Submitted:
30 September 2023
Posted:
01 October 2023
You are already at the latest version
Abstract
Keywords:
INTRODUCTION
BACKGROUND
Mitochondrial Interplay with Immune System
- Increased NADH/NAD+ ratio in matrix
- Highly rescued CoQ pool in conjugation with a maximal Δp and no ATP synthesis.
Molecular patterns associated mitochondrial damage
Metabolic repurposing of mitochondria to drive inflammatory macrophages
Immune cells and their mitochondrial metabolism
CONCLUSION
| Characteristic | Immune metabolism associated with Salmonella | Reference |
|---|---|---|
| Growth of Salmonella | Competes with the host nutrients such as Fe (iron) which is necessary for the growth of Salmonella within host. | Khan et al., 2014 |
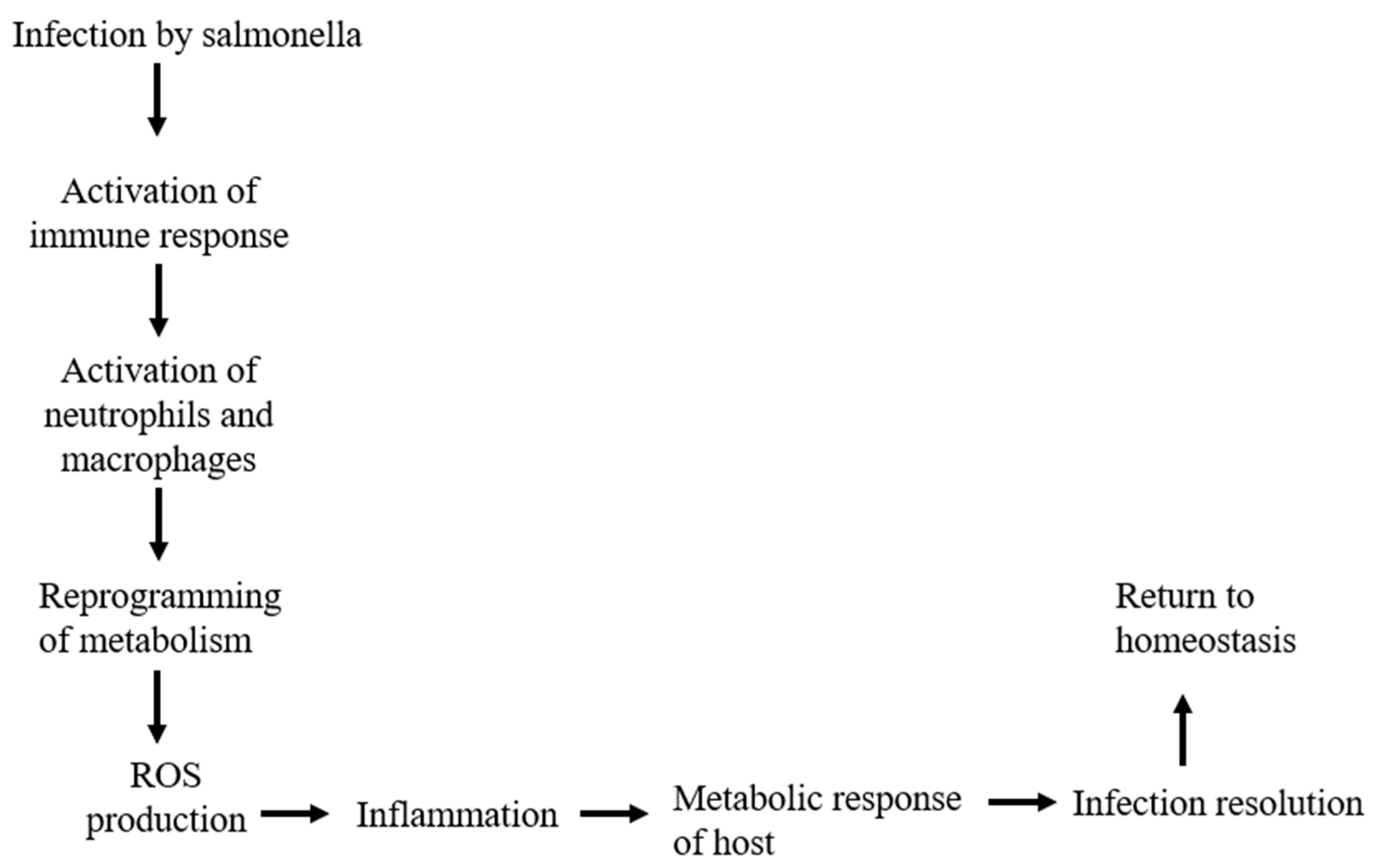
| Inflammation | Inflammation leads to the formation of inflammasome and which releases various pro-inflammatory cytokines. | Eckmann et al., 2001 |
| Infection response | Host cells seizes Fe availability. | Parrow et al., 2013 |
| Reprogramming of metabolism | Switch from glucose metabolism to fatty acid oxidation occurs during the course of infection. | Ganeshan & Chawla (2014) |
| Recruitment of immune cells | Immune response generated leads to the recruitment of various immune cells such as macrophages, dendritic cells, etc. in which chemotaxis play a major role. | Guak & Krawczyk (2020) |
| Infection resolution | After the resolution of infection homeostasis is established again and a switch to OXPHOS occurs so as to repair metabolic damage. | Ganeshan & Chawla (2014) |
| Characteristic | Mitochondrial dynamics associated with Salmonella infection | Reference |
|---|---|---|
| Targeting mitochondria | Salmonella targets various effector protein to translocate into the mitochondria. | Layton et al., 2005 |
| Fragmentation of mitochondria | Salmonella can lead to the fusion and fission of mitochondria which leads to altered mitochondrial dynamics. | Tiku et al., 2020 |
| Energy production | Energy is produced through OXPHOS which affects the function of mitochondria thus exerts influence on the host cells. | Ramond et al., 2019 |
| Production of ROS | Salmonella infection leads to the production of ROS which is one of the crucial productions during infection. | Rhen M. 2019 |
| Inflammatory response | Mitochondrial damage during the infection of Salmonella leads to the secretion of mt DNA and various proteins which elicits the immune response. | Missiroli et al., 2020 |
| Host defense | Mitochondrial dynamics plays the defense such the induction of mitophagy which leads to the elimination of damaged mitochondria. | Kubli et al., 2012 |
References
- Garai, P., Gnanadhas, D. P., & Chakravortty, D. (2012). Salmonella enterica serovars Typhimurium and Typhi as model organisms: revealing paradigm of host-pathogen interactions. Virulence, 3(4), 377–388. [CrossRef]
- Takaya, A., Yamamoto, T., & Tokoyoda, K. (2020). Humoral Immunity vs. Salmonella. Frontiers in immunology, 10, 3155. [CrossRef]
- Hurley, D., McCusker, M. P., Fanning, S., & Martins, M. (2014). Salmonella-host interactions - modulation of the host innate immune system. Frontiers in immunology, 5, 481. [CrossRef]
- Khan, S., Raj, D., Jaiswal, K., & Lahiri, A. (2020). Modulation of host mitochondrial dynamics during bacterial infection. Mitochondrion, 53, 140–149. [CrossRef]
- Xie, J. H., Li, Y. Y., & Jin, J. (2020). The essential functions of mitochondrial dynamics in immune cells. Cellular & molecular immunology, 17(7), 712–721. [CrossRef]
- Wang, Y., Branicky, R., Noë, A., & Hekimi, S. (2018). Superoxide dismutases: Dual roles in controlling ROS damage and regulating ROS signaling. The Journal of cell biology, 217(6), 1915–1928. [CrossRef]
- Pua, H. H., Guo, J., Komatsu, M., & He, Y. W. (2009). Autophagy is essential for mitochondrial clearance in mature T lymphocytes. Journal of immunology (Baltimore, Md. : 1950), 182(7), 4046–4055. [CrossRef]
- Angajala, A., Lim, S., Phillips, J. B., Kim, J. H., Yates, C., You, Z., & Tan, M. (2018). Diverse Roles of Mitochondria in Immune Responses: Novel Insights Into Immuno-Metabolism. Frontiers in immunology, 9, 1605. [CrossRef]
- Nakahira, K., Hisata, S., & Choi, A. M. (2015). The Roles of Mitochondrial Damage-Associated Molecular Patterns in Diseases. Antioxidants & redox signaling, 23(17), 1329–1350. [CrossRef]
- Page, M. J., Kell, D. B., & Pretorius, E. (2022). The Role of Lipopolysaccharide-Induced Cell Signalling in Chronic Inflammation. Chronic stress (Thousand Oaks, Calif.), 6, 24705470221076390. [CrossRef]
- Jiang, L., Wang, P., Song, X., Zhang, H., Ma, S., Wang, J., Li, W., Lv, R., Liu, X., Ma, S., Yan, J., Zhou, H., Huang, D., Cheng, Z., Yang, C., Feng, L., & Wang, L. (2021). Salmonella Typhimurium reprograms macrophage metabolism via T3SS effector SopE2 to promote intracellular replication and virulence. Nature communications, 12(1), 879. [CrossRef]
- Mills, E. L., Kelly, B., Logan, A., Costa, A. S. H., Varma, M., Bryant, C. E., Tourlomousis, P., Däbritz, J. H. M., Gottlieb, E., Latorre, I., Corr, S. C., McManus, G., Ryan, D., Jacobs, H. T., Szibor, M., Xavier, R. J., Braun, T., Frezza, C., Murphy, M. P., & O'Neill, L. A. (2016). Succinate Dehydrogenase Supports Metabolic Repurposing of Mitochondria to Drive Inflammatory Macrophages. Cell, 167(2), 457–470.e13. [CrossRef]
- Yoshimoto, T., Okamura, H., Tagawa, Y. I., Iwakura, Y., & Nakanishi, K. (1997). Interleukin 18 together with interleukin 12 inhibits IgE production by induction of interferon-gamma production from activated B cells. Proceedings of the National Academy of Sciences of the United States of America, 94(8), 3948–3953. [CrossRef]
- Gutiérrez, S., Fischer, J., Ganesan, R., Hos, N. J., Cildir, G., Wolke, M., Pessia, A., Frommolt, P., Desiderio, V., Velagapudi, V., & Robinson, N. (2021). Salmonella Typhimurium impairs glycolysis-mediated acidification of phagosomes to evade macrophage defense. PLoS pathogens, 17(9), e1009943. [CrossRef]
- Lopez-Castejon, G., & Brough, D. (2011). Understanding the mechanism of IL-1β secretion. Cytokine & growth factor reviews, 22(4), 189–195. [CrossRef]
- Dimeloe, S., Burgener, A. V., Grählert, J., & Hess, C. (2017). T-cell metabolism governing activation, proliferation and differentiation; a modular view. Immunology, 150(1), 35–44. [CrossRef]
- Zuo, J., Tang, J., Lu, M., Zhou, Z., Li, Y., Tian, H., Liu, E., Gao, B., Liu, T., & Shao, P. (2021). Glycolysis Rate-Limiting Enzymes: Novel Potential Regulators of Rheumatoid Arthritis Pathogenesis. Frontiers in immunology, 12, 779787. [CrossRef]
- Hoitzing, H., Johnston, I. G., & Jones, N. S. (2015). What is the function of mitochondrial networks? A theoretical assessment of hypotheses and proposal for future research. BioEssays : news and reviews in molecular, cellular and developmental biology, 37(6), 687–700. [CrossRef]
- Kaur, J., & Jain, S. K. (2012). Role of antigens and virulence factors of Salmonella enterica serovar Typhi in its pathogenesis. Microbiological research, 167(4), 199–210. [CrossRef]
- Khan C. M. (2014). The Dynamic Interactions between Salmonella and the Microbiota, within the Challenging Niche of the Gastrointestinal Tract. International scholarly research notices, 2014, 846049. [CrossRef]
- Eckmann, L., & Kagnoff, M. F. (2001). Cytokines in host defense against Salmonella. Microbes and Infection, 3(14-15), 1191-1200.
- Parrow, N. L., Fleming, R. E., & Minnick, M. F. (2013). Sequestration and scavenging of iron in infection. Infection and immunity, 81(10), 3503–3514. [CrossRef]
- Ganeshan, K., & Chawla, A. (2014). Metabolic regulation of immune responses. Annual review of immunology, 32, 609–634. [CrossRef]
- Guak, H., & Krawczyk, C. M. (2020). Implications of cellular metabolism for immune cell migration. Immunology, 161(3), 200–208. [CrossRef]
- Layton, A. N., Brown, P. J., & Galyov, E. E. (2005). The Salmonella translocated effector SopA is targeted to the mitochondria of infected cells. Journal of bacteriology, 187(10), 3565–3571. [CrossRef]
- Tiku, V., Tan, M. W., & Dikic, I. (2020). Mitochondrial Functions in Infection and Immunity. Trends in cell biology, 30(4), 263–275.
- Ramond, E., Jamet, A., Coureuil, M., & Charbit, A. (2019). Pivotal Role of Mitochondria in Macrophage Response to Bacterial Pathogens. Frontiers in immunology, 10, 2461. [CrossRef]
- Rhen M. (2019). Salmonella and Reactive Oxygen Species: A Love-Hate Relationship. Journal of innate immunity, 11(3), 216–226. [CrossRef]
- Missiroli, S., Genovese, I., Perrone, M., Vezzani, B., Vitto, V. A. M., & Giorgi, C. (2020). The Role of Mitochondria in Inflammation: From Cancer to Neurodegenerative Disorders. Journal of clinical medicine, 9(3), 740. [CrossRef]
- Kubli, D. A., & Gustafsson, Å. B. (2012). Mitochondria and mitophagy: the yin and yang of cell death control. Circulation research, 111(9), 1208–1221. [CrossRef]
- Thapa SB, Pandey RP, Bashyal P, Yamaguchi T, Sohng JK. Cascade biocatalysis systems for bioactive naringenin glucosides and quercetin rhamnoside production from sucrose. Appl Microbiol Biotechnol. 2019 Oct;103(19):7953-7969. [CrossRef]
- Pandey RP, Bashyal P, Parajuli P, Yamaguchi T, Sohng JK. Two Trifunctional Leloir Glycosyltransferases as Biocatalysts for Natural Products Glycodiversification. Org Lett. 2019 Oct 4;21(19):8058-8064. [CrossRef]
- Kyu-Min Kim, Jin-Soo Park, HaeRi Choi, Min-Seon Kim, Joo-Hyun Seo, Ramesh Prasad Pandey, Jin Woo Kim, Chang-Gu Hyun & Seung-Young Kim (2018) Biosynthesis of novel daidzein derivatives using Bacillus amyloliquefaciens whole cells, Biocatalysis and Biotransformation, 36:6, 469-475. [CrossRef]
- Parajuli P, Pandey RP, Trang NTH, Oh TJ, Sohng JK. Expanded acceptor substrates flexibility study of flavonol 7-O-rhamnosyltransferase, AtUGT89C1 from Arabidopsis thaliana. Carbohydr Res. 2015 Dec 11;418:13-19. [CrossRef]
- Shin JY, Pandey RP, Jung HY, Chu LL, Park YI, Sohng JK. In vitro single-vessel enzymatic synthesis of novel Resvera-A glucosides. Carbohydr Res. 2016 Apr 7;424:8-14. [CrossRef]
- Pandey, R., and M. M. Agarwal. "INFLUENCE OF FERTILITY LEVELS, VARIETIES AND TRANSPLANTING TIME ON RICE (ORYZA-SATIVA)." Indian Journal of Agronomy 36.4 (1991): 459-463.
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





