Submitted:
28 September 2023
Posted:
30 September 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
- (1)
- Our goal was to determine the optimal diet for weight loss in women with PCOS, and we addressed the following questions:
- (2)
- Does replacement of dietary fat with CHO vs. protein influence the amount of weight loss and body composition?
- (3)
- What are the acute metabolic and endocrine effects of CHO and protein intake in PCOS?
- (4)
- Do amino acid compositions of dietary proteins affect weight-loss and/or insulin resistance?
1.2. Comparing low-fat/high-CHO vs. low-fat/high-protein diets during weight loss in women with PCOS
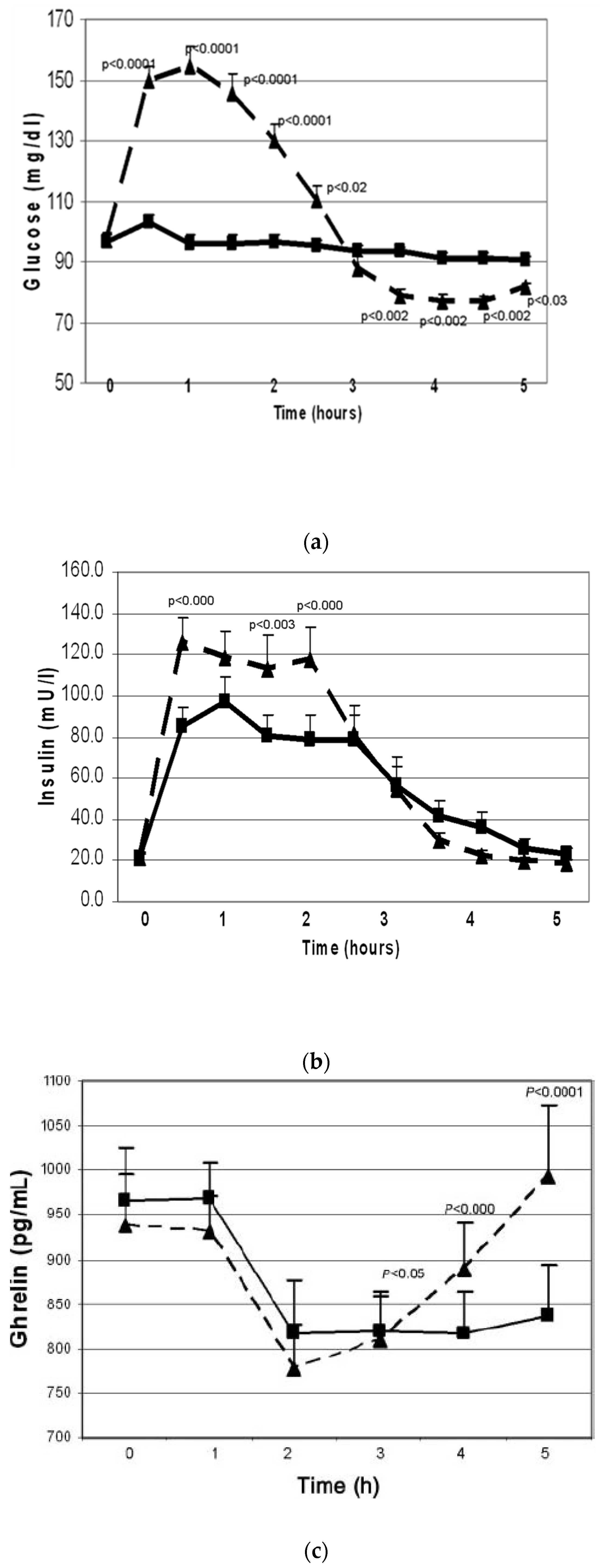
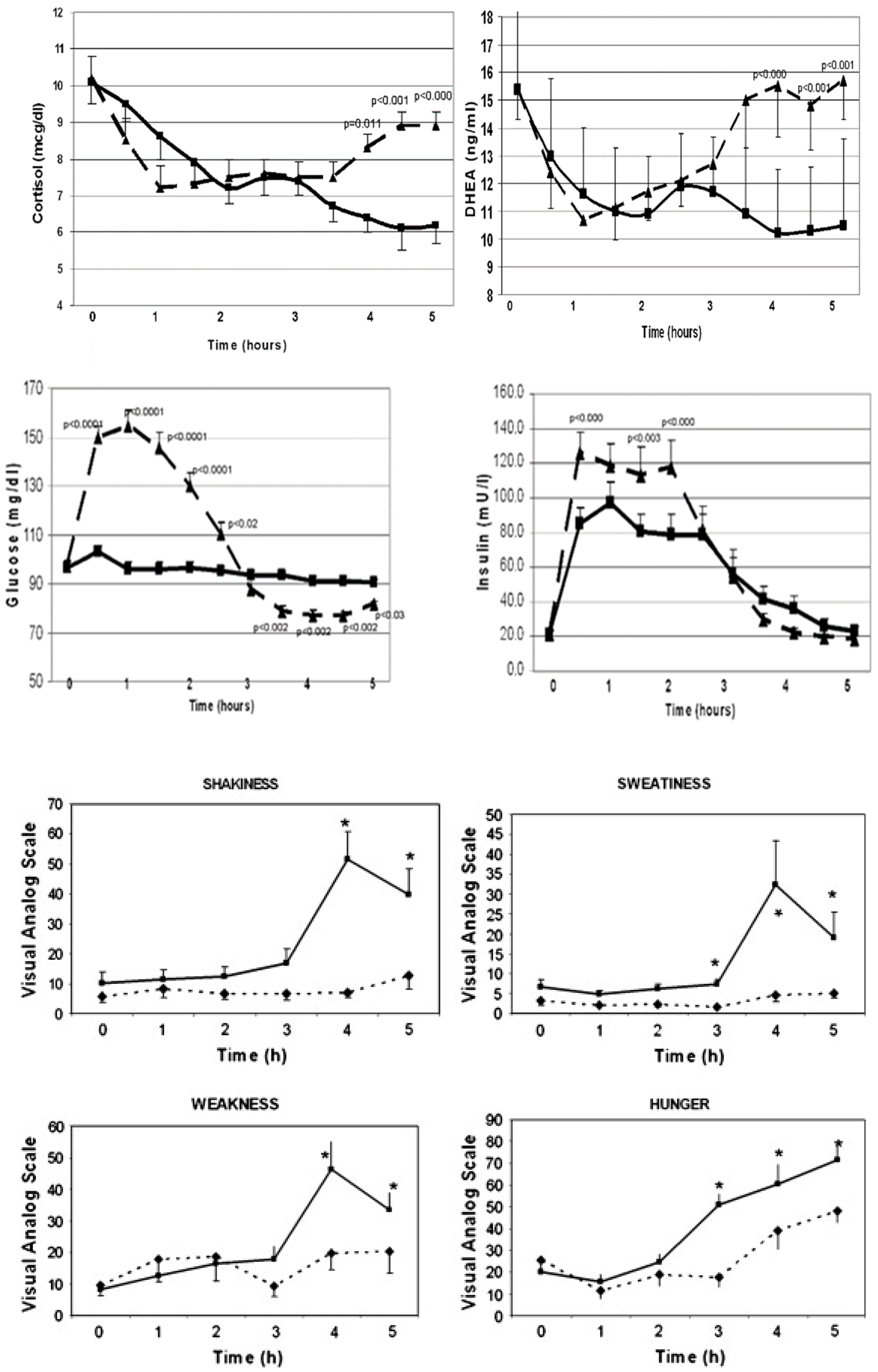
1.2.1. Acute effects of simple-CHO vs. protein
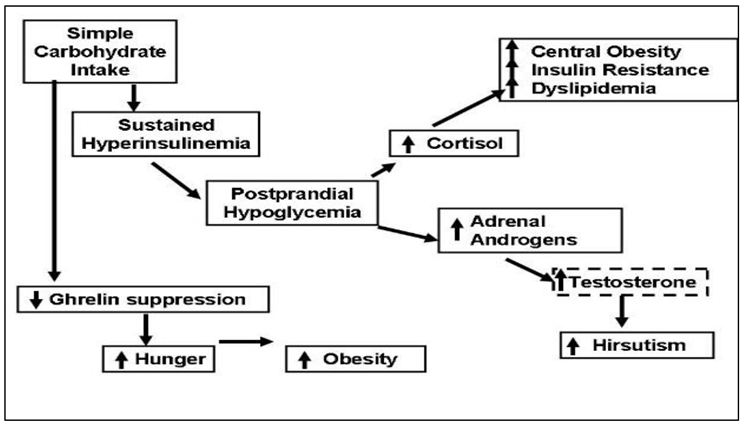
1.3. Postprandial hypoglycemia after simple-sugar vs. protein
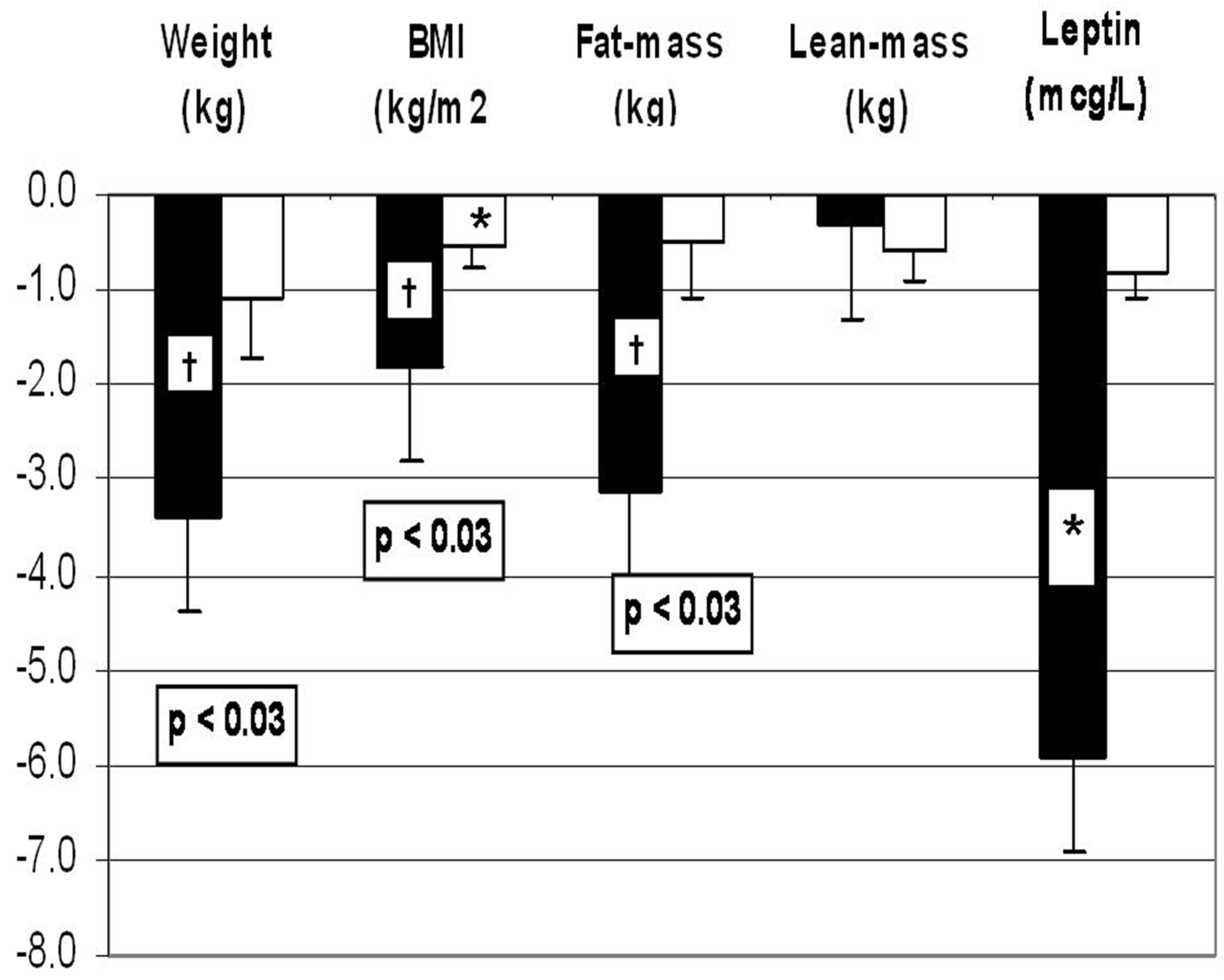
1.4. Effects of dietary CHO vs. protein on anthropometric outcomes during weight loss intervention:
1.5. Effects of amino acid composition of dietary protein on weight loss and metabolic parameters in women with PCOS
2. Discussion and a unifying hypothesis
- One third of the women with PCOS developed physiologically significant reactive hypoglycemia after simple-sugar intake and secreted of cortisol and adrenal androgens.
- Adrenal steroid secretion coincided with the hypoglycemic symptoms.
- Whey protein intake stimulated insulin secretion but did not cause hypoglycemia.
- Whey protein supplement suppressed the hunger signal ghrelin for a longer period as compared to simple-CHO supplement.
- A weight loss diet containing WP supplement was associated with greater weight loss and fat mass loss and decrease in leptin when compared to the diet containing simple-CHO supplement.
- When WP supplement was compared to gelatin supplement, there was no difference in the amount of weight loss or the improvement in insulin sensitivity, despite lower essential AA- and BCAA content of gelatin.
Conflicts of Interest
References
- Carmina E, Legro RS, Stamets K, Lowell J, and Lobo RA. Difference in body weight between American and Italian women with polycystic ovary syndrome: influence of the diet. Hum Reprod. 2003;18(11):2289-93.
- Glueck CJ, Papanna R, Wang P, Goldenberg N, and Sieve-Smith L. Incidence and treatment of metabolic syndrome in newly referred women with confirmed polycystic ovarian syndrome. Metabolism. 2003;52(7):908-15.
- Haase, C.L.; Varbo, A.; Laursen, P.N.; Schnecke, V.; Balen, A.H. Association between body mass index, weight loss and the chance of pregnancy in women with polycystic ovary syndrome and overweight or obesity: a retrospective cohort study in the UK. Hum Reprod 2023, 38, 471–481. [Google Scholar] [CrossRef]
- Marzouk, T.M.; Sayed Ahmed, W.A. Effect of Dietary Weight Loss on Menstrual Regularity in Obese Young Adult Women with Polycystic Ovary Syndrome. Journal of pediatric and adolescent gynecology 2015, 28, 457–461. [Google Scholar] [CrossRef]
- Ravn, P.; Haugen, A.G.; Glintborg, D. Overweight in polycystic ovary syndrome. An update on evidence based advice on diet, exercise and metformin use for weight loss. Minerva Endocrinol 2013, 38, 59–76. [Google Scholar]
- Thomson, R.L.; Buckley, J.D.; Moran, L.J.; Noakes, M.; Clifton, P.M.; Norman, R.J.; Brinkworth, G.D. The effect of weight loss on anti-Mullerian hormone levels in overweight and obese women with polycystic ovary syndrome and reproductive impairment. Hum Reprod 2009, 24, 1976–1981. [Google Scholar] [CrossRef]
- Mueller-Cunningham, W.M.; Quintana, R.; Kasim-Karakas, S.E. An ad libitum, very low-fat diet results in weight loss and changes in nutrient intakes in postmenopausal women. J Am Diet Assoc 2003, 103, 1600–1606. [Google Scholar] [CrossRef]
- Kasim-Karakas, S.E.; Tsodikov, A.; Singh, U.; Jialal, I. Responses of inflammatory markers to a low-fat, high-carbohydrate diet: effects of energy intake. Am J Clin Nutr 2006, 83, 774–779. [Google Scholar] [CrossRef]
- Gurusinghe, D.; Gill, S.; Almario, R.U.; Lee, J.; Horn, W.F.; Keim, N.L.; Kim, K.; Karakas, S.E. In polycystic ovary syndrome, adrenal steroids are regulated differently in the morning versus in response to nutrient intake. Fertil Steril 2010, 93, 1192–1199. [Google Scholar] [CrossRef]
- Kasim-Karakas, S.E.; Cunningham, W.M.; Tsodikov, A. Relation of nutrients and hormones in polycystic ovary syndrome. Am J Clin Nutr 2007, 85, 688–694. [Google Scholar] [CrossRef]
- Field, J.B. Hypoglycemia. Definition, clinical presentations, classification, and laboratory tests. Endocrinol Metab Clin North Am 1989, 18, 27–43. [Google Scholar] [CrossRef]
- Brun, J.F.; Fedou, C.; Mercier, J. Postprandial reactive hypoglycemia. Diabetes Metab 2000, 26, 337–351. [Google Scholar]
- Kasim-Karakas, S.E.; Almario, R.U.; Cunningham, W. Effects of protein versus simple sugar intake on weight loss in polycystic ovary syndrome (according to the National Institutes of Health criteria). Fertil Steril 2009, 92, 262–270. [Google Scholar] [CrossRef] [PubMed]
- Gorissen, S.H.M.; Crombag, J.J.R.; Senden, J.M.G.; Waterval, W.A.H.; Bierau, J.; Verdijk, L.B.; van Loon, L.J.C. Protein content and amino acid composition of commercially available plant-based protein isolates. Amino Acids 2018, 50, 1685–1695. [Google Scholar] [CrossRef] [PubMed]
- Newgard, C.B. Interplay between lipids and branched-chain amino acids in development of insulin resistance. Cell Metab 2012, 15, 606–614. [Google Scholar] [CrossRef] [PubMed]
- Newgard, C.B.; An, J.; Bain, J.R.; Muehlbauer, M.J.; Stevens, R.D.; Lien, L.F.; Haqq, A.M.; Shah, S.H.; Arlotto, M.; Slentz, C.A.; et al. A branched-chain amino acid-related metabolic signature that differentiates obese and lean humans and contributes to insulin resistance. Cell Metab 2009, 9, 311–326. [Google Scholar] [CrossRef]
- Eastoe, J.E. The amino acid composition of mammalian collagen and gelatin. Biochem J 1955, 61, 589–600. [Google Scholar] [CrossRef] [PubMed]
- Piccolo, B.D.; Comerford, K.B.; Karakas, S.E.; Knotts, T.A.; Fiehn, O.; Adams, S.H. Whey protein supplementation does not alter plasma branched-chained amino acid profiles but results in unique metabolomics patterns in obese women enrolled in an 8-week weight loss trial. J Nutr 2015, 145, 691–700. [Google Scholar] [CrossRef]
- Altuntas, Y.; Bilir, M.; Ucak, S.; Gundogdu, S. Reactive hypoglycemia in lean young women with PCOS and correlations with insulin sensitivity and with beta cell function. Eur J Obstet Gynecol Reprod Biol 2005, 119, 198–205. [Google Scholar] [CrossRef] [PubMed]
- Mumm, H.; Altinok, M.L.; Henriksen, J.E.; Ravn, P.; Glintborg, D.; Andersen, M. Prevalence and possible mechanisms of reactive hypoglycemia in polycystic ovary syndrome. Hum Reprod 2016, 31, 1105–1112. [Google Scholar] [CrossRef]
- Sam, S.; Vellanki, P.; Yalamanchi, S.K.; Bergman, R.N.; Dunaif, A. Exaggerated glucagon responses to hypoglycemia in women with polycystic ovary syndrome. Metabolism 2017, 71, 125–131. [Google Scholar] [CrossRef]
- Gennarelli, G.; Holte, J.; Stridsberg, M.; Niklasson, F.; Berne, C.; Backstrom, T. The counterregulatory response to hypoglycaemia in women with the polycystic ovary syndrome. Clin Endocrinol (Oxf) 1997, 46, 167–174. [Google Scholar] [CrossRef]
- Kishimoto, I. Subclinical Reactive Hypoglycemia with Low Glucose Effectiveness-Why We Cannot Stop Snacking despite Gaining Weight. Metabolites 2023, 13. [Google Scholar] [CrossRef] [PubMed]
- Ooi, D.S.Q.; Ling, J.Q.R.; Ong, F.Y.; Tai, E.S.; Henry, C.J.; Leow, M.K.S.; Khoo, E.Y.H.; Tan, C.S.; Chong, M.F.F.; Khoo, C.M.; et al. Branched Chain Amino Acid Supplementation to a Hypocaloric Diet Does Not Affect Resting Metabolic Rate but Increases Postprandial Fat Oxidation Response in Overweight and Obese Adults after Weight Loss Intervention. Nutrients 2021, 13. [Google Scholar] [CrossRef] [PubMed]
- Wycherley, T.P.; Moran, L.J.; Clifton, P.M.; Noakes, M.; Brinkworth, G.D. Effects of energy-restricted high-protein, low-fat compared with standard-protein, low-fat diets: a meta-analysis of randomized controlled trials. Am J Clin Nutr 2012, 96, 1281–1298. [Google Scholar] [CrossRef] [PubMed]
- Stamets, K.; Taylor, D.S.; Kunselman, A.; Demers, L.M.; Pelkman, C.L.; Legro, R.S. A randomized trial of the effects of two types of short-term hypocaloric diets on weight loss in women with polycystic ovary syndrome. Fertil Steril 2004, 81, 630–637. [Google Scholar] [CrossRef]
- Moran, L.J.; Ko, H.; Misso, M.; Marsh, K.; Noakes, M.; Talbot, M.; Frearson, M.; Thondan, M.; Stepto, N.; Teede, H.J. Dietary composition in the treatment of polycystic ovary syndrome: a systematic review to inform evidence-based guidelines. Journal of the Academy of Nutrition and Dietetics 2013, 113, 520–545. [Google Scholar] [CrossRef] [PubMed]
- Douglas, C.C.; Gower, B.A.; Darnell, B.E.; Ovalle, F.; Oster, R.A.; Azziz, R. Role of diet in the treatment of polycystic ovary syndrome. Fertil Steril 2006, 85, 679–688. [Google Scholar] [CrossRef]
- Marsh, K.A.; Steinbeck, K.S.; Atkinson, F.S.; Petocz, P.; Brand-Miller, J.C. Effect of a low glycemic index compared with a conventional healthy diet on polycystic ovary syndrome. Am J Clin Nutr 2010, 92, 83–92. [Google Scholar] [CrossRef]
- Galletly, C.; Moran, L.; Noakes, M.; Clifton, P.; Tomlinson, L.; Norman, R. Psychological benefits of a high-protein, low-carbohydrate diet in obese women with polycystic ovary syndrome--a pilot study. Appetite 2007, 49, 590–593. [Google Scholar] [CrossRef]
- Sorensen, L.B.; Soe, M.; Halkier, K.H.; Stigsby, B.; Astrup, A. Effects of increased dietary protein-to-carbohydrate ratios in women with polycystic ovary syndrome. Am J Clin Nutr 2012, 95, 39–48. [Google Scholar] [CrossRef]
- Newgard, C.B. Interplay between lipids and branched-chain amino acids in development of insulin resistance. Cell Metab 2012, 15, 606–614. [Google Scholar] [CrossRef]
- Ichikawa, R.; Takano, K.; Fujimoto, K.; Kobayashi, M.; Kitamura, T.; Shichiri, M.; Miyatsuka, T. Robust increase in glucagon secretion after oral protein intake, but not after glucose or lipid intake in Japanese people without diabetes. J Diabetes Investig 2023. [Google Scholar] [CrossRef]
- Ishikawa, E.; Aikawa, T.; Matsutaka, H. The roles of alanine as a major precursor among amino acids for hepatic gluconeogenesis and as a major end product of the degradation of amino acids in rat tissues. J Biochem 1972, 71, 1097–1099. [Google Scholar] [CrossRef] [PubMed]
- Moran, L.J.; Noakes, M.; Clifton, P.M.; Wittert, G.A.; Le Roux, C.W.; Ghatei, M.A.; Bloom, S.R.; Norman, R.J. Postprandial ghrelin, cholecystokinin, peptide YY, and appetite before and after weight loss in overweight women with and without polycystic ovary syndrome. Am J Clin Nutr 2007, 86, 1603–1610. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).



