1. Introduction
Balsam fir is a mid- to late-successional conifer species native to northeastern United States and Canada [
1]. Balsam fir can be used for lumber or fuel, but they are also a commonly used as Christmas tree species. Balsam fir Christmas trees are often harvested as early as October to meet export demand and are often a preferred species due to their unique fragrance, color, and high needle retention characteristics [
2]. However, postharvest needle retention has decreased over time, attributed to reduced cold acclimation time caused by earlier harvests and climate change [
3].
Cold stress and/or freezing temperatures can cause widespread metabolic dysfunction in plants, including reduced electron transport, increased oxidative stress, impaired water movement, changes in membrane fluidity, and eventual tissue death [
4,
5,
6]. Like many conifers, balsam fir is adept at tolerating freezing temperatures through cold acclimation. Cold acclimation is regulated by a complex network of signaling pathways triggered by environmental cues like light and temperature [
7]. Decreasing temperatures and shorter photoperiods trigger cryoprotective genes that ultimately improve protein stabilization, increase solute concentrations, and alter membrane composition [
8,
9].
Cold acclimation-induced membrane changes are manifested through shifts in the concentration of membrane lipids. One change is that the concentration of monogalactosyldiacylglycerol (MGDG) tend to decrease in tandem with an increase in digalactosyldiacylglycerol (DGDG) [
10,
11]. MGDG forms a single layer of lipids for greater efficiency of thylakoid membranes while DGDG forms a bilayer to create greater stability [
6]. A shift towards DGDG constitutes a shift towards membrane stability in the plant and helps protect plants from cold stress. A second shift is towards phospholipids (PLs) after cold acclimation [
12]. More specifically, there is an increase in phosphatidycholine (PC) and phoshatidylethanolamine (PE) after cold acclimation [
11,
13]. An increase in PLs is associated with a shift towards lipids containing unsaturated fatty acids to help maintain membrane fluidity in cold temperatures [
14].
Cold acclimation and tolerance can be calculated through many different methods, but in essence all methods operate by exposing plants to cold temperatures and assessing damage [
15]. The specific temperatures, exposure time, and damage assessment vary between studies and plant species [
15]. One technique is to assess plants for visual damage from freezing temperatures, though this can often be a tedious task [
15]. Alternatives include measuring electrolyte leakage [
16,
17] or chlorophyll fluorescence [
18,
19] to assess damage. Freezing tolerance is then often quantified using an LT50 value, or the temperature at which there is 50% electrolyte leakage or a 50% decrease in chlorophyll fluorescence. Freezing tolerance of
Abies species ranged from -25 to -70°C through visual observation [
20]. Freezing tolerance of
Abies procera was monitored throughout autumn and ranged from approximately -20°C in September to -38°C in December [
21].
Cold acclimation tends to be associated with improved postharvest needle retention in balsam fir trees. Needle retention increases throughout the autumn months and reaches a peak in November or December [
22,
23,
24,
25]. Though improved needle retention was strongly correlated with decreasing temperatures and photoperiod [
24], freezing tolerance of balsam fir has not been directly related to needle retention. Further, postharvest needle retention is linked to changes in lipids and fatty acids [
26]. Abscising needles had lower concentrations of MGDG and DGDG, but significantly higher concentrations of PC, lysophosphatidylglycerol (LPG), and phosphainositol (PI) than intact needles [
26]. It is reasonable to postulate that lipid changes in balsam fir are linked to both cold tolerance and postharvest needle retention. The objectives of this study are to (1) quantify changes in balsam fir cold tolerance throughout autumn, (2) determine changes in balsam fir needle polar lipids throughout autumn, and (3) to relate cold tolerance to changes in lipid concentration and needle retention in balsam fir.
2. Results
2.1. Confirming Cold Acclimation
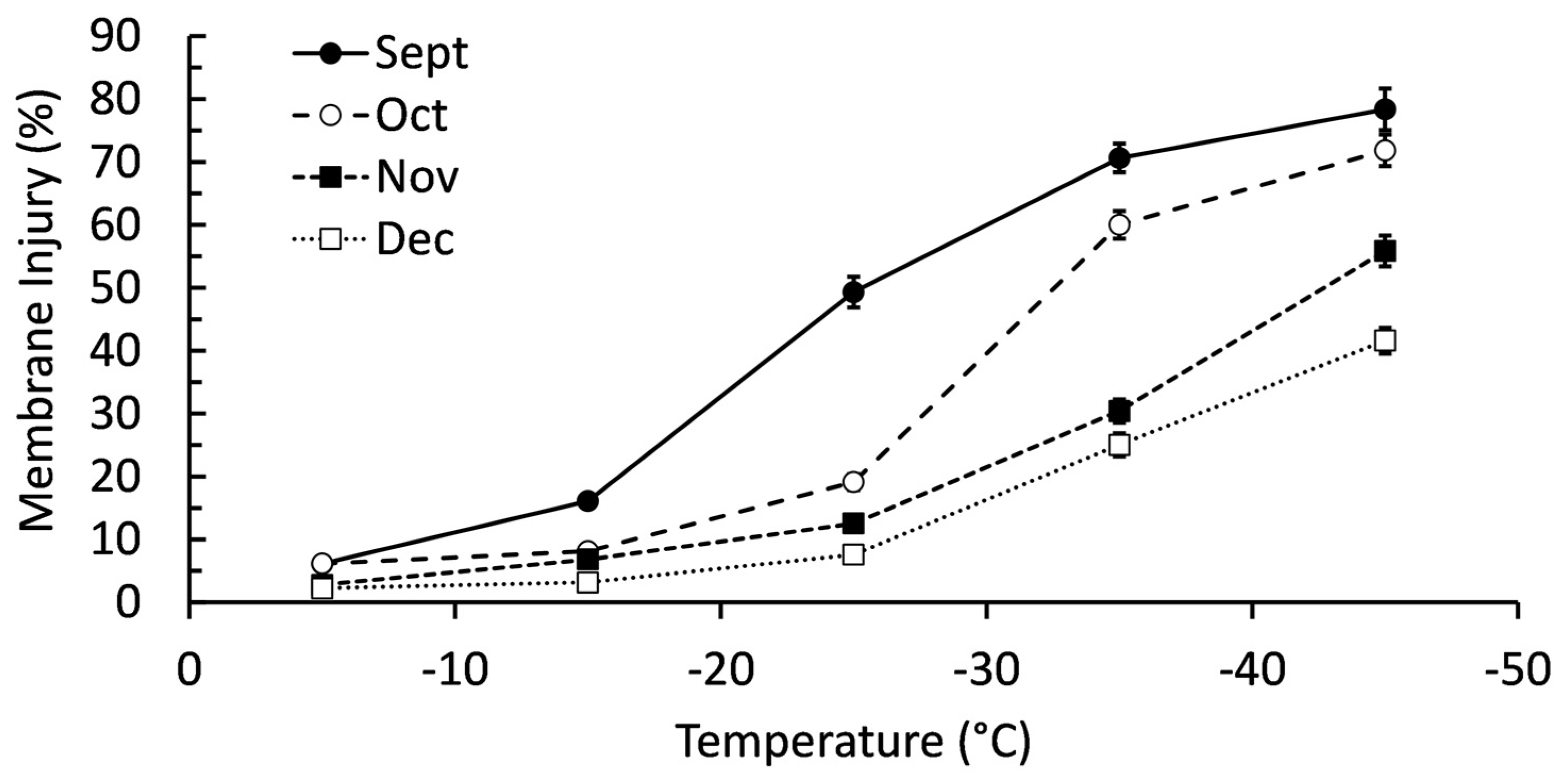
There was a significant interactive effect (P < 0.001) between collection month and freezing temperatures on membrane injury (
Figure 1). Branches collected in September had significantly more membrane injury once exposed to -15°C, a trend that continued until exposure to -45°C when there was no difference between branches collected in September and November. Exposure to -25°C was the point where there was very clear separation in membrane injury between sample months; highest to lowest membrane injury occurred in order of September, October, November, and December. Membrane injury was higher in all collection months when exposed to -35°C, though the order was identical to -25°C freezing. Ultimately, there was a clear trend of balsam fir having lower membrane injury in cold temperatures when harvested later in autumn. Blocking by genotype had minimal improvement on the statistical model (F = 0.94, P = 0.420).
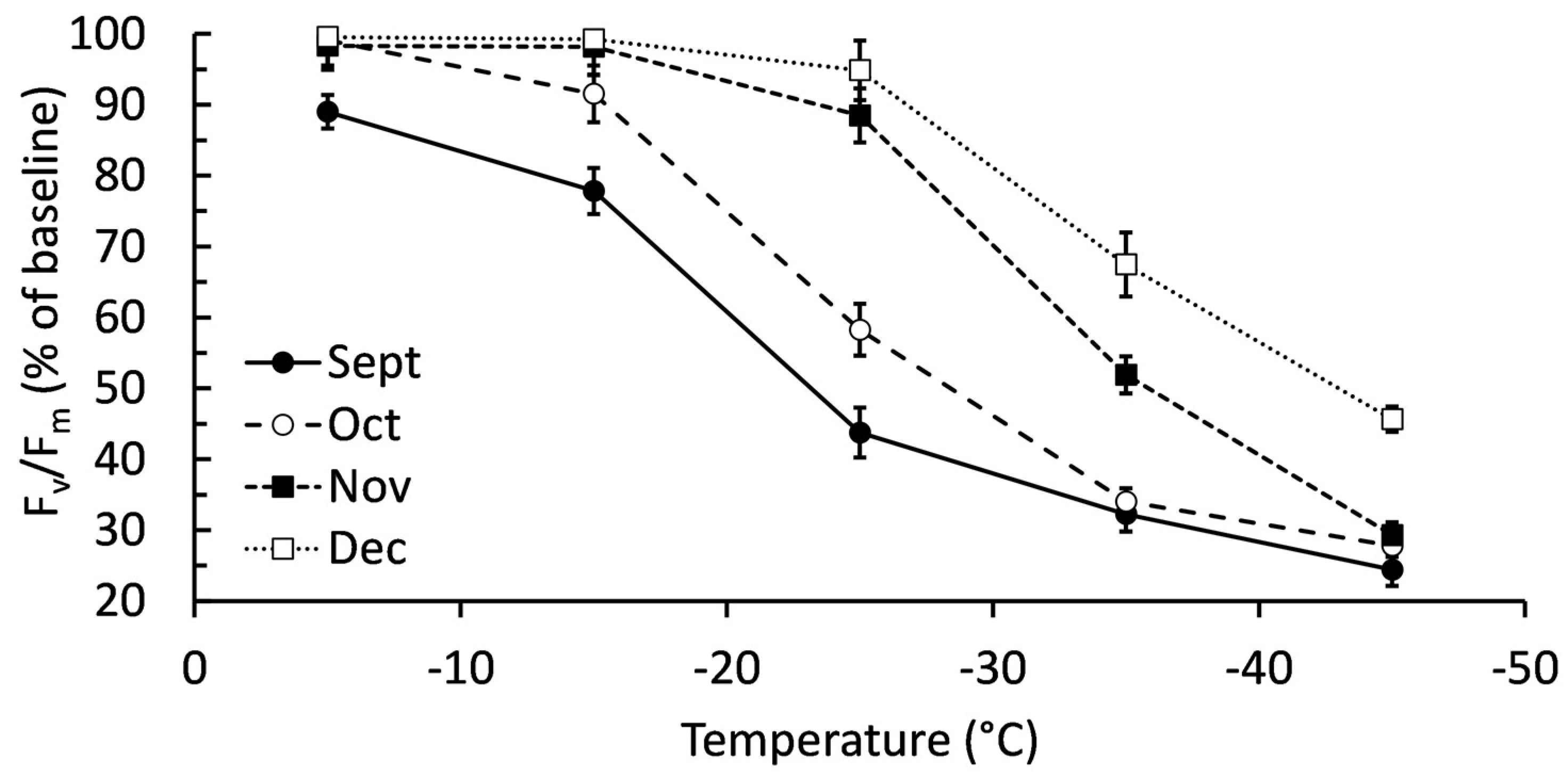
There was a significant interactive effect (P < 0.001) between collection month and freezing temperatures on chlorophyll fluorescence (
Figure 2). September had significantly lower fluorescence than other months when exposed to -5°C, though fluorescence was still maintained at approximately 90% of the baseline value. September was also the only sampling month with significantly lower fluorescence after -15°C exposure. Both September and October had significantly lower fluorescence after exposure to -25°C. December maintained significantly higher fluorescence than other months after exposure to -35°C and -45°C, though fluorescence had decreased compared to freeze tests at -5, -15, and -25°C. As with membrane injury, the general trend was balsam fir maintaining their chlorophyll fluorescence in cold temperatures when harvested later in autumn. Blocking by genotype improved the statistical model with respect to chlorophyll fluorescence (F = 3.57, P = 0.014).
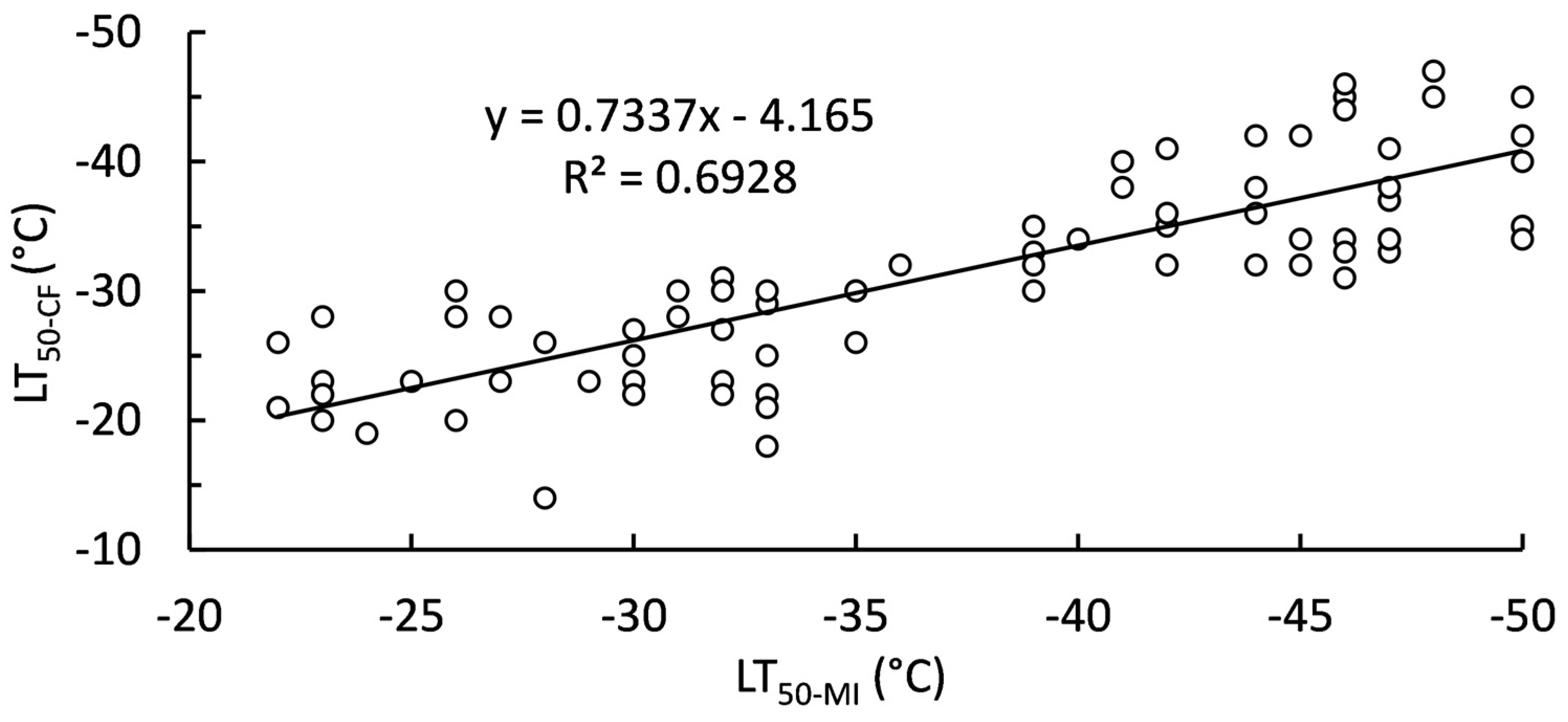
Sigmoidal curves fitted to membrane injury and chlorophyll fluorescence responses to freezing temperatures allowed for determination of LT50 values. However, LT50 values were not completely consistent between LT50 calculated from membrane injury (LT50-MI) and chlorophyll fluorescence (LT50-CF) (
Figure 3). A perfect relationship between LT50-MI and LT50-CF would have a slope of 1 compared to the calculated 0.7337. Instead, LT50-MI are lower than their respective LT50-CG estimates. Sampling month had a consistent significant (P < 0.001) effect on LT50, regardless of estimation method. September always had the highest LT50, while December always had the lowest LT50 (
Figure 4).
Sampling month had a significant (P < 0.001) effect on needle retention duration (NRD) (
Figure 4). September and October had the lowest NRD of 51 and 49 days, respectively. November and December had a 32-47% increase in NRD compared to September and October, though there was no significant difference between November and December. Although the overall trend was consistent to LT50 values per month, NRD was only weakly correlated to LT50-MI and LT50-CF (r = -0.365 and -0.290, respectively). Genotype contributed significant variation to NRD (F = 8.90, P < 0.001). A regression equation using genotype as a block improved the relationship between NRD and LT50-MI (R
2 = 35%) and between NRD and LT50-CF (R
2 = 36%).
2.2. Changes in Polar Lipids during Cold Acclimation
Sampling month had a significant effect (P < 0.05) on all polar lipid classes in balsam fir, except for LPG and LPE (
Table 1). Most polar lipids increased in relative concentration throughout autumn. There was a relative increase of 11.8% in DGDG, 30.3% in PC, 26.5% in PG, 81.7% in PE, and 23.1% in PI from September to December. The increases in the 5 lipid classes above were offset by a 26.2% decrease in MGDG over the same time span. LPG and PA did not have a consistent progression from September to December; instead, each reached their highest relative concentration in November before decreasing in December.
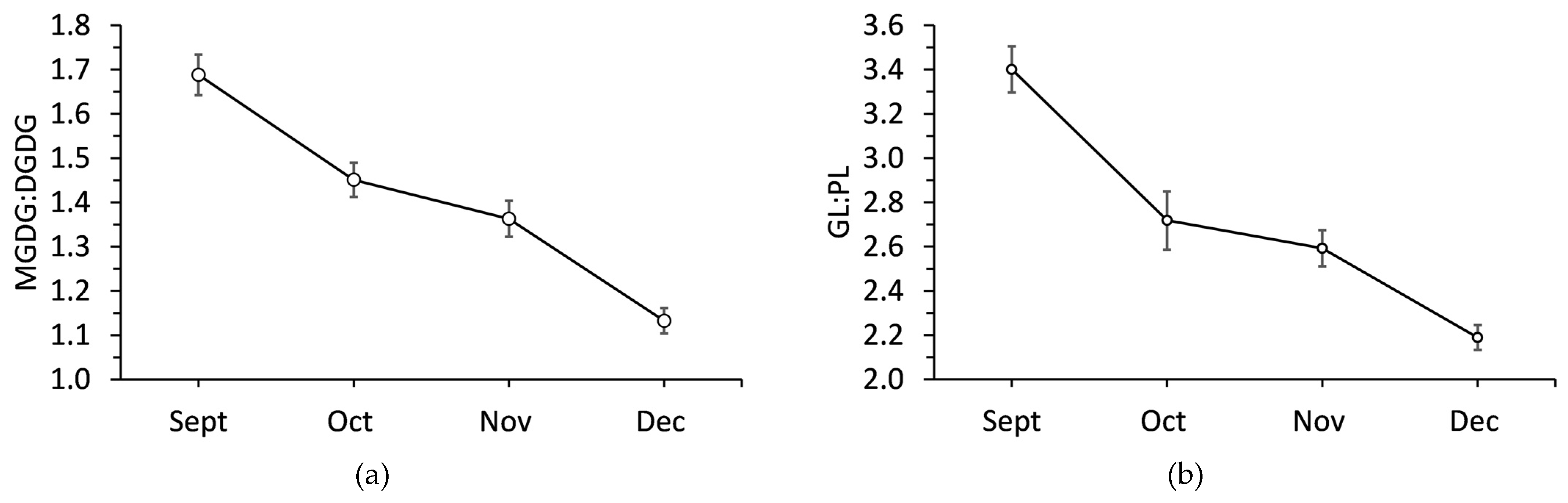
Sampling month had a significant effect (P <0.001) on MGDG:DGDG and GL:PL ratios (
Figure 5). The MGDG:DGDG was 1.69 in September and then significantly decreased each month. MGDG:DGDG decreased by 14.2% in October, 19.5% in November, and 33.1% in December all compared to September. GL:PL significantly decreased by 20.0% from September to October. There was further significant decrease in November, but by December GL:PL had decreased by 35.6% compared to its initial value.
2.3. Relationship of Polar Lipids and Lethal Temperatures
Almost all polar lipid classes had significant (P <0.05), linear relationships with LT50 values, except for PA, LPC, LPG, and LPE (
Table 2). MGDG had the strongest relationship with LT50, accounting for 55.0% of variation in LT50-MI and 42.7% of variation in LT50-CF. MGDG also had the only positive relationship with LT50 values. DGDG, PC, PE, and PI were weaker, accounting for 30.0-36.7% of the variation in LT50-MI and 22.9-29.5% of the variation in LT50-CF. PG accounted for less than 20% of the variation in each LT50 value.
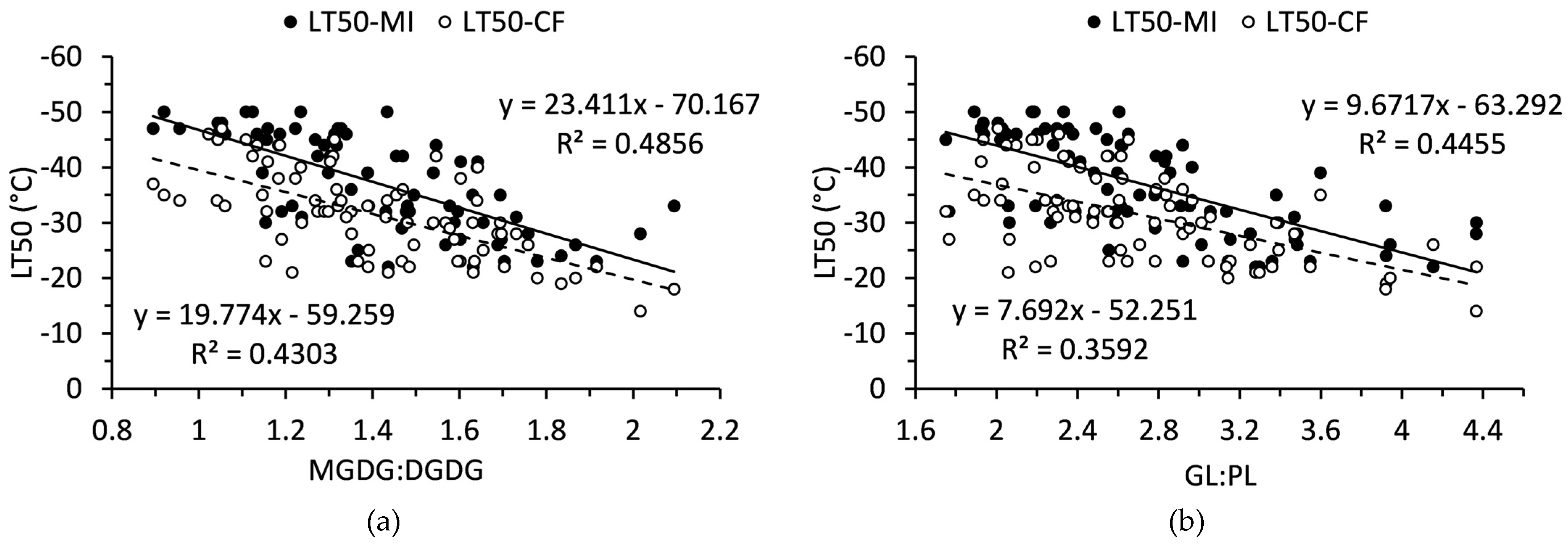
MGDG:DGDG and GL:PL ratios each had significant, linear relationship with LT50 values (
Figure 6). These relationships were stronger when LT50 was determined from membrane injury as opposed to chlorophyll fluorescence. The slope of each relationship was positive so that a shift towards DGDGs or PLs was related to lower LT50.
2.4. Relationship of Polar Lipids and Needle Abscission
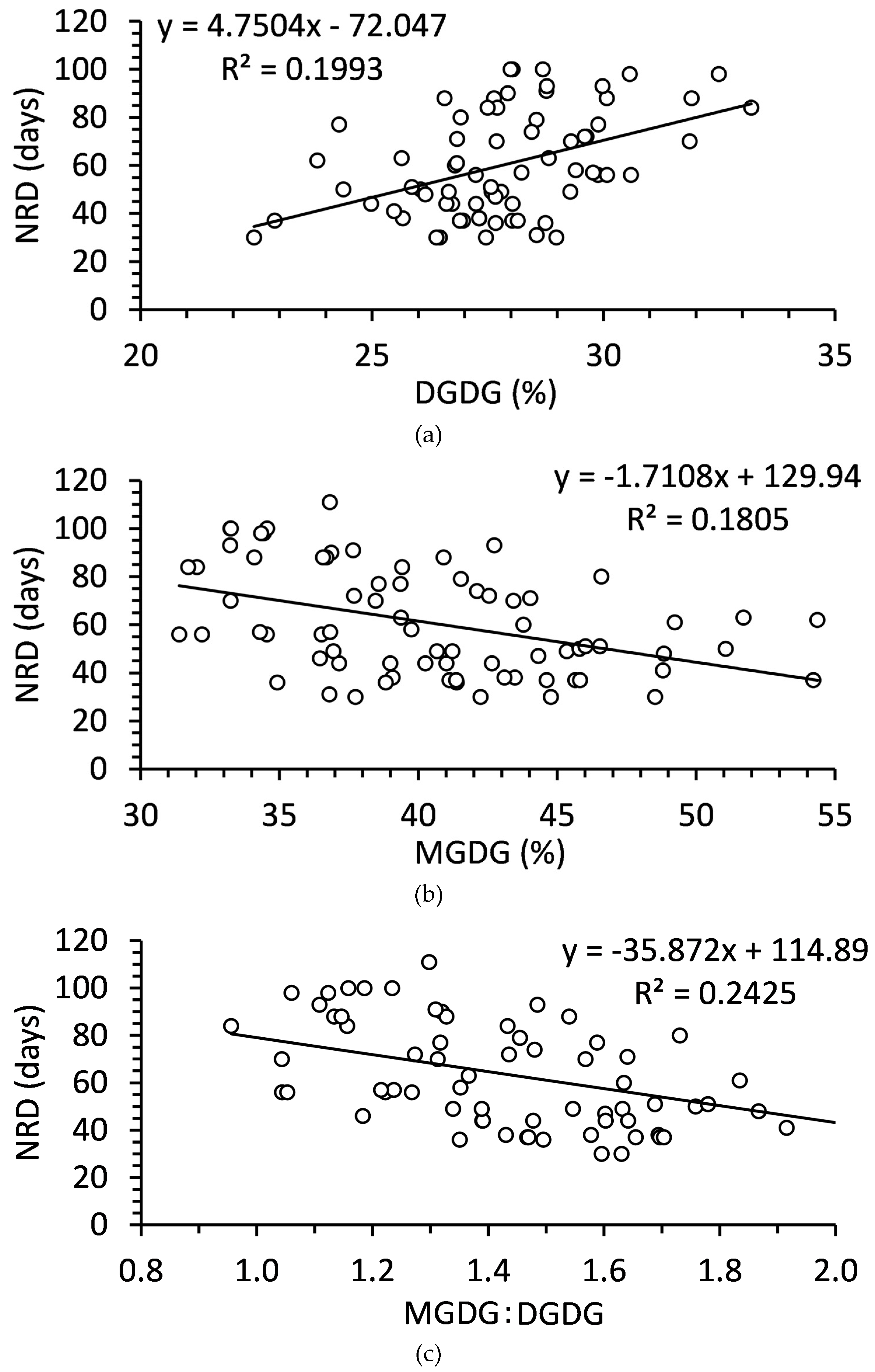
Only DGDG and MGDG were significantly related to NRD (
Figure 7). DGDG was positively related to NRD while MGDG was negatively related to NRD. Although each of the relationships was significant, they were relatively weak relationships that accounted for less than 20% of the variation in NRD. The MGDG:DGDG ratio slightly improved the model, accounting for 24% of the variation in NRD (
Figure 7).
3. Discussion
3.1. Cold Acclimation in Balsam Fir
There is sufficient evidence to support that balsam fir acclimates to cold temperatures throughout autumn in Nova Scotia. Balsam fir branches collected each month after September better resisted membrane injury and impaired chlorophyll fluorescence when subjected to freezing temperatures. The LT50 value for branches collected in December was between -40 and -50°C, almost twice as low as branches collected in September. Freezing tolerance of balsam fir fall well within the expected range of -25 to -70°C for
Abies species [
20].
The fact that balsam fir cold acclimates through autumn was expected. The minimum temperature observed in December during sampling was -23°C and continued to decrease in January and February. Although rare, it is possible to have minimum temperatures below -40°C in Nova Scotia where these branches were collected. Balsam fir would need to adapt to such freezing temperatures to survive the winter. However, it was important to confirm and quantify their ability to acclimate to freezing temperatures. One weakness of previous cold acclimation studies in balsam fir was that it was assumed that cold acclimation occurred, but the degree of that acclimation was not measured [
22,
23,
24,
25]. The LT50 values in this current student lend credence that the assumption of cold acclimation was likely correct in previous studies. It is noteworthy that accuracy of estimates in this current study could be improved with smaller increments in freezing temperatures tested [
15]. Also, a few branches from December did not have 50% membrane injury or a 50% decrease in chlorophyll fluorescence even at -45°C, although they were usually very close to that 50% mark. In those cases, LT50 values were extrapolated from a sigmoidal curve. Future evaluations of balsam fir freezing tolerance should include lower temperatures to avoid that problem.
3.2. Polar Lipids in Balsam Fir
Seasonal changes in lipid profiles have been studied extensively in other species, but there have been few studies of lipids in balsam fir. There are fewer major contributing species of polar lipids in balsam fir needles than in
Arabidopsis rosettes [
27]. GLs are the most abundant lipid classes in balsam fir needles, which can represent up to 80% of all polar lipids [
28]. In balsam fir, galactolipids (GLs) comprised 65-70 per cent of all polar lipids. The other major contributor was PC and to a lesser extent PG. A previous study found 33% MGDG and 28% DGDG in balsam fir harvested in December [
26]. Our study found 33% MGDG to 30% DGDG, resulting in a very similar ratio. This ratio is higher than reported in most plants, but that could be because plants in both studies were subjected to cold acclimation [
27].
The seasonal changes in balsam fir GLs are consistent with those in
Pinus sylvestris [
29]. MGDG decreased during the winter months and increased in the spring, while DGDG increased. When membranes of the chloroplast were isolated from rye leaves, there was a decrease in MGDG and increase in DGDG due to cold acclimation [
30]. Similar results were observed in
Arabidopsis [
27]. Studies have shown that MGDG, DGDG, sulfoquinovosyl diacylglycerol (SQDG), and PG are all involved in maintaining the thylakoid structure and for the proper functioning of photosystem II and related proteins [
10,
11]. Therefore, changes in GLs were expected during cold acclimation.
MGDG and DGDG are also known to stabilize the photosystem protein complexes in chloroplasts [
31]. Maintaining a constant MGDG:DGDG ratio in thylakoid membranes (at least under standard growth conditions) seems crucial for the stability and functional integrity of photosynthetic membranes [
28]. An increase in the bilayer forming galactolipid, DGDG, and a decrease in the monolayer forming MGDG during cold acclimation should help protect the chloroplast membranes from damage during the winter keeping the membrane more fluid [
28]. Due to the reduction of light and colder temperatures associated with winter, chloroplast membrane stabilization becomes ultimately important for survival. The ratio of MGDG to DGDG may vary according to the length of cold acclimation, as the biosynthesis of these compounds is tightly regulated to meet the needs of the cell under changing environmental conditions [
27]. MGDG content decreased more than DGDG increased in this study. This can be explained by the fact that it takes two MGDG to form one DGDG [
32]. The transition of MGDG to DGDG to maintain the appropriate ratio, which allows the reversible transition from the hexagonal II to lamellar α phase of the lipid bilayer, could be a very important factor in thylakoid biogenesis [
33].
Most PLs increased during balsam fir cold acclimation, with the largest increase observed in PC. An increase in the per cent PC during cold acclimation has been reported in two other conifer species,
Pinus silvestris and
Pinus nigra [
29,
34]. As MGDG combines to form DGDG, the proportion of GL decreases while PL increases. With respect to cold acclimation, PLs are often of interest due to shifts towards longer and unsaturated fatty acid chains [
30,
35].
Although PC was predominant PL, the relative increase in PLs was also a function of PG, PE, and PI. PI and PA have been identified to play a role in cell signaling [
36]. PA is closely related to an increased activity of phospholipase D (PLD), which catalyzes the hydrolysis of PC to PA [
37]. PA in balsam fir doubled from September to October but remained a minor contributor to overall PL concentration. Some studies with evidence of PLD activity have shown an increase from below 1 to 12 % in PA in a short time [
27]. The increase in PA never exceeded 1% in balsam fir. Other PLs have been linked to a variety of stress responses. For instance, PI is the first molecule in the phosphoinositide signaling pathway [
38] and increases have also been observed in drought and salinity stress [
39]. The exact role of each PL in response to stress signaling needs to be further explored in plants, including balsam fir.
3.3. Needle Retention in Balsam Fir
Needle retention increased throughout autumn with peak retention occurring in November and December, consistent with several previous studies [
22,
23,
24,
25]. Previous studies had speculated that the improvement in needle retention was due to cold acclimation. This current study supports the hypothesis that cold acclimation improves needle retention through a significant relationship between LT50 values and needle retention. However, the relationship between cold acclimation and needle retention was weak unless genotype was considered. Needle retention is highly variable between genotypes [
25,
40], which at one point was attributed to differences in the rate at which certain genotypes acclimated to cold temperatures [
25]. Even when genotype was included in the model, the model only explained 35-36% of the variation meaning there are likely other factors significantly affecting needle retention.
A decrease in MGDG and increase in DGDG were both associated with higher needle retention in balsam fir. As with cold acclimation in general, it is possible that increased stability of membranes due to higher proportion of DGDG also protects the plant from other stresses [
28]. Membrane damage occurs prior to and during abscission in balsam fir, likely as a stress response postharvest [
26]. Thus, any mechanism that protects membrane integrity might delay abscission. Although, the relationship between MGDG:DGDG ratio and needle retention was the strongest, it still only accounted for 24% of the variation. While shifting from MGDG to DGDG seems beneficial for needle retention, there must be other factors involved.
To only account for a portion of variation in needle retention should not diminish the importance of cold acclimation and the shift towards DGDG. Abscission of balsam fir, like other plants, is a complex physiological phenomenon. Multiple factors have been associated with abscission, with the prevailing theory being that water stress is the impetus for postharvest needle abscission [
2]. Synthesis of abscisic acid and ethylene occur postharvest, transpiration and water uptake decrease, and abscission occurs [
41,
42]. Meanwhile, having a high concentration of indole-3-acetic acid can delay abscission in balsam fir [
41]. Additional factors affecting balsam fir abscission include water quality [
43], volatiles [
44], nutrition [
23], and others. With such a multitude of other factors known to influence abscission, it makes sense that a shift in polar lipids is a part of the larger puzzle.
Cold acclimation could also affect postharvest abscission through other mechanisms than polar lipid shifts. Balsam fir accumulate carbohydrates and isopentenyladenosine during autumn, which were both associated with improved needle retention [
22,
25]. Other plants have shown a variety of cold acclimation specific proteins [
45,
46], which have never been assessed in balsam fir but could be linked to needle retention. There is also an established link between ethylene and cold acclimation in other species. Ethylene was synthesized during exposure to cold and upregulated cold acclimation in
Arabidopsis; plants that could not synthesize adequate amounts of ethylene did not acclimate as well [
47]. Where short-term exposure to ethylene helped promote cold tolerance in
Arabidopsis, short-term ethylene exposure delayed abscission in balsam fir [
48]. Any link between cold acclimation, ethylene, and needle abscission in balsam fir remains to be explored.
4. Materials and Methods
4.1. Sampling and Experimental Design
The plant material for this investigation was collected from the Debert clonal tree orchard (45 44’ N, -63 50’ W) located in Debert, NS, Canada from September to January. Sampling month was an explanatory variable, with specific sampling dates as Sept. 18, Oct. 28, Nov. 25, and Dec. 30. The Christmas tree germplasm collection has over 220 genotypes of balsam fir. Previous research demonstrated considerable variation in needle retention between genotypes [
25], so genotype was used as a blocking factor. Four genotypes were selected for levels of the blocking factor. The general experimental design included an explanatory variable with 4 levels, block with 4 levels, and was then replicated 5 times.
Branches with 2 years of growth were the experimental units for this study and were cut from approximately 20-year-old trees at an elevation of approximately 1m from ground level on the south facing side of the trees. Needle samples were taken on site, immediately immersed in liquid nitrogen, and stored at –80 °C until for later lipid analysis. Branches were taken to the lab for cold tolerance and needle retention assessment.
4.2. Environmental Conditions During Sampling Months
Temperatures and photoperiod from 30 days prior to the start of the experiment were recorded from the Debert Weather Station. Photoperiod was taken on the days of sampling only. However, temperatures leading up to the sampling days were used to calculate minimum temperature (T
min), maximum temperature (T
max), days exposure to freezing temperatures, and cold degree days (CDD), which are all shown in
Table 3. CDD was determined by using the following formula:
In the above equation, T
a is the daily mean air temperature calculated from the daily minimum and maximum temperatures. T
base is the threshold temperature of 5°C determined fir as the temperature at which plant growth no longer occurs in balsam fir [
49], and i is day of the period prior to sampling, with 1 being the first day and n being the last day.
4.3. Display Conditions and Needle Retention
Branches were transferred to an environmental controlled growth chamber at a constant temperature of 20°C and light intensity of 100µmol m
-2 s
-1. Light was supplied as a combination of incandescent and fluorescent. Both temperature and light conditions were selected to simulate household conditions as described by [
42]. Once in the lab, branches were given a fresh aseptic cut 2.5 cm above the previous cut while submerged in water to reduce risk of cavitation. Branches were placed into amber bottles and provided 100 mL of distilled water. The neck of each flask was plugged with cotton wool to reduce direct water evaporation and provide added stability to a branch.
Needle retention was evaluated by lightly holding the branch between thumb and index finger and sliding along the length of the branch. Care was taken to slide in the same direction as the needles were attached to the stem. The goal was to dislodge needles that had abscised and not to snap off needles that were healthy. Needles were dried at 80°C overnight and then weighed. This procedure was repeated every 2 days until complete needle shed. The day on which a branch lost 100% of its needles was referred to as its NRD.
4.4. Freeze Testing to Evaluate Cold Tolerance
Cold tolerance was assessed at 5 temperatures: -5, -15, -25, -35, and -45°C. The temperature range was determined partially by the typical range of regional winter temperatures as well as cold tolerance estimates from other
Abies species [
20,
21]. The freeze test protocol was based on previous work by [
50]. Branches were placed in a programmable freezer (Thermotron SM-32-C, Holland, MI) and the temperature was reduced 5°C h
-1 until reaching the target temperature. Branches were exposed to the target temperatures for 30 minutes, held at 0°C overnight, and then returned to the growth chamber until needles reached 20°C for evaluation of membrane injury and chlorophyll fluorescence.
Chlorophyll fluorescence (F
v/F
m), was determined using a MINI-PAM-II Photosynthesis Yield Analyzer (Heinz Walz, Effeltrich, Germany) based on [
24]. Plants were dark-adapted for 20 min before fluorescence measurements with a saturating light pulse of 8000µmol m
-2 s
-1. Three measurements were taken for each experimental unit; the average of these measurements was recorded as the F
v/F
m for each branch. Chlorophyll fluorescence was measured prior to freezing test to establish a baseline for fluorescence in each branch. Chlorophyll fluorescence was measured again after the freezing test.
Membrane injury was determined from the percentage of electrolytes leaked into solution versus the total electrolytes present. Test tubes were filled with 30 mL of distilled water and were allowed to adjust to room temperature (20 °C). The electrical conductivity of the distilled water (EC
w) alone was measured using a CDM 2e Conductivity Meter (Bach-Simpson, London, ON). Afterward, approximately 0.4 g of needles were removed from each branch and completely submerged in a centrifuge tube. The tubes were sealed and left at room temperature for 24 h. Initial conductivity (EC
0) was measured to determine electrolytes leaching into solution. Sealed tubes were then placed in a forced-air oven for 4 h at 90 °C to kill tissues and then cooled to room temperature. Final conductivity measurements (EC
f) were taken after equilibrating to 20 °C to determine maximum leakage. Membrane injury was then calculated using the following equation [
51]:
LT50 values were calculated from both chlorophyll fluorescence and membrane injury by fitting a sigmoidal curve to freeze test changes for each branch. From those sigmoidal curves, LT50-MI was the temperature at which balsam fir would have 50% membrane injury and LT50-CG was the temperature at which balsam fir would lose 50% of its initial chlorophyll fluorescence.
4.5. Lipid Extraction and Analysis
All lipids were extracted at the Kansas Lipidomic Research Center (Manhattan, KS, USA) using an extraction protocol for
Arabidopsis leaf tissue adapted from [
52]. Approximately 1g of frozen needles were cut into smaller pieces and incubated in 1.0mL of isopropanol with 0.01% butylated hydroxytoluene (BHT) at 75°C for 15min. Afterwards, 1.5mL of chloroform and 0.6mL of water were added to the solution. The solvent was shaken at room temperature for 1h and then transferred to a new glass tube with a Teflon-lined screw-cap using a Pasteur pipette. A total of 0.7 mL chloroform:methanol (2:1) was added, shaken for 30 min. The extraction was completed by adding 4 mL of chloroform: methanol (2:1) ten times, shaking for 1h and collecting the solvent. The solvent extracts were washed once with 1mL KCl (1.0M) and once with 0.66mL water. The solvent was evaporated under nitrogen and the lipid extract was quantified and dissolved in 1.0mL chloroform. The tissues, after lipid extraction, were dried in an oven at 105°C and dry weights were determined (3 –20mg).
Lipid extracts from were analyzed on a triple quadrupole mass spectrometer equipped for electrospray ionization (ESI–MS/MS; Applied Biosystems API 4000). Acquisition and ESI-MS/MS analysis parameters and are shown in
Table 4 and
Table 5, respectively. The lipids in each class were quantified in comparison to two internal standards of the class. Lipid species within each head group were identified by total carbon number and total double bonds. The molecular species of each head class were quantified by comparing with the signals of the internal standards [
27].
Average coefficient of variation (CoV) for lipid analytes is a function of corrections used in data processing. CoV is equal to the standard deviation of the measurements for each analyte divided by the average. CoV was calculated from the quality check samples, without any correction, using only the linear trend correction within each day's sample set, only the correction to the overall average across sample sets, or both corrections (as done on the experimental data).
4.6. Statistical Analysis
Freeze tests with membrane injury and chlorophyll fluorescence were analyzed using an analysis of variance in Minitab 19 software (Minitab, LLC., Pennsylvania State College, PA). Temperature (5 levels) and collection month (4 levels) were used an explanatory variables and genotype (4 levels) was used as a blocking factor. Analysis LT50 values, NRD, and lipids was also conducted using an analysis of variance with collection month as the only explanatory variable and genotype as the blocking factor. The relationships between response variables were assessed using linear regression. Genotype was included as a blocking variable in a regression model when it accounted for a significant amount of variation. Statistical assumption of normality, homogeneity, and independence were verified for each analysis.
5. Conclusions
This experiment was able to address each of the original objectives. Cold acclimation was confirmed in balsam fir, with trees able to tolerate colder temperatures throughout autumn assessed through decreased membrane injury and maintenance of chlorophyll fluorescence. LT50 values decreased from approximately -23°C in September to approximately -46°C in December. Polar lipids changed during this cold acclimation, with a significant shift from MGDG to DGDG and a shift from GLs to PLs. The highest increase of PLs was observed in PC, though most other PLs also increased significantly. Changes in lipids were significantly related to cold acclimation.
Needle retention improved throughout cold acclimation. Needle retention was also significantly related to cold tolerance and MGDG:DGDG. In general, higher cold acclimation or a shift from MDGD to DGDG also increased needle retention. Overall, it was concluded the cold acclimation is beneficial to needle retention possibly through increased stability of membranes from the increase in relative DGDG. The mechanism through which cold acclimation affects needle retention requires further work and factors beyond polar lipid shifts should also be considered moving forward.
Author Contributions
Conceptualization, all authors.; methodology, all authors.; software, M.M and G.M..; validation, G.M..; formal analysis, M.M. and G.M..; investigation, G.M..; resources, L.R..; data curation, M.M and G.M..; writing—original draft preparation, M.M.; writing—review and editing, all authors.; visualization, M.M.; supervision, R.L., C.C., C.U., M.M.; project administration, R.L..; funding acquisition, R.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by NSF grants MCB 0455318, MCB 092063, DBI 0521587, DBI 1228622, Kansas INBRE (NIH Grant P20 RR16475 from the INBRE program from the National Center for Research Resources, NSF EPSCoR grant EPS-0236913, Kansas Technology Enterprise Corporation, and Kansas State University
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data is contained within the article.
Acknowledgments
We thank the Nova Scotia Department of Natural resources for access to the clonal balsam fir orchard in Debert, NS. We also thank the Kansas Lipidomic Research Center at Kansas State University for assistance in lipid analysis.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Collier, J.; MacLean, D.A.; D’Orangeville, L.; Taylor, A.R. A Review of Climate Change Effects on the Regeneration Dynamics of Balsam Fir. The Forestry Chronicle 2022, 98, 54–65. [Google Scholar] [CrossRef]
- Lada, R.R.; MacDonald, M.T. Understanding the Physiology of Postharvest Needle Abscission in Balsam Fir. Front. Plant Sci. 2015, 6. [Google Scholar] [CrossRef] [PubMed]
- Thiagarajan, A.; MacDonald, M.T.; Lada, R. Environmental and Hormonal Physiology of Postharvest Needle Abscission in Christmas Trees. Critical Reviews in Plant Sciences 2016, 35, 1–17. [Google Scholar] [CrossRef]
- Beck, E.H.; Fettig, S.; Knake, C.; Hartig, K.; Bhattarai, T. Specific and Unspecific Responses of Plants to Cold and Drought Stress. J Biosci 2007, 32, 501–510. [Google Scholar] [CrossRef] [PubMed]
- Thakur, P.; Kumar, S.; Malik, J.A.; Berger, J.D.; Nayyar, H. Cold Stress Effects on Reproductive Development in Grain Crops: An Overview. Environmental and Experimental Botany 2010, 67, 429–443. [Google Scholar] [CrossRef]
- MacDonald, G.E.; Lada, R.R.; Caldwell, C.D.; Udenigwe, C.; MacDonald, M.T. Potential Roles of Fatty Acids and Lipids in Postharvest Needle Abscission Physiology. AJPS 2019, 10, 1069–1089. [Google Scholar] [CrossRef]
- Chang, C.Y.; Bräutigam, K.; Hüner, N.P.A.; Ensminger, I. Champions of Winter Survival: Cold Acclimation and Molecular Regulation of Cold Hardiness in Evergreen Conifers. New Phytologist 2021, 229, 675–691. [Google Scholar] [CrossRef]
- Vogg, G.; Heim, R.; Gotschy, B.; Beck, E.; Hansen, J. Frost Hardening and Photosynthetic Performance of Scots Pine (Pinus Sylvestris, L.). II. Seasonal Changes in the Fluidity of Thylakoid Membranes. Planta 1998, 204, 201–206. [Google Scholar] [CrossRef]
- Crosatti, C.; Rizza, F.; Badeck, F.W.; Mazzucotelli, E.; Cattivelli, L. Harden the Chloroplast to Protect the Plant. Physiologia Plantarum 2013, 147, 55–63. [Google Scholar] [CrossRef]
- Ivanova, A.P.; Stefanov, K.L.; Yordanov, I.T. Effect of Cytokinin 4-PU-30 on the Lipid Composition of Water Stressed Bean Plants. Biol. Plant. 1998, 41, 255–159. [Google Scholar] [CrossRef]
- Degenkolbe, T.; Giavalisco, P.; Zuther, E.; Seiwert, B.; Hincha, D.K.; Willmitzer, L. Differential Remodeling of the Lipidome during Cold Acclimation in Natural Accessions of Arabidopsis Thaliana: Lipidomics of Arabidopsis Cold Acclimation. The Plant Journal 2012, 72, 972–982. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, S.; Uemura, M. Protein and Lipid Compositions of Isolated Plasma Membranes from Orchard Grass ( Dactylis Glomerata L.) and Changes during Cold Acclimation. Plant Physiol. 1984, 75, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Nokhsorov, V.V.; Dudareva, L.V.; Senik, S.V.; Chirikova, N.K.; Petrov, K.A. Influence of Extremely Low Temperatures of the Pole of Cold on the Lipid and Fatty-Acid Composition of Aerial Parts of the Horsetail Family (Equisetaceae). Plants 2021, 10, 996. [Google Scholar] [CrossRef] [PubMed]
- MacDonald, G.E.; Lada, R.R.; Caldwell, C.D.; Udenigwe, C.C.; MacDonald, M.T. Linking Changes in Fatty Acid Composition to Postharvest Needle Abscission Resistance in Balsam Fir Trees. Forests 2022, 13, 800. [Google Scholar] [CrossRef]
- Atucha Zamkova, A.-A.; Steele, K.A.; Smith, A.R. Methods for Measuring Frost Tolerance of Conifers: A Systematic Map. Forests 2021, 12, 1094. [Google Scholar] [CrossRef]
- Murray, M.B.; Cape, J.N.; Fowler, D. Quantification of Frost Damage in Plant Tissues by Rates of Electrolyte Leakage. New Phytologist 1989, 113, 307–311. [Google Scholar] [CrossRef]
- Bachofen, C.; Wohlgemuth, T.; Ghazoul, J.; Moser, B. Cold Temperature Extremes during Spring Do Not Limit the Range Shift of Mediterranean Pines into Regions with Intermittent Frost. Functional Ecology 2016, 30, 856–865. [Google Scholar] [CrossRef]
- Adams, G.T.; Perkins, T.D. Assessing Cold Tolerance in Picea Using Chlorophyll Fluorescence. Environmental and Experimental Botany 1993, 33, 377–382. [Google Scholar] [CrossRef]
- Perks, M.P.; Osborne, B.A.; Mitchell, D.T. Rapid Predictions of Cold Tolerance in Douglas-Fir Seedlings Using Chlorophyll Fluorescence after Freezing. New Forests 2004, 28, 49–62. [Google Scholar] [CrossRef]
- Sakai, A. Comparative Study on Freezing Resistance of Conifers with Special Reference to Cold Adaptation and Its Evolutive Aspects. Can. J. Bot. 1983, 61, 2323–2332. [Google Scholar] [CrossRef]
- Norgaard Nielsen, C.C.; Rasmussen, H.N. Frost Hardening and Dehardening in Abies Procera and Other Conifers under Differing Temperature Regimes and Warm-Spell Treatments. Forestry 2009, 82, 43–59. [Google Scholar] [CrossRef]
- MacDonald, M.T.; Lada, R.R. Changes in Endogenous Hormone Levels Explains Seasonal Variation in Balsam Fir Needle Abscission Patterns. J Plant Growth Regul 2017, 36, 723–733. [Google Scholar] [CrossRef]
- MacDonald, M.T.; Lada, R.R. Seasonal Changes in Soil and Tissue Nutrition in Balsam Fir and Influence on Postharvest Needle Abscission. Scandinavian Journal of Forest Research 2018, 33, 426–436. [Google Scholar] [CrossRef]
- MacDonald, M.T.; Lada, R.R.; Veitch, R.S. Seasonal Changes in Balsam Fir Needle Abscission Patterns and Links to Environmental Factors. Scandinavian Journal of Forest Research 2017, 32, 438–445. [Google Scholar] [CrossRef]
- MacDonald, M.T.; Lada, R.R.; Veitch, R.S.; Thiagarajan, A.; Adams, A.D. Postharvest Needle Abscission Resistance of Balsam Fir ( Abies Balsamea ) Is Modified by Harvest Date. Can. J. For. Res. 2014, 44, 1394–1401. [Google Scholar] [CrossRef]
- MacDonald, G.E.; Lada, R.R.; Caldwell, C.D.; Udenigwe, C.; MacDonald, M. Lipid and Fatty Acid Changes Linked to Postharvest Needle Abscission in Balsam Fir, Abies Balsamea. Trees 2020, 34, 297–305. [Google Scholar] [CrossRef]
- Welti, R.; Li, W.; Li, M.; Sang, Y.; Biesiada, H.; Zhou, H.-E.; Rajashekar, C.B.; Williams, T.D.; Wang, X. Profiling Membrane Lipids in Plant Stress Responses. Journal of Biological Chemistry 2002, 277, 31994–32002. [Google Scholar] [CrossRef] [PubMed]
- Dörmann, P.; Benning, C. Galactolipids Rule in Seed Plants. Trends in Plant Science 2002, 7, 112–118. [Google Scholar] [CrossRef]
- Oquist, G. Seasonally Induced Changes in Acyl Lipids and Fatty Acids of Chloroplast Thylakoids of Pinus Silvestris: A Correlation between the Level of Unsaturation of Monogalactosyldiglyceride and the Rate of Electron Transport. Plant Physiol. 1982, 69, 869–875. [Google Scholar] [CrossRef]
- Uemura, M.; Steponkus, P.L. Effect of Cold Acclimation on the Lipid Composition of the Lnner and Outer Membrane of the Chloroplast Envelope Lsolated from Rye Leaves’. Plant Physiol. 1997, 114, 1493–1500. [Google Scholar] [CrossRef]
- Sakurai, I.; Mizusawa, N.; Wada, H.; Sato, N. Digalactosyldiacylglycerol Is Required for Stabilization of the Oxygen-Evolving Complex in Photosystem II. Plant Physiol. 2007, 145, 1361–1370. [Google Scholar] [CrossRef] [PubMed]
- Heemskerk, J.W.M.; Storz, T.; Schmidt, R.R.; Heinz, E. Biosynthesis of Digalactosyldiacylglycerol in Plastids from 16:3 and 18:3 Plants. Plant Physiol. 1990, 93, 1286–1294. [Google Scholar] [CrossRef] [PubMed]
- Rocha, J.; Nitenberg, M.; Girard-Egrot, A.; Jouhet, J.; Maréchal, E.; Block, M.A.; Breton, C. Do Galactolipid Synthases Play a Key Role in the Biogenesis of Chloroplast Membranes of Higher Plants? Front. Plant Sci. 2018, 9, 126. [Google Scholar] [CrossRef] [PubMed]
- Kojima, M.; Shiraki, H.; Ohnishi, N.; Ito, S. Seasonal Change Sin Glycolipids and Phospholipids in Pinus Nigra Needles. Research Bulletin of Obihiro 17, 13–19.
- Orlova, I.V.; Serebriiskaya, T.S.; Popov, V.; Merkulova, N.; Nosov, A.M.; Trunova, T.I.; Tsydendambaev, V.D.; Los, D.A. Transformation of Tobacco with a Gene for the Thermophilic Acyl-Lipid Desaturase Enhances the Chilling Tolerance of Plants. Plant Cell Physiol. 2003, 44, 447–450. [Google Scholar] [CrossRef]
- Van Meer, G.; Voelker, D.R.; Feigenson, G.W. Membrane Lipids: Where They Are and How They Behave. Nat. Rev. Mol. Cell. Biol. 2008, 9, 112–124. [Google Scholar] [CrossRef]
- Selvy, P.E.; Lavieri, R.R.; Lindsley, C.W.; Brown, H.A. Phospholipase D: Enzymology, Functionality, and Chemical Modulation. Chem. Rev. 2011, 111, 6064–6119. [Google Scholar] [CrossRef]
- Hou, Q.; Ufer, G.; Bartels, D. Lipid Signalling in Plant Responses to Abiotic Stress: Lipid Signalling in Plant Responses to Abiotic Stress. Plant Cell Environ. 2016, 39, 1029–1048. [Google Scholar] [CrossRef]
- Kiełbowicz-Matuk, A.; Banachowicz, E.; Turska-Tarska, A.; Rey, P.; Rorat, T. Expression and Characterization of a Barley Phosphatidylinositol Transfer Protein Structurally Homologous to the Yeast Sec14p Protein. Plant Science 2016, 246, 98–111. [Google Scholar] [CrossRef]
- MacDonald, M.T.; Lada, R.R.; Martynenko, A.I.; Pepin, S.; Desjardins, Y.; Dorais, M. Is There a Relationship between Ethylene Evolution, Ethylene Sensitivity, and Needle Abscission in Root-Detached Balsam Fir? Acta Hortic. 2012, 405–411. [Google Scholar] [CrossRef]
- MacDonald, M.T.; Lada, R.R. Biophysical and Hormonal Changes Linked to Postharvest Needle Abscission in Balsam Fir. J Plant Growth Regul 2014, 33, 602–611. [Google Scholar] [CrossRef]
- MacDonald, M.T.; Lada, R.R.; Dorais, M.; Pepin, S. Endogenous and Exogenous Ethylene Induces Needle Abscission and Cellulase Activity in Post-Harvest Balsam Fir (Abies Balsamea L.). Trees 2011, 25, 947–952. [Google Scholar] [CrossRef]
- Lada, R.R.; MacDonald, M.T.; West, R.R. Physiology of Postharvest Needle Abscission in Balsam Fir: Water Quality Modulates Postharvest Needle Abscission. Acta Hortic. 2016, 111–120. [Google Scholar] [CrossRef]
- Korankye, E.A.; Lada, R.R.; Asiedu, S.K.; Caldwell, C. Mechanical Shaking and Baling of Balsam Fir Trees Influence Postharvest Needle Senescence and Abscission. AJPS 2018, 09, 339–352. [Google Scholar] [CrossRef]
- Mohapatra, S.S.; Wolfraim, L.; Poole, R.J.; Dhindsa, R.S. Molecular Cloning and Relationship to Freezing Tolerance of Cold-Acclimation-Specific Genes of Alfalfa. Plant Physiol. 1989, 89, 375–380. [Google Scholar] [CrossRef]
- Pennycooke, J.C.; Cheng, H.; Stockinger, E.J. Comparative Genomic Sequence and Expression Analyses of Medicago Truncatula and Alfalfa Subspecies Falcata Cold-Acclimation-Specific Genes. Plant Physiol. 2008, 146, 1242–1254. [Google Scholar] [CrossRef]
- Catalá, R.; López-Cobollo, R.; Mar Castellano, M.; Angosto, T.; Alonso, J.M.; Ecker, J.R.; Salinas, J. The Arabidopsis 14-3-3 Protein RARE COLD INDUCIBLE 1A Links Low-Temperature Response and Ethylene Biosynthesis to Regulate Freezing Tolerance and Cold Acclimation. The Plant Cell 2014, 26, 3326–3342. [Google Scholar] [CrossRef]
- MacDonald, M.T.; Lada, R.R.; Martynenko, A.I.; Dorais, M.; Pepin, S.; Desjardins, Y. Ethylene Exposure Duration Affects Postharvest Needle Abscission in Balsam Fir (Abies Balsamea L.). HortScience 2011, 46, 260–264. [Google Scholar] [CrossRef]
- Hassan, Q.K. Spatial Mapping of Growing Degree Days: An Application of MODIS-Based Surface Temperatures and Enhanced Vegetation Index. J. Appl. Remote Sens 2007, 1, 013511. [Google Scholar] [CrossRef]
- Man, R.; Lu, P.; Dang, Q.-L. Cold Tolerance of Black Spruce, White Spruce, Jack Pine, and Lodgepole Pine Seedlings at Different Stages of Spring Dehardening. New Forests 2021, 52, 317–328. [Google Scholar] [CrossRef]
- Odlum, K.D.; Blake, T.J. A Comparison of Analytical Approaches for Assessing Freezing Damage in Black Spruce Using Electrolyte Leakage Methods. Can. J. Bot. 1996, 74, 952–958. [Google Scholar] [CrossRef]
- Bligh, E.G.; Dyer, W.J. A Rapid Method of Total Lipid Extraction and Purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef] [PubMed]
-
Plant Lipid Signaling Protocols; Munnik, T., Heilmann, I., Eds.; Methods in Molecular Biology; Humana Press: Totowa, NJ, 2013; Vol. 1009, ISBN 978-1-62703-400-5. [Google Scholar]
- Brügger, B.; Erben, G.; Sandhoff, R.; Wieland, F.T.; Lehmann, W.D. Quantitative Analysis of Biological Membrane Lipids at the Low Picomole Level by Nano-Electrospray Ionization Tandem Mass Spectrometry. Proc. Natl. Acad. Sci. U.S.A. 1997, 94, 2339–2344. [Google Scholar] [CrossRef]
- Taguchi, R.; Houjou, T.; Nakanishi, H.; Yamazaki, T.; Ishida, M.; Imagawa, M.; Shimizu, T. Focused Lipidomics by Tandem Mass Spectrometry. Journal of Chromatography B 2005, 823, 26–36. [Google Scholar] [CrossRef] [PubMed]
- Isaac, G.; Jeannotte, R.; Esch, S.; Welti, R. New Mass Spectrometry-Based Strategies for Lipids. In Genetic Engineering; Springer: Boston, MA, 2007; Vol. 28. [Google Scholar] [CrossRef]
Figure 1.
Membrane injury of balsam fir branches collected September to December then subjected to freeze tests at -5, -15, -25, -35, and -45°C. Each data point is a mean ± standard error as calculated from 20 replicates.
Figure 1.
Membrane injury of balsam fir branches collected September to December then subjected to freeze tests at -5, -15, -25, -35, and -45°C. Each data point is a mean ± standard error as calculated from 20 replicates.
Figure 2.
Chlorophyll fluorescence (Fv/Fm) of balsam fir branches collected September to December then subjected to freeze tests at -5, -15, -25, -35, and -45°C. Chlorophyll fluorescence is expressed as a percentage of the baseline values established by measuring branches that were not exposed to any freezing test. Each data point is a mean ± standard error as calculated from 20 replicates.
Figure 2.
Chlorophyll fluorescence (Fv/Fm) of balsam fir branches collected September to December then subjected to freeze tests at -5, -15, -25, -35, and -45°C. Chlorophyll fluorescence is expressed as a percentage of the baseline values established by measuring branches that were not exposed to any freezing test. Each data point is a mean ± standard error as calculated from 20 replicates.
Figure 3.
Linear relationship between LT50-MI and LT50-CF (N = 80).
Figure 3.
Linear relationship between LT50-MI and LT50-CF (N = 80).
Figure 4.
NRD and LT50 values estimated from membrane injury (LT50-MI) and chlorophyll fluorescence (LT50-CF) from balsam fir branches collected September to December. Bars represent mean ± standard error as calculated from 20 replicates.
Figure 4.
NRD and LT50 values estimated from membrane injury (LT50-MI) and chlorophyll fluorescence (LT50-CF) from balsam fir branches collected September to December. Bars represent mean ± standard error as calculated from 20 replicates.
Figure 5.
Ratio of (a) monogalactosyldiacylglycerol to digalactosyldiacylglycerol and (b) galactolipids to phospholipids in balsam fir co/llected from September to December. Each data point is a mean ± standard error as calculated from 20 replicates.
Figure 5.
Ratio of (a) monogalactosyldiacylglycerol to digalactosyldiacylglycerol and (b) galactolipids to phospholipids in balsam fir co/llected from September to December. Each data point is a mean ± standard error as calculated from 20 replicates.
Figure 6.
Relationships of (a) monogalactosyldiacylglycerol to digalactosyldiacylglycerol ratio and (b) galactolipid to phospholipid ratio to LT50 values in balsam fir collected from September to December. N = 80.
Figure 6.
Relationships of (a) monogalactosyldiacylglycerol to digalactosyldiacylglycerol ratio and (b) galactolipid to phospholipid ratio to LT50 values in balsam fir collected from September to December. N = 80.
Figure 7.
Relationships of (a) digalactosyldiacylglycerol, (b) monogalactosyldiacylglycerol, and (c) monogalactosyldiacylglycerol to digalactosyldiacylglycerol ratio to needle retention duration (NRD) in balsam fir collected September to December. N = 80 in each.
Figure 7.
Relationships of (a) digalactosyldiacylglycerol, (b) monogalactosyldiacylglycerol, and (c) monogalactosyldiacylglycerol to digalactosyldiacylglycerol ratio to needle retention duration (NRD) in balsam fir collected September to December. N = 80 in each.
Table 1.
Comparison of lipid classes by percentage of total lipids for balsam fir branches harvested at five different months. Values are expressed as mean ± standard error as calculated from 20 replicates. The P-value denotes whether there was a significant difference in at least one of the sampling dates for each class of lipids. DGDG, digalactosyldiacylglycerol; LPG, lysophosphatidylglycerol; LPE, lysophosphatidylethanolamine; LPC, lysophosphatidylcholine; MGDG, monogalactosyldiacylglycerol; PA, phosphatidic acid; PC, phosphatidycholine; PE: phoshatidylethanolamine; PG, phosphatidylglycerol; PI, phosphainositol.
Table 1.
Comparison of lipid classes by percentage of total lipids for balsam fir branches harvested at five different months. Values are expressed as mean ± standard error as calculated from 20 replicates. The P-value denotes whether there was a significant difference in at least one of the sampling dates for each class of lipids. DGDG, digalactosyldiacylglycerol; LPG, lysophosphatidylglycerol; LPE, lysophosphatidylethanolamine; LPC, lysophosphatidylcholine; MGDG, monogalactosyldiacylglycerol; PA, phosphatidic acid; PC, phosphatidycholine; PE: phoshatidylethanolamine; PG, phosphatidylglycerol; PI, phosphainositol.
Lipid
Class |
Sept. |
Oct. |
Nov. |
Dec. |
P-value |
| DGDG |
26.87 |
± |
0.36 |
27.10 |
± |
0.43 |
28.33 |
± |
0.38 |
30.04 |
± |
0.37 |
< 0.001 |
| MGDG |
45.90 |
± |
0.87 |
40.98 |
± |
1.17 |
38.37 |
± |
0.81 |
33.86 |
± |
0.58 |
< 0.001 |
| PC |
15.01 |
± |
0.39 |
18.12 |
± |
0.61 |
18.48 |
± |
0.44 |
19.56 |
± |
0.40 |
< 0.001 |
| PG |
4.04 |
± |
0.20 |
3.89 |
± |
0.16 |
3.81 |
± |
0.15 |
5.11 |
± |
0.15 |
< 0.001 |
| PE |
2.62 |
± |
0.17 |
3.74 |
± |
0.18 |
3.75 |
± |
0.21 |
4.76 |
± |
0.24 |
< 0.001 |
| PI |
3.86 |
± |
0.15 |
3.99 |
± |
0.16 |
4.64 |
± |
0.17 |
4.75 |
± |
0.11 |
< 0.001 |
| PA |
0.47 |
± |
0.06 |
0.93 |
± |
0.09 |
1.19 |
± |
0.34 |
0.66 |
± |
0.05 |
= 0.049 |
| LPC |
0.05 |
± |
0.01 |
0.12 |
± |
0.02 |
0.14 |
± |
0.01 |
0.09 |
± |
0.01 |
= 0.041 |
| LPG |
1.05 |
± |
0.19 |
0.96 |
± |
0.15 |
1.14 |
± |
0.15 |
1.00 |
± |
0.20 |
= 0.344 |
| LPE |
0.11 |
± |
0.01 |
0.15 |
± |
0.01 |
0.14 |
± |
0.01 |
0.14 |
± |
0.01 |
= 0.071 |
Table 2.
Strength, significance, and linear equation of relationships between relative polar lipid concentration and LT50 values. LT50 were estimated from membrane injury (LT50-MI) and chlorophyll fluorescence (LT50-CF). Regression calculated from 80 data points using LT50 as the dependent variable and relative lipid concentration as the independent variable. DGDG, digalactosyldiacylglycerol; LPG, lysophosphatidylglycerol; LPE, lysophosphatidylethanolamine; LPC, lysophosphatidylcholine; MGDG, monogalactosyldiacylglycerol; PA, phosphatidic acid; PC, phosphatidycholine; PE: phoshatidylethanolamine; PG, phosphatidylglycerol; PI, phosphainositol.
Table 2.
Strength, significance, and linear equation of relationships between relative polar lipid concentration and LT50 values. LT50 were estimated from membrane injury (LT50-MI) and chlorophyll fluorescence (LT50-CF). Regression calculated from 80 data points using LT50 as the dependent variable and relative lipid concentration as the independent variable. DGDG, digalactosyldiacylglycerol; LPG, lysophosphatidylglycerol; LPE, lysophosphatidylethanolamine; LPC, lysophosphatidylcholine; MGDG, monogalactosyldiacylglycerol; PA, phosphatidic acid; PC, phosphatidycholine; PE: phoshatidylethanolamine; PG, phosphatidylglycerol; PI, phosphainositol.
| Lipid Class |
LT50-MI |
LT50-CF |
| R2 (%) |
P-value |
Slope |
Constant |
|
R2 (%) |
P-value |
Slope |
Constant |
| DGDG |
31.5 |
< 0.001 |
-2.23 |
25.8 |
|
29.5 |
< 0.001 |
-1.87 |
21.3 |
| MGDG |
55.0 |
< 0.001 |
1.10 |
-80.8 |
|
42.7 |
< 0.001 |
0.86 |
-65.6 |
| PC |
36.7 |
< 0.001 |
-1.86 |
-3.80 |
|
26.0 |
< 0.001 |
-1.36 |
-7.01 |
| PG |
15.2 |
= 0.001 |
-3.86 |
-20.6 |
|
16.6 |
= 0.008 |
-3.43 |
-16.8 |
| PE |
35.4 |
< 0.001 |
-4.40 |
-20.6 |
|
27.7 |
< 0.001 |
-3.41 |
-18.6 |
| PI |
30.0 |
< 0.001 |
-6.13 |
-10.5 |
|
22.9 |
< 0.001 |
-4.56 |
-11.6 |
| PA |
2.5 |
= 0.180 |
-1.65 |
-35.5 |
|
3.4 |
= 0.411 |
-0.73 |
-30.7 |
| LPC |
5.1 |
= 0.060 |
-29.0 |
-34.0 |
|
7.8 |
= 0.193 |
24.2 |
-28.85 |
| LPG |
0.7 |
= 0.498 |
-0.69 |
-36.2 |
|
3.4 |
= 0.399 |
-1.10 |
-30.1 |
| LPE |
3.1 |
= 0.130 |
-30.3 |
-32.8 |
|
4.6 |
= 0.208 |
-28.4 |
-27.5 |
Table 3.
Temperature parameters 30 days prior to first sampling periods and between each sampling time. Temperature related parameters are CDD (cold degree days), Tmin (minimum temperature in the days prior to testing), Tmax (highest temperature experienced in the month prior and since the last sampling date), and cumulative days below 0°C. Photoperiod represents the daylight hours on the day of sampling.
Table 3.
Temperature parameters 30 days prior to first sampling periods and between each sampling time. Temperature related parameters are CDD (cold degree days), Tmin (minimum temperature in the days prior to testing), Tmax (highest temperature experienced in the month prior and since the last sampling date), and cumulative days below 0°C. Photoperiod represents the daylight hours on the day of sampling.
| Sampling Date |
CDD
(days) |
Tmin
(°C) |
Tmax
(°C) |
Days Below
0°C |
Photoperiod
(h) |
| Sept. 18 |
0 |
5 |
21 |
0 |
12.4 |
| Oct. 28 |
9 |
-4 |
18 |
7 |
10.3 |
| Nov. 25 |
102 |
-8 |
17 |
28 |
9.2 |
| Dec. 30 |
354 |
-23 |
8.3 |
60 |
8.8 |
Table 4.
Acquisition parameters of lipid classes using Applied Biosystems API 4000, triple quadrupole mass spectrometer.
Table 4.
Acquisition parameters of lipid classes using Applied Biosystems API 4000, triple quadrupole mass spectrometer.
| Parameter |
|
PA |
PC/LPC |
PE/LPE |
PG |
PI |
PS |
DGDG |
MGDG |
| Typical Scan Time (min) |
|
3.51 |
1.28 |
3.34 |
3.21 |
4.00 |
4.01 |
1.67 |
1.67 |
| Depolarization Potential (V) |
|
100 |
100 |
100 |
100 |
100 |
100 |
90 |
90 |
| Exit Potential (V) |
|
14 |
14 |
14 |
14 |
14 |
14 |
10 |
10 |
| Collision Energy (V) |
|
25 |
40 |
28 |
20 |
25 |
26 |
24 |
21 |
| Collision Exit Potential (V) |
|
14 |
14 |
14 |
14 |
14 |
14 |
23 |
23 |
Table 5.
ESI-MS/MS analysis parameters (using Applied Biosystems API 4000) for plant lipids. DGDG, digalactosyldiacylglycerol; ESI-MS/MS, electrospray ionization tandem mass spectrometry; MGDG, monogalactosyldiacylglycerol; PA, phosphatidic acid; PC, phosphatidylcholine; LPC, lyso- phosphatidylcholine; PE, phosphatidylethanolamine; LPE, lysophosphatidylethanolamine; PG, phosphatidylglycerol; LPG, lysophosphatidylglycerol; PI, phosphatidylinositol; PS, phosphatidylserine.
Table 5.
ESI-MS/MS analysis parameters (using Applied Biosystems API 4000) for plant lipids. DGDG, digalactosyldiacylglycerol; ESI-MS/MS, electrospray ionization tandem mass spectrometry; MGDG, monogalactosyldiacylglycerol; PA, phosphatidic acid; PC, phosphatidylcholine; LPC, lyso- phosphatidylcholine; PE, phosphatidylethanolamine; LPE, lysophosphatidylethanolamine; PG, phosphatidylglycerol; LPG, lysophosphatidylglycerol; PI, phosphatidylinositol; PS, phosphatidylserine.
| Class |
Ion Analyzed |
Positive Ion Scan Mode |
m/z range |
Reference |
| PA |
(M + NH4) -
|
NL of 115.00 |
500 – 850 |
[53] |
| PC/LPC |
(M + H) -
|
Pre of m/z 184.07 |
450 – 960 |
[54] |
| PE/LPE |
(M + H) -
|
NL of 141.02 |
420 – 920 |
[54] |
| PG |
(M + NH4) -
|
NL of 189.04 |
650 – 1,000 |
[55] |
| LPG |
(M-H) -
|
Prec 153 |
- |
[27] |
| PI |
(M + NH4) -
|
NL of 277.06 |
790 – 950 |
[55] |
| PS |
(M + H) -
|
NL of 185.01 |
600 – 920 |
[54] |
| DGDG |
(M + NH4) -
|
NL of 341.13 |
890 – 1,050 |
[56] |
| MGDG |
(M + NH4) -
|
NL of 179.08 |
700 – 900 |
[56] |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).