Submitted:
29 September 2023
Posted:
30 September 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
Cytomegalovirus and Oncoprotection – from T-Cells to Vaccines
At What Time Could CMV offer its Greatest Protection?
Arguments in Favor of CMV Oncogenesis
Study Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zuhair M, Smit GSA, Wallis G, Jabbar F, Smith C, Devleesschauwer B, Griffiths P. Estimation of the worldwide seroprevalence of cytomegalovirus: A systematic review and meta-analysis. Rev Med Virol. 2019, 29, e2034. [Google Scholar] [CrossRef] [PubMed]
- Fowler K, Mucha J, Neumann M, Lewandowski W, Kaczanowska M, Grys M, Schmidt E, Natenshon A, Talarico C, Buck PO, Diaz-Decaro J. A systematic literature review of the global seroprevalence of cytomegalovirus: possible implications for treatment, screening, and vaccine development. BMC Public Health. 2022, 22, 1659. [Google Scholar] [CrossRef]
- Pandey, JP. Immunoglobulin GM Genes, Cytomegalovirus Immunoevasion, and the Risk of Glioma, Neuroblastoma, and Breast Cancer. Front Oncol. 2014, 29, 236. [Google Scholar] [CrossRef] [PubMed]
- Nehme Z, Pasquereau S, Haidar Ahmad S, El Baba R, Herbein G. Polyploid giant cancer cells, EZH2 and Myc upregulation in mammary epithelial cells infected with high-risk human cytomegalovirus. EBioMedicine. 2022, 80, 104056. [Google Scholar] [CrossRef]
- Sarshari B, Mohebbi SR, Ravanshad M, Shahrokh S, Aghdaei HA, Zali MR. Detection and quantification of Epstein-Barr virus, cytomegalovirus, and human herpesvirus-6 in stomach frozen tissue of chronic gastritis and gastric cancer patients. Microbiol Immunol. 2022, 66, 379–385. [Google Scholar] [CrossRef]
- Doniger J, Muralidhar S, Rosenthal LJ. Human cytomegalovirus and human herpesvirus 6 genes that transform and transactivate. Clin Microbiol Rev. 1999, 12, 367–382. [Google Scholar] [CrossRef]
- Janković M, Knežević A, Todorović M, Đunić I, Mihaljević B, Soldatović I, Protić J, Miković N, Stoiljković V, Jovanović T. Cytomegalovirus infection may be oncoprotective against neoplasms of B-lymphocyte lineage: single-institution experience and survey of global evidence. Virol J. 2022, 19, 155. [Google Scholar] [CrossRef]
- Bigley AB, Baker FL, Simpson RJ. Cytomegalovirus: an unlikely ally in the fight against blood cancers? Clin Exp Immunol. 2018, 193, 265–274. [Google Scholar] [CrossRef]
- Rashid S, Ardeljan A, Frankel LR, Cardeiro M, Kim E, Nagel BM, Takabe K, Rashid O. Human Cytomegalovirus (CMV) Infection Associated With Decreased Risk of Bronchogenic Carcinoma: Understanding How a Previous CMV Infection Leads to an Enhanced Immune Response Against Malignancy. Cureus. 2023, 15, e37265. [Google Scholar] [CrossRef]
- Nagel B, Frankel L, Ardeljan A, Cardeiro M, Rashid S, Takabe K, Rashid OM. The Association of Human Cytomegalovirus Infection and Colorectal Cancer: A Clinical Analysis. World J Oncol. 2023, 14, 119–124. [Google Scholar] [CrossRef]
- Daei Sorkhabi A, Sarkesh A, Saeedi H, Marofi F, Ghaebi M, Silvestris N, Baradaran B, Brunetti O. The Basis and Advances in Clinical Application of Cytomegalovirus-Specific Cytotoxic T Cell Immunotherapy for Glioblastoma Multiforme. Front Oncol. 2022, 12, 818447. [Google Scholar] [CrossRef] [PubMed]
- Ahn J, Shin C, Kim YS, Park JS, Jeun SS, Ahn S. Cytomegalovirus-Specific Immunotherapy for Glioblastoma Treatments. Brain Tumor Res Treat. 2022, 10, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Schuessler A, Walker DG, Khanna R. Cytomegalovirus as a novel target for immunotherapy of glioblastoma multiforme. Front Oncol. 2014, 4, 275. [Google Scholar] [CrossRef]
- Available online: https://gco.iarc.fr/.
- Gustafsson RKL, Jeffery HC, Yaiw K-C, Wilhelmi V, Kostopoulou ON, Davoudi B, Rahbar A, Benard M, Renné T, Söderberg-Nauclér C & Butler LM. Direct infection of primary endothelial cells with human cytomegalovirus prevents angiogenesis and migration. J Gen Virol. 2015; 96(12).
- Ozel I, Duerig I, Domnich M, Lang S, Pylaeva E, Jablonska J. The Good, the Bad, and the Ugly: Neutrophils, Angiogenesis, and Cancer. Cancers (Basel). 2022, 14, 536. [Google Scholar] [CrossRef]
- Laane CJ, Murr AH, Mhatre AN, Jones KD, and Lalwan AK. Role of Epstein-Barr virus and cytomegalovirus in the etiology of benign parotid tumors. Head Neck. 2002, 24, 443–450. [Google Scholar] [CrossRef]
- Ingerslev K, Høgdall E, Skovrider-Ruminski W. et al. The prevalence of EBV and CMV DNA in epithelial ovarian cancer. Infect Agents Cancer. 2019;14(7).
- Huang TS, Lee JJ, & Cheng SP. No evidence of association between human cytomegalovirus infection and papillary thyroid cancer. World J Surg Onc. 2014;12(41).
- Vermeulen JF, van Hecke W, Jansen MK, Spliet WG, Broekhuizen R, Bovenschen N. No evidence for human cytomegalovirus infection in pediatric medulloblastomas. Neuro Oncol. 2016, 18, 1461–1462. [Google Scholar] [CrossRef]
- Wiemels JL, Talbäck M, Francis S, Feychting M. Early Infection with Cytomegalovirus and Risk of Childhood Hematologic Malignancies. Cancer Epidemiol Biomarkers Prev. 2019, 28, 1024–1027. [Google Scholar] [CrossRef]
- Thompson CH, Rose BR, Elliott PM. Cytomegalovirus and cervical cancer: failure to detect a direct association or an interaction with human papillomaviruses. Gynecol Oncol. 1994, 54, 40–46. [Google Scholar] [CrossRef]
- Koldehoff M, Ross SR, Dührsen U, Beelen DW, Elmaagacli AH. Early CMV-replication after allogeneic stem cell transplantation is associated with a reduced relapse risk in lymphoma. Leuk Lymphoma. 2017, 58, 822–833. [Google Scholar] [CrossRef]
- Elmaagacli AH, Steckel NK, Koldehoff M, Hegerfeldt Y, Trenschel R, Ditschkowski M, Christoph S, Gromke T, Kordelas L, Ottinger HD, Ross RS, Horn PA, Schnittger S, Beelen DW. Early human cytomegalovirus replication after transplantation is associated with a decreased relapse risk: evidence for a putative virus-versus-leukemia effect in acute myeloid leukemia patients. Blood. 2011, 118, 1402–1412. [Google Scholar] [CrossRef] [PubMed]
- Green ML, Leisenring WM, Xie H, Walter RB, Mielcarek M, Sandmaier BM, Riddell SR, Boeckh M. CMV reactivation after allogeneic HCT and relapse risk: evidence for early protection in acute myeloid leukemia. Blood. 2013, 122, 1316–1324. [Google Scholar] [CrossRef] [PubMed]
- Litjens NHR, van der Wagen L, Kuball J, Kwekkeboom J. Potential Beneficial Effects of Cytomegalovirus Infection after Transplantation. Front Immunol. 2018, 9, 389. [Google Scholar] [CrossRef] [PubMed]
- Inagaki J, Noguchi M, Kurauchi K, Tanioka S, Fukano R, Okamura J. Effect of Cytomegalovirus Reactivation on Relapse after Allogeneic Hematopoietic Stem Cell Transplantation in Pediatric Acute Leukemia. Biol Blood Marrow Transplant. 2016, 22, 300–306. [Google Scholar] [CrossRef] [PubMed]
- Peric Z, Wilson J, Durakovic N, Ostojic A, Desnica L, Vranjes VR, Marekovic I, Serventi-Seiwerth R, Vrhovac R. Early human cytomegalovirus reactivation is associated with lower incidence of relapse of myeloproliferative disorders after allogeneic hematopoietic stem cell transplantation. Bone Marrow Transplant. 2018, 53, 1450–1456. [Google Scholar] [CrossRef] [PubMed]
- Geris JM, Spector LG, Pfeiffer RM, Limaye AP, Yu KJ and Engels EA. Cancer risk associated with cytomegalovirus infection among solid organ transplant recipients in the United States. Cancer. 2022, 128, 3985–3994. [Google Scholar] [CrossRef]
- Erlach KC, Podlech J, Rojan A, Reddehase MJ. Tumor control in a model of bone marrow transplantation and acute liver-infiltrating B-cell lymphoma: an unpredicted novel function of cytomegalovirus. J Virol. 2002, 76, 2857–2870. [Google Scholar] [CrossRef]
- Kelly E, Russell SJ. History of oncolytic viruses: genesis to genetic engineering. Mol Ther. 2007, 15, 651–659. [Google Scholar] [CrossRef]
- Russell SJ, Peng KW, Bell JC. Oncolytic virotherapy. Nat Biotechnol. 2012, 30, 658–670. [Google Scholar] [CrossRef]
- Cao GD, He XB, Sun Q, Chen S, Wan K, Xu X, Feng X, Li PP, Chen B, Xiong MM. The Oncolytic Virus in Cancer Diagnosis and Treatment. Front Oncol. 2020, 10, 1786. [Google Scholar] [CrossRef]
- Ye S, Hu Y, Chen C, Chen S, Tong X, Zhu H, Deng B, Hu X, Sun X, Chen X, Shi X, Gu R, Xie W, Guo G, Xing D, Shen X, Xue X, Shen S. The Human Cytomegalovirus US31 Gene Predicts Favorable Survival and Regulates the Tumor Microenvironment in Gastric Cancer. Front Oncol. 2021, 11, 614925, Erratum in: Front Oncol. 2021 Jun 17;11:715746. [Google Scholar] [CrossRef]
- Herbein G, Nehme Z. Tumor Control by Cytomegalovirus: A Door Open for Oncolytic Virotherapy? Mol Ther Oncolytics. 2020, 17, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Erkes DA, Wilski NA, Snyder CM. Intratumoral infection by CMV may change the tumor environment by directly interacting with tumor-associated macrophages to promote cancer immunity. Hum Vaccin Immunother. 2017, 13, 1778–1785. [Google Scholar] [CrossRef] [PubMed]
- Wilski NA, Stotesbury C, Del Casale C, Montoya B, Wong E, Sigal LJ, Snyder CM. STING Sensing of Murine Cytomegalovirus Alters the Tumor Microenvironment to Promote Antitumor Immunity. J Immunol. 2020, 204, 2961–2972. [Google Scholar] [CrossRef] [PubMed]
- Erlach KC, Böhm V, Seckert CK, Reddehase MJ, Podlech J. Lymphoma cell apoptosis in the liver induced by distant murine cytomegalovirus infection. J Virol. 2006, 80, 4801–4819. [Google Scholar] [CrossRef]
- Jackson SE, Redeker A, Arens R, et al. CMV immune evasion and manipulation of the immune system with aging. GeroScience. 2017;39, 273–291.
- Kumar A, Coquard L, Pasquereau S, Russo L, Valmary-Degano S, Borg C, Pothier P, Herbein G. Tumor control by human cytomegalovirus in a murine model of hepatocellular carcinoma. Mol Ther Oncolytics. 2016, 3, 16012. [Google Scholar] [CrossRef]
- Koldehoff M, Lindemann M, Opalka B, Bauer S, Ross RS, Elmaagacli AH. Cytomegalovirus induces apoptosis in acute leukemia cells as a virus-versus-leukemia function. Leuk Lymphoma. 2015, 56, 3189–3197. [Google Scholar] [CrossRef]
- Erkes DA, Xu G, Daskalakis C, Zurbach KA, Wilski NA, Moghbeli T, Hill AB, Snyder CM. Intratumoral Infection with Murine Cytomegalovirus Synergizes with PD-L1 Blockade to Clear Melanoma Lesions and Induce Long-term Immunity. Mol Ther. 2016, 24, 1444–1455. [Google Scholar] [CrossRef]
- Wilski NA, Del Casale C, Purwin TJ, Aplin AE, Snyder CM. Murine Cytomegalovirus Infection of Melanoma Lesions Delays Tumor Growth by Recruiting and Repolarizing Monocytic Phagocytes in the Tumor. J Virol. 2019, 93, e00533–19. [Google Scholar] [CrossRef]
- Picarda G, Benedict CA; Cytomegalovirus: Shape-Shifting the Immune System. J Immunol 15 June 2018; 200 (12): 3881–3889. 15 June.
- Lee S, Doualeh M, Affandi JS, Makwana N, Irish A, Price P. Functional and clinical consequences of changes to natural killer cell phenotypes driven by chronic cytomegalovirus infections. J Med Virol. 2019, 91, 1120–1127. [Google Scholar] [CrossRef]
- Wilkinson GWG, Tomasec P, Stanton RJ, Armstrong M, Prod’homme V, Aicheler R, McSharry BP, Rickards CR, Cochrane D, Llewellyn-Lacey S, Wang ECY, Griffin CA, & Davison AJ. Modulation of natural killer cells by human cytomegalovirus. J Clin Virol. 2008, 41, 206–212. [Google Scholar] [CrossRef] [PubMed]
- Lisnić B, Lisnić VJ, Jonjić S. NK cell interplay with cytomegaloviruses. Curr Opin Virol. 2015, 15, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Jackson SE, Mason GM, Wills MR. Human cytomegalovirus immunity and immune evasion. Virus Res. 2011, 157, 151–160. [Google Scholar] [CrossRef] [PubMed]
- Tršan T, Vuković K, Filipović P, Brizić AL, Lemmermann NAW, Schober K, Busch DH, Britt WJ, Messerle M, Krmpotić A, & Jonjić S. Cytomegalovirus vector expressing RAE-1γ induces enhanced anti-tumor capacity of murine CD8+ T cells. Eur. J. Immunol. 2017, 47, 1354–1367. [Google Scholar] [CrossRef]
- Qiu Z, Huang H, Grenier JM, Perez OA, Smilowitz HM, Adler B, Khanna KM. Cytomegalovirus-Based Vaccine Expressing a Modified Tumor Antigen Induces Potent Tumor-Specific CD8(+) T-cell Response and Protects Mice from Melanoma. Cancer Immunol Res. 2015, 3, 536–546. [Google Scholar] [CrossRef]
- Quinn M, Erkes DA, Snyder CM. Cytomegalovirus and immunotherapy: opportunistic pathogen, novel target for cancer and a promising vaccine vector. Immunotherapy. 2016, 8, 211–221. [Google Scholar] [CrossRef]
- Hartman ZC, Wei J, Glass OK, Guo H, Lei G, Yang X-Y, Osada T, Hobeika A, Delcayre A, Le Pecq J-B, Morse MA, Clay TM, & Lyerly HK. Increasing vaccine potency through exosome antigen targeting. Vacc. 2011, 29, 9361–9367. [Google Scholar] [CrossRef]
- Liu J, Jaijyan DK, Tang Q, Zhu H. Promising Cytomegalovirus-Based Vaccine Vector Induces Robust CD8+ T-Cell Response. Int J Mol Sci. 2019, 20, 4457. [Google Scholar] [CrossRef]
- Mujoo K, Maneval DC, Anderson SC, Gutterman JU. Adenoviral-mediated p53 tumor suppressor gene therapy of human ovarian carcinoma. Oncogene. 1996, 12, 1617–1623. [Google Scholar]
- De Leon G, Nair S, Xie W, Drake J, Mitchell D. IT-06: PERSONALIZED IMMUNOTHERAPY FOR THE TREATMENT OF GLIOBLASTOMA. Neuro Oncol. 2014 Nov;16(Suppl 5):v111. [CrossRef]
- Rooney CM, Leen AM, Vera JF, & Heslop HE. T lymphocytes targeting native receptors. Immunol Rev. 2014, 257, 39–55. [Google Scholar] [CrossRef] [PubMed]
- Meng Q, Valentini D, Rao M, Dodoo E, Maeurer M. CMV and EBV targets recognized by tumor-infiltrating B lymphocytes in pancreatic cancer and brain tumors. Sci Rep. 2018, 8, 17079. [Google Scholar] [CrossRef] [PubMed]
- Hortal AM, Vermeulen JF, Van Hecke W, Bovenschen N. Oncogenic role of cytomegalovirus in medulloblastoma? Cancer Lett. 2017, 408, 55–59. [Google Scholar] [CrossRef] [PubMed]
- Daei Sorkhabi A, Sarkesh A, Saeedi H, Marofi F, Ghaebi M, Silvestris N, Baradaran B, Brunetti O. The Basis and Advances in Clinical Application of Cytomegalovirus-Specific Cytotoxic T Cell Immunotherapy for Glioblastoma Multiforme. Front Oncol. 2022, 12, 818447. [Google Scholar] [CrossRef] [PubMed]
- Düzgüneş N, Cheung J, Konopka K. Non-viral suicide gene therapy in cervical, oral and pharyngeal carcinoma cells with CMV- and EEV-plasmids. J Gene Med. 2018 Oct;20(10-11):e3054. [CrossRef]
- Wilski NA, Snyder CM. From Vaccine Vector to Oncomodulation: Understanding the Complex Interplay between CMV and Cancer. Vaccines (Basel). 2019, 7, 62. [Google Scholar] [CrossRef]
- Francis SS, Wallace AD, Wendt GA, Li L, Liu F, Riley LW, Kogan S, Walsh KM, de Smith AJ, Dahl GV, Ma X, Delwart E, Metayer C, Wiemels JL. In utero cytomegalovirus infection and development of childhood acute lymphoblastic leukemia. Blood. 2017, 129, 1680–1684. [Google Scholar] [CrossRef]
- Ahmed HG, Suliman RSA, Ashankyty IM, Albieh ZA, Warille AA. Role of human Cytomegalovirus in the etiology of nasopharyngeal carcinoma. J Cancer Res Ther. 2018, 14, 583–586. [Google Scholar] [CrossRef] [PubMed]
- Geder L, Sanford EJ, Rohner TJ, Rapp F. Cytomegalovirus and cancer of the prostate: in vitro transformation of human cells. Cancer Treatment Reports. 1977, 61, 139–146. [Google Scholar]
- Herbein, G. Tumors and Cytomegalovirus: An Intimate Interplay. Viruses. 2022, 14, 812. [Google Scholar] [CrossRef]
- Melnick M, Sedghizadeh PP, Allen CM, Jaskoll T. Human cytomegalovirus and mucoepidermoid carcinoma of salivary glands: cell-specific localization of active viral and oncogenic signaling proteins is confirmatory of a causal relationship. Exp Mol Pathol. 2012, 92, 118–125. [Google Scholar] [CrossRef] [PubMed]
- Nauclér CS, Geisler J, Vetvik K. The emerging role of human cytomegalovirus infection in human carcinogenesis: a review of current evidence and potential therapeutic implications. Oncotarget. 2019, 10, 4333–4347. [Google Scholar] [CrossRef]
- Cobbs, CS. Cytomegalovirus and brain tumor: epidemiology, biology and therapeutic aspects. Curr Opin Oncol. 2013, 25, 682–688. [Google Scholar] [CrossRef] [PubMed]
- Barami, K. Oncomodulatory mechanisms of human cytomegalovirus in gliomas. J Clin Neurosci. 2010, 17, 819–823. [Google Scholar] [CrossRef] [PubMed]
- Polz-Gruszka D, Stec A, Dworzański J, Polz-Dacewicz M. EBV, HSV, CMV and HPV in laryngeal and oropharyngeal carcinoma in Polish patients. Anticancer Res. 2015, 35, 1657–1661. [Google Scholar]
- Wolmer-Solberg, N.; Baryawno, N.; Rahbar, A.; Fuchs, D.; Odeberg, J.; Taher, C.; Wilhelmi, V.; Milosevic, J.; Mohammad, A.A.; Martinsson, T.; et al. Frequent detection of human cytomegalovirus in neuroblastoma: A novel therapeutic target? Int. J. Cancer 2013, 133, 2351–2361. [Google Scholar] [CrossRef]
- Herbein G, Kumar A. The oncogenic potential of human cytomegalovirus and breast cancer. Front Oncol. 2014, 4, 230. [Google Scholar] [CrossRef]
- Taher, Chato & Boniface, Jana & Mohammad, Abdul-Aleem & Religa, Piotr & Hartman, Johan & Yaiw, Koon-Chu & Frisell, Jan & Rahbar, Afsar & Söderberg-Nauclér, Cecilia. (2013). High Prevalence of Human Cytomegalovirus Proteins and Nucleic Acids in Primary Breast Cancer and Metastatic Sentinel Lymph Nodes. PloS one. 8. e56795. [CrossRef]
- Zhang L, Guo G, Xu J, Sun X, Chen W, Jin J, Hu C, Zhang P, Shen X, Xue X. Human cytomegalovirus detection in gastric cancer and its possible association with lymphatic metastasis. Diagn Microbiol Infect Dis. 2017, 88, 62–68. [Google Scholar] [CrossRef]
- Yang Z, Tang X, Hasing ME, Pang X, Ghosh S, McMullen TPW, Brindley DN, Hemmings DG. Human Cytomegalovirus Seropositivity and Viral DNA in Breast Tumors Are Associated with Poor Patient Prognosis. Cancers (Basel). 2022, 14, 1148. [Google Scholar] [CrossRef]
- Youssry S, Hussein A, Ramadan R, Alkarmouty A, Elsheredy A. The association of human cytomegalovirus with biomarkers of inflammation and immune activation in breast cancer. Breast Dis. 2022, 41, 229–239. [Google Scholar] [CrossRef] [PubMed]
- Touma J, Pantalone MR, Rahbar A, Liu Y, Vetvik K, Sauer T, Söderberg-Naucler C, Geisler J. Human Cytomegalovirus Protein Expression Is Correlated with Shorter Overall Survival in Breast Cancer Patients: A Cohort Study. Viruses. 2023, 15, 732. [Google Scholar] [CrossRef] [PubMed]
- Cui J, Wang Q, Wang HB, Wang B, Li L. Protein and DNA evidences of HCMV infection in primary breast cancer tissues and metastatic sentinel lymph nodes. Cancer Biomark. 2018, 21, 769–780. [Google Scholar] [CrossRef] [PubMed]
- Paradowska E, Jabłońska A, Studzińska M, Wilczyński M, Wilczyński JR. Detection and genotyping of CMV and HPV in tumors and fallopian tubes from epithelial ovarian cancer patients. Sci Rep. 2019, 9, 19935. [Google Scholar] [CrossRef]
- Fang Y, Wang Q, Huang K, Zhang M, Pei S, Li L, Peng Y, Lan L, Zheng X. Human cytomegalovirus-induced immune regulation is correlated with poor prognosis in patients with colorectal cancer. Clin Exp Med. 2023, 23, 427–436. [Google Scholar] [CrossRef]
- Chen HP, Chan YJ. The oncomodulatory role of human cytomegalovirus in colorectal cancer: implications for clinical trials. Front Oncol. 2014, 4, 314. [Google Scholar] [CrossRef]
- Marongiu L, Venturelli S, Allgayer H. Involvement of HHV-4 (Epstein-Barr Virus) and HHV-5 (Cytomegalovirus) in Inflammatory Bowel Disease and Colorectal Cancer: A Meta-Analysis. Cancers (Basel). 2022, 14, 5085. [CrossRef]
- Soroceanu L, Cobbs CS. Is HCMV a tumor promoter? Virus Res. 2011, 157, 193–203. [Google Scholar] [CrossRef]
- Harkins L, Volk AL, Samanta M, Mikolaenko I, Britt WJ, Bland KI, Cobbs CS. Specific localisation of human cytomegalovirus nucleic acids and proteins in human colorectal cancer. Lancet. 2002, 360, 1557–1563. [Google Scholar] [CrossRef]
- Michaelis M, Doerr HW, Cinatl J. The story of human cytomegalovirus and cancer: increasing evidence and open questions. Neoplasia. 2009, 11, 1–9. [Google Scholar] [CrossRef]
- Costa H, Xu X, Overbeek G, Vasaikar S, Patro CP, Kostopoulou ON, Jung M, Shafi G, Ananthaseshan S, Tsipras G, Davoudi B, Mohammad AA, Lam H, Strååt K, Wilhelmi V, Shang M, Tegner J, Tong JC, Wong KT, Söderberg-Naucler C, Yaiw KC. Human cytomegalovirus may promote tumor progression by upregulating arginase-2. Oncotarget. 2016, 7, 47221–47231. [Google Scholar] [CrossRef] [PubMed]
- Cobbs CS, Harkins L, Samanta M, Gillespie GY, Bharara S, King PH, Nabors LB, Cobbs CG, Britt WJ. Human cytomegalovirus infection and expression in human malignant glioma. Cancer Res. 2002, 62, 3347–3350. [Google Scholar]
- Joseph GP, McDermott R, Baryshnikova MA, Cobbs CS, Ulasov IV. Cytomegalovirus as an oncomodulatory agent in the progression of glioma. Cancer Lett. 2017, 384, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Farias KPRA, Moreli ML, Floriano VG, da Costa VG. Evidence based on a meta-analysis of human cytomegalovirus infection in glioma. Arch Virol. 2019, 164, 1249–1257. [Google Scholar] [CrossRef]
- Bhattacharjee B, Renzette N, Kowalik TF. Genetic analysis of cytomegalovirus in malignant gliomas. J Virol. 2012, 86, 6815–6824. [Google Scholar] [CrossRef] [PubMed]
- Maleki F, Sadigh ZA, Sadeghi F, Muhammadnejad A, Farahmand M, Parvin M, Shirkoohi R. Human cytomegalovirus infection in Iranian glioma patients correlates with aging and tumor aggressiveness. J Med Virol. 2020, 92, 1266–1276. [Google Scholar] [CrossRef] [PubMed]
- Yang T, Liu D, Fang S, Ma W, Wang Y. Cytomegalovirus and Glioblastoma: A Review of the Biological Associations and Therapeutic Strategies. Journal of Clinical Medicine. 2022, 11, 5221. [Google Scholar] [CrossRef]
- Libard S, Popova S. N., Amini R.-M, Kärjä V, Pietiläinen T, Hämäläinen K.M., Sundström C, Hesselager G, Bergqvist M, Ekman S, et al. Human Cytomegalovirus Tegument Protein pp65 Is Detected in All Intra- and Extra-Axial Brain Tumours Independent of the Tumour Type or Grade. PLoS ONE 2014, 9, e108861. [Google Scholar]
- Habibi Z, Hajizadeh M, Nozarian Z, Safavi M, Monajemzadeh M, Meybodi KT, Nejat F, Vasei M. Cytomegalovirus DNA in non-glioblastoma multiforme brain tumors of infants. Childs Nerv Syst. 2021, 37, 1581–1586. [Google Scholar] [CrossRef]
- Ranganathan P, Clark PA, Kuo JS, Salamat MS, Kalejta RF. Significant association of multiple human cytomegalovirus genomic Loci with glioblastoma multiforme samples. J Virol. 2012, 86, 854–864. [Google Scholar] [CrossRef]
- Carpagnano GE, Lacedonia D, Natalicchio MI, Cotugno G, Zoppo L, Martinelli D, Antonetti R, Foschino-Barbaro MP. Viral colonization in exhaled breath condensate of lung cancer patients: Possible role of EBV and CMV. Clin Respir J. 2018, 12, 418–424. [Google Scholar] [CrossRef]
- Nelson HH, Contestabile E, Hunter-Schlichting D, Koestler D, Pawlita M, Waterboer T, Christensen BC, Petersen CL, Miller JS, Kelsey KT. Human cytomegalovirus alters immune cell profile with potential implications for patient survival in head and neck cancer. Carcinogenesis. 2022, 43, 430–436. [Google Scholar] [CrossRef] [PubMed]
- Lv YL, Han FF, An ZL, Jia Y, Xuan LL, Gong LL, Zhang W, Ren LL, Yang S, Liu H, Liu LH. Cytomegalovirus Infection Is a Risk Factor in Gastrointestinal Cancer: A Cross-Sectional and Meta-Analysis Study. Intervirology. 2020;63(1-6):10-16. [CrossRef]
- Samanta M, Harkins L, Klemm K, Britt WJ, Cobbs CS. High prevalence of human cytomegalovirus in prostatic intraepithelial neoplasia and prostatic carcinoma. J Urol. 2003, 170, 998–1002. [Google Scholar] [CrossRef] [PubMed]
- Stevenson K, Macnab JC. Cervical carcinoma and human cytomegalovirus. Biomed Pharmacother. 1989, 43, 173–176. [Google Scholar] [CrossRef] [PubMed]
- Gaekwad SS, Gujjari SK. Cytomegalovirus occurrence in chronic periodontitis and in carcinoma of the cervix: an exploratory study. J Clin Diagn Res. 2012, 6, 1442–1447. [Google Scholar] [CrossRef]
- Yin M, Chen A, Zhao F, Ji X, Li C, Wang G. Detection of human cytomegalovirus in patients with epithelial ovarian cancer and its impacts on survival. Infect Agent Cancer. 2020, 15, 23. [Google Scholar] [CrossRef]
- D’Orazi G, Cordani M, Cirone M. Oncogenic pathways activated by pro-inflammatory cytokines promote mutant p53 stability: clue for novel anticancer therapies. Cell Mol Life Sci. 2021, 78, 1853–1860. [Google Scholar] [CrossRef]
- Grivennikov SI, Karin M. Inflammation and oncogenesis: a vicious connection. Curr Opin Genet Dev. 2010, 20, 65–71. [Google Scholar] [CrossRef]
- Greten FR, Grivennikov SI. Inflammation and Cancer: Triggers, Mechanisms, and Consequences. Immunity. 2019, 51, 27–41. [Google Scholar] [CrossRef]
- Qu X, Tang Y, Hua S. Immunological Approaches Towards Cancer and Inflammation: A Cross Talk. Front Immunol. 2018, 9, 563. [Google Scholar] [CrossRef] [PubMed]
- Singh N, Baby D, Rajguru JP, Patil PB, Thakkannavar SS, Pujari VB. Inflammation and cancer. Ann Afr Med. 2019, 18, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Balkwill F, Charles KA, Mantovani A. Smoldering and polarized inflammation in the initiation and promotion of malignant disease. Cancer Cell. 2005, 7, 211–217. [Google Scholar] [CrossRef]
- Colotta F, Allavena P, Sica A, Garlanda C, Mantovani A. Cancer-related inflammation, the seventh hallmark of cancer: links to genetic instability. Carcinogenesis. 2009, 30, 1073–1081. [Google Scholar] [CrossRef] [PubMed]
- Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 140(6):883–99.
- Michaelis M, Doerr HW, Cinatl J Jr. Oncomodulation by human cytomegalovirus: evidence becomes stronger. Med Microbiol Immunol. 2009, 198, 79–81. [Google Scholar] [CrossRef]
- Herbein, G. High-Risk Oncogenic Human Cytomegalovirus. Viruses. 2022, 14, 2462. [Google Scholar] [CrossRef]
- Cinatl J Jr, Vogel JU, Kotchetkov R, Wilhelm Doerr H. Oncomodulatory signals by regulatory proteins encoded by human cytomegalovirus: a novel role for viral infection in tumor progression. FEMS Microbiol Rev. 2004, 28, 59–77. [Google Scholar] [CrossRef]
- Goerig NL, Frey B, Korn K, Fleckenstein B, Überla K, Schmidt MA, Dörfler A, Engelhorn T, Eyüpoglu I, Rühle PF, Putz F, Semrau S, Gaipl US, Fietkau R. Frequent occurrence of therapeutically reversible CMV-associated encephalopathy during radiotherapy of the brain. Neuro Oncol. 2016, 18, 1664–1672. [Google Scholar] [CrossRef]
- Merchut-Maya JM, Bartek J Jr, Bartkova J, Galanos P, Pantalone MR, Lee M, Cui HL, Shilling PJ, Brøchner CB, Broholm H, Maya-Mendoza A, Söderberg-Naucler C, Bartek J. Human cytomegalovirus hijacks host stress response fueling replication stress and genome instability. Cell Death Differ. 2022, 29, 1639–1653. [Google Scholar] [CrossRef]
- Yang R, Liang J, Xu GX, Ding LM, Huang HM, Su QZ, Yan J, Li YC. Human cytomegalovirus glycoprotein B inhibits migration of breast cancer MDA-MB-231 cells and impairs TGF-β/Smad2/3 expression. Oncol Lett. 2018, 15, 7730–7738. [Google Scholar] [CrossRef]
- Geisler J, Touma J, Rahbar A, Söderberg-Nauclér C, Vetvik K. A Review of the Potential Role of Human Cytomegalovirus (HCMV) Infections in Breast Cancer Carcinogenesis and Abnormal Immunity. Cancers (Basel). 2019, 11, 1842. [Google Scholar] [CrossRef] [PubMed]
- Herbein, G. The Human Cytomegalovirus, from Oncomodulation to Oncogenesis. Viruses. 2018, 10, 408. [Google Scholar] [CrossRef] [PubMed]
- Cobbs, CS. Cytomegalovirus is a tumor-associated virus: armed and dangerous. Curr Opin Virol. 2019, 39, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Roche, J. The Epithelial-to-Mesenchymal Transition in Cancer. Cancers (Basel). 2018, 10, :52. [Google Scholar] [CrossRef] [PubMed]
- Marcucci F, Stassi G, De Maria R. Epithelial-mesenchymal transition: a new target in anticancer drug discovery. Nat Rev Drug Discov. 2016, 15, 311–325. [Google Scholar] [CrossRef]
- Maussang D, Verzijl D, van Walsum M, Leurs R, Holl J, Pleskoff O, Michel D, van Dongen GA, Smit MJ. Human cytomegalovirus-encoded chemokine receptor US28 promotes tumorigenesis. Proc Natl Acad Sci U S A. 2006, 103, :13068–73. [Google Scholar] [CrossRef]
- Wang, J. , Belcher, J., Marker, P. et al. Cytomegalovirus inhibits p53 nuclear localization signal function. J Mol Med 78, 642–647 (2001). [CrossRef]
- Hecker M, Qiu D, Marquardt K, Bein G, Hackstein H. Continuous cytomegalovirus seroconversion in a large group of healthy blood donors. Vox Sang. 2004, 86, 41–44. [Google Scholar] [CrossRef]
- Staras SAS, Dollard SC, Radford KW, Flanders WD, Pass RF, Cannon MJ. Seroprevalence of cytomegalovirus infection in the United States, 1988- 1994. Clin Infect Dis. 2006, 43, 1143–1151. [CrossRef]
- Ahlfors, K. IgG antibodies to cytomegalovirus in a normal urban Swedish population. Scand J Infect Dis. 1984, 16, 335–337. [Google Scholar] [CrossRef]
- Varga M, Görög D, Kári D, Környei E, Kis É, Túryné HJ, et al. Cytomegalovirus seroprevalence among solid organ donors in Hungary: correlations with age, gender, and blood group. Transplant Proc. 2011, 43, 1233–1235. [Google Scholar] [CrossRef] [PubMed]
- Bate SL, Dollard SC, Cannon MJ. Cytomegalovirus seroprevalence in the United States: the national health and nutrition examination surveys, 1988-2004. Clin Infect Dis. 2010, 50, 1439–1447. [CrossRef] [PubMed]
- Lachmann R, Loenenbach A, Waterboer T, Brenner N, Pawlita M, Michel A, et al. Cytomegalovirus (CMV) seroprevalence in the adult population of Germany. PLoS One. 2018, 13, e0200267. [Google Scholar] [CrossRef]
- Cannon MJ, Schmid DS, Hyde TB. Review of cytomegalovirus seroprevalence and demographic characteristics associated with infection. Rev Med Virol. 2010, 20, 202–213. [Google Scholar] [CrossRef]
- Marshall GS, Rabalais GP, Stewart JA, Dobbins JG. Cytomegalovirus seroprevalence in women bearing children in Jefferson County, Kentucky. Am J Med Sci. 1993, 305, 292–296. [Google Scholar] [CrossRef]
- Clarke CA, Glaser SL, Gomez SI, Wang SS, Keegan TH, Yang J, et al. Lymphoid malignancies in US Asians: incidence rate differences by birthplace and acculturation. Cancer Epidemiol Biomarkers Prev. 2011, 20, 1064–1077. [Google Scholar] [CrossRef]
- Li Y, Wang Z, Yi D, Ma S. Racial Differences in Three mayor NHL Subtypes: Descriptive epidemiology. Cancer Epidemiol. 2015, 39, 8–13. [Google Scholar] [CrossRef]
- Janković M, Milićević O, Todorović-Balint M, Đunić I, Mihaljević B, Jovanović T, Knežević A. Cytomegalovirus seropositivity relates inversely to cancer incidences across races and ethnicities: implications for oncoprevention. medRxiv 2023.08.26.23294534. [CrossRef]
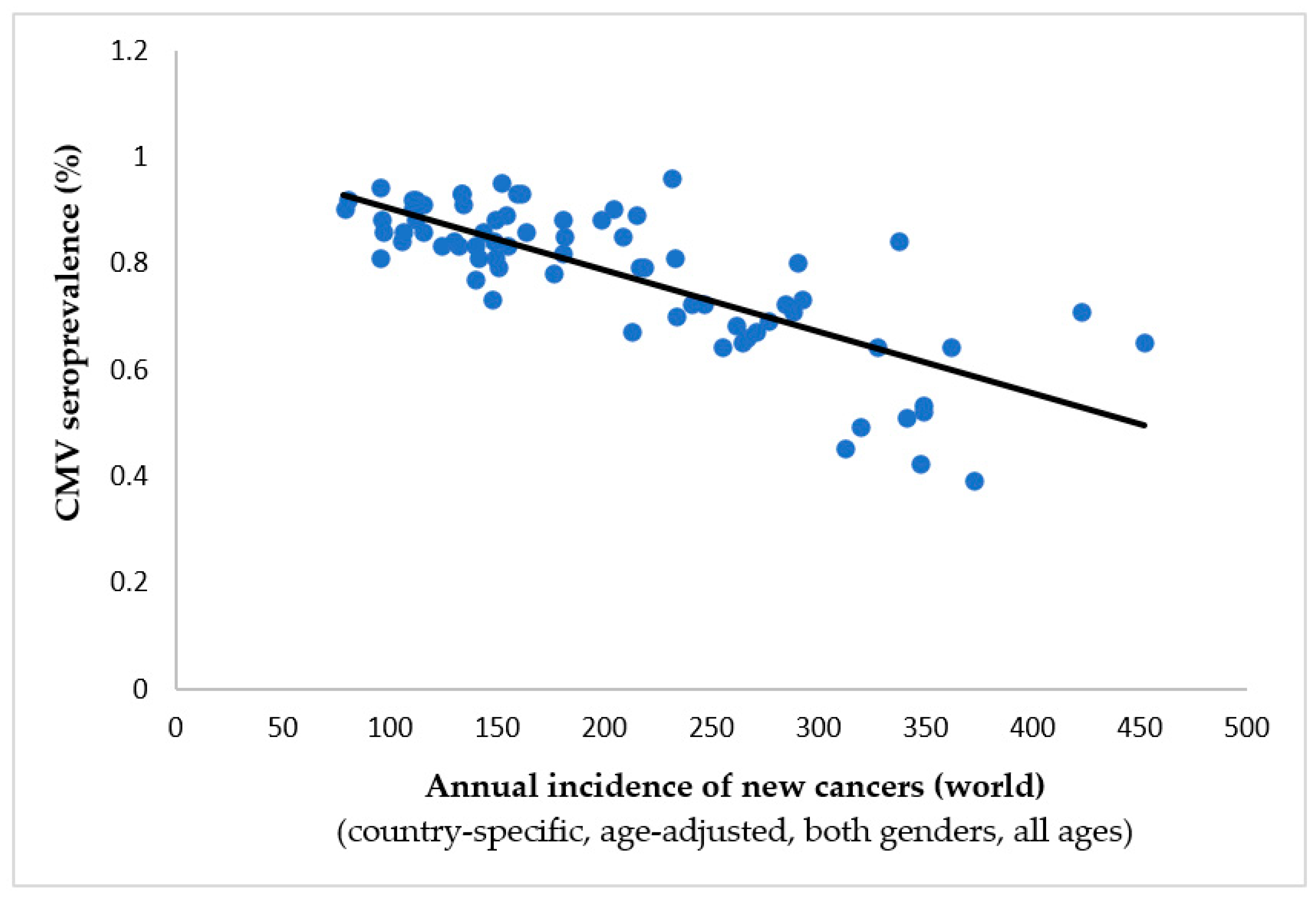
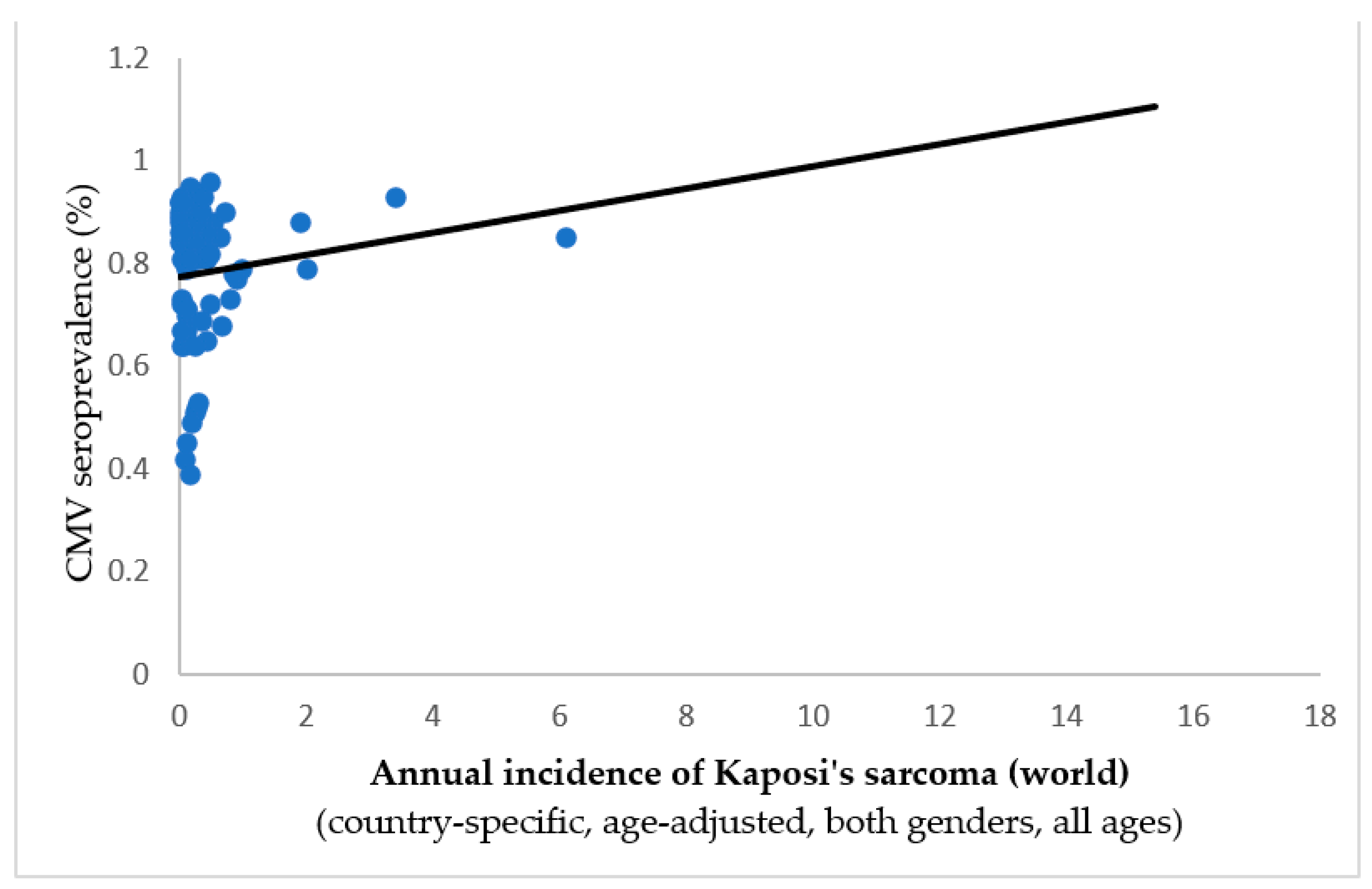
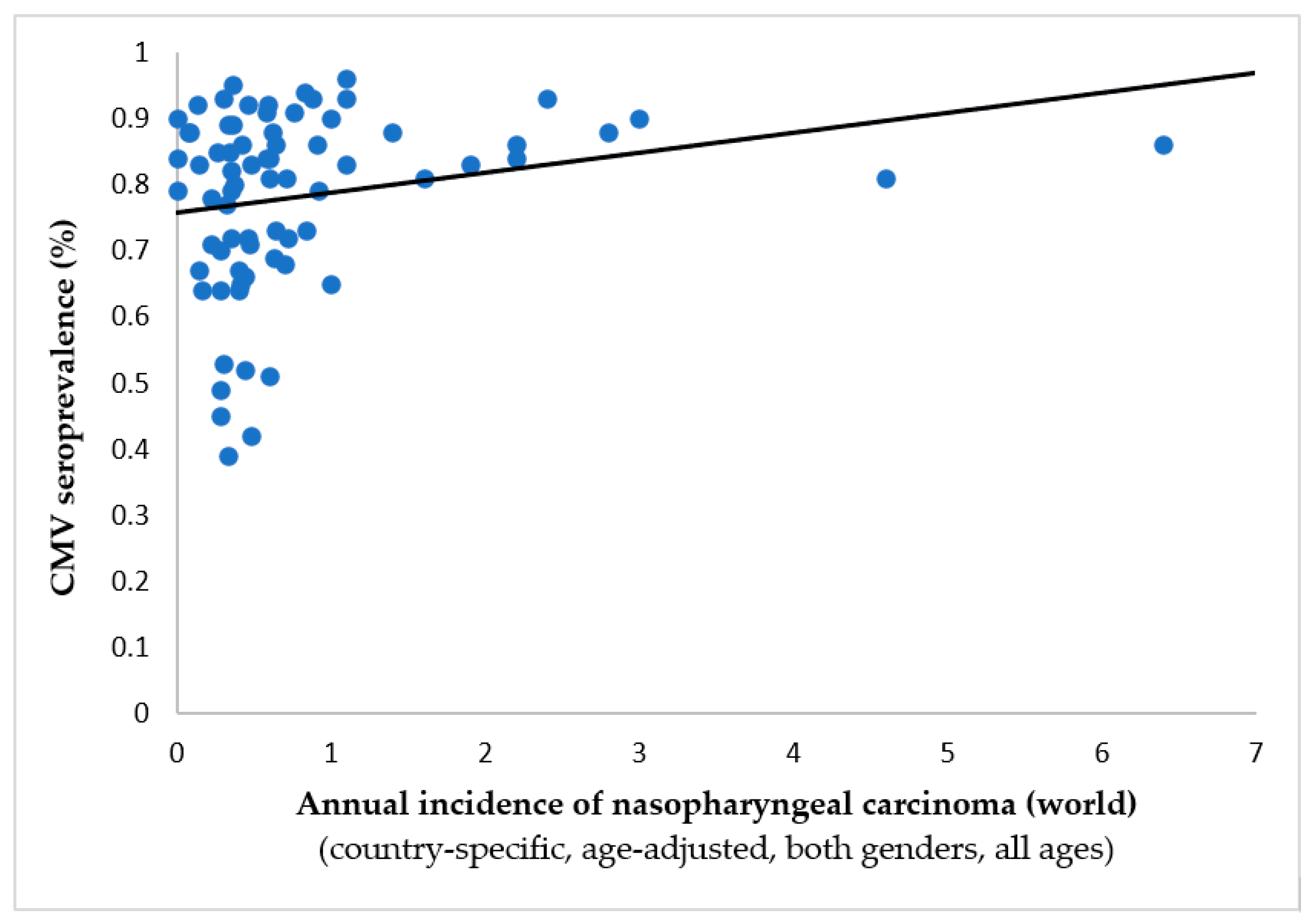
| Tumor/localization | Spearman’s ρ | p-value | Inverse correlation to CMV prevalence |
|---|---|---|---|
|
-0.763 | 0.001* | Yes |
|
-0.754 | 0.001* | Yes |
|
-0.732 | 0.001* | Yes |
|
-0.726 | 0.001* | Yes |
|
-0.719 | 0.001* | Yes |
|
-0.711 | 0.001* | Yes |
|
-0.692 | 0.001* | Yes |
|
-0.671 | 0.001* | Yes |
|
-0.665 | 0.001* | Yes |
|
-0.663 | 0.001* | Yes |
|
-0.656 | 0.001* | Yes |
|
-0.651 | 0.001* | Yes |
|
-0.633 | 0.001* | Yes |
|
-0.633 | 0.001* | Yes |
|
-0.632 | 0.001* | Yes |
|
-0.618 | 0.001* | Yes |
|
-0.617 | 0.001* | Yes |
|
-0.574 | 0.001* | Yes |
|
-0.551 | 0.001* | Yes |
|
-0.548 | 0.001* | Yes |
|
-0.541 | 0.001* | Yes |
|
-0.532 | 0.001* | Yes |
|
-0.519 | 0.001* | Yes |
|
-0.461 | 0.001* | Yes |
|
-0.432 | 0.001* | Yes |
|
-0.377 | 0.001* | Yes |
|
-0.35 | 0.002* | Yes |
|
0.316 | 0.006* | No |
|
0.266 | 0.023* | No |
|
-0.224 | 0.056 | Yes |
|
-0.165 | 0.164 | Yes |
|
-0.149 | 0.208 | Yes |
|
0.118 | 0.319 | No |
|
-0.085 | 0.473 | Yes |
|
-0.007 | 0.953 | Yes |
|
0.007 | 0.951 | No |
| Tumor* | Statistical measures | Age intervals (years) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 0-9 | 10-19 | 20-29 | 30-39 | 40-49 | 50-59 | 60-69 | ≥70 | ||
| Gallbladder | Spearman’s ρ | N/A | .154 | .297 | .394 | .416 | .423 | .290 | .147 |
| p-value | N/A | .193 | .011 | .001 | <.001 | <.001 | .013 | .215 | |
| Bladder† | Spearman’s ρ | -.082 | .120 | -.185 | -.167 | -.254 | -.427 | -.517 | -.552 |
| p-value | .490 | .311 | .118 | .158 | .030 | <.001 | <.001 | <.001 | |
| Colorectum† | Spearman’s ρ | .089 | -.394 | -.205 | -.373 | -.604 | -.648 | -.648 | -.662 |
| p-value | .452 | .001 | .082 | .001 | <.001 | <.001 | <.001 | <.001 | |
| Kaposi’s sarcoma† | Spearman’s ρ | -.642 | .218 | -.083 | -.242 | -.101 | .044 | .006 | .031 |
| p-value | <.001 | .064 | .486 | .040 | .394 | .709 | .959 | .792 | |
| Cervix uteri† | Spearman’s ρ | -.016 | -.017 | -.427 | -.220 | .036 | .249 | .373 | .400 |
| p-value | .895 | .884 | <.001 | .061 | .761 | .034 | .001 | <.001 | |
| Corpus uteri† | Spearman’s ρ | -.196 | -.071 | -.180 | -.294 | -.576 | -.644 | -.672 | -.655 |
| p-value | .096 | .549 | .128 | .012 | <.001 | <.001 | <.001 | <.001 | |
| Hypopharynx† | Spearman’s ρ | .050 | .205 | .307 | .187 | -.235 | -.335 | -.425 | -.378 |
| p-value | .674 | .081 | .008 | .114 | .045 | .004 | <.001 | .001 | |
| Larynx† | Spearman’s ρ | .377 | .219 | .359 | .047 | -.103 | -.212 | -.236 | -.004 |
| p-value | .001 | .063 | .002 | .693 | .388 | .072 | .045 | .972 | |
| Lip/Oral† | Spearman’s ρ | .375 | .244 | -.218 | -.438 | -.501 | -.536 | -.528 | -.528 |
| p-value | .001 | .038 | .064 | <.001 | <.001 | <.001 | <.001 | <.001 | |
| Liver† | Spearman’s ρ | -.378 | .076 | .239 | .353 | .088 | -.056 | -.071 | .057 |
| p-value | .001 | .523 | .042 | .002 | .458 | .639 | .548 | .631 | |
| Lung† | Spearman’s ρ | -.092 | -.120 | -.312 | -.308 | -.373 | -.558 | -.579 | -.501 |
| p-value | .438 | .311 | .007 | .008 | .001 | <.001 | <.001 | <.001 | |
| Melanoma† | Spearman’s ρ | -.242 | -.692 | -.769 | -.786 | -.746 | -.749 | -.722 | -.722 |
| p-value | .039 | <.001 | <.001 | <.001 | <.001 | <.001 | <.001 | <.001 | |
| Mesothelioma† | Spearman’s ρ | .129 | -.090 | .076 | -.235 | -.373 | -.516 | -.544 | -.595 |
| p-value | .278 | .448 | .524 | .045 | .001 | <.001 | <.001 | <.001 | |
| Non-melanoma skin cancer† | Spearman’s ρ | .173 | -.099 | -.345 | -.411 | -.531 | -.589 | -.633 | -.709 |
| p-value | .144 | .403 | .003 | <.001 | <.001 | <.001 | <.001 | <.001 | |
| Nasopharynx | Spearman’s ρ | .210 | .233 | .157 | .138 | .184 | .150 | .138 | .425 |
| p-value | .074 | .047 | .185 | .246 | .119 | .204 | .244 | <.001 | |
| Oropharynx† | Spearman’s ρ | .389 | .239 | .180 | -.423 | -.582 | -.643 | -.657 | -.611 |
| p-value | .001 | .042 | .127 | <.001 | <.001 | <.001 | <.001 | <.001 | |
| Esophagus† | Spearman’s ρ | .298 | .406 | .208 | .192 | -.091 | -.211 | -.243 | -.025 |
| p-value | .011 | <.001 | .077 | .104 | .445 | .073 | .039 | .833 | |
| Pancreas† | Spearman’s ρ | .052 | -.230 | -.155 | -.360 | -.594 | -.649 | -.620 | -.611 |
| p-value | .662 | .050 | .189 | .002 | <.001 | <.001 | <.001 | <.001 | |
| Penis† | Spearman’s ρ | .171 | -.021 | .037 | -.188 | -.317 | -.337 | -.453 | -.495 |
| p-value | .148 | .863 | .756 | .112 | .006 | .004 | <.001 | <.001 | |
| Prostate† | Spearman’s ρ | -.058 | .029 | .185 | -.126 | -.602 | -.663 | -.708 | -.542 |
| p-value | .628 | .807 | .118 | .289 | <.001 | <.001 | <.001 | <.001 | |
| Salivary glands† | Spearman’s ρ | .198 | -.078 | -.226 | -.276 | -.417 | -.359 | -.126 | -.429 |
| p-value | .093 | .509 | .055 | .018 | <.001 | .002 | .287 | <.001 | |
| Testis† | Spearman’s ρ | -.319 | -.653 | -.694 | -.707 | -.728 | -.728 | -.486 | -.097 |
| p-value | .006 | <.001 | <.001 | <.001 | <.001 | <.001 | <.001 | .415 | |
| Thyroid† | Spearman’s ρ | -.266 | -.489 | -.467 | -.491 | -.499 | -.550 | -.544 | -.363 |
| p-value | .023 | <.001 | <.001 | <.001 | <.001 | <.001 | <.001 | .002 | |
| Vulva† | Spearman’s ρ | .044 | -.003 | -.192 | -.384 | -.549 | -.544 | -.642 | -.745 |
| p-value | .709 | .980 | .103 | .001 | <.001 | <.001 | <.001 | <.001 | |
| All cancers† | Spearman’s ρ | -.642 | -.672 | -.731 | -.784 | -.756 | -.726 | -.720 | -.678 |
| p-value | <.001 | <.001 | <.001 | <.001 | <.001 | <.001 | <.001 | <.001 | |
| Vagina† | Spearman’s ρ | -.033 | .126 | <.001 | -.125 | -.257 | -.103 | -.189 | -.376 |
| p-value | .785 | .287 | .999 | .291 | .028 | .385 | .110 | .001 | |
| Stomach | Spearman’s ρ | .283 | .145 | .079 | .039 | -.042 | -.055 | -.081 | -.097 |
| p-value | .015 | .221 | .504 | .746 | .723 | .644 | .496 | .413 | |
| Ovary† | Spearman’s ρ | -.121 | -.181 | -.071 | -.188 | -.284 | -.395 | -.517 | -.454 |
| p-value | .308 | .124 | .551 | .111 | .015 | .001 | <.001 | <.001 | |
| Brain/CNS† | Spearman’s ρ | -.649 | -.577 | -.654 | -.607 | -.529 | -.477 | -.455 | -.452 |
| p-value | <.001 | <.001 | <.001 | <.001 | <.001 | <.001 | <.001 | <.001 | |
| All cancers excl. non-melanoma skin† | Spearman’s ρ | -.645 | -.667 | -.733 | -.785 | -.753 | -.726 | -.717 | -.656 |
| p-value | <.001 | <.001 | <.001 | <.001 | <.001 | <.001 | <.001 | <.001 | |
| Breast† | Spearman’s ρ | .050 | .182 | -.239 | -.645 | -.674 | -.696 | -.700 | -.661 |
| p-value | .674 | .124 | .042 | <.001 | <.001 | <.001 | <.001 | <.001 | |
| Kidney† | Spearman’s ρ | -.493 | -.016 | -.365 | -.693 | -.736 | -.731 | -.728 | -.744 |
| p-value | <.001 | .893 | .002 | <.001 | <.001 | <.001 | <.001 | <.001 | |
| Hodgkin lymphoma† | Spearman’s ρ | .174 | -.622 | -.676 | -.681 | -.575 | -.478 | -.389 | -.229 |
| p-value | .141 | <.001 | <.001 | <.001 | <.001 | <.001 | .001 | .051 | |
| Non-Hodgkin lymphoma† | Spearman’s ρ | -.036 | -.005 | -.437 | -.590 | -.593 | -.618 | -.610 | -.552 |
| p-value | .762 | .968 | <.001 | <.001 | <.001 | <.001 | <.001 | <.001 | |
| Multiple myeloma† | Spearman’s ρ | .235 | .110 | .055 | -.356 | -.483 | -.577 | -.644 | -.627 |
| p-value | .045 | .355 | .645 | 0.002 | <.001 | <.001 | <.001 | <.001 | |
| Leukemia† | Spearman’s ρ | -.495 | -.236 | .108 | -.165 | -.472 | -.630 | -.645 | -.543 |
| p-value | <.001 | .044 | .364 | .164 | <.001 | <.001 | <.001 | <.001 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





