1. Introduction
Activated sludge systems, which enable to degrade the organic matter by the ability of aerobic bacteria are used worldwide as a general wastewater treatment method [
1,
2]. However, a large amount of waste sewage sludge (WSS) has been daily produced through the activated sludge systems [
3]. In addition, it is desirable that WSS should be used effectively for a sustainable social system because a large amount of WSS is stably generated daily [
4], therefore, the development of a facile technology to effectively reduce the amount of WSS is an urgent issue. Anaerobic digestion process has been known as one of the efficient reuse methods of WSS because it can produce methane gas as bioenergy [
5]. However, the anaerobic digestion process is mostly operated in dark conditions, and there are limited studies of the anaerobic digestion process while illuminated by light. This is because the type of biogas to be targeted and the type of biomass resources to be used are different between the photo and dark fermentation systems. In other words, the target of gas in the photo-fermentation system is biohydrogen instead of methane, and the biomass resources used are lignocellulosic biomass such as food waste [
6].
Photo-fermentation systems are able to obtain biohydrogen from lignocellulosic biomass [
7]. The presence of sugars is important for biohydrogen production [
8], and lignocellulosic biomass (such as corn, sugarcane bagasse, and wheat straw) is characterized by its high carbohydrate content as cellulose and hemicellulose [
9]. In addition, the aim of the photo-fermentation system is the growth of photosynthetic bacteria, which produce hydrogen production from volatile organic acids [
10], and it has been also reported that light energy increases the catalytic reaction efficiency of nitrogenase in photosynthetic bacteria, then promotes hydrogen production [
11]. On the other hand, WSS is also a biomass resource consisting of polymeric compounds. Since most of the WSS may have dead microorganisms, it contains a large amount of protein [
12]. The anaerobic digestion process using WSS mainly consists of three stages: (a) hydrolysis stage to degrade the high molecular weight compounds (such as protein, carbohydrate, and lipid) which are components of WSS to low molecular weight; (b) acidogenesis stage to produce organic acids such as acetic acid from low molecular weight compounds; and (c) methanogenesis stage to produce methane from organic acids and other substances, which proceed even in the absence of light energy [
13].
For the improvement of anerobic digestion of WSS, various additives and approaches have been investigated, and detailed mechanisms have been clarified so far [
14,
15,
16]. The antibiotic azithromycin positively affected the acidogenic process and promoted methanogenesis [
17], while chloramphenicol decreased archaeal activity and inhibited methanogenesis [
18], and the inorganic material sodium tungstate and the metallic material aluminum oxide increased methane production by promoting the activity of acetoclastic methanogens and the hydrolysis stage, respectively [
19,
20]. In a study focusing on quorum sensing (QS) as bacterial interaction, 5-fluorouracil as QS inhibitor reduced the activity of acetoclastic methanogens, while the quorum quenching enzyme AiiM increased the percentage of Gram-positive bacteria in the WSS and caused a decrease in methanogenesis, respectively [
21,
22]. In addition, it has been also reported that pretreatments such as thermal alkali, ultrasound, and decomposition enzymes for WSS have resulted in improved methane production [
23,
24,
25]. Therefore, understanding the microbial interactions within the anaerobic digestion process using WSS has the potential to not only improve the anaerobic digestion process, but also to support the production of other useful substances (such as acetate, lactic acid, and bioethanol) from WSS and find new technological offerings.
The goal of this study is to investigate the effect of photo-fermentation (photo-irradiation) on the anaerobic digestion process using WSS in terms of microbial interactions and the effect of photo-irradiation on hydrogen sulfide removal in anaerobic digestion. Methane production was suppressed and the amount of hydrogen sulfide in the sample was significantly reduced by photo-irradiation during anaerobic digestion. We provide the effect of photo-irradiation on microbial interactions within anaerobic digestion systems.
2. Materials and Methods
2.1. WSS (Waste Sewage Sludge) Preparation
WSS was kindly provided from Hiagari wastewater treatment plant in Kitakyushu City, Japan. The WSS composition was previously reported to be 3.3–4.0% of total solid (TS), 2.6-3.1% of volatile solid (VS), 30-41% of suspended solid (SS), 26-33% of volatile suspended solid (VSS), and 44-51 g/L of chemical oxygen demand (COD). The main components of WSS are protein (40%–45%), carbohydrate (12%–14%), and lipid (11%–13% of total solid) [
12]. The fresh WSS was washed three times by centrifugation at 8,000 × g for 10 min at 4°C, and the pellet was resuspended in purified water prior to adjusting its concentration to 50% (w/w) with the water. Then, The WSS was finally adjusted to the concentration of 5% (w/w) in the purified water.
2.2. Methane and Hydrogen Sulfide Productions with Photo-Irradiation
WSS (30 mL) was prepared in 66 mL vials, and the vials were tightly sealed with rubber stoppers and crimped with aluminum caps. Then, nitrogen gas was sparged at 0.02 MPa for 15 seconds to create an anaerobic condition, and the vials were incubated at 37°C for 13 days at 120 rpm with photo-irradiation at 2000 - 4000 lux illuminance using white LED (Light Emitting Diode) lamps (OHM Electric Inc, LDA7N-G AG5, Japan). The vials completely covered with aluminum foil were prepared as controls. For the experiments of dark/light fermentation, the vials were originally incubated for 52 days under dark conditions and 0.5 mL of WSS with green sulfur bacteria (WSS, which was incubated with photo-irradiation for 7 days) was added to the WSS sample. The mixture was additionally incubated for 6 days under the light conditions. Each experiment was conducted at least in triplicate.
The amount of methane gas was measured by injecting 50 μL of headspace gas from each vial into a GC-3200 gas chromatograph (GL Science, Japan) as previously described [
26]. In addition, the amount of hydrogen sulfide in the headspace of each vial was measured using the GASTEC system (GASTEC Inc, GV-110S, Japan) as previously described [
27]. The amount of both gases was calculated based on volatile solid (VS) of WSS.
2.3. Chlorophyll Measurement
The amount of chlorophyll was measured to evaluate the growth level of photosynthetic bacteria using the method described previously [
28]. WSS samples with or without photo-irradiation (1 mL) were centrifuged at 13,000 rpm for 1 min, and the pellets and 100% methanol were mixed and incubated at room temperature for 5 min. After centrifugation at 10,000 rpm for 10 min, the absorbance was detected at 650 nm and 655 nm using the supernatants.
2.4. Analytical Methods
WSS samples with or without the photo-irradiation were centrifuged at 13,000 rpm for 1 min, and the supernatants which were filtered by a 0.2-μm membrane syringe filter were used for the protease activities and organic acids.
Protease activity: Protease activity was measured using casein as substrate, as described previously [
29]. 4% casein solution (4 g in 100 mL of 0.4 M Tris-HCl, pH 8.5) was prepared. The casein solution (0.1 mL) and enzyme solution (0.3 mL) were mixed and incubated at 37°C for 120 min. After incubation, 0.44 M trichloroacetic acid (0.4 mL) was added to stop the reaction, and incubated at room temperature for 30 min. After centrifugation at 14,000 rpm for 10 min at 4°C, the supernatant (0.2 mL), Folin reagent (0.2 mL), and 0.4 M sodium carbonate (1 mL) were mixed and incubated at 37℃ for 30 min. After incubation, the absorbance was detected at 660 nm. One unit of protease activity was defined as the amount of tyrosine (μmol) produced from casein per minute by 1 mg of enzyme.
Organic acid: Organic acids (acetate, propionate, isobutyrate, and butyrate) were analyzed using high-performance liquid chromatography (Shimadzu LC-10AD) as described previously [
12,
30].
2.5. Activity Measurement of Acetoclastic Methanogen and Sulfur-Reducing Bacteria
Acetoclastic methanogen and sulfur-reducing bacteria activities were analyzed to evaluate the effect of photo-irradiation on the microorganisms that produce methane gas and hydrogen sulfide.
Acetoclastic methanogen: WSS samples with or without the photo-irradiation were incubated at 37°C for 7 days at 120 rpm, and each sample was mixed with four antibiotics (to be the final concentration as follows: vancomycin 60 mg/mL, ampicillin 60 mg/mL, streptomycin 150 mg/mL, and benzylpenicillin 150 mg/mL) to inactivate bacterial activity [
31]. Then, sodium acetate was added as substrate for acetoclastic methanogen to be the final concentration of 20 mM, and tightly sealed with rubber stoppers and crimped with aluminum caps and sparged nitrogen gas. The vials were additionally incubated at 37°C for 7 days at 120 rpm, and the amount of methane gas was measured.
Sulfur-reducing bacteria: WSS samples incubated 7 days with or without the photo-irradiation were washed three times using purified water by centrifugation at 8,000 × g for 10 min at 4°C to remove the products (e.g., organic acids, and soluble proteins). The pellets were resuspended in substrate solution to be the final WSS concentration to 5% (w/w), tightly sealed with rubber stoppers, and crimped with aluminum caps and sparged nitrogen gas. The substrate solution contained sodium formate and sodium sulfate, each final concentration was 0.016 mM in the 5% WSS. The WSS sample adjusted to 5% using purified water was prepared as blank. The vials were additionally incubated at 37°C for 24 hours at 120 rpm, and the amount of hydrogen sulfide was measured.
2.6. DNA Extractions
DNA was extracted from the pellets of the WSS samples with or without the photo-irradiation using a DNeasy PowerSoil Kit (Qiagen, Hilden, Germany) following the manufacturer's protocols. The extracted DNA samples were stored at -70°C and used a template to investigate bacterial communities using MiSeq.
2.7. RNA Extraction and cDNA Synthesis
Total RNA was extracted from the pellets of the WSS samples with or without the photo-irradiation using the RNeasy kit (Qiagen, Valencia, CA) as described previously [
30], and the complementary deoxyribonucleic acid (cDNA) was synthesized using the PrimeScript RT Reagent Kits (TAKARA Bio Inc., Shiga, Japan) as described previously [
18]. The cDNA was used as a template to quantitate the archaeal population using quantitative real-time polymerase chain reaction (qRT-PCR).
2.8. qRT-PCR and High-Throughput 16S rRNA Sequencing and Data Processing
The qRT-PCR quantification for archaea in the samples was executed by using StepOne Real-Time PCR System (Applied Biosystem) for amplification and detection of fluorescence by specific primers and probes of TaqMan system. The real-time PCR mixture and cycling conditions were set as described previously [
32]. 16S rRNA genes were amplified using the forward primer 341F (5'-CCTACGGGNGGCWGCAG-3') and the reverse primer 785R (5'-GACTACHVGGGTATCTAATCC-3') targeting the V3 and V4 regions [
33]. All steps for sequencing were proceeded based on the Illumina protocol for 16S ribosomal RNA gene sequencing library preparation for the Illumina MiSeq system as previously described [
34]. The data obtained were de-multiplexed and the reads were then classified to different taxonomic levels. The raw sequence data were deposited in the National Center for Biotechnology International (NCBI) Sequence Reads Archive (SRA) database under the Accession number of SRP072534. The obtained data were processed as previously described [
34].
3. Results and Discussion
3.1. Effect of Photo-Irradiation on Methane and Hydrogen Sulfide Productions
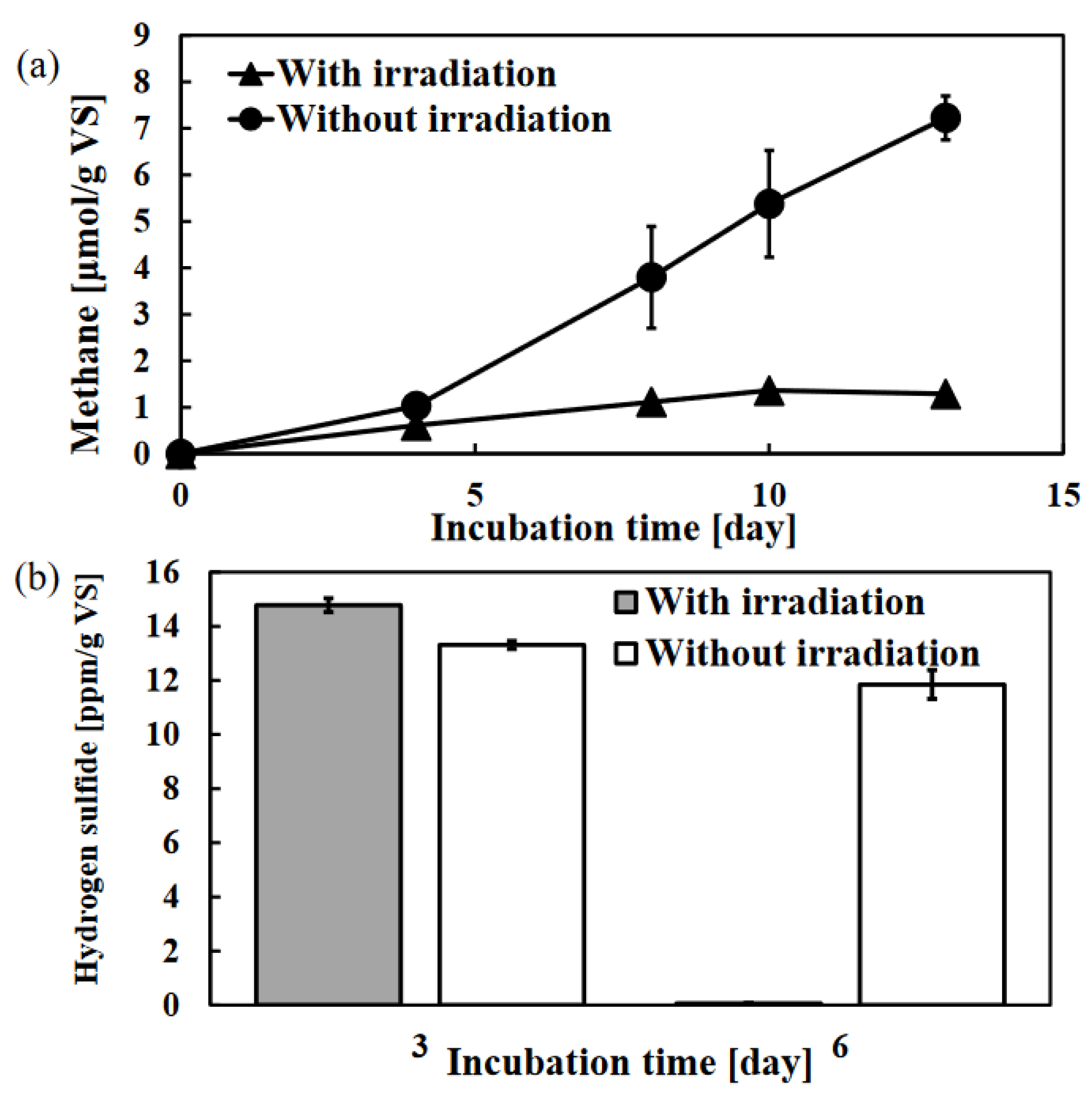
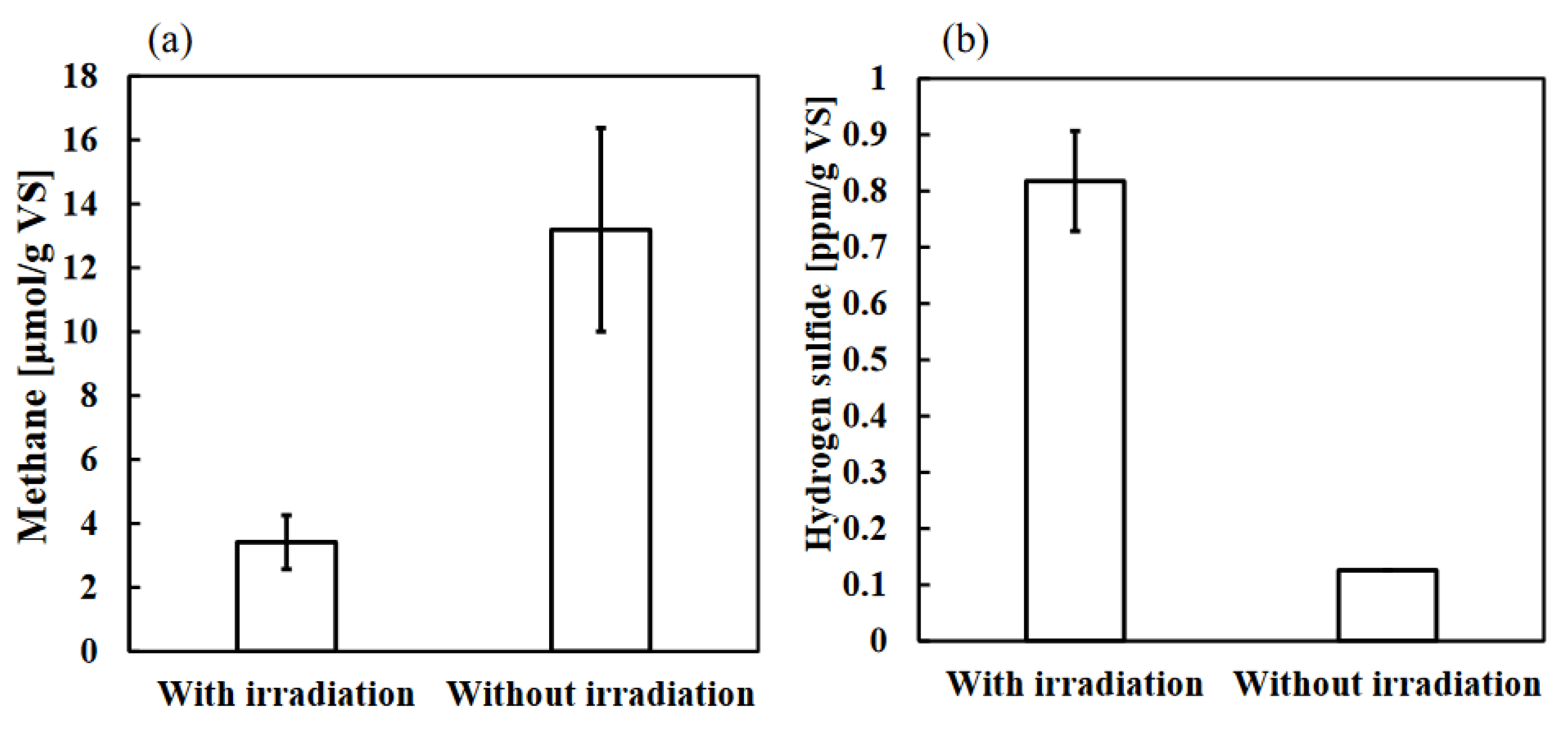
First, the effect of photo-irradiation during the anaerobic digestion was investigated by focusing on the amount of methane and hydrogen sulfide produced. Methane production was suppressed, and the amount of hydrogen sulfide in the vial was significantly reduced by the photo-irradiation as shown in
Figure 1. The methane production was observed after 4 days of incubation whereas photo-irradiation suppressed methane production, and each amount of methane with photo-irradiation and the control sample on 13 days of incubation was 1.29 ± 0.03 vs. 7.2 ± 0.5 μmol/g vs. which is 5.6-fold difference (
Figure 1a). In addition, the hydrogen sulfide production was observed after 3 days of incubation with or without the photo-irradiation, each amount of hydrogen sulfide with photo-irradiation and the control sample was 14.8 ± 0.3 vs. 13.3 ± 0.1 ppm/g vs. whereas the amount of hydrogen sulfide with photo-irradiation sample was almost undetectable after 6 days of incubation, recorded as 0.06 ± 0.02 ppm/g vs. (
Figure 1b). These results indicate that photo-irradiation has different effects on methane and hydrogen sulfide production during anaerobic digestion. In other words, methane production is suppressed from the beginning by photo-irradiation whereas the decrease in hydrogen sulfide is caused during the incubation.
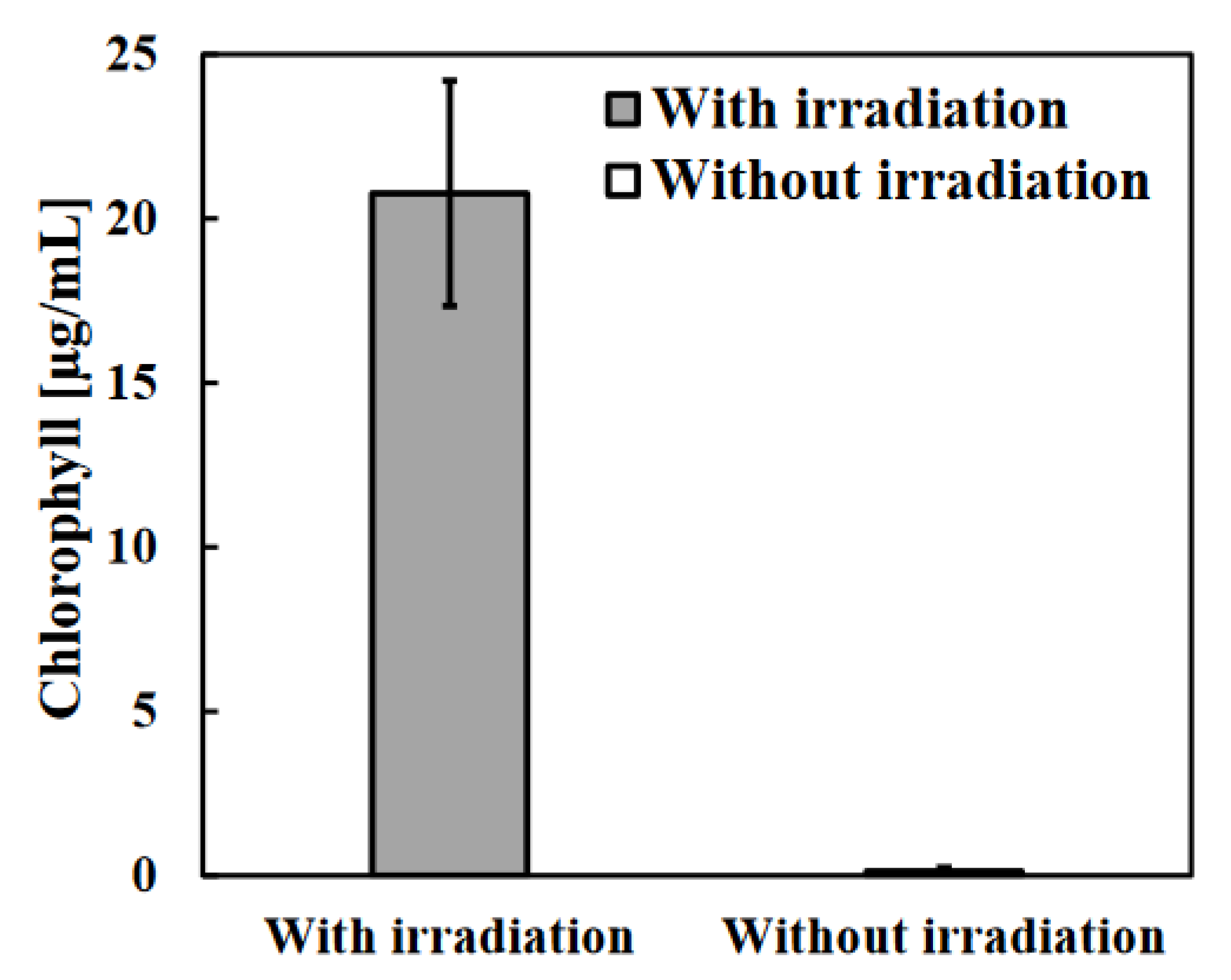
The change in the appearance of the photo-irradiation sample was remarkable and no change was observed after 3 days of incubation whereas the discoloration to green was observed after 6 days of incubation due to the effect of photo-irradiation (data not shown). This discoloration was caused by the growth of green sulfur bacteria, and the amount of chlorophyll in the photo-irradiation sample was significantly high (
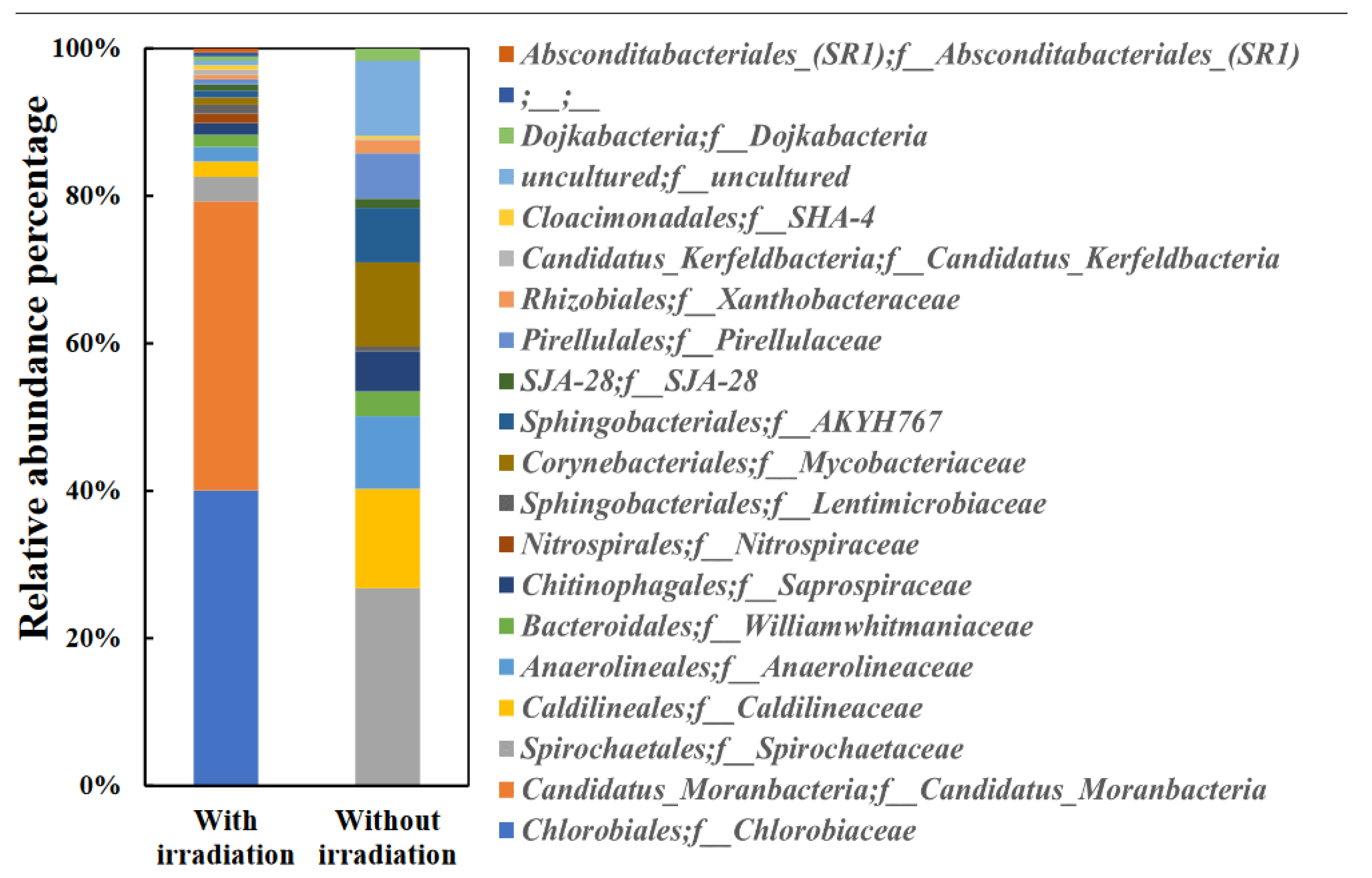
Figure 2). In addition, the bacterial community structure with photo-irradiation and the control sample on 13 days of incubation were analyzed, and it was indicated that Chlorobiaceae, which includes green sulfur bacterium was the most abundant bacterial family in the photo-irradiation sample (
Figure 3). Green sulfur bacteria have been known as photosynthetic sulfur bacteria along with purple sulfur bacteria, and these bacterial communities are capable of anoxygenic photosynthesis [
35]. Various sulfur compounds including hydrogen sulfide are used as electron donors during anoxygenic photosynthesis by photosynthetic sulfur bacteria [
36]. Hence, as shown in Fig.1b, the reason why no decrease in hydrogen sulfide was observed on the 3 days of incubation was that the green sulfur bacteria were not growing, and it is considered that the anoxygenic photosynthesis by the green sulfur bacteria occurred on the 6 days of incubation which the discoloration to green was observed, and the hydrogen sulfide was consumed as electron donors.
Thus, the photo-irradiation suppressed methane production during anaerobic digestion, while it accelerated the growth of green sulfur bacteria and caused hydrogen sulfide consumption through anoxygenic photosynthesis.
3.2. Effect of Photo-Irradiation on Anaerobic Digestion
It was described earlier that methane production in 5% WSS has been suppressed by photo-irradiation (
Figure 1a). The anaerobic digestion process using WSS mainly consists of three stages: (a) hydrolysis stage, (b) acidogenesis stage, and (c) methanogenesis stage [
13]. To clarify the suppression mechanism for methane production by photo-irradiation, hydrolysis, acidogenesis, and methanogenesis stages during the methane production using WSS were evaluated. Since protein is the main component of WSS [
12], protease activity was measured to evaluate the hydrolysis stage.
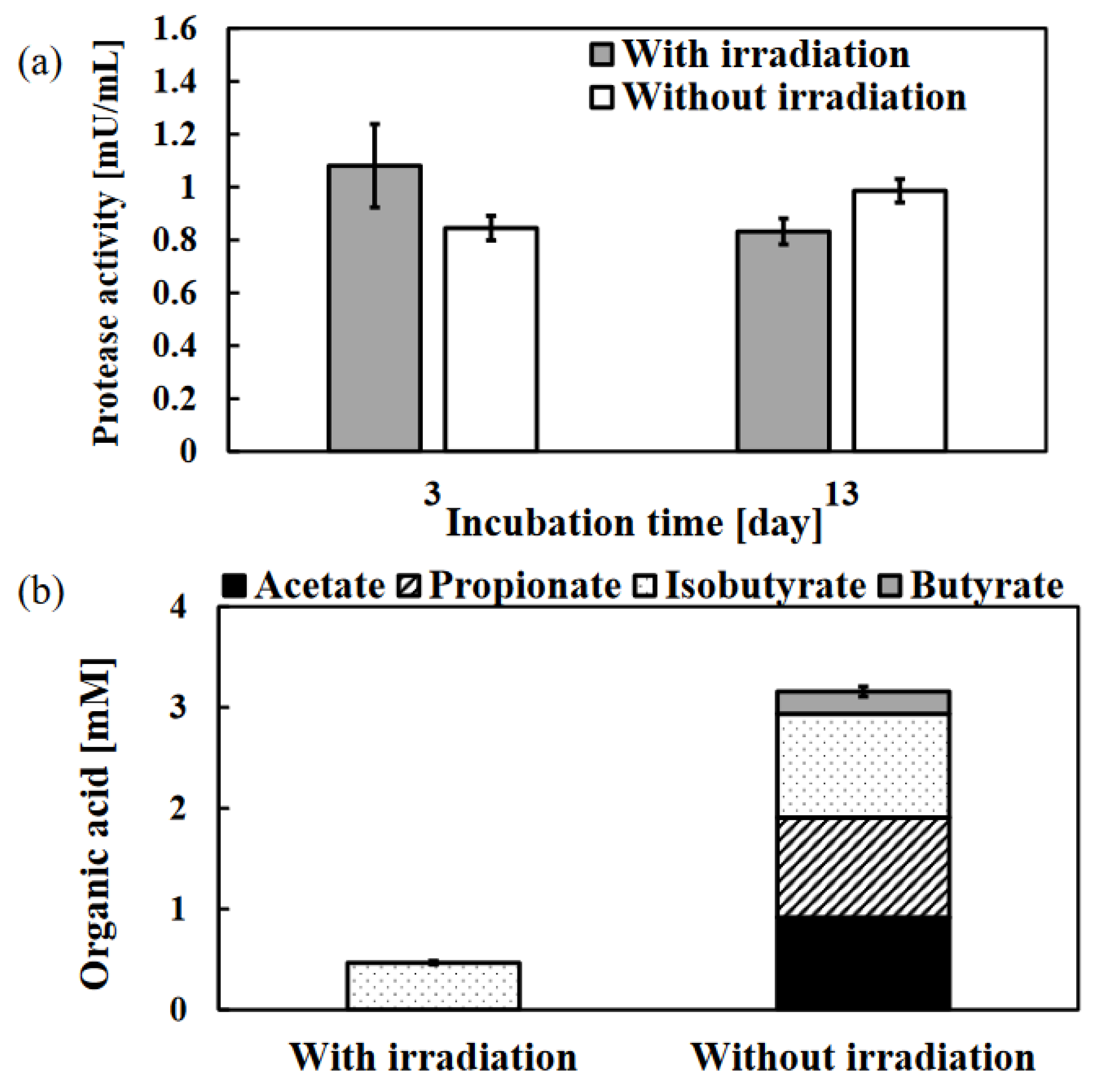
The protease activity was slightly high in the photo-irradiation sample on the 3 days of incubation whereas no significant difference in protease activity was observed between the photo-irradiation sample and the control sample on the 13 days of incubation (
Figure 4a). Bacteria in sludge often use degrading enzymes (protease, amylase, cellulase, and lipase) to degrade the high molecular weight compounds in the WSS for their growth [
37,
38]. However, the presence of alternative nutrients may suppress the expression of degrading enzymes [
29]. Chlorobiaceae grown by photo-irradiation are green sulfur bacteria (
Figure 3), and their main growth strategy is anoxygenic photosynthesis using sulfur compounds [
35]. In addition, there was hardly any knowledge about the production of degradative enzymes by green sulfur bacteria. Therefore, it was indicated that the hydrolysis stage during the methane production was not affected by photo-irradiation.
Figure 4b shows the amount of organic acids in each sample after 13 days of incubation with or without photo-irradiation. The main organic acids detected were acetate, propionate, isobutyrate, and butyrate, and the results indicate that photo-irradiation significantly reduced the accumulation of organic acids (
Figure 4b). Acetate, propionate, and butyrate accumulated in the control sample, while only isobutyrate was detected in the photo-irradiation sample. The presence of acetate is important for methanogenesis from organic acids, and methanogens such as Methanosarcina and Methanosaeta produce methane using acetate as a substrate [
39]. In addition, propionate and isobutyrate have been reported to be converted to acetate by bacteria such as Syntrophobacter and Smithella [
40,
41], and the presence of acetate is one of the key points in methane production. Since methane production was suppressed by photo-irradiation (
Figure 1a), it is clear that the organic acids in the photo-irradiation sample were not used for methane production. In addition, it has been reported that organic acids were utilized as nutrients by red non-sulfur bacteria, which are included in photosynthetic sulfur bacteria [
42]. However, since the growth of green sulfur bacteria was promoted by photo-irradiation in this experimental system (
Figure 3), it is unlikely that organic acids were consumed by the sulfur bacteria. Therefore, although the detailed effects on organic acid-producing bacteria are unknown, it was indicated that photo-irradiation had a negative impact on the acidogenesis stage, and suppressed organic acid accumulation. In addition, the archaeal activity in the samples on 13 days of incubation with or without photo-irradiation was quantified using qRT-PCR, and it was found that the archaeal activity of the photo-irradiation sample was 4.8 times lower than that of the control sample (Figure not shown).
Thus, the photo-irradiation during anaerobic digestion caused suppression of organic acid production and decrease in archaeal activity, then methane production was suppressed.
3.3. Effect of Photo-Irradiation on Acetoclastic Methanogens and Sulfate-Reducing Bacteria
The amount of hydrogen sulfide and methane gas during the anaerobic digestion was markedly changed by photo-irradiation, and it was indicated that the growth of green sulfur bacteria and negative effects on the acidogenesis stage were involved. In order to further clarify the effects of photo-irradiation on anaerobic digestion, the activities of acetoclastic methanogens and sulfate-reducing bacteria were evaluated. The respective activities of acetoclastic methanogens and sulfate-reducing bacteria were evaluated as the amount of conversion to metabolic products (methane for acetoclastic methanogens and hydrogen sulfide for sulfate-reducing bacteria) when equal amounts of each substrate were added.
Figure 5a shows acetoclastic methanogen activity in each sample after 7 days of incubation with or without photo-irradiation. The results showed that each amount of methane with photo-irradiation and the control sample was 3.4 ± 0.8 vs. 13 ± 3 μmol/g VS, methane production of the photo-irradiation sample from an equal amount of substrate was 3.9 times lower than that of the control sample (
Figure 5a). No difference between the photo-irradiation sample and the control sample was observed in the activity of hydrogenotrophic methanogenesis, which produces methane using hydrogen as a substrate (data not shown). The low concentration of acetate in the photo-irradiation sample (
Figure 4b) may also cause the reduced activity of acetoclastic methanogen. Hence, it was confirmed that photo-irradiation reduced acetoclastic methanogen activity in particular. In addition, Fig.5b shows sulfate-reducing bacteria activity in each sample after 7 days of incubation with or without photo-irradiation. Interestingly, hydrogen sulfide production of the photo-irradiation sample from an equal amount of substrate was 6.5 times higher than that of the control sample (
Figure 5b). This result indicated that despite the amount of hydrogen sulfide in the photo-irradiation sample markedly reduced (
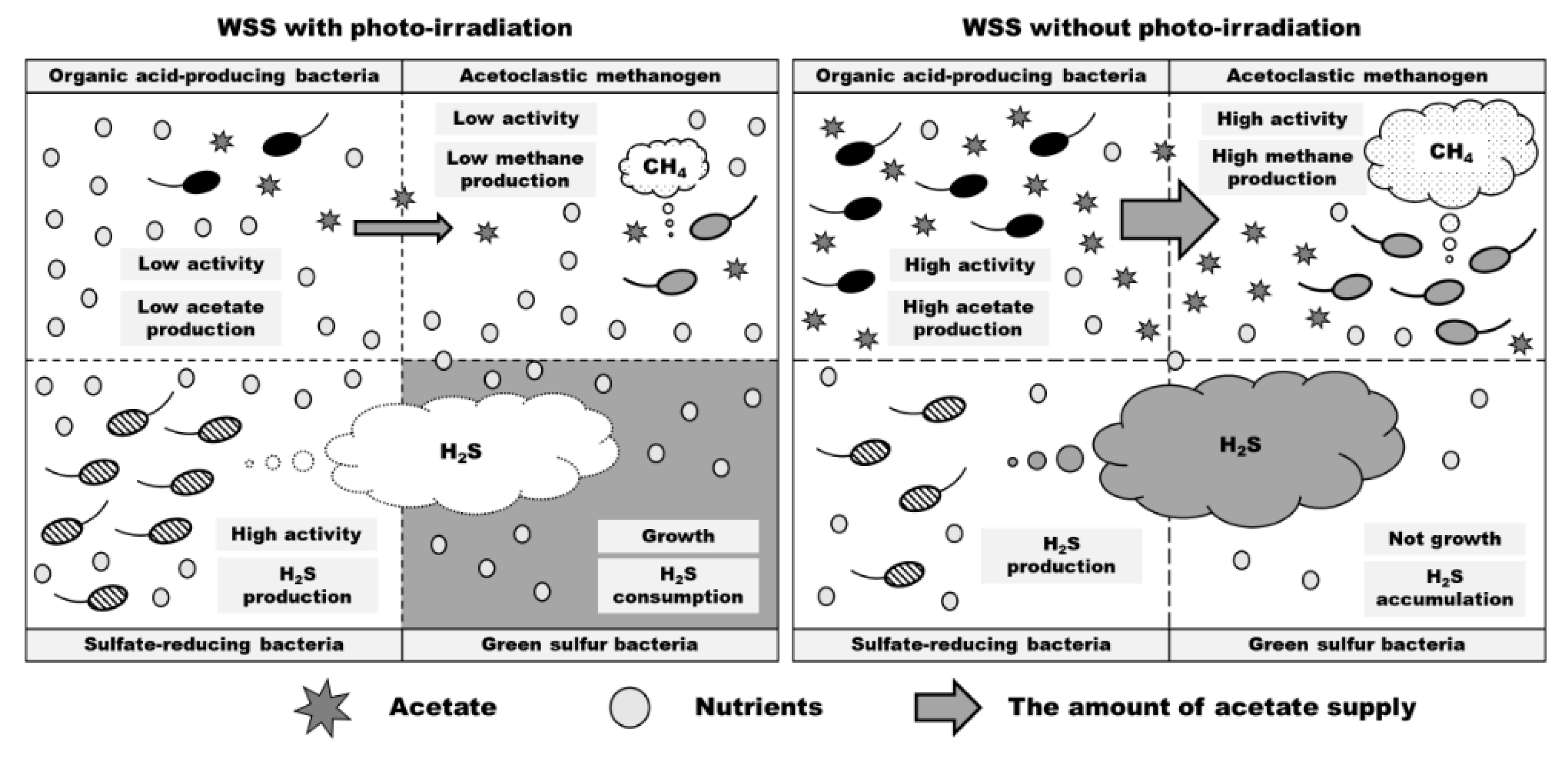
Figure 1b), the sulfate-reducing bacteria themselves were not affected by photo-irradiation. From these results, the effect of photo-irradiation on anaerobic digestion is summarized in
Figure 6.
Since diverse microorganisms are present in WSS, the microorganisms are thought to establish direct or indirect relationships (such as mutualism, predation, parasitism, competition, commensalism, suppression, and neutralism) with each other [
43]. To reveal the various bacterial interactions present in environmental samples is important because it provides new considerations. This time, it was suggested that effects and interrelationships on four microbial groups (organic acid-producing bacteria, acetoclastic methanogen, sulfate-reducing bacteria, and green sulfur bacteria) by photo-irradiation of anaerobic digestion system. First, it is noted that the purpose of these microorganisms is not to produce each metabolite (acetate, methane, and hydrogen sulfide) but to obtain energy using the metabolic pathways by which each metabolite is produced. As shown in
Figure 4b, photo-irradiation significantly suppressed the accumulation of organic acids, and this suggests that photo-irradiation reduced the activity of organic acid-producing bacteria. Therefore, it also caused a decrease in the concentration of acetate in the WSS (
Figure 4b). Next, the reduced concentration of acetate in the WSS may have created an adverse environment for acetoclastic methanogens, which use acetate as a substrate to produce methane. Hence, methane production and acetoclastic methanogen activity in the photo-irradiation sample decreased (
Figure 1a,
Figure 5a). In addition, the decrease in activity of these microbial groups may have increased the relative amount of nutrients available to the sulfate-reducing bacteria in the WSS. Therefore, the activity of sulfate-reducing bacteria was high in the photo-irradiation sample (
Figure 5b). However, hydrogen sulfide produced by sulfate-reducing bacteria was consumed by anoxygenic photosynthesis of green sulfur bacteria, whose growth was promoted by photo-irradiation. Thus, the amount of hydrogen sulfide was low in the photo-irradiation sample (
Figure 1b).
3.4. Removal of Hydrogen Sulfide during Anaerobic Digestion by Dark/Light Switching
Photo-irradiation of the anaerobic digestion system was shown to significantly reduce hydrogen sulfide. Since the presence of hydrogen sulfide during anaerobic digestion has a negative impact on methanogenesis efficiency [
44], the removal of hydrogen sulfide is essential in long-term anaerobic digestion processes. However, it is undesirable to adversely affect methanogenesis because methane gas is a type of biogas [
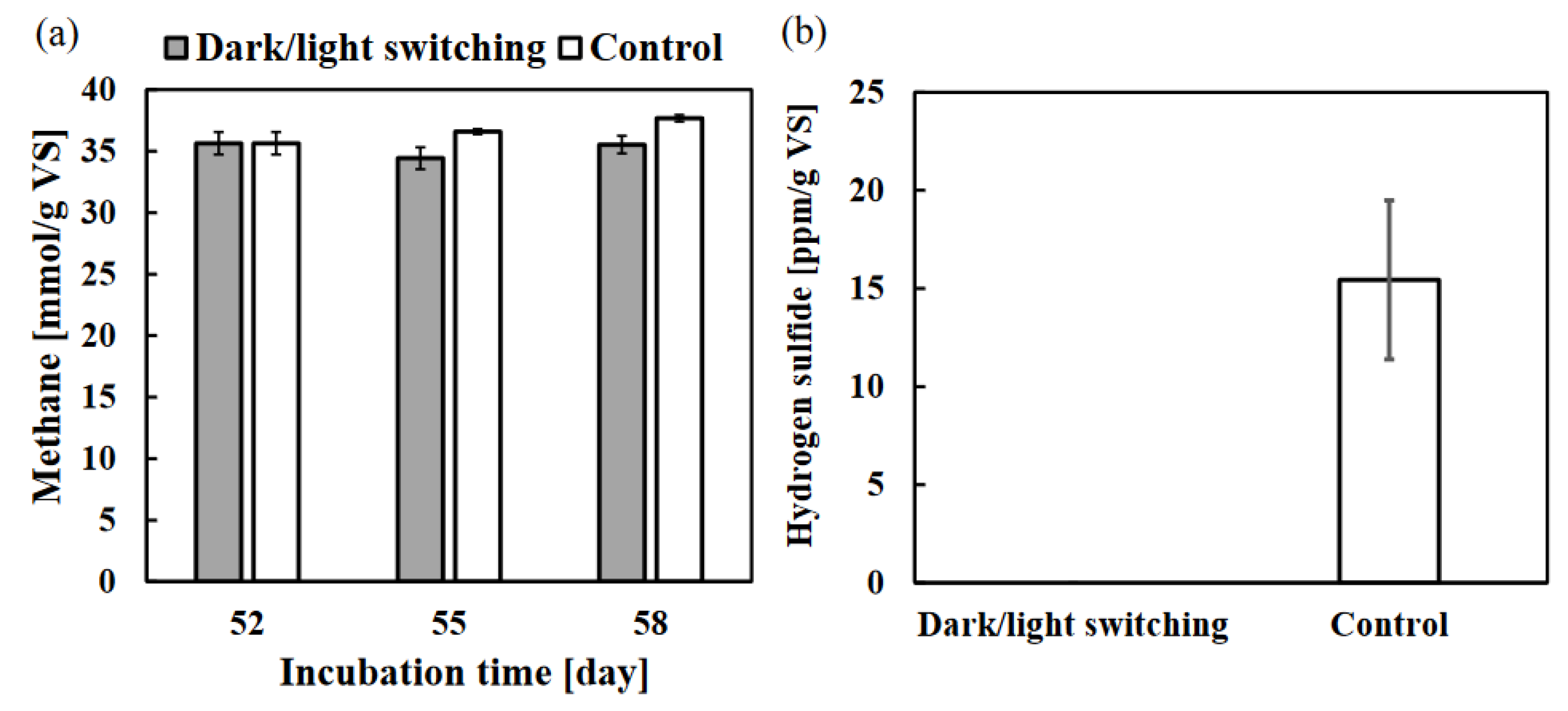
5]. Hence, a practical usage of dark/light switching for anaerobic digestion using WSS was investigated. After 52 days of anaerobic digestion without photo-irradiation, the WSS samples were further incuated under light conditions. As a result, there was no reduction of hydrogen sulfide (the color of vials did not turn green). On the other hand, the supply of WSS with green sulfur bacteria and photo-irradiation was able to only reduce hydrogen sulfide while methane kept almost the same (
Figure 7). Compared to the sample of WSS after 58 days of anaerobic digestion without photo-irradiation (control sample), no change in the amount of existing methane level in the dark/light switch sample was observed after 6 days of incubation (
Figure 7a). In addition, the amount of hydrogen sulfide at 58 days of incubation was not detected in the sample with the dark/light switch, even though the control sample contained 15 ± 4 ppm/g vs. of hydrogen sulfide (
Figure 7b). Thus, dark/light switching in long-term anaerobic digestion may efficiently remove only hydrogen sulfide without affecting the methane produced. However, continuous methane production during the short-term anaerobic digestion was suppressed by photo-irradiation (
Figure 1a). In addition, since hydrogen sulfide in anaerobic digestion causes corrosion of the digester tank, its early removal is desirable [
44]. Therefore, a contactless anaerobic digestion system of photo-irradiated and without photo-irradiated WSS is proposed, then it should be further investigated in the future. It has already been reported in our previous research that efficient hydrogen sulfide removal in a contactless anaerobic digestion system using inorganic materials [
27].
4. Conclusions
The effect of photo-irradiation on anaerobic digestion was investigated in comparison to dark fermentation sample. It was indicated that the activity of organic acid-producing bacteria in WSS was decreased by photo-irradiation and markedly suppressed the accumulation of organic acids in WSS. Accompanying this, the activity of acetoclastic methanogen in the WSS was particularly reduced, and overall methane production was suppressed. In addition, it was suggested that the activity of sulfate-reducing bacteria in the WSS was not directly affected by photo-irradiation, but the activity may have increased indirectly in terms of nutrient acquisition. Photo-irradiation to the anaerobic digestion system promoted the growth of green sulfur bacteria and caused significant consumption of hydrogen sulfide through anoxygenic photosynthesis. Light/dark switching in long-term anaerobic digestion system efficiently removed only hydrogen sulfide without affecting the methane gas produced. Therefore, this study not only provided the effects of photo-irradiation on microbial interactions within the anaerobic digestion system but also provided the potential of photo-irradiation as a method for efficient hydrogen sulfide removal.
Author Contributions
Conceptualization, T.M.; methodology, S.T., K.Y. and T.M; investigation, S.T., K.Y. and S.I..; data curation, T.M..; writing—original draft preparation, S.T.; writing—review and editing, T.M..; supervision, T.M..; project administration, T.M.; funding acquisition, T.M.. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Research Foundation for Opto-Science and Technology.
Institutional Review Board Statement
Not applicable because this study is not involving humans or animals.
Informed Consent Statement
Not applicable because this study is not involving humans.
Data Availability Statement
All the data are available upon the request.
Acknowledgments
We are grateful to Research Foundation for Opto-Science and Technology for supporting this study.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Shchegolkova, N.M.; Krasnov, G.S.; Belova, A.A.; Dmitriev, A.A.; Kharitonov, S.L.; Klimina, K.M.; Melnikova, N. V.; Kudryavtseva, A. V. Microbial Community Structure of Activated Sludge in Treatment Plants with Different Wastewater Compositions. Frontiers in Microbiology 2016, 7, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Modin, O.; Alam, S.S.; Persson, F.; Wilén, B.M. Sorption and Release of Organics by Primary, Anaerobic, and Aerobic Activated Sludge Mixed with Raw Municipal Wastewater. PLoS ONE 2015, 10, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Hara, K.; Mino, T. Environmental assessment of sewage sludge recycling options and treatment processes in Tokyo. Waste Management 2008, 28, 2645–2652. [Google Scholar] [CrossRef] [PubMed]
- Kacprzak, M.; Neczaj, E.; Fijałkowski, K.; Grobelak, A.; Grosser, A.; Worwag, M.; Rorat, A.; Brattebo, H.; Almås, Å.; Singh, B.R. Sewage sludge disposal strategies for sustainable development. Environmental Research 2017, 156, 39–46. [Google Scholar] [CrossRef]
- Weiland, P. Biogas production: current state and perspectives. Applied Microbiology and Biotechnology 2010, 85, 849–860. [Google Scholar] [CrossRef] [PubMed]
- Hitam, C.N.C.; Jalil, A.A. A review on biohydrogen production through photo-fermentation of lignocellulosic biomass. Biomass Conversion and Biorefinery 2023, 13, 8465–8483. [Google Scholar] [CrossRef]
- Saha, R.; Bhattacharya, D.; Mukhopadhyay, M. Enhanced production of biohydrogen from lignocellulosic feedstocks using microorganisms: A comprehensive review. Energy Conversion and Management: X 2022, 13, 100153. [Google Scholar] [CrossRef]
- Zabed, H.M.; Akter, S.; Yun, J.; Zhang, G.; Awad, F.N.; Qi, X.; Sahu, J.N. Recent advances in biological pretreatment of microalgae and lignocellulosic biomass for biofuel production. Renewable and Sustainable Energy Reviews 2019, 105, 105–128. [Google Scholar] [CrossRef]
- Okolie, J.A.; Nanda, S.; Dalai, A.K.; Kozinski, J.A. Chemistry and Specialty Industrial Applications of Lignocellulosic Biomass. Waste and Biomass Valorization 2021, 12, 2145–2169. [Google Scholar] [CrossRef]
- Yadav, M.; Paritosh, K.; Vivekanand, V. Lignocellulose to bio-hydrogen: An overview on recent developments. International Journal of Hydrogen Energy 2020, 45, 18195–18210. [Google Scholar] [CrossRef]
- Sabourin-Provost, G.; Hallenbeck, P.C. High yield conversion of a crude glycerol fraction from biodiesel production to hydrogen by photofermentation. Bioresource Technology 2009, 100, 3513–3517. [Google Scholar] [CrossRef] [PubMed]
- Maeda, T.; Yoshimura, T.; Shimazu, T.; Shirai, Y.; Ogawa, H.I. Enhanced production of lactic acid with reducing excess sludge by lactate fermentation. Journal of Hazardous Materials 2009, 168, 656–663. [Google Scholar] [CrossRef]
- Maeda, T.; Sabidi, S.; Sanchez-Torres, V.; Hoshiko, Y.; Toya, S. Engineering anaerobic digestion via optimizing microbial community: effects of bactericidal agents, quorum sensing inhibitors, and inorganic materials. Applied Microbiology and Biotechnology 2021, 105, 7607–7618. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, Y.; Li, Z.; Zhao, Z.; Quan, X.; Zhao, Z. Adding granular activated carbon into anaerobic sludge digestion to promote methane production and sludge decomposition. Journal of Cleaner Production 2017, 149, 1101–1108. [Google Scholar] [CrossRef]
- Roy, C.K.; Hoshiko, Y.; Toya, S.; Maeda, T. Effect of different concentrations of sodium selenite on anaerobic digestion of waste sewage sludge. Environmental Technology and Innovation 2022, 27, 102403. [Google Scholar] [CrossRef]
- Dong, D.; Wang, R.; Geng, P.; Li, C.; Zhao, Z. Enhancing effects of activated carbon supported nano zero-valent iron on anaerobic digestion of phenol-containing organic wastewater. Journal of Environmental Management 2019, 244, 1–12. [Google Scholar] [CrossRef]
- Nguyen, M.T.; Maeda, T.; Mohd Yusoff, M.Z.; Ogawa, H.I. Effect of azithromycin on enhancement of methane production from waste activated sludge. Journal of Industrial Microbiology and Biotechnology 2014, 41, 1051–1059. [Google Scholar] [CrossRef]
- Mustapha, N.A.; Sakai, K.; Shirai, Y.; Maeda, T. Impact of different antibiotics on methane production using waste-activated sludge: mechanisms and microbial community dynamics. Applied Microbiology and Biotechnology 2016, 100, 9355–9364. [Google Scholar] [CrossRef]
- Roy, C.K.; Toya, S.; Hoshiko, Y.; Sabidi, S.; Mustapha, N.A.; Miyazaki, T.; Maeda, T. Effect of sodium tungstate on anaerobic digestion of waste sewage sludge: Enhanced methane production via increased acetoclastic methanogens. Journal of Environmental Chemical Engineering 2022, 10, 107524. [Google Scholar] [CrossRef]
- Chen, Y.; Yang, Z.; Zhang, Y.; Xiang, Y.; Xu, R.; Jia, M.; Cao, J.; Xiong, W. Effects of different conductive nanomaterials on anaerobic digestion process and microbial community of sludge. Bioresource Technology 2020, 304, 123016. [Google Scholar] [CrossRef]
- Hoshiko, Y.; Hirano, R.; Mustapha, N.A.; Nguyen, P.D.T.; Fujie, S.; Sanchez-Torres, V.; Maeda, T. Impact of 5-fluorouracil on anaerobic digestion using sewage sludge. Chemosphere 2022, 298, 134253. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, P.D.T.; Mustapha, N.A.; Kadokami, K.; Garcia-Contreras, R.; Wood, T.K.; Maeda, T. Quorum sensing between Gram-negative bacteria responsible for methane production in a complex waste sewage sludge consortium. Applied Microbiology and Biotechnology 2019, 103, 1485–1495. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Xu, Q.; Wang, D.; Yang, Q.; Wu, Y.; Li, Y.; Fu, Q.; Yang, F.; Liu, Y.; Ni, B.J.; et al. Thermal-alkaline pretreatment of polyacrylamide flocculated waste activated sludge: Process optimization and effects on anaerobic digestion and polyacrylamide degradation. Bioresource Technology 2019, 281, 158–167. [Google Scholar] [CrossRef]
- Li, X.; Guo, S.; Peng, Y.; He, Y.; Wang, S.; Li, L.; Zhao, M. Anaerobic digestion using ultrasound as pretreatment approach: Changes in waste activated sludge, anaerobic digestion performances and digestive microbial populations. Biochemical Engineering Journal 2018, 139, 139–145. [Google Scholar] [CrossRef]
- Kainthola, J.; Kalamdhad, A.S.; Goud, V. V.; Goel, R. Fungal pretreatment and associated kinetics of rice straw hydrolysis to accelerate methane yield from anaerobic digestion. Bioresource Technology 2019, 286, 121368. [Google Scholar] [CrossRef] [PubMed]
- Mohd Yasin, N.H.; Maeda, T.; Hu, A.; Yu, C.P.; Wood, T.K. CO2 sequestration by methanogens in activated sludge for methane production. Applied Energy 2015, 142, 426–434. [Google Scholar] [CrossRef]
- Mustapha, N.A.; Toya, S.; Maeda, T. Effect of Aso limonite on anaerobic digestion of waste sewage sludge. AMB Express 2020, 10. [Google Scholar] [CrossRef]
- Tanaka, K.; Shimakawa, G.; Tabata, H.; Kusama, S.; Miyake, C.; Nakanishi, S. Quantification of NAD(P)H in cyanobacterial cells by a phenol extraction method. Photosynthesis Research 2021, 148, 57–66. [Google Scholar] [CrossRef]
- Maeda, T.; Yoshimura, T.; García-Contreras, R.; Ogawa, H.I. Purification and characterization of a serine protease secreted by Brevibacillus sp. KH3 for reducing waste activated sludge and biofilm formation. Bioresource Technology 2011, 102, 10650–10656. [Google Scholar] [CrossRef]
- Mohd Yusoff, M.Z.; Maeda, T.; Sanchez-Torres, V.; Ogawa, H.I.; Shirai, Y.; Hassan, M.A.; Wood, T.K. Uncharacterized Escherichia coli proteins YdjA and YhjY are related to biohydrogen production. International Journal of Hydrogen Energy 2012, 37, 17778–17787. [Google Scholar] [CrossRef]
- Battumur, U.; Yoon, Y.; Bae, G.S.; Kim, C.H. Isolation and characterization of new Methanosarcina mazei strains KOR-3, -4, -5, and -6 from an anaerobic digester using pig slurry. Asian-Australasian Journal of Animal Sciences 2017, 30, 1198–1203. [Google Scholar] [CrossRef] [PubMed]
- Mustapha, N.A.; Sharuddin, S.S.; Mohd Zainudin, M.H.; Ramli, N.; Shirai, Y.; Maeda, T. Inhibition of methane production by the palm oil industrial waste phospholine gum in a mimic enteric fermentation. Journal of Cleaner Production 2017, 165, 621–629. [Google Scholar] [CrossRef]
- Klindworth, A.; Pruesse, E.; Schweer, T.; Peplies, J.; Quast, C.; Horn, M.; Glöckner, F.O. Evaluation of general 16S ribosomal RNA gene PCR primers for classical and next-generation sequencing-based diversity studies. Nucleic Acids Research 2013, 41, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Mustapha, N.A.; Hu, A.; Yu, C.P.; Sharuddin, S.S.; Ramli, N.; Shirai, Y.; Maeda, T. Seeking key microorganisms for enhancing methane production in anaerobic digestion of waste sewage sludge. Applied Microbiology and Biotechnology 2018, 102, 5323–5334. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.H.; Wu, H.; Xu, C.; Liu, X.C.; Huang, Z.; Chang, S.; Wang, W.; Han, G.; Kuang, T.; Shen, J.R.; et al. Architecture of the photosynthetic complex from a green sulfur bacterium. Science 2020, 370. [Google Scholar] [CrossRef]
- Wu, B.; Liu, F.; Fang, W.; Yang, T.; Chen, G.H.; He, Z.; Wang, S. Microbial sulfur metabolism and environmental implications. Science of the Total Environment 2021, 778. [Google Scholar] [CrossRef]
- Moreno, R.; Rojo, F. Features of pseudomonads growing at low temperatures: another facet of their versatility. Environmental Microbiology Reports 2014, 6, 417–426. [Google Scholar] [CrossRef]
- Tang, J.; Wang, X.; Hu, Y.; Zhang, Y.; Li, Y. Lactic acid fermentation from food waste with indigenous microbiota: Effects of pH, temperature and high OLR. Waste Management 2016, 52, 278–285. [Google Scholar] [CrossRef]
- Kurade, M.B.; Saha, S.; Salama, E.S.; Patil, S.M.; Govindwar, S.P.; Jeon, B.H. Acetoclastic methanogenesis led by Methanosarcina in anaerobic co-digestion of fats, oil and grease for enhanced production of methane. Bioresource Technology 2019, 272, 351–359. [Google Scholar] [CrossRef]
- Wang, T.; Zhu, G.; Kuang, B.; Jia, J.; Liu, C.; Cai, G.; Li, C. Novel insights into the anaerobic digestion of propionate via Syntrophobacter fumaroxidans and Geobacter sulfurreducens: Process and mechanism. Water Research 2021, 200, 117270. [Google Scholar] [CrossRef]
- Zhang, C.; Yuan, Q.; Lu, Y. Inhibitory effects of ammonia on syntrophic propionate oxidation in anaerobic digester sludge. Water Research 2018, 146, 275–287. [Google Scholar] [CrossRef]
- Monroy, I.; Buitrón, G. Production of polyhydroxybutyrate by pure and mixed cultures of purple non-sulfur bacteria: A review. Journal of Biotechnology 2020, 317, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Xu, P. Dynamics of microbial competition, commensalism, and cooperation and its implications for coculture and microbiome engineering. Biotechnology and Bioengineering 2021, 118, 199–209. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Jiang, X.; Li, X.; Jiang, W. The control of H2S in biogas using iron ores as in situ desulfurizers during anaerobic digestion process. Applied Microbiology and Biotechnology 2016, 100, 8179–8189. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).