Submitted:
30 September 2023
Posted:
01 October 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Material design and contact lenses fabrication
2.1. Main Materials used in contact lenses fabrication
2.2. Manufacturing methods
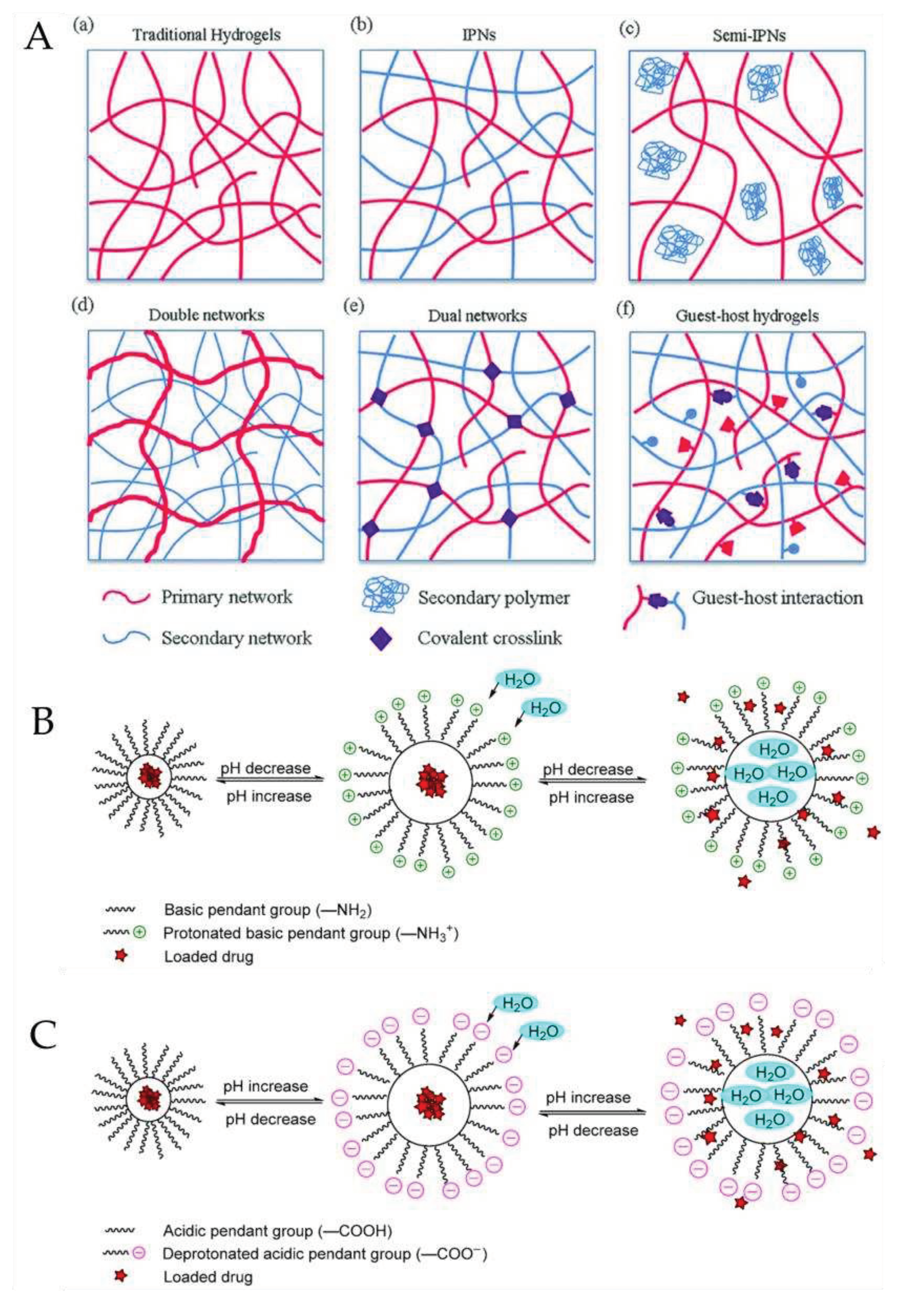
3. Embodying bioactive molecules into contact lenses
3.1. Soaking Method
3.2. Incorporation of Functional Molecules within Contact Lenses
3.3. Molecular Imprinted Contact Lenses
3.4. Supercritical fluid method
3.5. Colloidal nanoparticles
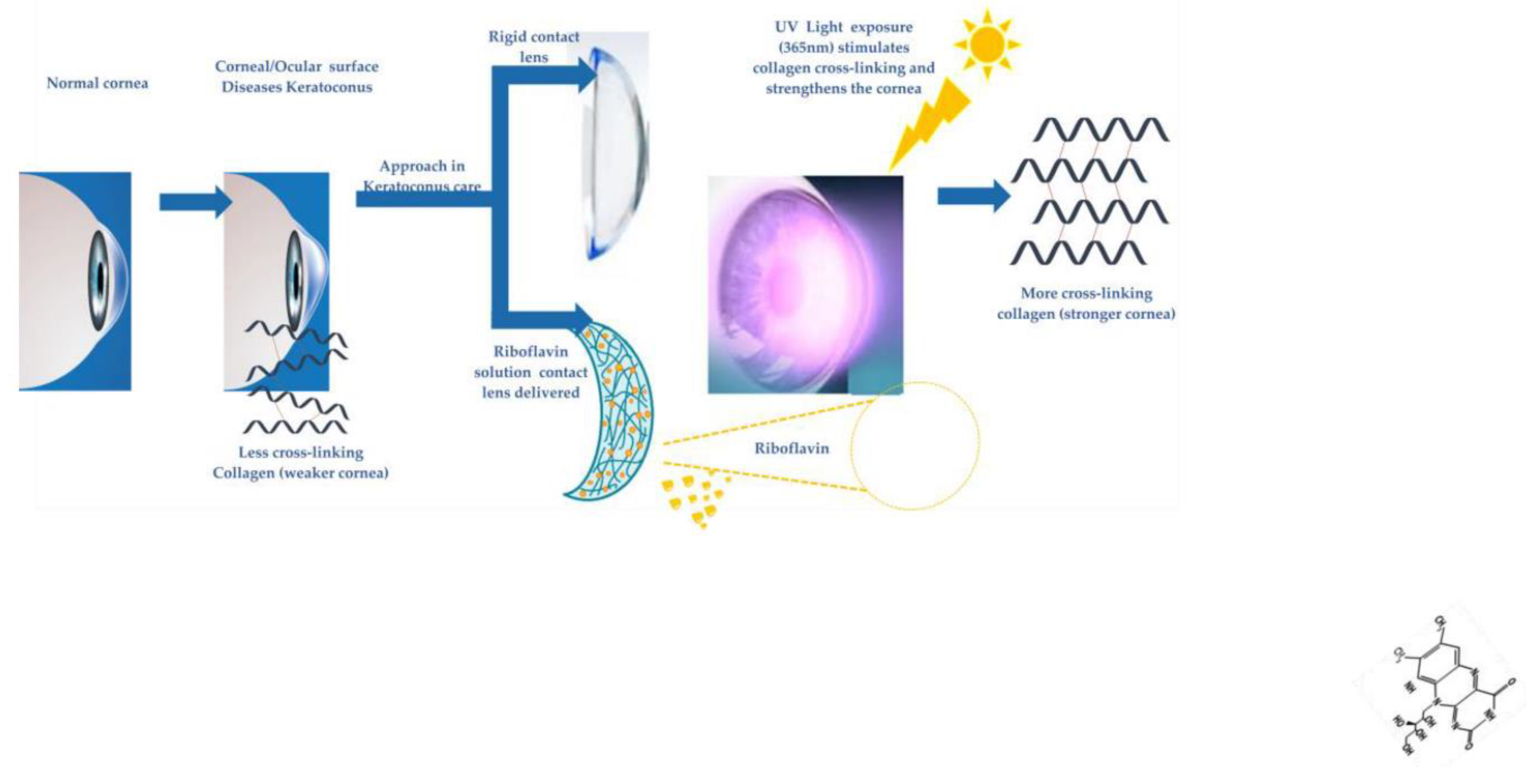
4. Non-interventional future perspectives in keratoconus
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- P. Dixon, C.S., S. Gause, K. H. Hsu, K. C. Powell, and A. Chauhan. Therapeutic contact lenses: a patent review. Oct. 2015, vol. 25. review. Oct. 2015, vol. 25. [CrossRef]
- Choi, S.W.; Kim, J. Therapeutic Contact Lenses with Polymeric Vehicles for Ocular Drug Delivery: A Review. Materials 2018, 11. [Google Scholar] [CrossRef] [PubMed]
- Janagam, D.R.; Wu, L.; Lowe, T.L. Nanoparticles for drug delivery to the anterior segment of the eye. Adv Drug Deliv Rev 2017, 122, 31–64. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Prausnitz, M.R.; Edwards, A. Model of transient drug diffusion across cornea. J Control Release 2004, 99, 241–258. [Google Scholar] [CrossRef] [PubMed]
- Holgado, M.A.; Anguiano-Domínguez, A.; Martín-Banderas, L. Contact lenses as drug-delivery systems: a promising therapeutic tool. Archivos de la Sociedad Española de Oftalmología (English Edition) 2020, 95, 24–33. [Google Scholar] [CrossRef]
- Chatterjee, S.; Upadhyay, P.; Mishra, M.; M, S.; Akshara, M.R.; N, K.; Zaidi, Z.S.; Iqbal, S.F.; Misra, S.K. Advances in chemistry and composition of soft materials for drug releasing contact lenses. RSC Adv 2020, 10, 36751–36777. [Google Scholar] [CrossRef] [PubMed]
- Lim L, L.E. Therapeutic Contact Lenses in the Treatment of Corneal and Ocular Surface Diseases-A Revie. Asia Pac J Ophthalmol 2020. [CrossRef]
- Lu, D.; Aksimentiev, A.; Shih, A.Y.; Cruz-Chu, E.; Freddolino, P.L.; Arkhipov, A.; Schulten, K. The role of molecular modeling in bionanotechnology. Phys Biol 2006, 3, S40–S53. [Google Scholar] [CrossRef]
- Muraru, S.; Ionita, M. Super Carbonaceous Graphene-based structure as a gas separation membrane: A Non-Equilibrium Molecular Dynamics Investigation. Composites Part B: Engineering 2020, 196. [Google Scholar] [CrossRef]
- Filipecka-Szymczyk, K.; Makowska-Janusik, M.; Marczak, W. Molecular Dynamics Simulation of Hydrogels Based on Phosphorylcholine-Containing Copolymers for Soft Contact Lens Applications. Molecules 2023, 28. [Google Scholar] [CrossRef]
- Tonner, R. Molecular Modeling Basics. By Jan H. Hensen. ChemPhysChem 2011, 12, 2352. [Google Scholar] [CrossRef]
- Joshua, B. Fernandes, Y.Y., Jeffery B. Klauda. Molecular dynamics simulations of the human ocular lens with age and cataract. 2022, Volume 1864. [CrossRef]
- Esteban-Ibañez, E.; Montagud-Martínez, D.; Sawides, L.; Zaytouny, A.; de Castro, A.; Sisó-Fuertes, I.; Barcala, X.; Piñero, D.P.; Furlan, W.D.; Dorronsoro, C.; et al. 2023. [CrossRef]
- Behnam Abdi, M.M., Fatemeh Hassanpour, Emel Kirbas Cilingir, Sepideh K. Kalajahi, Paria H. Milani, Mahsa Ghanbarzadeh, Daddi Fadel, Melissa Barnett, Christopher N. Ta, Roger M. Leblanc, Anuj Chauhan, Farhang Abbasi. Therapeutic contact lenses for the treatment of corneal and ocular surface diseases: Advances in extended and targeted drug delivery. International Journal of Pharmaceutics 2023, Volume 638. [CrossRef]
- Franco, P.; De Marco, I. Contact Lenses as Ophthalmic Drug Delivery Systems: A Review. Polymers 2021, 13. [Google Scholar] [CrossRef]
- Rykowska, I.; Nowak, I.; Nowak, R. Soft Contact Lenses as Drug Delivery Systems: A Review. Molecules 2021, 26. [Google Scholar] [CrossRef] [PubMed]
- Varela-Garcia, A.; Gomez-Amoza, J.L.; Concheiro, A.; Alvarez-Lorenzo, C. Imprinted Contact Lenses for Ocular Administration of Antiviral Drugs. Polymers 2020, 12. [Google Scholar] [CrossRef]
- Musgrave, C.S.A.; Fang, F. Contact Lens Materials: A Materials Science Perspective. Materials 2019, 12. [Google Scholar] [CrossRef] [PubMed]
- Bailey, J.; Morgan, P.; Gleeson, H.; Jones, J. Switchable Liquid Crystal Contact Lenses for the Correction of Presbyopia. Crystals 2018, 8. [Google Scholar] [CrossRef]
- Jones, J.C.; Wahle, M.; Bailey, J.; Moorhouse, T.; Snow, B.; Sargent, J. Polarisation independent liquid crystal lenses and contact lenses using embossed reactive mesogens. Journal of the Society for Information Display 2020, 28, 211–223. [Google Scholar] [CrossRef]
- Milton, H.E.; Morgan, P.B.; Clamp, J.H.; Gleeson, H.F. Electronic liquid crystal contact lenses for the correction of presbyopia. Opt Express 2014, 22, 8035–8040. [Google Scholar] [CrossRef]
- Toader G, P.A. , Diacon A, Rusen E, Mocanu A, Brincoveanu O, Alexandru M, Zorila FL, Bacalum M, Albota F, Gavrila AM, Trica B, Rotariu T, Ionita M, Istrate M. Nanocomposite Hydrogel Films Based on Sequential Interpenetrating Polymeric Networks as Drug Delivery Platforms. Polymers 2023. [Google Scholar] [CrossRef]
- Panteli, P.A.; Patrickios, C.S. Multiply Interpenetrating Polymer Networks: Preparation, Mechanical Properties, and Applications. Gels 2019, 5. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.; Kim, H.J.; Noh, H. pH Sensitive Soft Contact Lens for Selective Drug-Delivery. Macromolecular Research 2018, 26, 278–283. [Google Scholar] [CrossRef]
- Maulvi, F.A.; Choksi, H.H.; Desai, A.R.; Patel, A.S.; Ranch, K.M.; Vyas, B.A.; Shah, D.O. pH triggered controlled drug delivery from contact lenses: Addressing the challenges of drug leaching during sterilization and storage. Colloids Surf B Biointerfaces 2017, 157, 72–82. [Google Scholar] [CrossRef]
- Toader, G.; Podaru, I.A.; Rusen, E.; Diacon, A.; Ginghina, R.E.; Alexandru, M.; Zorila, F.L.; Gavrila, A.M.; Trica, B.; Rotariu, T.; et al. Nafcillin-Loaded Photocrosslinkable Nanocomposite Hydrogels for Biomedical Applications. Pharmaceutics 2023, 15. [Google Scholar] [CrossRef]
- Vega, S.L.; Kwon, M.Y.; Burdick, J.A. Recent advances in hydrogels for cartilage tissue engineering. Eur Cell Mater 2017, 33, 59–75. [Google Scholar] [CrossRef]
- Tan, R.Y.H.; Lee, C.S.; Pichika, M.R.; Cheng, S.F.; Lam, K.Y. PH Responsive Polyurethane for the Advancement of Bio medical and Drug Delivery. Polymers (Basel) 2022, 14. [Google Scholar] [CrossRef]
- Chang, H.-C.; Hsu, M.-Y.; Hsiao, W.-T.; Shum, P.J.-T. Finite Element Modeling of an Elderly Person’s Cornea and Rigid Gas Permeable Contact Lenses for Presbyopic Patients. Applied Sciences 2018, 8. [Google Scholar] [CrossRef]
- Garcia-Millan, E.; Koprivnik, S.; Otero-Espinar, F.J. Drug loading optimization and extended drug delivery of corticoids from pHEMA based soft contact lenses hydrogels via chemical and microstructural modifications. Int J Pharm 2015, 487, 260–269. [Google Scholar] [CrossRef]
- Xue Y, Z.J. , Chen Z, Xue F, Zeng L, Qu X, Zhou X. Factors Affecting Long-Term Compliance with Rigid Gas-Permeable Contact Lens Wear in Patients with Keratoconus. J Clin Med. 2022. [Google Scholar] [CrossRef] [PubMed]
- Tran, N.P.; Yang, M.C. Synthesis and Characterization of Silicone Contact Lenses Based on TRIS-DMA-NVP-HEMA Hydrogels. Polymers (Basel) 2019, 11. [Google Scholar] [CrossRef]
- Moore, J.; Lopes, B.T.; Eliasy, A.; Geraghty, B.; Wu, R.; White, L.; Elsheikh, A.; Abass, A. Simulation of the Effect of Material Properties on Soft Contact Lens On-Eye Power. Bioengineering (Basel) 2019, 6. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Xue, Y.; Hu, G.; Lin, T.; Gou, J.; Yin, T.; He, H.; Zhang, Y.; Tang, X. A comprehensive review on contact lens for ophthalmic drug delivery. J Control Release 2018, 281, 97–118. [Google Scholar] [CrossRef]
- Reindel, W.; Mosehauer, G.; Rah, M.; Proskin, H.; Steffen, R. Clinical Performance of Samfilcon A, a Unique Silicone Hydrogel Lens, on a 7-Day Extended Wear Basis. Clin Ophthalmol 2020, 14, 3457–3464. [Google Scholar] [CrossRef] [PubMed]
- Gulsen, D.; Chauhan, A. Ophthalmic drug delivery through contact lenses. Invest Ophthalmol Vis Sci 2004, 45, 2342–2347. [Google Scholar] [CrossRef] [PubMed]
- Gause, S.; Hsu, K.H.; Shafor, C.; Dixon, P.; Powell, K.C.; Chauhan, A. Mechanistic modeling of ophthalmic drug delivery to the anterior chamber by eye drops and contact lenses. Adv Colloid Interface Sci 2016, 233, 139–154. [Google Scholar] [CrossRef] [PubMed]
- Efron, N. Rigid Lens Materials. In Contact Lens Practice; 2018; pp. 115-122.e111.
- Tetsunosuke Kunitomo, K.H.K., Yokohama; Syoji Nagaoka, Kamakura; Takeshi; Yoshioka, K.H.; Tanzawa, K., all of Japan. CROSS-LINKED N-VNYL PYRROL DONE POLYMER COMPOSITION SUITABLE FOR CONTACT LENSEs. United States Patent 1976.
- Kakisu, K.; Matsunaga, T.; Kobayakawa, S.; Sato, T.; Tochikubo, T. Development and efficacy of a drug-releasing soft contact lens. Invest Ophthalmol Vis Sci 2013, 54, 2551–2561. [Google Scholar] [CrossRef] [PubMed]
- Nguyen-Phuong-Dung Tran, M.-C.Y. , Nur Hasanah, Phuong Lan Tran-Nguyen. Effect of poly(ethylene glycol) methacrylate on the ophthalmic properties of silicone hydrogel contact lenses. Colloids and Surfaces B: Biointerfaces. [CrossRef]
- Efron, C.M.-C.a.N. Impact of manufacturing technology and material composition on the mechanical properties of hydrogel contact lenses. Ophthalmic and Physiological Optics 2004. [Google Scholar] [CrossRef]
- Chia, H.N.; Wu, B.M. Recent advances in 3D printing of biomaterials. J Biol Eng 2015, 9, 4. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Leow, W.R.; Chen, X. 3D Printing of Flexible Electronic Devices. Small Methods 2018, 2. [Google Scholar] [CrossRef]
- Hittini, S.; Salih, A.E.; Alam, F.; Shanti, A.; Lee, S.; Polychronopoulou, K.; AlSafar, H.; Almaskari, F.; Butt, H. Fabrication of 3D-Printed Contact Lenses and Their Potential as Color Blindness Ocular Aids. Macromolecular Materials and Engineering 2023, 308. [Google Scholar] [CrossRef]
- Alam, F.; Elsherif, M.; AlQattan, B.; Salih, A.; Lee, S.M.; Yetisen, A.K.; Park, S.; Butt, H. 3D Printed Contact Lenses. ACS Biomater Sci Eng 2021, 7, 794–803. [Google Scholar] [CrossRef]
- Vaidya, N.; Solgaard, O. 3D printed optics with nanometer scale surface roughness. Microsyst Nanoeng 2018, 4, 18. [Google Scholar] [CrossRef]
- Vallejo-Melgarejo, L.D.; Reifenberger, R.G.; Newell, B.A.; Narváez-Tovar, C.A.; Garcia-Bravo, J.M. Characterization of 3D-printed lenses and diffraction gratings made by DLP additive manufacturing. Rapid Prototyping Journal 2019, 25, 1684–1694. [Google Scholar] [CrossRef]
- Toffoletto, N.; Saramago, B.; Serro, A.P. Therapeutic Ophthalmic Lenses: A Review. Pharmaceutics 2020, 13. [Google Scholar] [CrossRef] [PubMed]
- Anthony Soluri*, A.H.; , a. L.J. Delivery of Ketotifen Fumarate by Commercial Contact Lens Materials. American Academy of Optometry 2012. [Google Scholar] [CrossRef]
- Lokendrakumar C Bengani, K.-H.H. , Samuel Gause &; Chauhan†, A. Contact lenses as a platform for ocular drug delivery. Expert Opin. Drug Delivery 2013. [Google Scholar] [CrossRef]
- Maulvi, F.A.; Soni, T.G.; Shah, D.O. A review on therapeutic contact lenses for ocular drug delivery. Drug Deliv 2016, 23, 3017–3026. [Google Scholar] [CrossRef]
- Hsu, K.H.; Carbia, B.E.; Plummer, C.; Chauhan, A. Dual drug delivery from vitamin E loaded contact lenses for glaucoma therapy. Eur J Pharm Biopharm 2015, 94, 312–321. [Google Scholar] [CrossRef] [PubMed]
- Zhen Liu, M.O. , Anuj Chauhan Transport of Vitamin E from Ethanol/Water Solution into Contact Lenses and Impact on Drug Transport. Drug Transport. J Ocul Pharmacol Ther. 2022. [Google Scholar] [CrossRef]
- Uchida, R.; Sato, T.; Tanigawa, H.; Uno, K. Azulene incorporation and release by hydrogel containing methacrylamide propyltrimenthylammonium chloride, and its application to soft contact lens. J Control Release 2003, 92, 259–264. [Google Scholar] [CrossRef]
- Bengani, L.C.; Chauhan, A. Extended delivery of an anionic drug by contact lens loaded with a cationic surfactant. Biomaterials 2013, 34, 2814–2821. [Google Scholar] [CrossRef]
- Phan, C.M.; Subbaraman, L.N.; Jones, L. In vitro drug release of natamycin from beta-cyclodextrin and 2-hydroxypropyl-beta-cyclodextrin-functionalized contact lens materials. J Biomater Sci Polym Ed 2014, 25, 1907–1919. [Google Scholar] [CrossRef]
- dos Santos, J.F.; Torres-Labandeira, J.J.; Matthijs, N.; Coenye, T.; Concheiro, A.; Alvarez-Lorenzo, C. Functionalization of acrylic hydrogels with alpha-, beta- or gamma-cyclodextrin modulates protein adsorption and antifungal delivery. Acta Biomater 2010, 6, 3919–3926. [Google Scholar] [CrossRef]
- Garcia-Fernandez, M.J.; Tabary, N.; Martel, B.; Cazaux, F.; Oliva, A.; Taboada, P.; Concheiro, A.; Alvarez-Lorenzo, C. Poly-(cyclo)dextrins as ethoxzolamide carriers in ophthalmic solutions and in contact lenses. Carbohydr Polym 2013, 98, 1343–1352. [Google Scholar] [CrossRef]
- Wang, Z.L., Ting; Li, Xinhua; Wu, Haitao; Li, Yuhang; Hao, Lingyun. Preparation of Molecularly Imprinted Hydrogel Contact Lenses for Extended Atropine Eluting. Journal of Biomedical Nanotechnology 2023, Volume 19. 19. [CrossRef]
- Yanez, F.; Martikainen, L.; Braga, M.E.; Alvarez-Lorenzo, C.; Concheiro, A.; Duarte, C.M.; Gil, M.H.; de Sousa, H.C. Supercritical fluid-assisted preparation of imprinted contact lenses for drug delivery. Acta Biomater 2011, 7, 1019–1030. [Google Scholar] [CrossRef]
- ElShaer, A.; Mustafa, S.; Kasar, M.; Thapa, S.; Ghatora, B.; Alany, R.G. Nanoparticle-Laden Contact Lens for Controlled Ocular Delivery of Prednisolone: Formulation Optimization Using Statistical Experimental Design. Pharmaceutics 2016, 8. [Google Scholar] [CrossRef] [PubMed]
- Zerillo, L.; Polvere, I.; Varricchio, R.; Madera, J.R.; D’Andrea, S.; Voccola, S.; Franchini, I.; Stilo, R.; Vito, P.; Zotti, T. Antibiofilm and repair activity of ozonated oil in liposome. Microb Biotechnol 2022, 15, 1422–1433. [Google Scholar] [CrossRef] [PubMed]
- Maulvi, F.A.; Parmar, R.J.; Desai, A.R.; Desai, D.M.; Shukla, M.R.; Ranch, K.M.; Shah, S.A.; Shah, D.O. Tailored gatifloxacin Pluronic(R) F-68-loaded contact lens: Addressing the issue of transmittance and swelling. Int J Pharm 2020, 581, 119279. [Google Scholar] [CrossRef] [PubMed]
- Hiratani, H.; Mizutani, Y.; Alvarez-Lorenzo, C. Controlling drug release from imprinted hydrogels by modifying the characteristics of the imprinted cavities. Macromol Biosci 2005, 5, 728–733. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.H.; Li, Y.; Jin, R.; Shrestha, T.; Choi, J.S.; Lee, W.J.; Moon, M.J.; Ju, H.T.; Choi, W.; Yoon, K.C. The Efficiency of Cyclosporine A-Eluting Contact Lenses for the Treatment of Dry Eye. Curr Eye Res 2019, 44, 486–496. [Google Scholar] [CrossRef] [PubMed]
- Hassan Hashemi, M., Samira Heydarian, PhD, Elham Hooshmand, MSc,Mohammad Saatchi, PhD,; Abbasali Yekta, P., Mohamadreza Aghamirsalim, MD,k Mehrnaz Valadkhan, M.Sc,; Mehdi Mortazavi, M., Alireza Hashemi, MD, and Mehdi Khabazkhoob, PhD. The Prevalence and Risk Factors for Keratoconus: A Systematic Review and Meta-Analysis. Cornea 2019, 1. [CrossRef]
- Ashar, J.N., & Vadavalli, P. K. Long-term Results of Riboflavin Ultraviolet A Corneal Collagen Cross-linking for Keratoconus in Italy: The Siena Eye Cross Study. American Journal of Ophthalmology 2010. [CrossRef]
- Hwang, J.S.; Lee, J.H.; Wee, W.R.; Kim, M.K. Effects of multicurve RGP contact lens use on topographic changes in keratoconus. Korean J Ophthalmol 2010, 24, 201–206. [Google Scholar] [CrossRef]
- Zohar Meiri, B.S.K., MD, Amir Rosenblatt, MD, Tal Sarig, MSc,; Liat Shenhav, B., and David Varssano, MD. Efficacy of Corneal Collagen Cross-Linking for the Treatment of Keratoconus: A Systematic Review and Meta-Analysis. Cornea 2016. [CrossRef]
- Sykakis, E.; Karim, R.; Evans, J.R.; Bunce, C.; Amissah-Arthur, K.N.; Patwary, S.; McDonnell, P.J.; Hamada, S. Corneal collagen cross-linking for treating keratoconus. Cochrane Database Syst Rev 2015, CD010621. [Google Scholar] [CrossRef]
- Hovakimyan, M.; Guthoff, R.F.; Stachs, O. Collagen cross-linking: current status and future directions. J Ophthalmol 2012, 2012, 406850. [Google Scholar] [CrossRef] [PubMed]
- Adamiak, K.; Sionkowska, A. Current methods of collagen cross-linking: Review. Int J Biol Macromol 2020, 161, 550–560. [Google Scholar] [CrossRef]
- tissue, I.o.c.-l.i.c. Induction of cross-links in corneal tissue. Experimental Eye Research 1998. [Google Scholar] [CrossRef]
- McCall, A.S.; Kraft, S.; Edelhauser, H.F.; Kidder, G.W.; Lundquist, R.R.; Bradshaw, H.E.; Dedeic, Z.; Dionne, M.J.; Clement, E.M.; Conrad, G.W. Mechanisms of corneal tissue cross-linking in response to treatment with topical riboflavin and long-wavelength ultraviolet radiation (UVA). Invest Ophthalmol Vis Sci 2010, 51, 129–138. [Google Scholar] [CrossRef]
- Seiler, T.G.; Ehmke, T.; Fischinger, I.; Zapp, D.; Stachs, O.; Seiler, T.; Heisterkamp, A. Two-Photon Fluorescence Microscopy for Determination of the Riboflavin Concentration in the Anterior Corneal Stroma When Using the Dresden Protocol. Invest Ophthalmol Vis Sci 2015, 56, 6740–6746. [Google Scholar] [CrossRef] [PubMed]
- Atalay, E.; Ozalp, O.; Yildirim, N. Advances in the diagnosis and treatment of keratoconus. Ther Adv Ophthalmol 2021, 13, 25158414211012796. [Google Scholar] [CrossRef] [PubMed]
| Lens Type | Material(monomer) | Features | Reference |
|---|---|---|---|
| Rigid | Methyl methacrylate | -low permeability -stiff |
[31] |
| Cellulose acetate butyrate | -superior gas permeability to PMMA -stiff |
[34] | |
| Siloxy methacrylate | -exceptional gas permeability -low surface wetness -lipid surface deposits |
[32] | |
| Fluoro-siloxymethacrylate | -gas permeability higher than PMMA -improved wettability -no clinically considerable balance of advantages over PMMA |
[38] | |
| Soft | Hydroxyethyl-methacrylate | -not enough O2 permeability | [30,33] |
| N-vinyl pyrrolidone | -high water content -increase the relative evaporation rate of water -beneficial effect on drug loading and release |
[39] | |
| HEMA-co-NVP | -high water content when compared to pure polymer -higher O2permeability -improved drug loading and more optimal drug release |
[30] | |
| HEMA-co-HEMA-co-MPTS | -excellent cationic drug loading -improved drug release (Gatifloxacin and Moxifloxacin) compared to commercial etafilcon A and polymacon and eye drops |
[40] | |
| TRIS-DMA-NVP-HEMA | -best balance oxygen permeability, equilibrium water content, hydrophilicity and reduced protein film formation compared to simpler formulations | [32] | |
| TRIS-NVP-MAA-PEGMA | -the overall oxygen permeability, friction coefficient, water absorption capacity, contact angle, modulus and protein adsorption are superior to some of the commercial contact lenses (e.g.Acuvue Advances or Cooper vision). | [41] |
| Lens Type | Backbone Monomers | Features | Drug | Drug loading techniques | Ref. |
|---|---|---|---|---|---|
| 1 | ACUVUE TruEye CLs with vitamin E barriers | -vitamin E modification increases the release duration of both drugs to about 2 days -reduces IOP with lower drug dose compared to eye drops |
timolol and dorzolamide simultameously loaded | Soaking Method | [53] |
| 2 | ACUVUE TruEye CLs with vitamin E barriers | -effective at sustaining release of timolol -reduced swelling which reduce lens damage |
timolol | Soaking Method | [54] |
| 3 | HEMA/MAPTAC /MOEP/MAA | -prevent the size change -efficient drug delivery |
azulene | Incorporation of Functional Molecules | [55] |
| 4 | HEMA/ cetalkonium chloride |
-drug release duration (50h) -good wettability -low protein absorption -excellent transparency of lenses |
dexamethasone 21-disodium phosphate | Incorporation of Functional Molecules | [56] |
| 5 | HEMA / DMA/TRIS/CDs | -improve the water solubility of natamycin -more effective to deliver natamycin |
natamycin / methacrylated beta-cyclodextrin (Mβ-CD) natamycin / methacrylated 2-hydroxypropyl-β-cyclodextrin |
Incorporation of Functional Molecules | [57] |
| 6 | HEMA / HEMA–co-GMA/α-, β- and γ-cyclodextrins functionalized | -reduced protein sorptin -HEMA γ-cyclodextrins has the higest loading for miconazole -sustained miconazole delivery for over 14days -high efficiency for against biofilm formation |
miconazole | Incorporation of Functional Molecules | [58] |
| 7 | poly-CDs-HEMA | -high drug doses loaded -sustained drug release for 6 days |
ethoxzolamide | Incorporation of Functional Molecules | [59] |
| 8 | MAA and methacrylamide (MAm) functional comonomers | - atropine release for up to 72 h -good balance light transmission, water content, and contact angle |
atropine | Molecularly Imprinted | [60] |
| 9 | MAA/HEMA/EGDMA | -ACV-imprinted hydrogels were not effective in terms of drug loading -VACV-imprinted hydrogels has a sustained release profile for 10 h, -relevant amount of VACV is accumulated in the cornea. -promising for delivery to the posterior segment |
acyclovir valacyclovir | Molecularly Imprinted | [17] |
| 10 | Hilafilcon B commercial | -higher flurbiprofen loaded -sustained release profiles |
flurbiprofen | supercritical fluid (SCF)-assisted molecular imprinting | [61] |
| 11 | HEMA/MAA EGDMA / Prednisolone loaded PLGA nanoparticles | -slow drug release of drug over 24 h -release of 10.8% encapsulated drug -insignificant changes in light transmission, wettability, and hydration by loading Prednisolone loaded PLGA nanoparticles |
Prednisolone | Colloidal nanoparticles | [62] |
| 12 | Dailies AquaComfort PLUS | -inhibit / eradication the formation of Pseudomonas aeruginosa and Staphylococcus biofilm | Ozodrop® Ozodrop® gel |
Liposome | [63] |
| 13 | DMA/siloxane/ NVP/EGDMA/ HEMA loaded Pluronic® F-68/gatifloxacin | -Pluronic® F-68 improves the drug uptake and sustained drug delivery -excellent optical transmittance, swelling and mechanical features |
Gatifloxacin/ Pluronic® F-68 | Micelles | [64] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




