Submitted:
01 October 2023
Posted:
02 October 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Materials and reagents
2.2. Synthesis of (3-mercaptopropyl)trimethoxysilane stabilized AuNCs (MPTS-AuNCs)
2.3. One-pot synthesis of MPTS-AuNCs in MSNs (MPTS-AuNCs@MSN)
2.4. Preparation of MnFe2O4 magnetic nanoparticles (MnFe2O4 NPs)
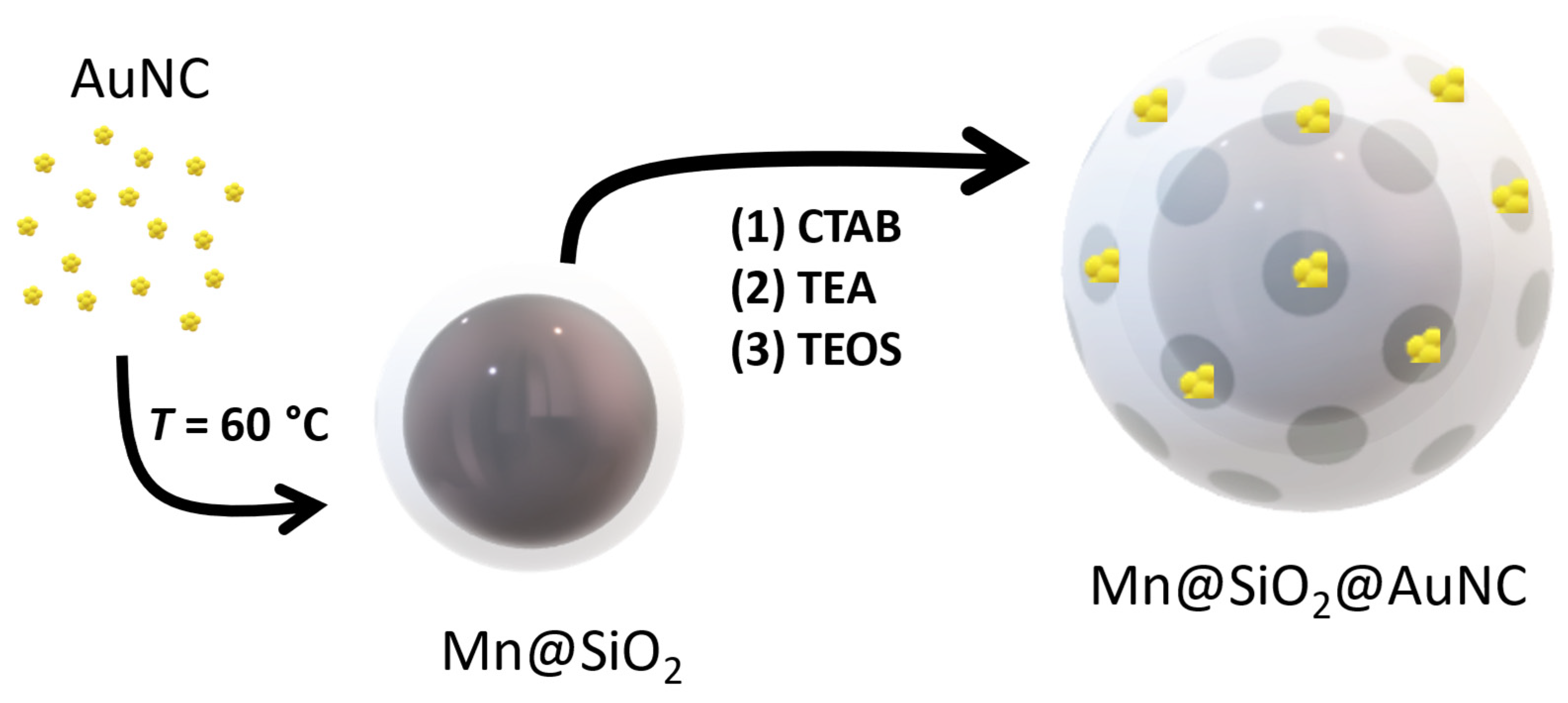
2.5. Silica coating of MnFe2O4 magnetic nanoparticles (Mn@SiO2)
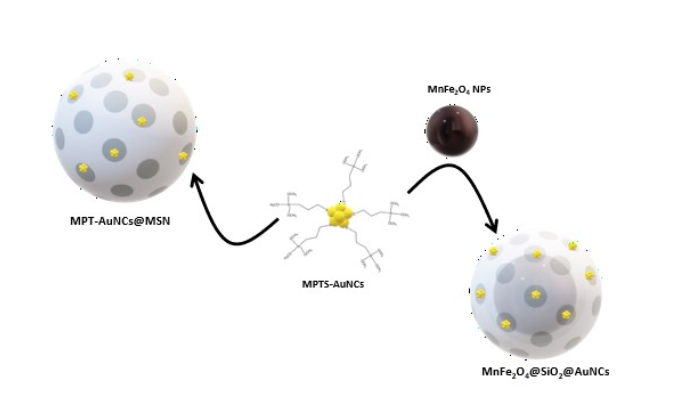
2.6. Hybrid nanocomposite conjugating Mn@SiO2 and MPTS-AuNCs (Mn@SiO2@AuNCs)
2.7. Characterization of the materials
3. Results and discussion
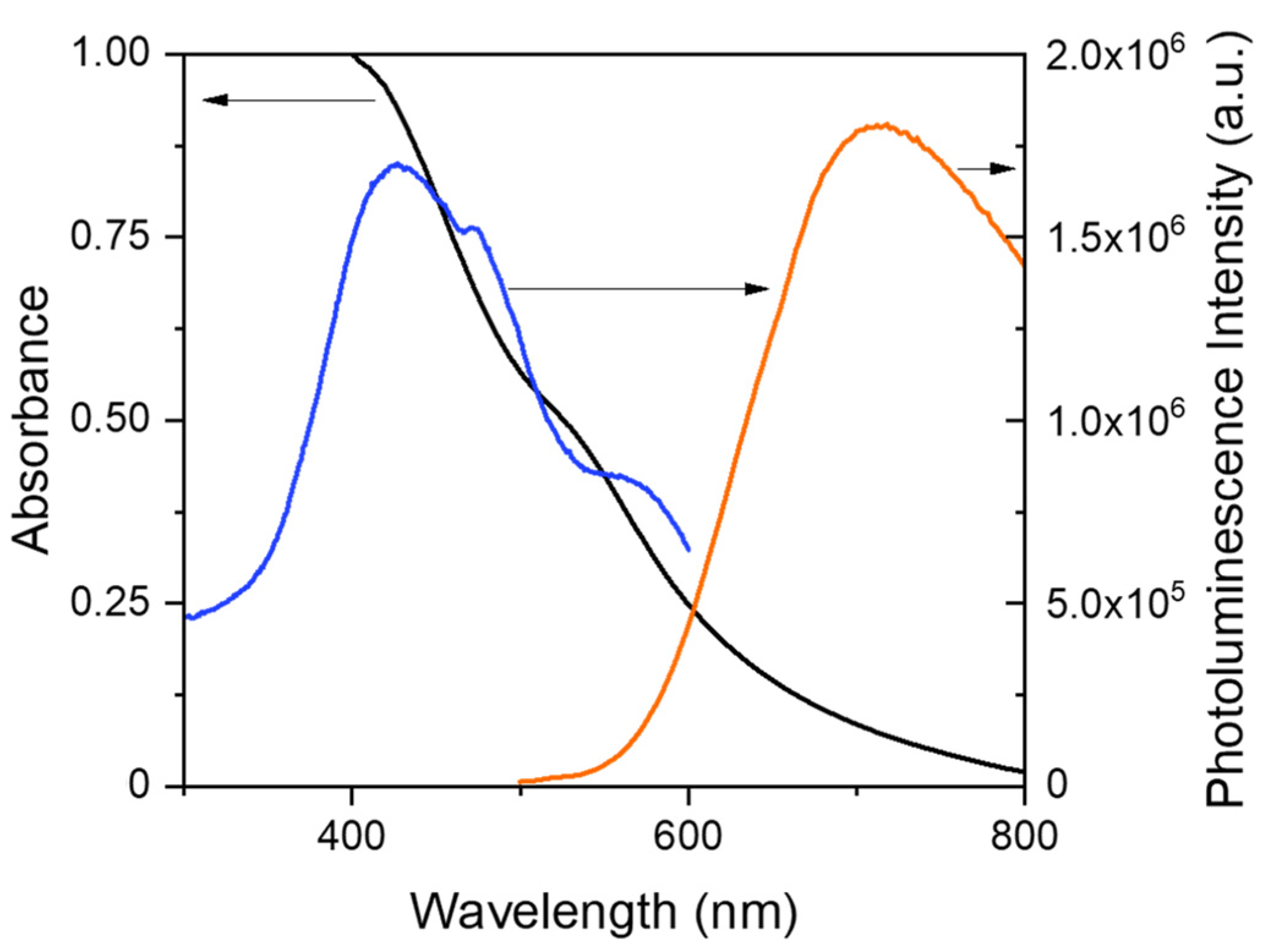
3.1. Synthesis and characterization of AuNCs stabilized with MPTS
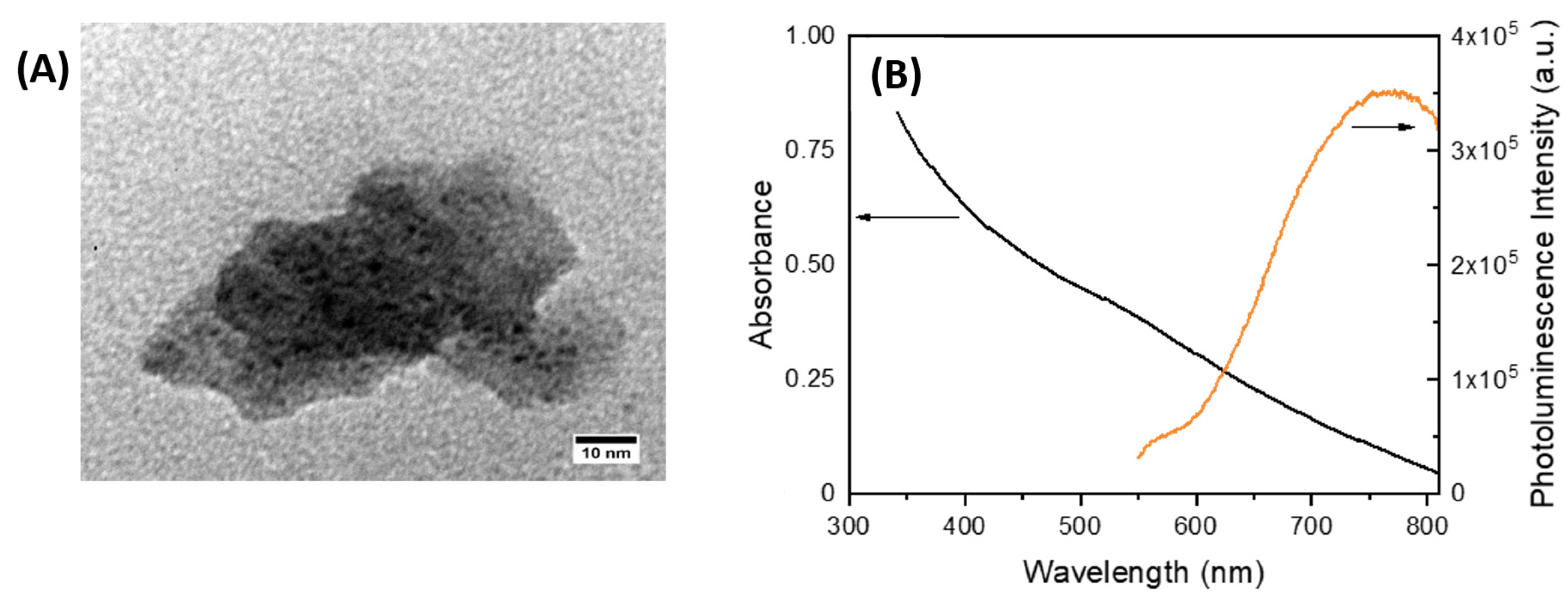
3.2. One-pot synthesis of AuNC in MSN (AuNCs@MSN)
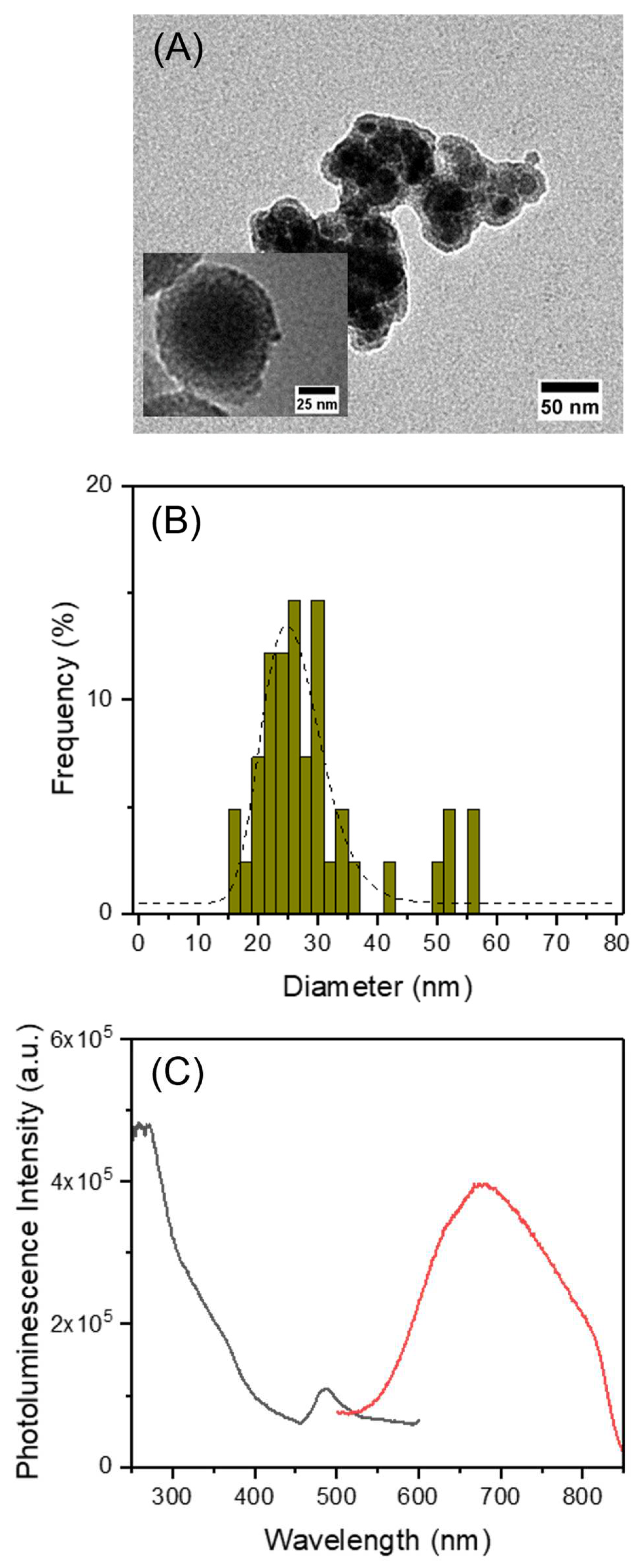
3.3. Incorporation of AuNCs and MnFe2O4 in MSNs
4. Conclusions
Acknowledgements
References
- Piñeiro, Y.; Rivas, J.; López-Quintela, M. A. : Amsterdam, 2014; pp 81-105.Clusters. In Colloidal Foundations of Nanoscience; Berti, D., Palazzo, G., Eds.; Elsevier: Amsterdam, 2014. [Google Scholar]
- Sahoo, A. K.; Banerjee, S.; Ghosh, S. S.; Chattopadhyay, A. : Simultaneous RGB Emitting Au Nanoclusters in Chitosan Nanoparticles for Anticancer Gene Theranostics. ACS Applied Materials & Interfaces 2014, 6, 712–724. [Google Scholar]
- Chen, D.; Luo, Z.; Li, N.; Lee, J. Y.; Xie, J.; Lu, J. : Amphiphilic Polymeric Nanocarriers with Luminescent Gold Nanoclusters for Concurrent Bioimaging and Controlled Drug Release. Adv. Funct. Mater. 2013, 23, 4324–4331. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, D.; Zhao, Y.; Zhao, T.; Sun, H.; Li, X.; Wang, C.; Yang, B.; Lin, Q. : Polycation-functionalized gold nanodots with tunable near-infrared fluorescence for simultaneous gene delivery and cell imaging. Nano Research 2018, 11, 2392–2404. [Google Scholar] [CrossRef]
- Chen, Y.; Zheng, X.; Wang, X.; Wang, C.; Ding, Y.; Jiang, X. : Near-Infrared Emitting Gold Cluster–Poly(acrylic acid) Hybrid Nanogels. ACS Macro Letters 2014, 3, 74–76. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-A. J.; Yang, T.-Y.; Lee, C.-H.; Huang, S. H.; Sperling, R. A.; Zanella, M.; Li, J. K.; Shen, J.-L.; Wang, H.-H.; Yeh, H.-I.; Parak, W. J.; Chang, W. H. : Synthesis, Characterization, and Bioconjugation of Fluorescent Gold Nanoclusters toward Biological Labeling Applications. ACS Nano 2009, 3, 395–401. [Google Scholar] [CrossRef]
- Wu, X.; Li, C.; Liao, S.; Li, L.; Wang, T.; Su, Z.; Wang, C.; Zhao, J.; Sui, C.; Lin, J. : Silica-Encapsulated Gd3+-Aggregated Gold Nanoclusters for In Vitro and In Vivo Multimodal Cancer Imaging. Chemistry – A European Journal 2014, 20, 8876–8882. [Google Scholar] [CrossRef]
- Wu, X. T.; Li, L.; Zhang, L. Y.; Wang, T. T.; Wang, C. G.; Su, Z. M. : Multifunctional spherical gold nanocluster aggregate@polyacrylic acid@mesoporous silica nanoparticles for combined cancer dual-modal imaging and chemo-therapy. Journal of Materials Chemistry B 2015, 3, 2421–2425. [Google Scholar] [CrossRef]
- Hembury, M.; Chiappini, C.; Bertazzo, S.; Kalber, T. L.; Drisko, G. L.; Ogunlade, O.; Walker-Samuel, S.; Krishna, K. S.; Jumeaux, C.; Beard, P.; Kumar, C.; Porter, A. E.; Lythgoe, M. F.; Boissiere, C.; Sanchez, C.; Stevens, M. M. : Gold-silica quantum rattles for multimodal imaging and therapy. Proceedings of the National Academy of Sciences of the United States of America 2015, 112, 1959–1964. [Google Scholar] [CrossRef]
- Shin, T.-H.; Choi, Y.; Kim, S.; Cheon, J. : Recent advances in magnetic nanoparticle-based multi-modal imaging. Chemical Society Reviews 2015, 44, 4501–4516. [Google Scholar] [CrossRef]
- Wahsner, J.; Gale, E. M.; Rodríguez-Rodríguez, A.; Caravan, P. : Chemistry of MRI Contrast Agents: Current Challenges and New Frontiers. Chemical Reviews 2019, 119, 957–1057. [Google Scholar] [CrossRef]
- Xiao, Y.-D.; Paudel, R.; Liu, J.; Ma, C.; Zhang, Z.-S.; Zhou, S.-K. : MRI contrast agents: Classification and application (Review). Int J Mol Med 2016, 38, 1319–1326. [Google Scholar] [CrossRef]
- Rocha, M.; Fernandes, C.; Pereira, C.; Rebelo, S. L. H.; Pereira, M. F. R.; Freire, C. : Gold-supported magnetically recyclable nanocatalysts: a sustainable solution for the reduction of 4-nitrophenol in water. RSC Advances 2015, 5, 5131–5141. [Google Scholar] [CrossRef]
- Pereira, C.; Pereira, A. M.; Rocha, M.; Freire, C.; Geraldes, C. F. G. C. : Architectured design of superparamagnetic Fe3O4 nanoparticles for application as MRI contrast agents: mastering size and magnetism for enhanced relaxivity. Journal of Materials Chemistry B 2015, 3, 6261–6273. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, C.; Pereira, C.; Fernandez-Garcia, M. P.; Pereira, A. M.; Guedes, A.; Fernandez-Pacheco, R.; Ibarra, A.; Ibarra, M. R.; Araujo, J. P.; Freire, C. : Tailored design of CoxMn1-xFe2O4 nanoferrites: a new route for dual control of size and magnetic properties. Journal of Materials Chemistry C 2014, 2, 5818–5828. [Google Scholar] [CrossRef]
- Ravichandran, M.; Velumani, S. : Manganese ferrite nanocubes as an MRI contrast agent. Materials Research Express 2020, 7, 016107–10. [Google Scholar] [CrossRef]
- Islam, K.; Haque, M.; Kumar, A.; Hoq, A.; Hyder, F.; Hoque, S. M. : Manganese Ferrite Nanoparticles (MnFe2O4): Size Dependence for Hyperthermia and Negative/Positive Contrast Enhancement in MRI. Nanomaterials 2020, 10, 2297–10. [Google Scholar] [CrossRef]
- Gorgizadeh, M.; Behzadpour, N.; Salehi, F.; Daneshvar, F.; Vais, R. D.; Nazari-Vanani, R.; Azarpira, N.; Lotfi, M.; Sattarahmady, N. : A MnFe2O4/C nanocomposite as a novel theranostic agent in MRI, sonodynamic therapy and photothermal therapy of a melanoma cancer model. Journal of Alloys and Compounds 2020, 816, 152597–10. [Google Scholar] [CrossRef]
- Hu, J.; Dong, Y.-l.; Chen, X.-j.; Zhang, H.-j.; Zheng, J.-m.; Wang, Q.; Chen, X.-g. : A highly efficient catalyst: In situ growth of Au nanoparticles on graphene oxide–Fe3O4 nanocomposite support. Chemical Engineering Journal 2014, 236, 1–8. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, B.; Zhang, B.; Bian, G.; Qi, Y.; Yang, X.; Li, C. : Synthesis of monodisperse magnetic sandwiched gold nanoparticle as an easily recyclable catalyst with a protective polymer shell. Colloids and Surfaces A: Physicochemical and Engineering Aspects 2015, 466, 210–218. [Google Scholar] [CrossRef]
- Saire-Saire, S.; Barbosa, E. C. M.; Garcia, D.; Andrade, L. H.; Garcia-Segura, S.; Camargo, P. H. C.; Alarcon, H. : Green synthesis of Au decorated CoFe2O4 nanoparticles for catalytic reduction of 4-nitrophenol and dimethylphenylsilane oxidation. RSC Advances 2019, 9, 22116–22123. [Google Scholar] [CrossRef]
- Silvestri, A.; Mondini, S.; Marelli, M.; Pifferi, V.; Falciola, L.; Ponti, A.; Ferretti, A. M.; Polito, L. : Synthesis of Water Dispersible and Catalytically Active Gold-Decorated Cobalt Ferrite Nanoparticles. Langmuir 2016, 32, 7117–7126. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Li, B.; Ren, X.; Tan, L.; Huang, Z.; Tang, F. : One-pot gradient solvothermal synthesis of Au–Fe3O4 hybrid nanoparticles for magnetically recyclable catalytic applications. Journal of Materials Chemistry A 2013, 1, 10513–10517. [Google Scholar] [CrossRef]
- Herzing, A. A.; Kiely, C. J.; Carley, A. F.; Landon, P.; Hutchings, G. J. : Identification of Active Gold Nanoclusters on Iron Oxide Supports for CO Oxidation. Science 2008, 321, 1331–1335. [Google Scholar] [CrossRef]
- Fratila, R. M.; Mitchell, S. G.; del Pino, P.; Grazu, V.; de la Fuente, J. M. : Strategies for the Biofunctionalization of Gold and Iron Oxide Nanoparticles. Langmuir 2014, 30, 15057–15071. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Park, S.; Stojanović, Z.; Han, H.-S.; Lee, J.; Seok, H. K.; Uskoković, D.; Lee, K. H. : Facile Solvothermal Preparation of Monodisperse Gold Nanoparticles and Their Engineered Assembly of Ferritin–Gold Nanoclusters. Langmuir 2013, 29, 15698–15703. [Google Scholar] [CrossRef] [PubMed]
- Ye, M.; Wei, Z.; Hu, F.; Wang, J.; Ge, G.; Hu, Z.; Shao, M.; Lee, S.-T.; Liu, J. : Fast assembling microarrays of superparamagnetic Fe3O4@Au nanoparticle clusters as reproducible substrates for surface-enhanced Raman scattering. Nanoscale 2015, 7, 13427–13437. [Google Scholar] [CrossRef]
- Saikova, S.; Pavlikov, A.; Trofimova, T.; Mikhlin, Y.; Karpov, D.; Asanova, A.; Grigoriev, Y.; Volochaev, M.; Samoilo, A.; Zharkov, S.; Velikanov, D. : Hybrid Nanoparticles Based on Cobalt Ferrite and Gold: Preparation and Characterization. Metals 2021, 11, 705–10. [Google Scholar] [CrossRef]
- Binaymotlagh, R.; Hajareh Haghighi, F.; Aboutalebi, F.; Mirahmadi-Zare, S. Z.; Hadadzadeh, H.; Nasr-Esfahani, M.-H. : Selective chemotherapy and imaging of colorectal and breast cancer cells by a modified MUC-1 aptamer conjugated to a poly(ethylene glycol)-dimethacrylate coated Fe3O4–AuNCs nanocomposite. New Journal of Chemistry 2019, 43, 238–248. [Google Scholar] [CrossRef]
- Le Guével, X.; Prinz, E.-M.; Müller, R.; Hempelmann, R.; Schneider, M. : Synthesis and characterization of superparamagnetic nanoparticles coated with fluorescent gold nanoclusters. Journal of Nanoparticle Research 2012, 14, 727–10. [Google Scholar] [CrossRef]
- Huang, C.-L.; Hsieh, W.-J.; Lin, C.-W.; Yang, H.-W.; Wang, C.-K. : Multifunctional liposomal drug delivery with dual probes of magnetic resonance and fluorescence imaging. Ceramics International 2018, 44, 12442–12450. [Google Scholar] [CrossRef]
- Guo, H.; Zhang, Y.; Liang, W.; Tai, F.; Dong, Q.; Zhang, R.; Yu, B.; Wong, W.-Y. : An inorganic magnetic fluorescent nanoprobe with favorable biocompatibility for dual-modality bioimaging and drug delivery. Journal of Inorganic Biochemistry 2019, 192, 72–81. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Yao, Y.; Song, Q. : Gold nanoclusters decorated with magnetic iron oxide nanoparticles for potential multimodal optical/magnetic resonance imaging. Journal of Materials Chemistry C 2015, 3, 5910–5917. [Google Scholar] [CrossRef]
- Xu, Y.; Palchoudhury, S.; Qin, Y.; Macher, T.; Bao, Y. : Make Conjugation Simple: A Facile Approach to Integrated Nanostructures. Langmuir 2012, 28, 8767–8772. [Google Scholar] [CrossRef] [PubMed]
- Luo, S.; Liu, Y.; Rao, H.; Wang, Y.; Wang, X. : Fluorescence and magnetic nanocomposite Fe3O4@SiO2@Au MNPs as peroxidase mimetics for glucose detection. Analytical Biochemistry 2017, 538, 26–33. [Google Scholar] [CrossRef]
- Kim, D.; Lee, B. : Fluorescence detection of bisphenol A in aqueous solution using magnetite core-shell material with gold nanoclusters prepared by molecular imprinting technique. Korean J. Chem. Eng. 2019, 36, 1509–1517. [Google Scholar] [CrossRef]
- Ribeiro, T.; Rodrigues, A. S.; Calderon, S.; Fidalgo, A.; Gonçalves, J. L. M.; André, V.; Teresa Duarte, M.; Ferreira, P. J.; Farinha, J. P. S.; Baleizão, C. : Silica nanocarriers with user-defined precise diameters by controlled template self-assembly. Journal of Colloid and Interface Science 2020, 561, 609–619. [Google Scholar] [CrossRef]
- Calderon V, S.; Ribeiro, T.; Farinha, J. P. S.; Baleizão, C.; Ferreira, P. J. : On the Structure of Amorphous Mesoporous Silica Nanoparticles by Aberration-Corrected STEM. Small 2018, 14, 1802180–10. [Google Scholar] [CrossRef]
- Gonçalves, J. L. M.; J. Castanheira, E.; P. C. Alves, S.; Baleizão, C.; Farinha, J. P.: Grafting with RAFT—gRAFT Strategies to Prepare Hybrid Nanocarriers with Core-shell Architecture. Polymers 2020, 12, 2175–10. [Google Scholar] [CrossRef]
- Ribeiro, T.; Coutinho, E.; Rodrigues, A. S.; Baleizão, C.; Farinha, J. P. S. : Hybrid mesoporous silica nanocarriers with thermovalve-regulated controlled release. Nanoscale 2017, 9, 13485–13494. [Google Scholar] [CrossRef]
- Tavares, M. T.; Oliveira, M. B.; Gaspar, V. M.; Mano, J. F.; Farinha, J. P.; Baleizão, C. : Efficient Single-Dose Induction of Osteogenic Differentiation of Stem Cells Using Multi-Bioactive Hybrid Nanocarriers. 2020, 4, 2000123–10.
- Gonçalves, J. L. M.; Lopes, A. B. C.; Baleizão, C.; Farinha, J. P. S. : Mesoporous Silica Nanoparticles Modified inside and out for ON:OFF pH-Modulated Cargo Release. Pharmaceutics 2021, 13, 716. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, J. L. M.; Crucho, C. I. C.; Alves, S. P. C.; Baleizão, C.; Farinha, J. P. S. : Hybrid Mesoporous Nanoparticles for pH-Actuated Controlled Release. Nanomaterials 2019, 9, 483–10. [Google Scholar] [CrossRef] [PubMed]
- Baleizão, C.; Farinha, J. P. S. : Hybrid smart mesoporous silica nanoparticles for theranostics. Nanomedicine 2015, 10, 2311–2314. [Google Scholar] [CrossRef] [PubMed]
- Saroj, S.; Rajput, S. J. : Composite smart mesoporous silica nanoparticles as promising therapeutic and diagnostic candidates: Recent trends and applications. Journal of Drug Delivery Science and Technology 2018, 44, 349–365. [Google Scholar] [CrossRef]
- Liu, D.; Wang, J.; Zhang, Y.; Liu, J.; Li, H.; Zhou, L.; Wu, S.; Gao, X. : Preparation of core–shell structured Au@SiO2 nanocomposite catalyst with Au core size below 2 nm without high-temperature calcination procedure. Journal of Materials Science 2018, 53, 8086–8097. [Google Scholar] [CrossRef]
- Yan, W.; Chen, B.; Mahurin, S. M.; Hagaman, E. W.; Dai, S.; Overbury, S. H. : Surface Sol−Gel Modification of Mesoporous Silica Materials with TiO2 for the Assembly of Ultrasmall Gold Nanoparticles. The Journal of Physical Chemistry B 2004, 108, 2793–2796. [Google Scholar] [CrossRef]
- Liu, Y.; Tsunoyama, H.; Akita, T.; Tsukuda, T. : Preparation of ∼1 nm Gold Clusters Confined within Mesoporous Silica and Microwave-Assisted Catalytic Application for Alcohol Oxidation. The Journal of Physical Chemistry C 2009, 113, 13457–13461. [Google Scholar] [CrossRef]
- Casteleiro, B.; Martinho, J. M. G.; Farinha, J. P. S. : Encapsulation of gold nanoclusters: stabilization and more. Nanoscale 2021, 13, 17199–17217. [Google Scholar] [CrossRef]
- He, F.; Yang, G.; Yang, P.; Yu, Y.; Lv, R.; Li, C.; Dai, Y.; Gai, S.; Lin, J. : A New Single 808 nm NIR Light-Induced Imaging-Guided Multifunctional Cancer Therapy Platform. Adv. Funct. Mater. 2015, 25, 3966–3976. [Google Scholar] [CrossRef]
- Pereira, C.; Pereira, A. M.; Fernandes, C.; Rocha, M.; Mendes, R.; Fernández-García, M. P.; Guedes, A.; Tavares, P. B.; Grenèche, J.-M.; Araújo, J. P.; Freire, C. : Superparamagnetic MFe2O4 (M = Fe, Co, Mn) Nanoparticles: Tuning the Particle Size and Magnetic Properties through a Novel One-Step Coprecipitation Route. Chemistry of Materials 2012, 24, 1496–1504. [Google Scholar] [CrossRef]
- Seybold, P. G.; Gouterman, M. : Porphyrins: XIII: Fluorescence spectra and quantum yields. Journal of Molecular Spectroscopy 1969, 31, 1–13. [Google Scholar] [CrossRef]
- Brust, M.; Walker, M.; Bethell, D.; Schiffrin, D. J.; Whyman, R. : Synthesis of thiol-derivatised gold nanoparticles in a two-phase Liquid-Liquid system. Journal of the Chemical Society, Chemical Communications 1994, 801–802. [Google Scholar] [CrossRef]
- Zhu, M.; Lanni, E.; Garg, N.; Bier, M. E.; Jin, R. : Kinetically Controlled, High-Yield Synthesis of Au25 Clusters. Journal of the American Chemical Society 2008, 130, 1138–1139. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.; Murray, R. W. : Visible luminescence of water-soluble monolayer- protected gold clusters. Journal of Physical Chemistry B 2001, 105, 12498–12502. [Google Scholar] [CrossRef]
- Chen, L.-Y.; Wang, C.-W.; Yuan, Z.; Chang, H.-T. : Fluorescent Gold Nanoclusters: Recent Advances in Sensing and Imaging. Analytical Chemistry 2015, 87, 216–229. [Google Scholar] [CrossRef]
- Casteleiro, B.; Ribeiro, T.; Mariz, I.; Martinho, J. M. G.; Farinha, J. P. S. : Encapsulation of gold nanoclusters by photo-initiated miniemulsion polymerization. Colloids and Surfaces A: Physicochemical and Engineering Aspects 2022, 129410. [Google Scholar] [CrossRef]
- Khan, Z.; Singh, T.; Hussain, J. I.; Hashmi, A. A. : Au(III)–CTAB reduction by ascorbic acid: Preparation and characterization of gold nanoparticles. Colloids and Surfaces B: Biointerfaces 2013, 104, 11–17. [Google Scholar] [CrossRef]
- Kubo, S.; Kosuge, K. : Salt-Induced Formation of Uniform Fiberlike SBA-15 Mesoporous Silica Particles and Application to Toluene Adsorption. Langmuir 2007, 23, 11761–11768. [Google Scholar] [CrossRef]
- Pereira, C.; Pereira, A. M.; Quaresma, P.; Tavares, P. B.; Pereira, E.; Araújo, J. P.; Freire, C. : Superparamagnetic γ-Fe2O3@SiO2 nanoparticles: a novel support for the immobilization of [VO(acac)2]. Dalton Transactions 2010, 39, 2842–2854. [Google Scholar] [CrossRef]
- Fidalgo, A.; Ilharco, L. M. : The defect structure of sol–gel-derived silica/polytetrahydrofuran hybrid films by FTIR. Journal of Non-Crystalline Solids 2001, 283, 144–154. [Google Scholar] [CrossRef]
- Dupuis, R.; Pellenq, R.; Champenois, J.-B.; Poulesquen, A. : Dissociation Mechanisms of Dissolved Alkali Silicates in Sodium Hydroxide. The Journal of Physical Chemistry C 2020, 124, 8288–8294. [Google Scholar] [CrossRef]
- Safdar, M. H.; Hasan, H.; Anees, M.; Hussain, Z. : FOLIC ACID-CONJUGATED DOXORUBICIN-LOADED PHOTOSENSITIZING MANGANESE FERRITE NANOPARTICLES: SYNTHESIS, CHARACTERIZATION AND ANTICANCER ACTIVITY AGAINST HUMAN CERVICAL CARCINOMA CELL LINE (HELA). International Journal of Pharmacy and Pharmaceutical Sciences 2017, 9, 60–67. [Google Scholar] [CrossRef]
- León-Félix, L.; Chaker, J.; Parise, M.; Coaquira, J. A. H.; De Los Santos Valladares, L.; Bustamante, A.; Garg, V. K.; Oliveira, A. C.; Morais, P. C. : Synthesis and characterization of uncoated and gold-coated magnetite nanoparticles. Hyperfine Interactions 2014, 224, 179–188. [Google Scholar] [CrossRef]
- Banerjee, S.; Raja, S. O.; Sardar, M.; Gayathri, N.; Ghosh, B.; Dasgupta, A. : Iron oxide nanoparticles coated with gold: Enhanced magnetic moment due to interfacial effects. Journal of Applied Physics 2011, 109, 123902–10. [Google Scholar] [CrossRef]
- Wang, L.; Park, H.-Y.; Lim, S. I. I.; Schadt, M. J.; Mott, D.; Luo, J.; Wang, X.; Zhong, C.-J. : Core@shell nanomaterials: gold-coated magnetic oxide nanoparticles. Journal of Materials Chemistry 2008, 18, 2629–2635. [Google Scholar] [CrossRef]
- Khan, G. G.; Sarkar, D.; Singh, A. K.; Mandal, K. : Enhanced band gap emission and ferromagnetism of Au nanoparticle decorated α-Fe2O3 nanowires due to surface plasmon and interfacial effects. RSC Advances 2013, 3, 1722–1727. [Google Scholar] [CrossRef]
- Agrachev, M.; Antonello, S.; Dainese, T.; Ruzzi, M.; Zoleo, A.; Aprà, E.; Govind, N.; Fortunelli, A.; Sementa, L.; Maran, F. : Magnetic Ordering in Gold Nanoclusters. ACS Omega 2017, 2, 2607–2617. [Google Scholar] [CrossRef]
- Tuboltsev, V.; Savin, A.; Pirojenko, A.; Räisänen, J. : Magnetism in Nanocrystalline Gold. ACS Nano 2013, 7, 6691–6699. [Google Scholar] [CrossRef]
- Krishna, K. S.; Tarakeshwar, P.; Mujica, V.; Kumar, C. S. S. R. : Chemically Induced Magnetism in Atomically Precise Gold Clusters. Small 2014, 10, 907–911. [Google Scholar] [CrossRef]
- Pereira, A. M.; Pereira, C.; Silva, A. S.; Schmool, D. S.; Freire, C.; Grenèche, J.-M.; Araújo, J. P. : Unravelling the effect of interparticle interactions and surface spin canting in γ-Fe2O3@SiO2 superparamagnetic nanoparticles. Journal of Applied Physics 2011, 109, 114319–10. [Google Scholar] [CrossRef]
- Huang, R.; Tang, T. : Assembly of Magnetic Nano-Fe3O4@GSH-Au NCs Core–Shell Microspheres for the Visualization of Latent Fingerprints. Nano 2018, 13, 1850128–10. [Google Scholar] [CrossRef]
- Xiao, X.; Yang, H.; Jiang, P.; Chen, Z.; Ji, C.; Nie, L. : Multi-Functional Fe3O4@mSiO2-AuNCs Composite Nanoparticles Used as Drug Delivery System. Journal of Biomedical Nanotechnology 2017, 13, 1292–1299. [Google Scholar] [CrossRef]
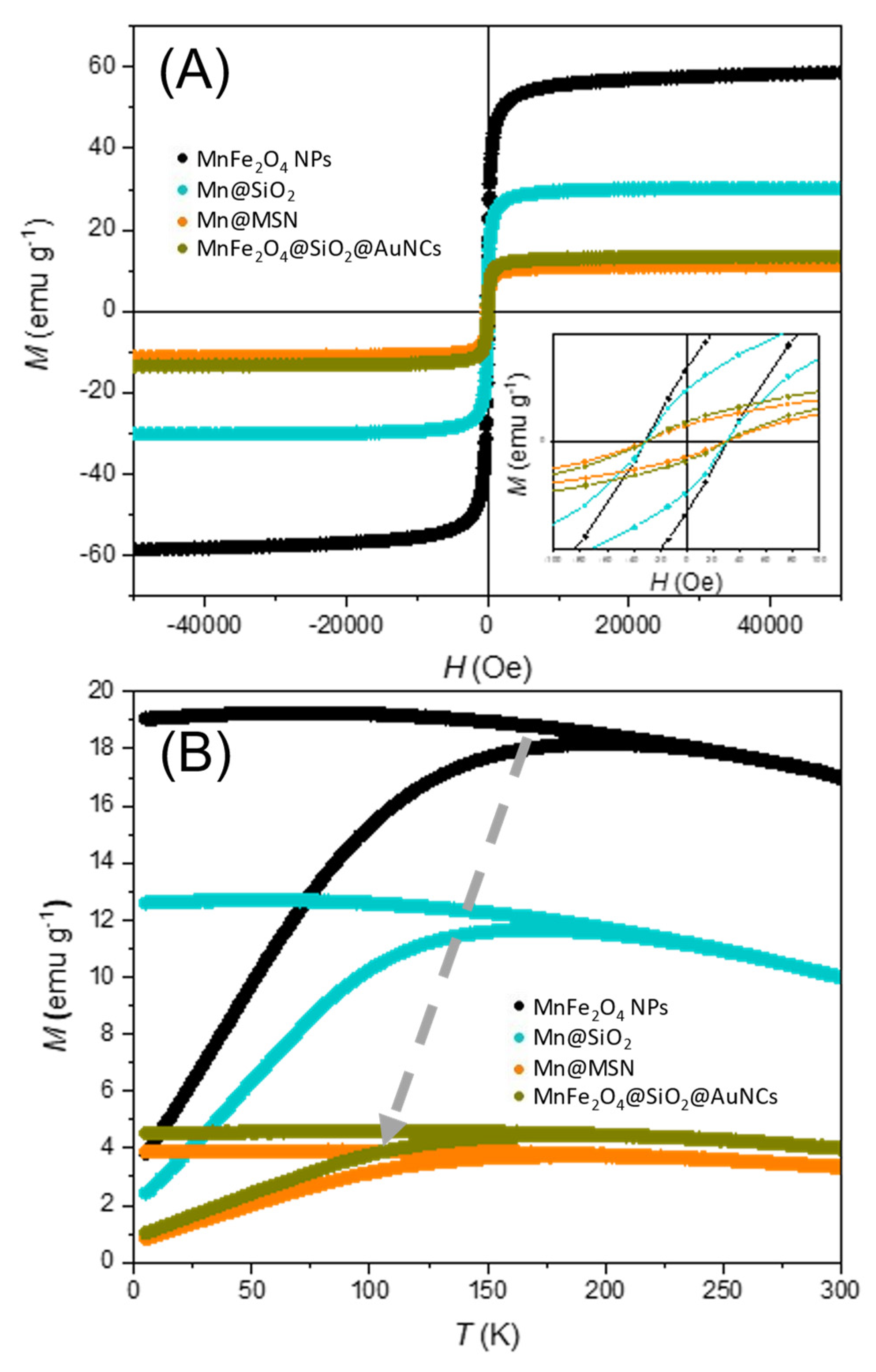
| Sample | MS @ 5 K (emu/g) | MS @ 300 K (emu/g) | HC @ 300 K (Oe) | Trev(K) |
| MnFe2O4 | 85.4 | 58.7 | 31.0 | 253 |
| Mn@SiO2 | 44.7 | 30.1 | 30.3 | 245 |
| Mn@SiO2@MSN | 17.3 | 11.2 | 30.3 | 217 |
| Mn@SiO2@AuNCs | 19.0 | 13.4 | 30.2 | 241 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).





