Influenza and other respiratory infections are the leaders among the exacerbations of bronchopulmonary diseases [
1,
2]. There is no doubt that exacerbations in patients with chronic obstructive pulmonary disease (COPD) are combined with an increase in inflammation activity, therefore prolonging the post-exacerbation recovery period [
3,
4]. In patients with frequent exacerbations, the QoL (Quality of life) is worse, the reduction in pulmonary function is faster, and mortality rates are higher than in patients with less frequent exacerbations [
5,
6,
7,
8].
Patients with COPD showed statistically significant changes in FEV1 during the ARI, while influenza was associated with a greater decrease in FEV1 than other ARIs [
9,
10,
11]. A significant deterioration in FEV1 was noted among patients with laboratory-confirmed influenza, in contrast to patients without laboratory confirmation of infection (48% vs. 24%). Each infection leads to the further deterioration of pulmonary function [
12]. The most formidable signs of severe influenza are the rapid progression to acute respiratory failure and the development of multi-lobe lung damage. Markers of bad prognosis are: reduction in SatO2 less than 90%, tachypnea above 25 breaths/min, hemoptysis, hypotension, diarrhea, laboratory tests—thrombocytopenia, increased lactate dehydrogenase, creatine phosphokinase and creatinine [
13].
Every fourth patient with asthma after acute respiratory infections (ARIs) experiences a deterioration of pulmonary function, symptoms of bronchial obstruction and poor asthma control for 4-6 weeks [
14]. Influenza can often be the cause of hospitalizations.
To date, vaccination against influenza, which has been used for decades, has proven its efficacy in reducing the frequency of exacerbations and the severity of COPD, asthma, and other comorbidities [
13,
15,
16,
17].
Vaccination has a double effect: specific and non-specific. The specific mechanism includes the activation of humoral and cellular protection factors against the antigens that make up the vaccine composition [
18,
19,
20,
21]. The non-specific mechanism includes the stimulation of the phagocytic activity of macrophages and neutrophils, as well as the secretion of lactoferrin, lysozyme, and interferon, which increases protective potential of the respiratory system regardless of the antigenic characteristics of microorganisms [
22,
23]. That is, vaccination reduces the incidence not only of influenza but also of the other ARIs, both among adults and children by 25-65% [
24,
25].
However, despite the discovery of many positive clinical and immunological effects of the influenza vaccination, investigations of immunization effect on the cytokine profile, C-reactive protein (CRP), which characterize the inflammatory process in patients with diseases of the bronchopulmonary system, as well as the relationship with clinical and functional parameters in patients with asthma and COPD vaccinated against influenza are currently limited. Studying these parameters will expand the concept of not only direct preventive, but also indirect therapeutic efficacy of the influenza vaccination.
Purpose of this study is to investigate dynamics of CRP, serum cytokines (IL-2, IL-6, IL-10, IL-17) in patients with asthma and COPD, as well as to perform a correlation analysis with the clinical manifestations of the diseases within a year after vaccination.
Materials
Study Design
Primary objectives of the study were to assess baseline levels of CRP, serum cytokines (IL-2, IL-6, IL-10, IL-17) during a period of remission in patients with asthma and COPD, and to study a history of the clinical course of the diseases for the previous 12 months. Secondary objectives were to analyze influenza vaccine’s effect on the dynamics of the studied immune parameters (after 6-12 months) and the clinical effects of influenza immunization on the course of the underlying disease in patients with bronchial obstructive syndrome for 12 months.
The total number of individuals participating in the study was 82 patients, including 36 patients with asthma, 20 patients with COPD and 26 healthy subjects vaccinated against influenza.
Stage IV (postmarketing): an open-label, non-randomized, comparative, controlled study of observers was conducted in three centers in Russia. Patients were enrolled in the study group during flu season 2009-2010 at the Research Institute of Pulmonology of the FMBA of Russia (Moscow), Pulmonology Department of Kirov Regional State Budgetary Healthcare Institution Kirov Regional Clinic Hospital (Kirov) and FSBSI I. Mechnikov Research Institute of Vaccines and Sera (Moscow). All patients were monitored by a pulmonologist and received treatment in accordance with the regulated protocols of clinical guidelines approved in the Russian Federation (RF).
After preliminary studies and examination by a physician, taking into account indications and contraindications according to the instructions for the vaccine usage, as well as the official recommendations for immunization in the Russian Federation, informed consent was obtained from a participating patient, and the patient was sent to the immunization room. Vaccination was carried out by a nurse in compliance with aseptic and antiseptic measures, as well as the rules for the drug administration. After the vaccination, the patient was observed in a medical institution for 45 minutes to exclude the development of immediate reactions to the vaccine. A physician in charge asked the vaccinated person daily about his/her health and filled out the list of possible adverse reactions for the next 7 days. Within the next 12 months investigator—a pulmonologist, followed up all vaccinated patients and clinically assessed asthma and COPD course with repeated blood sampling and immunological studies in 6 and 12 months. All information about the vaccination, examination and the study data was recorded in the official, standard, individual medical documentation of the patient, which can be used by an attending physician and also healthcare organizers to monitor the performed studies.
Legal and Ethical Aspects of the Study
The study was conducted following the Declaration of Helsinki, the Guidelines of the International Council on Harmonization for Good Clinical Practice, and Russian regulatory requirements.
Vaccination of patients with asthma and COPD was carried out under the National Calendar of Prophylactic Vaccinations in the Russian Federation—a regulatory legal act, establishing the timing and procedure for conducting preventive vaccinations for citizens (Federal Law No. 157-FZ of September 17, 1998); Article 20 “Informed Voluntary Consent to Medical Intervention and Refusal of Medical Intervention” (Federal Law No. 323-FZ of November 1, 2011 “On Basics of Health Protection of the Citizens in the Russian Federation” (revised on April 3, 2017); Methodical Guidelines (MG) 3.3.1.1123-02 “Monitoring of Post-Vaccination Complications and their Prevention” approved by Chief State Sanitary Inspector of the Russian Federation on May 26, 2002).
The study protocol was approved by the local Ethics Committee of the Federal State Budgetary Scientific Institution I. Mechnikov Research Institute of Vaccines. Written informed assent was obtained from the patients before their enrolment in the study. All research was performed in accordance with relevant guidelines/regulations.
Inclusion Criteria
Men and women over 18 years of age; patients with moderate or severe asthma or COPD, receiving bronchodilator and anti-inflammatory therapy with no signs of exacerbation at the time of inclusion in the study; informed consent signed by the patient.
Exclusion Criteria
Acute infectious diseases, including tuberculosis; active phase of chronic viral hepatitis; mental disorders; renal or hepatic impairment; hypersensitivity to vaccine components; severe complications to previous vaccinations; pregnancy; inability of the patient to understand the essence of the study or give consent to participate in it.
Study Groups
Group I: Asthma patients vaccinated against influenza (n = 34); Group II (n = 20): COPD patients; Group III (n = 26): healthy vaccinated subjects (as a reference group to assess changes in immunological parameters after vaccine administration).
The asthma diagnosis, its severity and control were confirmed by the history, clinical presentation and functional diagnostics (GINA).
The diagnosis of COPD was confirmed by the medical history, clinical presentation, X-ray, and functional diagnostics (GOLD). According to the GOLD criteria (FEV1/FVC <70% and 30% ≤FEV1 <80% of the reference value), the inclusion criteria are the following: patients with moderate to severe COPD aged >45 years, with a smoking history of >10 packs/year.
Patients were enrolled in the study after a relieved exacerbation, which occurred during the inpatient treatment in the pulmonary department of the Regional Clinical Hospital or were followed up on an outpatient basis. Patients with asthma and COPD were vaccinated within the period of 2-4 weeks of remission, against the background of maintenance basic therapy.
The characteristics of patients with asthma /COPD and healthy subjects included in the study are presented in
Table 1. The groups were comparable in terms of age, sex and a history of the disease (p >0.05).
Table 1.
Characteristics of patients with asthma /COPD and healthy subjects included in the study. Note: Continuous variables are presented as Mean ± SD, categorical variables are presented as number / percentage (%).
Table 1.
Characteristics of patients with asthma /COPD and healthy subjects included in the study. Note: Continuous variables are presented as Mean ± SD, categorical variables are presented as number / percentage (%).
| Characteristics |
Asthma group |
COPD group |
Healthy |
| N=34 |
N=20 |
N=26 |
| Males, abs./% |
14 / 39% |
19 / 95% |
8 / 31% |
| Females, abs./% |
22 / 61% |
1 / 5% |
18 / 69% |
| Age, years |
51.05 ± 1.73 |
58.5 ± 1.37 |
39.27 ± 3.25 |
| Disease history, years |
18.36 ± 2 |
15.7 ± 1.83 |
- |
| Moderate, abs./% |
29 / 81% |
13 / 65% |
- |
| Severe, abs./% |
7 / 19% |
7 / 35% |
- |
| Steroid-dependent patients, abs./% |
6 / 17% |
- |
- |
| FEV1, abs. |
1.71 ± 0.13 |
1.08 ± 0.12 |
- |
| FEV1, % due |
56.71 ± 3.57 |
32.25 ± 3.24 |
- |
| FVC, abs. |
2.14 ± 0.19 |
1.48 ± 0.15 |
- |
| FVC, % due |
55.70 ± 3.52 |
35.06 ± 3.29 |
- |
| FEV1/FVC, % due |
68.33 ± 4.49 |
50.78 ± 3.49 |
- |
| FEV1/FVC, % due (post-bronchodilator) |
83.38 ± 6.97 |
51 ± 2.59 |
- |
| O2 saturation,% |
96.66 ± 0.25 |
95.62 ± 0.76 |
- |
| 6-MX test, m |
378.94 ± 14.58 |
351.44 ± 14.53 |
- |
During the 1-year follow-up period after the vaccination, one patient with severe COPD withdrew from the study due to death caused by decompensated chronic respiratory and heart failure.
Vaccine
Grippol Plus, a trivalent polymer-subunit vaccine (NPO Petrovax Pharm LLC, Russia), was used for vaccination. The study participants to get immunized received a 0.5 mL dose of the vaccine. The vaccine includes protective antigens isolated from virus-containing allantoic fluid of chicken embryos, which are linked with immunoadjuvant azoximer bromide (Polyoxidonium). The vaccine contains 5 µg of hemagglutinin, epidemically relevant strains of influenza virus subtypes A (H1N1, AH3N2) and type B (total 15 µg of HA), and 500 µg of azoximer bromide. Vaccine composition in 2009-2010: A/H1N1/Brisbane/59/2007, A/H3N2/Brisbane/10/2007, B/Brisbane/60/2008. Pharmacological action of the vaccine: anti-influenza, immunomodulatory. The vaccine forms a high-level specific immunity against influenza. The protective effect after vaccination usually occurs in 8-12 days and lasts up to 12 months. Azoximer bromide, included in the vaccine, provides an increase in immunogenicity and stability of antigens, allows to increase immunological memory and significantly reduces the vaccination dose of antigens [
26]. Polyoxidonium as an adjuvant has proven itself in various in vivo and in vitro studies, especially among patients with immunosuppressive conditions [
11,
27,
28,
29,
30,
31,
32,
33,
34].
Methods
Clinical Methods
Assessment of vaccine tolerance in patients with asthma and COPD
According to the study protocol, all local and systemic post-vaccination reactions were recorded within 7 days after the immunization.
Evaluation of clinical parameters before and after vaccination
After 6 and 12 months, the patients underwent a clinical and physical examination, including the collection of complaints and medical history, examination, frequency and duration of exacerbations of the underlying disease, courses of antibacterial chemotherapy and systemic corticosteroids, frequency and duration of hospitalization, days of work incapacity, frequency of outpatient visits during the year before and 12 months after the vaccination.
Spirometry
The spirometry study was performed using the Spiroanalyzer ST-95 following the recommendations of the European Respiratory Society at the baseline and 12 months after the vaccination. The main evaluated parameters were forced expiratory volume in one second (FEV1), forced vital vessel (FVC), ratio (FEV1/FVC), vital vessel (VC). The studied values were expressed as a percentage of the due, which simplifies the comparison of different groups of patients, eliminating the need for standardization by age, sex, weight, or height.
Immunological Method
To study the levels of CRP and cytokines, donor blood was taken into disposable plastic vacuum sterile tubes with a volume of a minimum of 10 mL before the vaccination, and after 6 and 12 months after the vaccination. The tubes with blood were centrifuged at 3,000 rpm. The obtained blood serum samples from donors were frozen at -25 °C.
Assay of the CRP level was performed by ELISA using the CRP–ELISA–Best (high sensitivity) reagent kit, Russia. Determination of IL-2, IL-6, IL-10, and IL-17 was performed by ELISA using Vector Best reagent kits, Russia, following the attached instructions for the generally accepted procedure.
The work was performed using licensed equipment of the Shared Use Center of the Federal State Budgetary Scientific Institution I. Mechnikov Research Institute of Vaccines and Sera.
Statistics
Descriptive statistics were used to process the numerical data. All numerical data are presented as Mean SD. To compare the data before and after the vaccination, the paired Wilcoxon test was used. To compare data at 3 time points (baseline, 6 months post-vaccination and 12 months), Friedman’s test with Dunn’s multiple comparison was used. The differences were considered statistically significant at p <0.05.
Statistical processing of the results was performed using GraphPad Prism (v.9.3.0 license GPS-1963924).
Results
Post-Vaccination
The administration of the influenza vaccine in patients with asthma and COPD was not accompanied by the development of serious adverse events, and the incidence of moderate local reactions in the form of pain, redness, and induration at the injection site within 3 days after vaccination was observed only in 3 (8.3%) patients with asthma that do not require therapeutic measures. Systemic reactions were recorded in 4 (11.1%) vaccinated patients with asthma and in one patient with COPD (1.72%), which were characterized by the appearance of catarrhal events from the upper respiratory tract, but it was impossible to exclude episodes of intercurrent diseases within 7 days after vaccination. No patient needed to intensify the basic therapy. In healthy subjects, the post-vaccination period proceeded without any unusual events.
The follow-up of patients with asthma and COPD for a year after the vaccination revealed a significant reduction in the frequency and duration of exacerbations of the bronchial obstructive syndrome (BF) as well as the frequency and duration of hospitalizations (
Table 2). In addition, patients with asthma showed a decrease in the need for outpatient care, a decrease in the frequency of prescription of systemic corticosteroids courses for 1 year; the number of patients who required course prescription of systemic corticosteroids during exacerbations decreased by 16.7%. In patients with COPD, in contrast to the observed patients with asthma, a decrease in the need for antibiotics was established as compared to the previous year before the vaccination.
Table 2.
Analysis of medical care requests in patients with asthma and COPD against influenza. Note: Continuous variables are presented as Mean ± SD, categorical variables are presented as number / percentage (%). 1 observation was 12 months before and 12 months after vaccination. 2 the paired Wilcoxon’s test was used
Table 2.
Analysis of medical care requests in patients with asthma and COPD against influenza. Note: Continuous variables are presented as Mean ± SD, categorical variables are presented as number / percentage (%). 1 observation was 12 months before and 12 months after vaccination. 2 the paired Wilcoxon’s test was used
| Parameter |
Before
Vaccination 1
|
After
vaccination |
P value 2
|
| Asthma group (N=34) |
| Frequency of BF exacerbations, episodes/year |
3.1 ± 0.2 |
1.6 ± 0.2 |
<0.001 |
| Duration of exacerbations, days |
42.7 ± 3.3 |
19.6 ± 2.5 |
<0.001 |
| Frequency of outpatient visits, cases/year |
14.0 ± 1.1 |
8.9 ± 0.9 |
0.002 |
| Frequency of hospitalizations, episodes/year |
2.5 ± 0.25 |
1.2 ± 0.2 |
<0.001 |
| Duration of hospitalization, days |
36.4 ± 3.5 |
16.0 ± 2.7 |
<0.001 |
| Number of courses of antibiotics per year |
1.4 ± 0.2 |
1.3 ± 0.2 |
0.92 |
| Number of systemic corticosteroids courses during the year |
2.1 ± 0.3 |
1.5 ± 0.2 |
0.04
|
| COPD group (N=20) |
| Frequency of BF exacerbations, episodes/year |
2.9 ± 0.3 |
1.5 ± 0.2 |
0.01 |
| Duration of exacerbations, days |
45.4 ± 4.5 |
17.5 ± 3.4 |
0.004 |
| Frequency of outpatient visits, cases/year |
11.1 ± 1.4 |
8.9 ± 1.5 |
0.13 |
| Frequency of hospitalizations, episodes/year |
2.8 ± 0.3 |
1.1 ± 0.3 |
0.001 |
| Duration of hospitalization, days |
44.8 ± 4.9 |
15.1 ± 3.6 |
0.002 |
| Number of courses of antibiotics per year |
2.8 ± 0.6 |
0.8 ± 0.2 |
0.03 |
| Number of systemic corticosteroids courses during the year |
1.3 ± 0.3 |
0.8 ± 0.2 |
0.09 |
Spirometry Parameters in Vaccinated Subjects
Analysis of spirometry parameters in asthma and COPD patients before vaccination and after 12 months of observation is presented in
Table 3. In vaccinated asthma patients after 12 months, there is a tendency to an increase in both volumetric and velocity parameters compared to the baseline. In COPD patients, an increase in VC was detected within the specified period compared to the baseline.
Table 3.
Pre-bronchodilation parameters of spirometry in patients with asthma and COPD vaccinated against influenza. Note: Continuous variables are presented as Mean ± SD. 1 the paired Wilcoxon’s test was used.
Table 3.
Pre-bronchodilation parameters of spirometry in patients with asthma and COPD vaccinated against influenza. Note: Continuous variables are presented as Mean ± SD. 1 the paired Wilcoxon’s test was used.
| Parameter |
Baseline |
After 12 months |
P value1
|
| Asthma group (N=34) |
| VC, abc |
2.55 ± 0.18 |
2.84 ± 0.17 |
0.09 |
| VC, % |
63.21 ± 3.91 |
70.28 ± 2.57 |
0.06 |
| FVC, abc |
2.15 ± 0.24 |
2.49 ± 0.23 |
0.19 |
| FVC, % |
52.37 ± 3.91 |
58.84 ± 3.67 |
0.11 |
| FEV1, abc |
1.71 ± 0.17 |
1.86 ± 0.16 |
0.26 |
| FEV1, % |
52 ± 3.65 |
56.2 ± 3.46 |
0.08 |
| FEV1/FVC |
68.34 ± 4.49 |
64.59 ± 2.75 |
0.54 |
| COPD group (N=20) |
| VC, abc |
2.08 ± 0.14 |
2.45 ± 0.15 |
0.01 |
| VC, % |
50.24 ± 2.75 |
60.06 ± 2.75 |
0.04 |
| FVC, abc |
1.62 ± 0.18 |
1.71 ± 0.13 |
0.28 |
| FVC, % |
37.69 ± 3.61 |
42 ± 2.74 |
0.07 |
| FEV1, abc |
1.13 ± 0.13 |
1.17 ± 0.08 |
0.49 |
| FEV1, % |
33.65 ± 3.43 |
35.76 ± 2.16 |
0.33 |
| FEV1/FVC |
52.7 ± 3.42 |
46.46 ± 3.2 |
0.06 |
Correlation analysis of clinical, immunological, and functional parameters in asthma and COPD patients before vaccination against influenza
Asthma Patients
In asthma patients before the vaccination, the IL-6 level is directly correlated with the level of serum CRP (r = 0.68; p <0.05. IL-6 also has a direct moderate correlation with the duration of exacerbations of the underlying disease (r = 0.54; p <0.05) and the number of systemic corticosteroids courses during exacerbations (r = 0.58; p < 0.05). A similar direct moderate correlation dependence was found between pro-inflammatory IL-2 and the frequency of systemic corticosteroids prescription (r = 0.68; p < 0.05). Moderate inverse correlations between the IL-17 level and FEV1 were revealed (r = -0.65; p <0.05).
There was a direct strong correlation between the number of exacerbations and their duration (r = 0.91; p <0.05), the number of hospitalizations for exacerbations (r = 0.74; p <0.05), a direct moderate correlation with the number of exacerbations of the underlying disease with the frequency of outpatient visits (r = 0.39; p <0.05), the duration of hospitalizations (r = 0.68; p <0.05), the number of courses of antibiotic therapy (r = 0.42; p <0.05), and the number of SGCS courses (r = 0.54; p <0.05).
COPD Patients
In COPD patients before the vaccination against influenza, a strong inverse correlation between the pro-inflammatory cytokine IL-2 and the frequency of outpatient visits was revealed (r = -0.81; p<0.05).
A direct strong correlation was observed between the clinical parameters: the frequency of COPD exacerbations with the duration of exacerbations (r = 0.85; p <0.05), the frequency of outpatient visits (r = 0.73; p <0.05), and the frequency of hospitalizations (r = 0.78; p <0.05). The serum CRP level was directly correlated with the rate of FEV1 (r = 0.51; p <0.05). The frequency of hospitalizations had a direct moderate correlation with the frequency of prescription of antibacterial chemotherapy (r = 0.66; p <0.05).
Cytokine Profile
Cytokine profile of patients with bronchial asthma, COPD and healthy vaccinated subjects is presented in
Table 4. The level of CRP exceeded the conditional norm (up to 8 mg/L) in all study periods in asthma and COPD patients. In healthy subjects, the CRP was within the reference values.
Table 4.
Cytokine profile of patients with bronchial asthma and chronic obstructive pulmonary disease and healthy subjects. Note: Continuous variables are presented as Mean ± SD. 1 the Friedman test was used, with Dunn’s multiple comparison, where: p6 – comparison at 6 months with baseline, p12 – comparison at 12 months with baseline.
Table 4.
Cytokine profile of patients with bronchial asthma and chronic obstructive pulmonary disease and healthy subjects. Note: Continuous variables are presented as Mean ± SD. 1 the Friedman test was used, with Dunn’s multiple comparison, where: p6 – comparison at 6 months with baseline, p12 – comparison at 12 months with baseline.
| Parameter |
Baseline |
After
6 months |
After
12 months |
P value1
|
| Asthma group |
| CRP (N=32) |
10.79 ± 1.88 |
9.27 ± 2.04 |
9.6 ± 1.94 |
p=0.67 |
| IL-2 (N=18) |
0.58 ± 0.31 |
0.35 ± 0.15 |
0.15 ± 0.1 |
p=0.06 |
| IL-6 (N=18) |
8.3 ± 3.08 |
6.61 ± 2.43 |
4.64 ± 2.0 |
p=0.004: p6=0.17, p12=0.003
|
| IL-17 (N=18) |
0.98 ± 0.2 |
1.02 ± 0.29 |
0.42 ± 0.28 |
p=0.06 |
| IL-10 (N=18) |
2.91 ± 0.77 |
3.4 ± 0.66 |
1.48 ± 0.44 |
p=0.08 |
| COPD group |
| CRP (N=16) |
22.44 ± 7.33 |
31.4 ± 11.95 |
17.71 ± 3.38 |
p=0.21 |
| IL-2(N=16) |
5.49 ± 4.19 |
1.41 ± 0.61 |
0.6 ± 0.27 |
p=0.07 |
| IL-6(N=18) |
12.13 ± 5.69 |
5.06 ± 0.93 |
2.16 ± 0.77 |
p=0.01: p6=0.53, p12=0.004
|
| IL-17(N=15) |
0.48 ± 0.14 |
0.33 ± 0.14 |
0.46 ± 0.32 |
p=0.31 |
| IL-10(N=18) |
3.72 ± 0.73 |
3.65 ± 1.0 |
0.75 ± 0.51 |
p=0.002: p6=0.46, p12=0.01
|
| Healthy |
| CRP (N=18) |
4.89 ± 1.29 |
4.61 ± 1.58 |
5.79 ± 2.17 |
p=0,82 |
| IL-2 (N=13) |
1.11 ± 0.47 |
0.55 ± 0.33 |
0.2 ± 0.11 |
p=0,11 |
| IL-6 (N=12) |
6.67 ± 1.16 |
5.22 ± 0.82 |
3.75 ± 0.71 |
p=0.04: p6=0.24, p12=0.02
|
| IL-17 (N=11) |
0.04 ± 0.04 |
0.12 ± 0.06 |
0.09 ± 0.07 |
p=0.78 |
| IL-10 (N=15) |
5.15 ± 0.33 |
4.46 ± 0.5 |
2.91 ± 0.73 |
p=0.007: p6=0.25, p12=0.006
|
The level of IL-2 in COPD and asthma patients and healthy vaccinated subjects during the entire follow-up period (12 months) was within the normal range (less than 10 pg/mL). In all groups, there was a tendency to a decrease in the level of IL-2 after 6 and 12 months (p >0.05), especially in COPD patients.
The baseline of IL-6 level in COPD patients was above the normal (more than 10 pg/mL) and amounted to 12.13 ± 5.69 pg/mL. In asthma patients and healthy subjects, the IL-6 level was within the normal range (
Figure 1). After 6 and 12 months after the vaccination against influenza, the IL-6 level in all groups was within the normal range, but its significant reduction compared to the baseline values was observed after the 12 months.
Figure 1.
Evolution of IL-6 levels in asthma and COPD patients and healthy individuals before and after influenza vaccination (normal values less than 10 pg/mL); Note: * p< 0.05 ** p<0.005—significance of differences relative to the baseline values.
Figure 1.
Evolution of IL-6 levels in asthma and COPD patients and healthy individuals before and after influenza vaccination (normal values less than 10 pg/mL); Note: * p< 0.05 ** p<0.005—significance of differences relative to the baseline values.
The IL-17 level in all groups before vaccination, as well as after 6 and 12 months, was within the reference values (less than 5 pg/mL), although it was higher in asthma patients than in COPD patients and healthy vaccinated subjects.
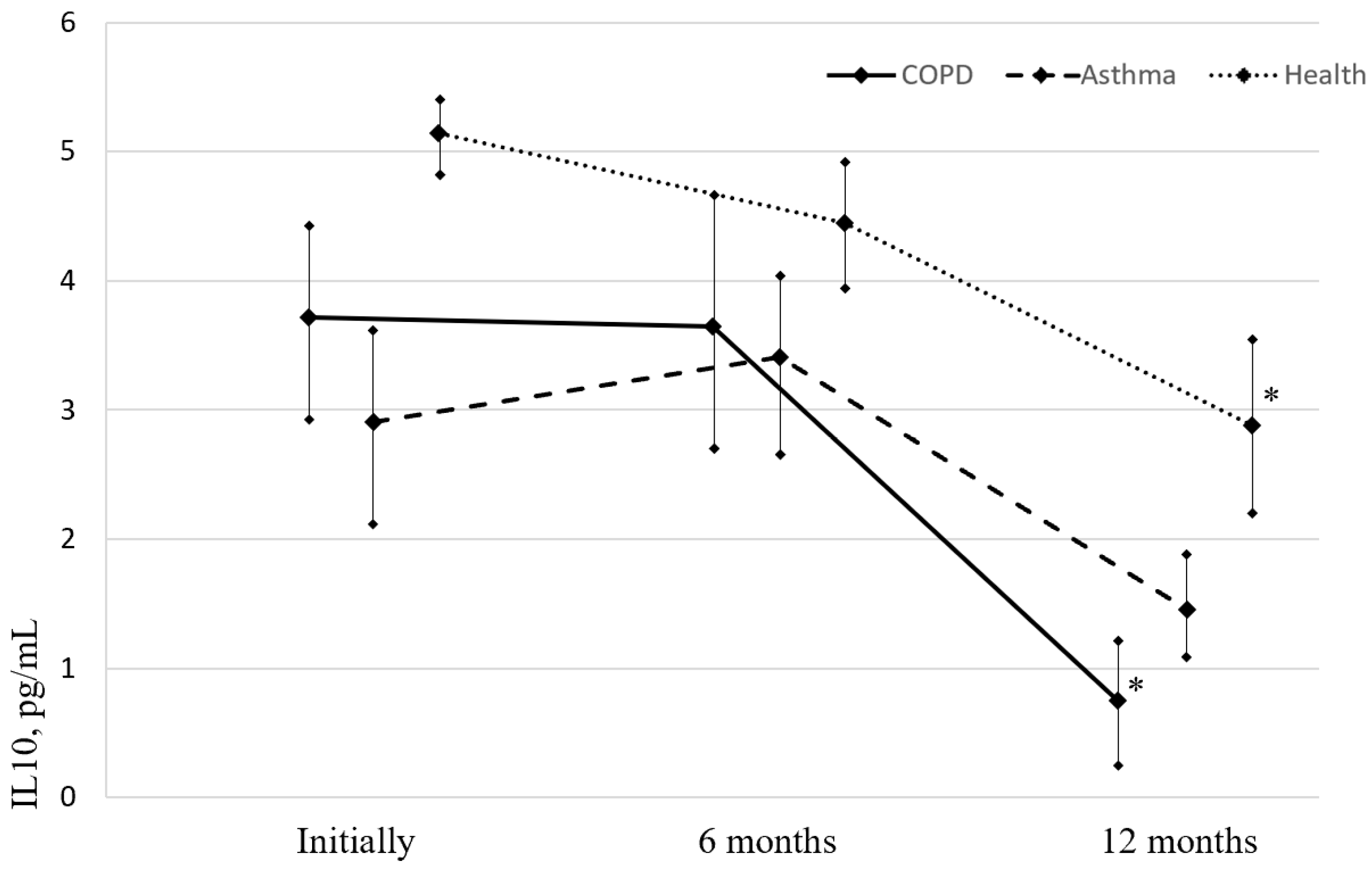
IL-10 level in all groups at the time of vaccination, as well as after 6 and 12 months, was below the permissible values (less than 20 pg/mL) (
Figure 2). During the first 6 months after vaccination, there were no statistically significant changes in the IL-10 concentration in any group. Over the next 6-12 months, there was a significant reduction in the level of IL-10 compared to baseline in the group of patients with COPD and healthy vaccinated people.
Figure 2.
Evolution of IL-10 (normal values less than 20 pg/mL) levels in asthma and COPD patients and healthy individuals before and after influenza vaccination; Note: * p< 0.05—significance of differences relative to the baseline values. (Image acquisition tool and image processing software package: Microsoft Office 2019, PowerPoint, Diagrams).
Figure 2.
Evolution of IL-10 (normal values less than 20 pg/mL) levels in asthma and COPD patients and healthy individuals before and after influenza vaccination; Note: * p< 0.05—significance of differences relative to the baseline values. (Image acquisition tool and image processing software package: Microsoft Office 2019, PowerPoint, Diagrams).
Discussion
Grippol Plus, the Russian trivalent polymer-subunit vaccine with a reduced (3-fold) content of epidemically relevant strains of influenza virus subtypes A (H1N1, H3N2) and type B (total 15 µg HA) and azoximer bromide (500 µg), was used in asthma and COPD patients. The vaccine has been used for more than 25 years within the framework of the National Immunization Schedule in children from 6 months of age without age restrictions, patients with various pathologies [
35,
36,
37], and pregnant women [
30,
33,
39,
40]. Asthma and COPD patients were also vaccinated with this drug product, but it was not previously studied whether vaccination is accompanied by a change in the content of inflammatory mediators in the post-vaccination period and the considered immunological parameters.
During the follow-up period of asthma and COPD patients vaccinated against influenza, there were no unusual events that would be accompanied by a deterioration in the condition of patients, both in the early stage after the vaccination and the long-term period (12 months of the follow-up). On the contrary, within a year after the vaccination against influenza, was indicated a favorable effect on the clinical course of the disease with a 1.9-2-fold decrease in the frequency of BF exacerbations in asthma and COPD patients, respectively. In the same period, duration of exacerbations decreased by 2.2-2.5 times, frequency of hospitalizations decreased by 2.0-2.5 times, duration of hospitalizations decreased by 2.3-3 times, and the need for outpatient care decreased by 1.6 timesin asthma patients and 20% (p >0.05) in the COPD patients. The obtained results are consistent with the data of other researchers who studied the vaccine efficacy against influenza in patients with bronchopulmonary pathology [
41,
42,
43].
Unlike asthma patients, the group of COPD patients within 12 months after the vaccination showed a 3.6-fold decrease in the number of courses of antibacterial drugs, which may indicate a decrease in infection-dependent COPD exacerbations. Similar results were obtained by Poole P.J. during vaccination of COPD patients against influenza [
44] and Sumitani during combined vaccination against influenza and pneumococcal infection [
45]. A.D. Protasov used combined vaccination against influenza, hemophilic and pneumococcal infections in COPD patients and obtained an even more significant clinical effect: the number of exacerbations per year decreased by 3.7 times, and the number of courses of antimicrobial chemotherapy decreased by 4.3 times [
46,
47,
48].
Having been traditionally utilized as a marker of infection and cardiovascular events, there is now growing evidence that CRP plays important roles in inflammatory processes and host responses to infection including the complement pathway, apoptosis, phagocytosis, nitric oxide (NO) release, and the production of cytokines, particularly interleukin-6 and tumor necrosis factor-α [
49,
50]. In unvaccinated patients the CRP level has a direct moderate correlation with the duration of exacerbations of the underlying disease with FEV1, which indicates that a reduction of inflammation in the airways leads to a decrease in acute-phase proteins, therefore, to a decrease in the duration of exacerbation.
The analysis of the CRP level as a marker of systemic inflammation and a predictor of prognosis in COPD within a year after the vaccination against influenza showed that in patients with BF the level of CRP exceeds the permissible values (especially in patients with COPD) as compared to the healthy vaccinated subjects. No statistically significant changes in CRP parameters, after 6 and 12 months after the vaccination, in comparison with the baseline vaules, were revealed in any of the study groups, which may indicate a stable persistence of the chronic inflammatory process in the vaccinated BF patients.
IL-2, IL-6, and IL-17 are systemic pro-inflammatory cytokines, and IL-10 is an anti-inflammatory cytokine, the concentration of which increases in acute inflammatory processes and exacerbations of chronic diseases caused by a viral or bacterial infection.
In patients before the vaccination, the level of IL-6 is directly correlated with the level of serum CRP, which is explained by the fact that the synthesis of CRP is carried out in hepatocytes and is regulated by interleukins IL-1 and IL-6. A direct correlation dependence of moderate strength was revealed between pro-inflammatory IL-2 and the frequency of systemic corticosteroids prescription. Moderate inverse correlations between the IL-17 level and FEV1 were revealed. This confirms the fact that excessive synthesis of IL-17 can lead to the chronicity of the inflammatory process and a decrease in FEV1.
The study of cytokine levels in asthma patients and COPD patients vaccinated against influenza showed, that most parameters at the time of vaccination and during the study were within the proper values. A decrease in the level of the pro-inflammatory cytokine IL-6 and a tendency to a decrease in IL-2, IL-17 over time indicates a decrease in the systemic inflammatory process in vivo.
In asthma patients, the concentration of IL-17 was significantly higher than in COPD patients and healthy subjects, which confirms the role of this cytokine in the development of allergic reactions characteristic of asthma. After 12 months post-vaccination in asthma patients, there was a tendency to a decrease in IL-17. In COPD patients, the level of IL-17 practically did not change during the observation period. In healthy subjects, the content of IL-17 was lower than in COPD and asthma patients and did not exceed 0.12 ± 0.06 pg/mL.
We consider the decrease in anti-inflammatory IL-10, after the administration of the influenza vaccine, being a response to the reduction in pro-inflammatory cytokines, since the regulation of the inflammatory response is carried out according to the principle of negative feedback [
51]. We observed a tendency towards a decrease in the level of pro-inflammatory cytokines IL-2, IL-6 in the first 6 months after the vaccination, which led to a decrease in the level of anti-inflammatory IL-10 in the next 6 months. We also found positive clinical and functional changes in the course of asthma and COPD during the follow-up period.
Of note, the study sample is small, which makes it less representative, meaning that the study results are relevant for smaller homogeneous populations. To ensure homogeneity of the study population, we applied stricter inclusion and exclusion criteria compared to those used in larger studies (all study subjects were included within one epidemic period, they showed no signs of asthma or COPD exacerbation at the time of inclusion, etc.).
It should also be noted that, although the study sample is small, it ensures
100% power for each study group (the study power was calculated based on the
IL-6 level measured 12 months after the start of the study, with the effect size (d)
equaling 1.4 in the asthma group, 2.5 in the COPD group, and 3.03 in the group of
Thus, the analysis of immunological, clinical, and functional parameters in asthma and COPD patients confirms that vaccination against influenza in BF patients is effective and has a positive impact on the clinical, immunological, and functional parameters of the disease.
Conclusion
Development of the new vaccine technologies, using adjuvants or other substances that enhance the efficacy of the drug product, requires further study, including the mechanisms of the immune response that confirm their safety. The study evaluated the effect of a trivalent polymer-subunit vaccine with a reduced number of antigens associated with the azoximer bromide immunoadjuvant. The obtained results confirm the clinical vaccine efficacy for reducing the frequency of exacerbations, hospitalizations and increasing the remission period, as with other influenza vaccines. Vaccination may be accompanied by a decrease in the level of inflammatory mediators, which, in our opinion, is an indirect effect, that is caused by a decrease in respiratory infections, the achievement of persistent remission of chronic bronchopulmonary pathology in the post-vaccination period leading to the normalization of certain parameters of the immune system. Namely, the preventive effect of the vaccination against influenza is reflected in the clinical course of asthma and COPD. It is anticipated that this study will become an opening to this problem investigation on a larger scale and will be taken into account by our colleagues.
Author contributions statement
M.K.- head of the study, design, description of results, A.Chu - study coordinator, scientific advisor, A. Che - vaccination, observation of clinical status of patients, assessment of patients’ functional status, evaluation of long-term results of vaccination, N.A. - conducting immunological studies, V.P. - collection of literature materials, preparation of bibliography, N.K. – collecting and processing the material, responsible for correspondence with reviewers, A.K. - responsible for preparation of tables, translations, A.V. - statistical analysis, A.C – definition and collection of material, organization of transport of material, samples and collection of data I.S. -collection of materials, organization in the collection of materials, samples and data. - M.L. – verification of the text and revision of the article, evaluation of long-term results of vaccination, A.S. - conducting immunological studies. L.G – description of results, collection and processing of results, statistical analysis, Y.A. -checking the translation of the text and rereading the article in English, revision and correction of the references in the bibliography, publication of the article.
Availability of materials and data
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Additional information
The authors declare no competing interests.
References
- Papi, A., Bellettato C.M., Braccioni, F., et al. Infections and airway inflammation in chronic obstructive pulmonary disease severe exacerbations. Am. J. Respir. Crit. Care Med. 2006; 173(10):1114-21. https://doi.org/10.1164/rccm.200506-859OC. [CrossRef]
- Wilkinson, T.M.A., Aris E., Bourne1S. et al. A prospective, observational cohort study of the seasonal dynamics of airway pathogens in the aetiology of exacerbations in COPD. Thorax. 2017; 72(10):919-27. https://doi.org/. [CrossRef]
- Perera, W.R., Hurst J. R., Wilkinson T. M. A., et al. Inflammatory changes, recovery and recurrence at COPD exacerbation. Eur. Respir. J. 2017;29(3):527-34. https://doi.org/. [CrossRef]
- Anzueto, A. Impact of exacerbations on COPD. Eur. Respir. Rev. 2010; 19(116):113-8. https://doi.org/. [CrossRef]
- Miravitlles, M., Ferrer, M., Pont À., et al. Effect of exacerbations on quality of life in patients with chronic obstructive pulmonary disease: a 2 year follow up study. Thorax. 2004;59(5):387-95. https://doi.org/. [CrossRef]
- Decramer, M., Celli, B., Kesten, S., et al. Frequency of exacerbations adversely impacts the course of COPD. Am. J. Respir. Crit. Care Med. 2010;181(5):A.1526.
- Donaldson, G.C. & Wedzicha, J.A. COPD exacerbations. 1: Epidemiology. Thorax. 2006;61(2):164-8. https://doi.org/. [CrossRef]
- Soler-Cataluña, J.J., Martínez-García1 M. Á., Román-Sánchez P. et al. Severe acute exacerbations and mortality in patients with chronic obstructive pulmonary disease. Thorax. 2005;60(11):925-31. https://doi.org/. [CrossRef]
- Kuz’mina, T.Yu., Tikhonova, E.P., Upirova, A.A. & Strokina, G.G. ORVI i gripp v epidsezon 2009 goda. In: Materialy II Ezhegodnogo Vserossiyskogo Kongressa po infektsionnym boleznyam Mar 29-31 (Moscow). p. 165. In Russian (Izdatel’stvo “Dinastiya 2010).
- Chuchalin, A.G., Briko N.I., С.Н., Abdief S.N., et al. Vaccine prophylaxis of respiratory diseases in the framework of primary health care. Clinical recommendations in Pulmonologiya Suppl. 2015;2:1-19. In Russian.
- Kostinov, M.P. & Chuchalin AG. Rukovostvo po klinicheskoy immunologii v respiratornoy meditsine (ed. Kostinov, M.P. & Chuchalin AG). Izd. 1-e. Moscow. 128 p. Russian. (ATMO, 2016).
- Benfield, T., Lange, P. & Vestbo, J. COPD stage and risk of hospitalization for infectious disease. Chest. 2008;134(1):46-53. https://doi.org/. [CrossRef]
- Chuchalin, A.G. Respiratornaya meditsina in Rukovodstvo v 3 tomakh. (ed. Chuchalin, A.G.). Izd. 2-e, pererabotannoe i dop. Moscow. Vol. 2. 544 p. In Russian. (Litterra, 2017).
- Wood, L.G., Powell H., Grissell T., et al. Persistent airway obstruction after virus infection is not associated with airway inflammation. Chest. 2007;131(2):415-23. https://doi.org/. [CrossRef]
- Sanei, F. & Wilkinson, T. Influenza vaccination for patients with chronic obstructive pulmonary disease: understanding immunogenicity, efficacy and effectiveness. Ther. Adv. Respir. Dis. 2016;10(4):349-67. https://doi.org/. [CrossRef]
- Bekkat-Berkani, R., Wilkinson T., Buchyet P. al. Seasonal influenza vaccination in patients with COPD: a systematic literature review. BMC Pulm. Med. 2017;17(1):79. https://doi.org/. [CrossRef]
- Kostinov, M.P. & Zverev, V.V. Vaktsinatsiya protiv gepatita B, grippa i krasnukhi vzroslykh patsientov s khronicheskimi zabolevaniyami (ed. Kostinov, M.P., Zverev, V.V.) 196 p. In Russian (MDV, 2009).
- Burel, J.G., Nath K., Pritchard A.L. et al. Evaluation of immune responses to influenza vaccination in chronic obstructive pulmonary disease. J. Vaccines Vaccin. 2012;S4:001. https://doi.org/. [CrossRef]
- Li, Y., Ma, Y., An, Z., et al. Immunogenicity of trivalent seasonal influenza vaccine in patients with chronic obstructive pulmonary disease. Hum. Vaccin. Immunother. 2021;17(9):3131-6. https://doi.org/. [CrossRef]
- Staples, K.J., Williams, N. P., Bonduelle,O., et al. Acquired immune responses to the seasonal trivalent influenza vaccination in COPD. Clin. Exp. Immunol. 2019;198(1):71-82. https://doi.org/. [CrossRef]
- Bonduelle, O., Carrat, F., Luyt, C.,et al. Characterization of pandemic influenza immune memory signature after vaccination or infection. J. Clin. Invest. 2014;124(7):3129-36. https://doi.org/. [CrossRef]
- Khromova, E.A., Semochkine, I.A., AKHMATOVA, E.A., et al. Immunophenotype of lymphocytes under the influence of immunoadjuvanted and unadjuvanted influenza vaccines in Rossijskij immunologicheskij zhurnal. 2016;10:503–5. In Russian. Available online: https://www.elibrary.ru/item.asp?id=29124209.
- Khromova, E.A., Akhmatova, E.A., Skhodova, S.A., et al. Effect of influenza vaccines on subpopulations of blood dendritic cells. Zhurnal mikrobiologii, epidemiologii i immunobiologii. 2016;5:23–8. In Russian. https://doi.org/. [CrossRef]
- Hak, E., Wei, F., Nordin, J.,et al. Development and validation of a clinical prediction rule for hospitalization due to pneumonia or influenza or death during influenza epidemics among community-dwelling elderly persons. J. Infect. Dis. 2004;189(3):450-8. https://doi.org/. [CrossRef]
- Kostinov, M.P. Vaktsinatsiya vzroslykh s bronkholegochnoy patologiey in Rukovodstvo dlya vrachey (ed. Kostinov, M.P.). 112 p. In Russian. (Art studiya «Sozvezdie» 2013).
- Instruktsiya po meditsinskomu primeneniyu preparata Grippol® plyus. Russian. Available online: https://petrovax.com/medication/catalog/grippallplus/ (2022).
- Zverev, V.V. & Khaitov, R.M. Vaktsiny i vaktsinatsiya in Natsional’noe rukovodstvo (ed. Zverev, V.V., Khaitov, R.M.). 640 p. In Russian. (GEOTAR-Media, 2014).
- Kostinov, M.P. & Tarasova, A.A. Vaktsinoprofilaktika pnevmokokkovoy infektsii i grippa pri autoimmunnykh zabolevaniyakh in Rukovodstvo dlya vrachey. 252 p. In Russian. (MDV, 2009).
- Kostinov, M.P. & Cherdantsev, A.P. Health state of children born from pregnant women vaccinated against influenza. Journal Pediatria n.a. G.N. Speransky. 2016;95 (1):67-71. In Russian. https://doi.org/. [CrossRef]
- Zverev, V.V., Kostinov, M.P., Cherdancev, A.P. et al. Vakcinacija beremennyh protiv grippa in Federal’nye klinicheskie rekomendacii. 42 p. In Russian. (Remedium Privolzh’e, 2015).
- Kostinov, M.P., Cherdantsev, A.P., Semenova, S.S., et al. Obstetric and perinatal outcomes among pregnant women after influenza vaccination and after transferred respiratory infection. Gynecology 2015;17(4):43-6. In Russian. [CrossRef]
- Kostinov, M.P., Cherdantsev, A.P., Savis’ko, A.A., et al. True and false reactions in pregnant women to introduction of influenza vaccine. Gynecology, Obstetrics and Perinatology. 2011;10(6):44-8. In Russian.
- Cherdantsev, A.P., Kostinov, M.P., Kuselman A.I.,et al. A study of the clinical safety of influenza vaccination in pregnant women. Medical almanac. 2011;4:120-2. In Russian.
- Kostinov, M.P. & Cherdantsev, A.P. The clinical and immunological safety of inactivated immunologic adjuvant influenza subunit vaccine for pregnant women. Obstetrics and Gynecology. 2016;2:64–9. In Russian.
- Zhang, J., Zengfeng Zhang,Z., Fan, X., al. 2009 pandemic H1N1 influenza virus replicates in human lung tissues. J. Infect. Dis. 2010;201(10):1522-6. https://doi.org/. [CrossRef]
- Kostinova, A.M., Akhmatova,N.K., Latysheva, E.A., et al. Assessment of immunogenicity of adjuvanted influenza vaccine in healthy people and patients with common variable immune deficiency Front. Immunol. 2020;11:1876. https://doi.org/10.3389/fimmu.2020.01876. [CrossRef]
- Kostinov, M.P., Latysheva, E.A., Kostinova, A.M., et al. Immunogenicity and safety of the quadrivalent adjuvant subunit influenza vaccine in seropositive and seronegative healthy people and patients with common variable immunodeficiency. Vaccines (Basel). 2020;8(4):640. https://doi.org/. [CrossRef]
- Kostinov, M.P., Cherdantsev, A.P. & Pakhomov, D.V. Anti-influenza antibody level in mother-infant pairs depending on trimester of vaccination of pregnant women using immunoadjivant vaccine. J Vaccines Vaccin. 2015;6(5): 1000297. https://doi.org/. [CrossRef]
- Kostinov, M.P., Cherdantsev, A.P., Akhmatova, N.K., et al. Immunogenicity and safety of subunit influenza vaccines in pregnant women. ERJ Open Res. 2018;4(2):00060-2017. https://doi.org/. [CrossRef]
- Kostinov, M.P., Cherdantsev, A.P., Kuselman,A.I., et al. Prospective randomized open-label comparative study of immunogenicity after subunit and polymeric subunit influenza vaccines administration among mothers and infants. Hum. Vaccin. Immunother 2018;14(12):2971-8. https://doi.org/. [CrossRef]
- Petrova, T.I., Andreeva, N.P. & Kostinov, M.P.. Urgency of vaccinal prevention of the flu at children with diseases of respiratory apparatus. Practical medicine. 2009;7(39):63–5. In Russian.
- Gushchina, Y.S., Markelova, E.V., Kostinov, M.P. & Ibragimova, E.M. Vaccination of children with bronchial asthma. Pacific Medical Journal 2009;4:17–9. In Russian.
- Nekrasov, A.V., Puchkova, N.G. & Kostinov, M.P. Jeffektivnost’ i bezopasnost’ vakciny Grippol® pljus u raznyh kontingentov. Consiliummedicum. Pril. Pediatrija 2010;3:30-3. In Russian.
- Poole, P.J., Chacko, E., Wood-Baker, R.W. & Cates, C.J. Influenza vaccine for patients with chronic obstructive pulmonary disease. Cochrane Database Syst. Rev. 2006;1:CD002733. https://doi.org/. [CrossRef]
- Sumitani, M., Tochino, Y., Kamimori, T., et al. Additive inoculation of influenza vaccine and 23-valent pneumococcal polysaccharide vaccine to prevent lower respiratory tract infections in chronic respiratory disease patients. Intern. Med. 2008;47(13):1189-97. https://doi.org/. [CrossRef]
- Protasov, A.D., Zhestkov, A.V., Lavrentyeva, N.E., et al. Effect of complex vaccination against pneumococcal, haemophilus influenzae type b infections and influenza in patients with chronic obstructive pulmonary disease. Zhurnal mikrobiologii, epidemiologii i immunobiologii. 2011;4: 80–4. In Russian.
- Kostinov, M.P., Protasov, A.D., Zhestkov, A.V., et al. Post-vaccination immunity to pneumococcal, haemophilus influenzae type B infection and influenza in patients with chronic obstructive pulmonary disease (COPD). J. Vaccines Vaccin. 2014;5(2):1000221. https://doi.org/. [CrossRef]
- Protasov, A.D., Kostinov, M.P., Zhestkov, A.V., et al. Chronic obstructive pulmonary disease (COPD): clinical and immunological effects of mono-vaccination against influenza using an immunoadjvant vaccine of a new class versus combined administration S.pneumoniae, H. influenza and Influenza vaccines in Steps forwards in diagnosing and controlling influenza (ed. Daddour, M.M.). Chapter 11. p. 239-253. https://doi.org/ (Rijeka: InTech 2016). [CrossRef]
- Sproston, N.R. & Ashworth, J.J. Role of C-Reactive Protein at Sites of Inflammation and Infection. Front. Immunol. 2018;9:754. https://doi.org/. [CrossRef]
- Prins, H.J., Duijkers, R., Valk, P., et al. CRP-guided antibiotic treatment in acute exacerbations of COPD in hospital admissions. Eur. Respir. J. 2019;23;53(5):1802014. https://doi.org/. [CrossRef]
- Gereng, Ye.A., Sukhodolo, I., Pleshko, R. I., et al. Molecular markers of an inflammation in bronchial contents at various phenotypes of a serious bronchial asthma in Bulletin of Siberian medicine. 2011;3:24-9. In Russian.
- Cohen, J. (1988). Statistical power analysis for the behavioral sciences. Routledge: https://doi.org/10.4324/9780203771587. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).