increasingly used for the treatment of varicose veins. Endoluminal catheters can be used for this purpose, which technically allow mechano-chemical obliteration, but other endovenous procedures like cryostripping allow even venous removal. Although known for several years, it is not yet clear what changes such a catheter induces in the venous wall (Kendler et al., 2013) [
26]. The authors mentioned above proposed a four-grade score on the injuries produced by the catheter. Lesions were reported only for endothelium, not for the media and adventitia. The three basic endothelial cell markers, namely CD31, factor VIII and CD34 gave similar results, which is why we only used CD34. The reduction in factor VIII expression reported by Kendler et al. (2013) is most likely the result of limited expression of this marker in venous endothelial cells. In the light of these data, we considered it appropriate to analyze the possibilities of harvesting venous grafts by cryostripping. However, taking into account the principle of the method, which involves freezing the vein at -85 Celsius degrees in order to be harvested, we considered mandatory an analysis of the viability of the surgically excluded venous segment, as well as the possible options for temporary preservation until the grafting surgery. The present study investigated the effect of cryostripping and storage in physiological solution on the viability and proliferation of endothelial cells in venous specimens obtained from patients with varicose veins.
Immunohistochemistry is the most common application of immunostaining. It involves the process of selectively identifying antigens in cells of a tissue section by exploiting the principle of antibodies binding specifically to antigens in biological tissues [
27,
28]. In this study, CD34 which is a transmembrane glycoprotein expressed on early lymphohematopoietic stem cells, progenitor cells, and endothelial cells [
29] and Ki-67, which is an excellent marker to determine the growth fraction of a given cell population [
30], were used. Evaluation of small vessels in the saphenous adventitia with thrombophlebitis is not new, having been reported by previous published papers [
31,
32,
33]. Although the authors state that they studied the proliferation of endothelial cells in the vasa vasorum, they only applied the immunoreaction for CD34 [
31], which does not reflect this aspect. We applied the reaction for Ki67 which marks only proliferating cells or immediately after division. This aspect – even if it requires reconfirmation – is new to the literature as we have not found any published articles on this topic. The conclusion of the mentioned study actually reveals the density of the microvessels in the adventitia of the saphenous vein with thrombophlebitis, the proliferation being induced by the pathological process. Moreover, the stimulation of the endothelium and the induction of new vessel formation was also demonstrated in an experimental model (Nascimento et al., 2014) [
34,
35].
The vascular adventitia contains progenitor cells involved in vascular re-modeling. Progenitor cells are recruited into venous thrombi and can induce neovascularization, as shown in the experimental model (Covas et al., 2005) [
36], which is why a similar hypothesis has been proposed in saphenous vein thrombosis.The fact that the majority of CD34+CD117+ cells, considered progenitors, were identified in the adventitia supports our data which showed that most cases show positive immunoreaction for Ki67 in the adventitia and much less often in the other layers. In the last decades cells with a progenitor role have been identified in both rodents and humans, being located in a niche of the adventitia. Until now, there are no known data regarding the role of progenitor cells in varicose disease, and their reporting on human material is a novelty, especially under the conditions of the freezing technique. The transformation of adventitial progenitor cells and endothelial cells has been hypothesized, but there is no solid evidence for this to date. Our study opens the perspective of the molecular characterization of these cells as well as their isolation and the creation of specific cell lines. The mode of migration of adventitial progenitor cells into the intima and their transformation into endothelial cells under pathological conditions is currently only unverified speculation, even if this possibility has been demonstrated in vitro (Covas et al., 2005) [
36].
Our study explored the impact of cryostripping and subsequent storage in physiological solution on the viability and proliferation of endothelial cells in venous specimens from varicose veins patients. We observed that cryostripping effectively preserves the viability of venous specimens immediately. However, storage for more than 48 hours in physiological solution prior to fixation led to decreased CD34 and Ki67 expression, suggesting endothelial damage and an inhibitory effect on cell proliferation. The marked reduction in temperature during storage appears to inhibit cell divisions, as evidenced by the rarity of Ki67-positive cells in such specimens. These findings question the utility of Ki67 staining as a method for assessing vein graft viability.
The findings of the present study have several implications for future research and clinical practice. First, the findings suggest that cryostripping is a viable method for harvesting venous grafts for revascularization procedures. However, the findings also suggest that venous grafts should not be stored in physiological solution for more than 48 hours before grafting, as this can damage the endothelium and inhibit cell proliferation. Second, the findings suggest that cryostripped varicose veins may be a potential source of progenitor cells for vascular tissue engineering [
37]. While this study provides valuable insights, it is not without limitations. Further research is needed to substantiate the theories surrounding the transformation of adventitial progenitor cells into endothelial cells and their significance in varicose disease. Additionally, the study underscores the importance of employing meticulous specimen preservation techniques in venous research.
In conclusion, this study sheds light on the role of immunohistochemistry in understanding the behavior of endothelial cells and progenitor cells in venous specimens from patients with varicose veins. It also highlights the importance of specimen preservation methods in ensuring accurate research outcomes. Further investigations into the potential transformation of adventitial progenitor cells and their implications in varicose disease could open new avenues for understanding and treating this condition.
5. Conclusions
Our results support the use of CD34 for testing the morphological and molecular integrity of the excised venous specimen. Its expression is also dependent in part on the time between harvesting and fixation, the density of the reaction being reduced after 48 hours in physiological serum. We report the reaction of adventitial cells considered to have migratory potential, which mobilize and invade the media in cases with severe lesions. The immunohistochemical reaction for Ki67 is negative in all fixed and embedded specimens after 48 hours. Our data do not support the use of the Ki67 reaction to determine vein graft viability.
At the same time, corroborating all the data obtained, this study supports the use of cryostripping in harvesting healthy veins to be used as a graft. However, to be sure of the viability of the graft, it is recommended to keep it in saline solution and use it in the first hours after harvesting, with a maximum threshold of 24 hours.
Author Contributions
Conceptualization, A.P. and M.R.; methodology, A.R.C. and P.G.; validation, A.R.C., A.P. and P.G.; formal analysis, A.R.C. and P.G.; investigation, A.R.C. and A.P.; resources, A.P. and A.R.C.; writing—original draft preparation, A.R.C., A.P. and M.R.; writing—review and editing, A.R.C, M.R. and P.G.; visualization, A.P., A.R.C., P.G. and M.R.; supervision, A.R.C and M.R. All authors considered to have equally contribution to this work. All authors have read and agreed to the published version of the manuscript.
Funding
Publication fee for the paper was supported by Victor Babeş University of Medicine and Pharmacy and Angiogenesis Research Center, Timisoara, Romania from the research internal funds.
Institutional Review Board Statement
The principles of the Declaration of Helsinki were respected, and the study was approved by the Institutional Review Board of Scientific Research Ethic Committee ‘’Victor Babes’’ University of Medicine and Pharmacy, Timisoara, no. 66_22/22.03.2023.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Acknowledgments
We are grateful to Victor Babeş University of Medicine and Pharmacy, Timişoara for the research infrastructure. Many thanks to Emanuel Ciprian Onica (histotechnologist, for primary processing of the specimens).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Matei, S.-C.; Dumitru, C.Ș.; Radu, D. Measuring the Quality of Life in Patients with Chronic Venous Disease before and Short Term after Surgical Treatment—A Comparison between Different Open Surgical Procedures. J. Clin. Med. 2022, 11, 7171. [Google Scholar] [CrossRef] [PubMed]
- Matei SC, Matei M, Anghel FM, Carabenciov E, Murariu MS, Olariu, S. Utility of routine laboratory tests in the assessment of chronic venous disease progression in female patients. Exp. Ther. Med. 2022, 24, 571. [CrossRef] [PubMed]
- Guntani, A. , Yoshiga, R., Mii, S. Distal bypass with a varicose vein graft for critical limb ischemia: Report of a case. Surg. Case Rep. 2019, 5, 193. [Google Scholar] [CrossRef] [PubMed]
- Altshuler P, Nahirniak P, Welle NJ. Saphenous Vein Grafts. [Updated 2022 Aug 29]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK537035/.
- Matei, S.-C.; Dumitru, C.Ș.; Oprițoiu, A.-I.; Marian, L.; Murariu, M.-S.; Olariu, S. Female Gonadal Venous Insufficiency in a Clinical Presentation Which Suggested an Acute Abdomen—A Case Report and Literature Review. Medicina 2023, 59, 884. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud SA, Widrich, J. Endoscopic Vein Harvesting. [Updated 2023 Jul 25]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023 Jan-. Available online: https://www.ncbi.nlm.nih.gov/books/NBK553206/.
- Matei, S.-C.; Matei, M.; Anghel, F.M.; Murariu, M.-S.; Olariu, S. Cryostripping—A Safe and Efficient Alternative Procedure in Chronic Venous Disease Treatment. J. Clin. Med. 2022, 11, 5028. [Google Scholar] [CrossRef]
- Gullà, P.; Breccolotto, F. Technic for using the saphenous vein in the creation of a femoro-popliteal bypass in cases of anatomic changes of the saphenous vein. Minerva Chir. 1984, 39, 1029–1033. [Google Scholar]
- Corcos, L.; Peruzzi, G.P.; Romeo, V.; Procacci, T.; Dini, S. Peripheral venous biopsy in the selection of autologous venous grafts. Angiologia 1991, 43, 98–100. [Google Scholar] [PubMed]
- Bostoen, H.; Allegaert, W. On frozen vena saphena magna as an allograft in peripheral vascular surgery. Report of a case. Acta Chir. Belg. 1975, 74, 93–105. [Google Scholar]
- Sidney, L.E.; Branch, M.J.; Dunphy, S.E.; Dua, H.S.; Hopkinson, A. Concise review: Evidence for CD34 as a common marker for diverse progenitors. Stem Cells 2014, 32, 1380–1389. [Google Scholar] [CrossRef]
- Siemerink MJ, Klaassen I, Vogels IM, Griffioen AW, Van Noorden CJ, Schlingemann RO. CD34 marks angiogenic tip cells in human vascular endothelial cell cultures. Angiogenesis 2012, 15, 151–163. [CrossRef]
- Li, L.T.; Jiang, G.; Chen, Q.; Zheng, J.N. Ki67 is a promising molecular target in the diagnosis of cancer (review). Mol. Med. Rep. 2015, 11, 1566–1572. [Google Scholar] [CrossRef] [PubMed]
- Raluca, B.A.; Cimpean, A.M.; Cioca, A.; Cretu, O.; Mederle, O.; Ciolofan, A.; Gaje, P.; Raica, M. Endothelial Cell Proliferation and Vascular Endothelial Growth Factor Expression in Primary Colorectal Cancer and Corresponding Liver Metastases. Asian Pac. J. Cancer Prev. 2015, 16, 4549–4553. [Google Scholar] [CrossRef] [PubMed]
- Dumitru, C.S.; Ceausu, A.R.; Gaje, N.P.; Suciu, C.S.; Raica, M. Proliferating Lymphatic Endothelial Cells as a Prognostic Marker in Head and Neck Squamous Cell Carcinoma. Int. J. Mol. Sci. 2022, 23, 9793. [Google Scholar] [CrossRef] [PubMed]
- Lutsey PL, Zakai NA. Epidemiology and prevention of venous thromboembolism. Nat. Rev. Cardiol. 2023, 20, 248–262. [Google Scholar] [CrossRef] [PubMed]
- Kiefer, J. , Mazzeffi, M. Complications of Vascular Disease. Anesthesiol. Clin. 2022, 40, 587–604. [Google Scholar] [CrossRef]
- Ilijevski N, Atanasijević I, Lozuk B, Gajin P, Matić P, Babić S, Sagić D, Unić-Stojanović, D, Tanasković, S. Direct Ischemic Postconditioning After Carotid Endarterectomy in the Prevention of Postoperative Cerebral Ischemic Complications-Observational Case-Control Study. J. Cardiovasc. Pharmacol. Ther. 2022, 27. [CrossRef]
- Tanaskovic S, Sagic D, Radak D, Antonic Z, Kovacevic V, Vukovic M, Aleksic N, Radak S, Nenezic D, Cvetkovic S, Isenovic E, Vucurevic G, Lozuk B, Babic A, Babic S, Matic P, Gajin P, Unic-Stojanovic, D, Ilijevski, N. Carotid Restenosis Rate After Stenting for Primary Lesions Versus Restenosis After Endarterectomy With Creation of Risk Index. J. Endovasc. Ther. 2023, 30, 580–591.
- Zivkovic I, Krasic S, Milačić P, Vukovic P, Milicic M, Jovanovic M, Tabakovic Z, Sagic D, Ilijevski N, Peric, M, Bojic, M., Micovic, S. Long-term results after simultaneous carotid and coronary revascularisation. Asian Cardiovasc. Thorac. Ann. 2022, 30, 977–984.
- Seo J, Ramachandra AB, Boyd J, Marsden AL, Kahn AM. Computational Evaluation of Venous Graft Geometries in Coronary Artery Bypass Surgery. Semin. Thorac. Cardiovasc. Surg. 2022, 34, 521–532. [CrossRef]
- Shuck, J. , Masden DL. Options for revascularization: Artery versus vein: Technical considerations. Hand Clin. 2015, 31, 85–92. [Google Scholar] [CrossRef]
- Harky A, MacCarthy-Ofosu B, Grafton-Clarke, C., Pousios, D., Muir AD. Long saphenous vein harvesting techniques and their effect on graft patency. J, Card Surg. 2019, 34, 821–828.
- Caliskan E, de Souza DR, Böning A, Liakopoulos OJ, Choi YH, Pepper J, Gibson CM, Perrault LP, Wolf RK, Kim KB, Emmert MY. Saphenous vein grafts in contemporary coronary artery bypass graft surgery. Nat, Rev, Cardiol. 2020, 17, 155–169.
- Manninen HI, Jaakkola P, Suhonen M, Rehnberg S, Vuorenniemi R, Matsi PJ. Angiographic predictors of graft patency and disease progression after coronary artery bypass grafting with arterial and venous grafts. Ann, Thorac, Surg. 1998, 66, 1289–1294. [CrossRef] [PubMed]
- Kendler, M.; Averbeck, M.; Simon, J.C.; Ziemer, M. Histology of saphenous veins after treatment with the ClariVein® device - an ex-vivo experiment. J. DtschDermatolGes. 2013, 11, 348–352. [Google Scholar] [CrossRef] [PubMed]
- Dumitru, C.Ș.; Balica, N.C. Subglottotracheal Adenoid Cystic Carcinoma in a 16-Year-Old Female—A Case Report. Medicina 2023, 59, 1140. [Google Scholar] [CrossRef] [PubMed]
- Dumitru, C.S.; Ceausu, A.R.; Comsa, S.; Raica, M. Loss of E-Cadherin Expression Correlates With Ki-67 in Head and Neck Squamous Cell Carcinoma. In Vivo 2022, 36, 1150–1154. [Google Scholar] [CrossRef]
- Sidney LE, Branch MJ, Dunphy SE, Dua HS, Hopkinson, A. Concise review: Evidence for CD34 as a common marker for diverse progenitors. Stem Cells. 2014, 32, 1380–1389. [CrossRef]
- Li LT, Jiang, G, Chen Q, Zheng JN. Ki67 is a promising molecular target in the diagnosis of cancer (review). Mol. Med. Rep. 2015, 11, 1566–1572. [CrossRef]
- Chu, H.; Yan, F.; Zhao, J.; Xu, Y.; Wang, T.; Li, K.; Tang, J.; Guo, W. Assessment of vasa vasorum in the walls of thrombophlebitic saphenous vein. IntAngiol. 2013, 32, 459–464. [Google Scholar]
- Matei, S.C.; Matei, M.; Anghel, F.M.; Derban, M.D.; Olariu, A.; Olariu, S. Impact of statin treatment on patients diagnosed with chronic venous disease. Morphological analysis of the venous wall and clinical implications. Phlebology 2022, 37, 188–195. [Google Scholar] [CrossRef]
- Matei SC, Matei M, Anghel FM, Olariu A, Olariu, S. Great saphenous vein giant aneurysm. Acta Phlebol. 2022, 23, 87–92.
- Santos, Nascimento, D.; Mosqueira, D.; Sousa, L.M.; Teixeira, M.; Filipe, M.; Resende, T.P.; Araújo, A.F.; Valente, M.; Almeida, J.; Martins, J.P.; Santos, J.M.; Bárcia, R.N.; Cruz, P.; Cruz, H.; Pinto-do-Ó., P. Human umbilical cord tissue-derived mesenchymal stromal cells attenuate remodeling after myocardial infarction by proangiogenic, antiapoptotic, and endogenous cell-activation mechanisms. Stem Cell Res. Ther. 2014, 10, 5.
- Ling, S.; Ma, Z.; Teng, Y.; Jiang, X.; Cheng, J.; Li, R.; Zhang, M.; Luo, H.; Chen, Y. Adventitial Progenitor Cells of Human Great Saphenous Vein Enhance the Resolution of Venous Thrombosis via Neovascularization. Stem Cells Int. 2021, 2021, 8816763. [Google Scholar] [CrossRef] [PubMed]
- Covas, D.T.; Piccinato, C.E.; Orellana, M.D.; Siufi, J.L.; Silva, W.A., Jr.; Proto-Siqueira, R.; Rizzatti, E.G.; Neder, L.; Silva, A.R.; Rocha, V.; et al. Mesenchymal stem cells can be obtained from the human saphena vein. Exp. Cell Res. 2005, 309, 340–344. [Google Scholar] [CrossRef] [PubMed]
- Bajpai, V.K.; Andreadis, S.T. Stem cell sources for vascular tissue engineering and regeneration. Tissue Eng Part B Rev. 2012, 18, 405–425. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).