Submitted:
01 October 2023
Posted:
02 October 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Outcomes
2.3. Parameters
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Zhang, Y.; Lan, F.; Zhang, L. Advances and highlights in allergic rhinitis. Allergy 2021, 76, 3383–3389. [Google Scholar] [CrossRef] [PubMed]
- Bousquet, J.; Melén, E.; Haahtela, T.; Koppelman, G.H.; Togias, A.; Valenta, R.; Akdis, C.A. Rhinitis associated with asthma is distinct from rhinitis alone: The ARIA-MeDALL hypothesis. Allergy 2023, 78, 1169–1203. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Wang, Z.; Zhu, Y.; Zhu, X.; Guo, L.; Fu, Y.; Zhang, Q.; Mou, X.; Liu, Y. MiR-223-3p regulates the eosinophil degranulation and enhances the inflammation in allergic rhinitis by targeting FBXW7. Int Immunopharmacol 2023, 118, 110007. [Google Scholar] [CrossRef] [PubMed]
- Ricca, V.; Landi, M; Ferrero, P.; Bairo, A.; Tazzer, C.; Ciprandi, G. Minimal persistent inflammation is also present in patients with seasonal allergic rhinitis. J Allergy Clin Immunol 2000, 105, 54–57. [CrossRef] [PubMed]
- Siddiqui, Z.A.; Walker, A.; Pirwani, M.M.; Tahiri, M.; Syed, I. Allergic rhinitis: diagnosis and management. Br J Hosp Med 2022, 83, 1–9. [Google Scholar] [CrossRef]
- Li, A.R.; Zhang, K.; Reddy, P.D.; Nguyen, S.A.; Miglani, A.; Fried, J.; Nguyen, M.I.; Schlosser, R.J. Systematic review of measures of disease severity in rhinitis. Int Forum Allergy Rhinol 2021, 11, 1367–1377. [Google Scholar] [CrossRef]
- Ciprandi, G.; Mora, F.; Cassano, M.; Gallina, A.M.; Mora, R. Visual analog scale (VAS) and nasal obstruction in persistent allergic rhinitis. Otolaryngol Head Neck Surg 2009, 141, 527–529. [Google Scholar] [CrossRef]
- Mora, F.; Cassano, M.; Mora, R.; Gallina, A.M.; Ciprandi, G. V.A.S. the follow-up of turbinectomy. Rhinology 2009, 47, 450–453. [Google Scholar]
- Tosca, M.A.; Silvestri, M.; Olcese, R.; Pistorio, A.; Rossi, G.A.; Ciprandi, G. Breathlessness perception assessed by visual analogue scale and lung function in children with asthma: a real-life study. Pediatr Allergy Immunol 2012, 23, 537–542. [Google Scholar] [CrossRef]
- Tosca, M.A.; Silvestri, M.; Rossi, G.A.; Ciprandi, G. Perception of bronchodilation assessed by Visual Analogue Scale in children with asthma. Allergol Immunopathol (Madr) 2013, 41, 359–363. [Google Scholar] [CrossRef]
- Bousquet, J.; Schünemann, H.J.; Togias, A.; Bachert, C.; Erhola, M.; Hellings, P.W.; Klimek, L.; Pfaar, O.; Wallace, D.; Ansotegui, I.; et al. Next-generation Allergic Rhinitis and Its Impact on Asthma (ARIA) guidelines for allergic rhinitis based on Grading of Recommendations Assessment, Development and Evaluation (GRADE) and real-world evidence. J Allergy Clin Immunol 2020, 145, 70–80. [Google Scholar] [CrossRef] [PubMed]
- Global Initiative for Asthma Global Strategy for Asthma Management and Prevention (2023). 2023. Available online: www.ginasthma.org (accessed on 13 September 2023).
- Tosca, M.A.; Del Barba, P.; Licari, A.; Ciprandi, G. . The Measurement of Asthma and Allergic Rhinitis Control in Children and Adolescents. Children (Basel) 2020, 7, 43. [Google Scholar] [CrossRef] [PubMed]
- Tosca, M.A.; Marseglia, G.L.; Ciprandi, G. The real-world "ControL'Asma" study: a nationwide taskforce on asthma control in children and adolescents. Allergologia et Immunopathologia 2021, 49, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Schuler IV, C.F.; Montejo, J.M. Allergic Rhinitis in Children and Adolescents. Immunol Allergy Clin North Am 2021, 41, 613–625. [Google Scholar] [CrossRef]
- Meltzer, E.O.; Blaiss, M.S.; Naclerio, R.M. Burden of allergic rhinitis: allergies in America, Latin America, and Asia-Pacific adult surveys. Allergy Asthma Proc 2012, 33 (Suppl 1), S113–41. [Google Scholar] [CrossRef]
- Meltzer, E.O. Allergic rhinitis: burden of illness, quality of life, comorbidities, and control. Immunol Allergy Clin North Am 2016, 36, 235–248. [Google Scholar] [CrossRef]
- Muliol, J.; Maurer, M.; Bousquet, J. Sleep and allergic rhinitis. J Investig Allergol Clin Immunol 2008, 18, 415–419. [Google Scholar]
- Colas, C.; Galera, H.; Anibarro, B. Disease severity impairs sleep quality in allergic rhinitis (The SOMNIAAR study). Clin Exp Allergy 2012, 42, 1080–1087. [Google Scholar] [CrossRef]
- Bousquet, J.; Anto, J.M.; Bachert, C.; Baiardini, I.; Bosnic-Anticevich, S.; Melén, E.; Palomares, O.; Scadding, G.K.; Togias, A. , Toppila-Salmi, S. Allergic rhinitis. Nat Rev Dis Primers 2020, 6, 95. [Google Scholar] [CrossRef]
- Carr, W.W.; Yawn, B.P. Management of allergic rhinitis in the era of effective over-the-counter treatments. Postgrad Med 2017, 129, 572–580. [Google Scholar] [CrossRef]
- Bousquet, J.; Schunemann, H.J.; Fonseca, J.; Samolinski, B.; Bachert,, C. MACVIA-ARIA Sentinel NetworK for Allergic Rhinitis (MASK-Rhinitis): The New Generation Guideline Implementation. Allergy 2015, 70, 1372–1392. [CrossRef] [PubMed]
- Del Cuvillo, A.; Santos, V.; Montoro, J.; Bartra, J.; Davila, I.; Ferrer, M.; Jauregui, I.; Sastre, J.; Mullol, J.; Valero, A. Allergic rhinitis severity can be assessed using a visual analogue scale in mild, moderate and severe. Rhinology 2017, 55, 34–38. [Google Scholar] [CrossRef] [PubMed]
- Tosca, M.A.; Pistorio, A.; Silvestri, M.; Marseglia, G.L.; Ciprandi, G. The comparison between children and adolescents with asthma provided by the real-world "ControL'Asma" study. J Asthma 2022, 59, 1531–1536. [Google Scholar] [CrossRef]
- Abdullah, B.; Snidvongs, K.; Lestari Poerbonegoro, N.; Sutikno, B. Reshaping the Management of Allergic Rhinitis in Primary Care: Lessons from the COVID-19 Pandemic. Int J Environ Res Pub Health 2022, 19, 13632. [Google Scholar] [CrossRef] [PubMed]
- Thong, H.K.; Wong, D.K.C.; Gendeh, H.S.; Saim, L.; Athar, P.P.; Saim, A. Perception of telemedicine among medical practitioners in Malaysia during COVID-19. J Med Life 2021, 14, 468–480. [Google Scholar] [CrossRef] [PubMed]
- Thomas, I.; Siew, L.; Rutkowski, K. Synchronous Telemedicine in Allergy: Lessons Learned and Transformation of Care During the COVID-19 Pandemic. J Allergy Clin Immunol Pract 2021, 9, 170–176.e1. [Google Scholar] [CrossRef]
- Waibel, K.H.; Bickel, R.A.; Brown, T. Outcomes from a Regional Synchronous Tele-Allergy Service. J Allergy Clin Immunol Pract 2019, 7, 1017–1021. [Google Scholar] [CrossRef]
- Tan, R.; Cvetkovski, B.; Kritikos, R.; O’Hehir, R.E.; Lourenco, O.; Bousquet, J. Identifying an effective mobile health application for the self-management of allergic rhinitis and asthma in Australia. J Asthma 2019, 57, 1–15. [Google Scholar] [CrossRef]
- Thakkar, J.; Kurup, R.; Laba, T.L.; Santo, K.; Thiagalingam, A.; Rodgers, A. Mobile telephone text messaging for medication adherence in chronic disease: A meta-analysis. JAMA Intern Med 2016, 176, 340–349. [Google Scholar] [CrossRef]
- Bousquet J, Addis A, Adcock I, Agache I, Agusti A, Alonso A, et al. Integrated care pathways for airway diseases (AIRWAYS-ICPs). Eur Respir J 2014, 44, 304–323.
- Bousquet, J.; Arnavielhe, S.; Bedbrook, A.; Bewick, M.; Laune, D.; Mathieu-Dupas, E. MASK 2017: ARIA digitally-enabled, integrated, person-centred care for rhinitis and asthma multimorbidity using real-world-evidence. Clin Transl Allergy 2018, 8, 45. [Google Scholar] [CrossRef] [PubMed]
- Menditto, E.; Costa, E.; Midao, L.; Bosnic-Anticevich, S.; Novellino, E.; Bialek, S. Adherence to treatment in allergic rhinitis using mobile technology. The MASK Study. Clin Exp Allergy 2019, 49, 442–460. [Google Scholar] [CrossRef] [PubMed]
- Bedard, A.; Basagana, X.; Anto, J.M.; Garcia-Aymerich, J.; Devillier, P.; Arnavielhe, S. Mobile technology offers novel insights on control and treatment of allergic rhinitis. The MASK study. J Allergy Clin Immunol 2019, 144, 135–143.e6. [Google Scholar] [CrossRef] [PubMed]
- Sousa-Pinto, B.; Sa-Sousa, A.; Vieira, R.J.; Amaral, R.; Klimek, L.; Czarlewski, W. Behavioural patterns in allergic rhinitis medication in Europe: A study using MASK-air((R)) real-world data. Allergy 2022, 77, 2699–2711. [Google Scholar] [CrossRef] [PubMed]
- Vandenplas, O.; Suarthana, E.; Rifflart, C.; Lemiere, C.; Le Moual, N.; Bousquet, J. The Impact of Work-Related Rhinitis on Quality of Life and Work Productivity: A General Workforce-Based Survey. J Allergy Clin Immunol Pract 2020, 8, 1583–1591.e5. [Google Scholar] [CrossRef]
- Mitsias, D.I.; Dimou, M.V.; Lakoumentas, J.; Alevizopoulos, K.; Sousa-Pinto, B.; Fonseca, J.A.; Bousquet, J. Effect of nasal irrigation on allergic rhinitis control in children; complementarity between CARAT and MASK outcomes. Clin Transl Allergy 2020, 10, 9. [Google Scholar] [CrossRef]
- Brown, T. Diagnosis and Management of Allergic Rhinitis in Children. Pediatr Ann 2019, 48, e485–e488. [Google Scholar] [CrossRef]
- Meltzer, E.O.; Wallace, D.; Friedman, H.S.; Navaratnam, P.; Scott, E.P.; Nolte, H. Meta-analyses of the efficacy of pharmacotherapies and sublingual allergy immunotherapy tablets for allergic rhinitis in adults and children. Rhinology 2021, 59, 422–432. [Google Scholar] [CrossRef]
- Ciprandi, G.; Cirillo, I.; Vizzaccaro, A.; Milanese, M.; Tosca, M.A. Nasal obstruction in patients with seasonal allergic rhinitis: relationships between allergic inflammation and nasal airflow. Int Arch Allergy Immunol 2004, 134, 34–40. [Google Scholar] [CrossRef]
- Weaver-Agostoni, J.; Kosak, Z.; Bartlett, S. Allergic Rhinitis: Rapid Evidence Review. Am Fam Physician 2023, 107, 466–473. [Google Scholar]
- Paller, A.S.; Spergel, J.M.; Mina-Osorio, P.; Irvine, AD. The atopic march and atopic multimorbidity: Many trajectories, many pathways. J Allergy Clin Immunol 2019, 143, 46–55. [Google Scholar] [CrossRef] [PubMed]
- Passalacqua, G.; Ciprandi, G.; Pasquali, M.; Guerra, L.; Canonica, G.W. An update on the asthma-rhinitis link. Curr Opin Allergy Clin Immunol 2004, 4, 177–183. [Google Scholar] [CrossRef] [PubMed]
- Bousquet, J.; Melén, E.; Haahtela, T.; Koppelman, G.H.; Togias, A.; Valenta, R.; Akdis, C.A.; Czarlewski, W.; Rothenberg, M.; Valiulis, A.; et al. Rhinitis associated with asthma is distinct from rhinitis alone: The ARIA-MeDALL hypothesis. Allergy 2023, 78, 1169–1203. [Google Scholar] [CrossRef] [PubMed]
| Sex | Female | 56 (37.3%) |
|---|---|---|
| Male | 94 (62.7%) | |
| Age | 11.8 ± 3.06 | |
| VAS for nasal symptoms’ severity (perceived by patients) | 6.3 (5.0 - 8.0) | |
| VAS for doctor’s assessment of rhinitis | 7.0 (6.0 - 8.0) | |
| Antihistamines | 75 (50.0%) | |
| Intranasal Corticosteroids | 24 (16.0%) | |
| Specific allergen immunotherapy (AIT) | 26 (17.3%) | |
| Sensitizations | Mono-sensitization | 10 (6.7%) |
| Poly-sensitization | 140 (93.3%) | |
| Asthma comorbidity | 100 (67.1%) | |
| Treatment for asthma | 46 (46.0%) | |
| VAS for asthma symptoms’ severity (perceived by patients with asthma) | 7.0 (6.0 - 8.5) | |
| VAS for medical assessment of asthma | 7.0 (5.0 - 9.0) | |
| Abnormal (N = 75) | Normal (N = 75) | p | ||
|---|---|---|---|---|
| Sex | Female | 30 (40.0%) | 26 (34.7%) | 0.50 |
| Male | 45 (60.0%) | 49 (65.3%) | ||
| Age | 12.1 ± 2.98 | 11.6 ± 3.15 | 0.29 | |
| Antihiostamines | No | 35 (46.7%) | 40 (53.3%) | 0.41 |
| Yes | 40 (53.3%) | 35 (46.7%) | ||
| Intranasal Corticosteroids | No | 61 (81.3%) | 65 (86.7%) | 0.37 |
| Yes | 14 (18.7%) | 10 (13.3%) | ||
| Specific allergen immunotherapy (AIT) | No | 63 (84.0%) | 61 (81.3%) | 0.67 |
| Yes | 12 (16.0%) | 14 (18.7%) | ||
| Mono- or poly-sensitization | Mono-sensitization | 3 (4.0%) | 7 (9.3%) | 0.19 |
| Poly-sensitization | 72 (96.0%) | 68 (90.7%) | ||
| Asthma comorbidity | No | 27 (36.0%) | 22 (29.7%) | 0.42 |
| Yes | 48 (64.0%) | 52 (70.3%) | ||
| Treatment for asthma | No | 27 (55.1%) | 27 (52.9%) | 0.83 |
| Yes | 22 (44.9%) | 24 (47.1%) | ||
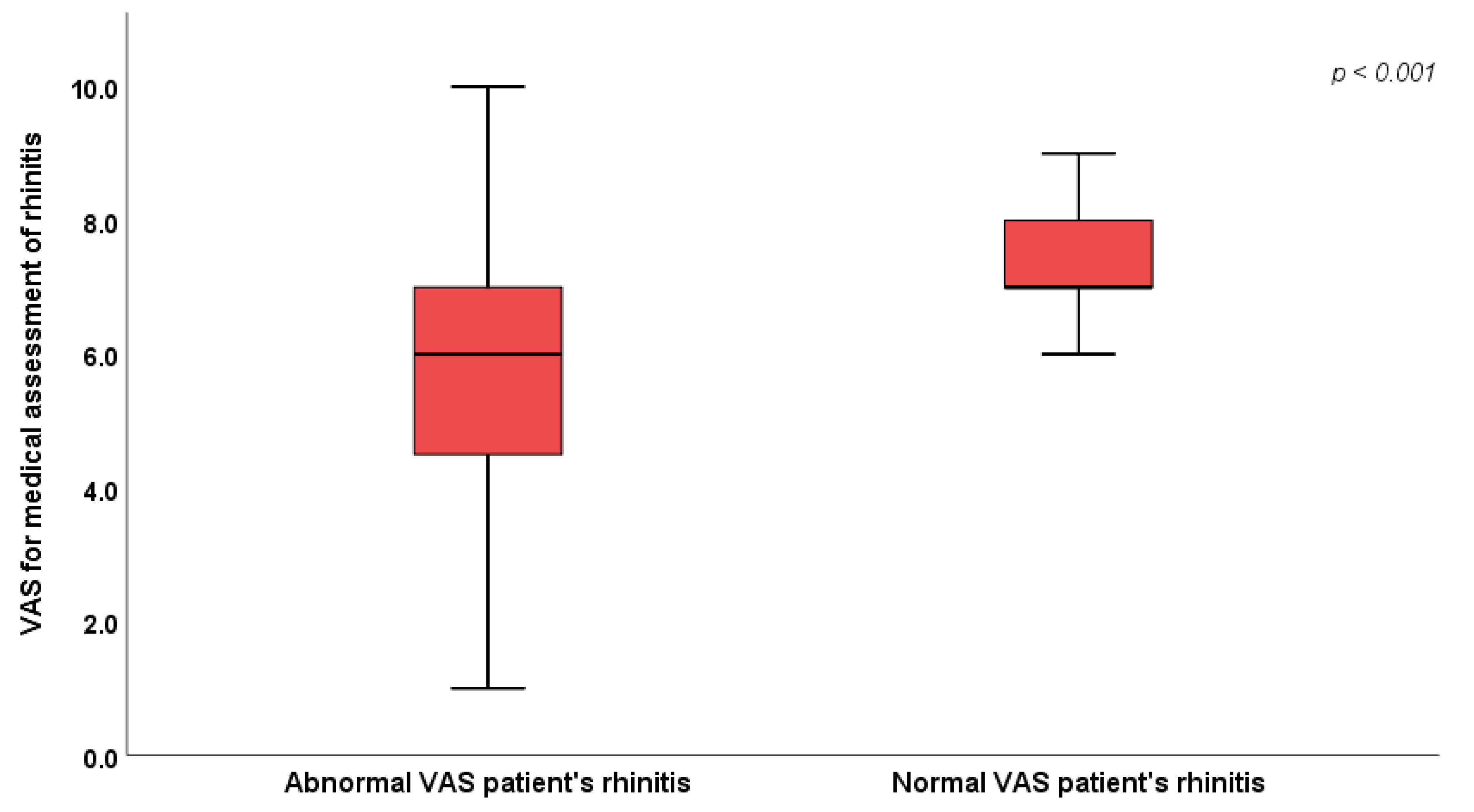
| VAS for medical assessment of rhinitis | 6.0 (4.0 – 7.0) | 7.0 (7.0 – 8.0) | <0.001* | |
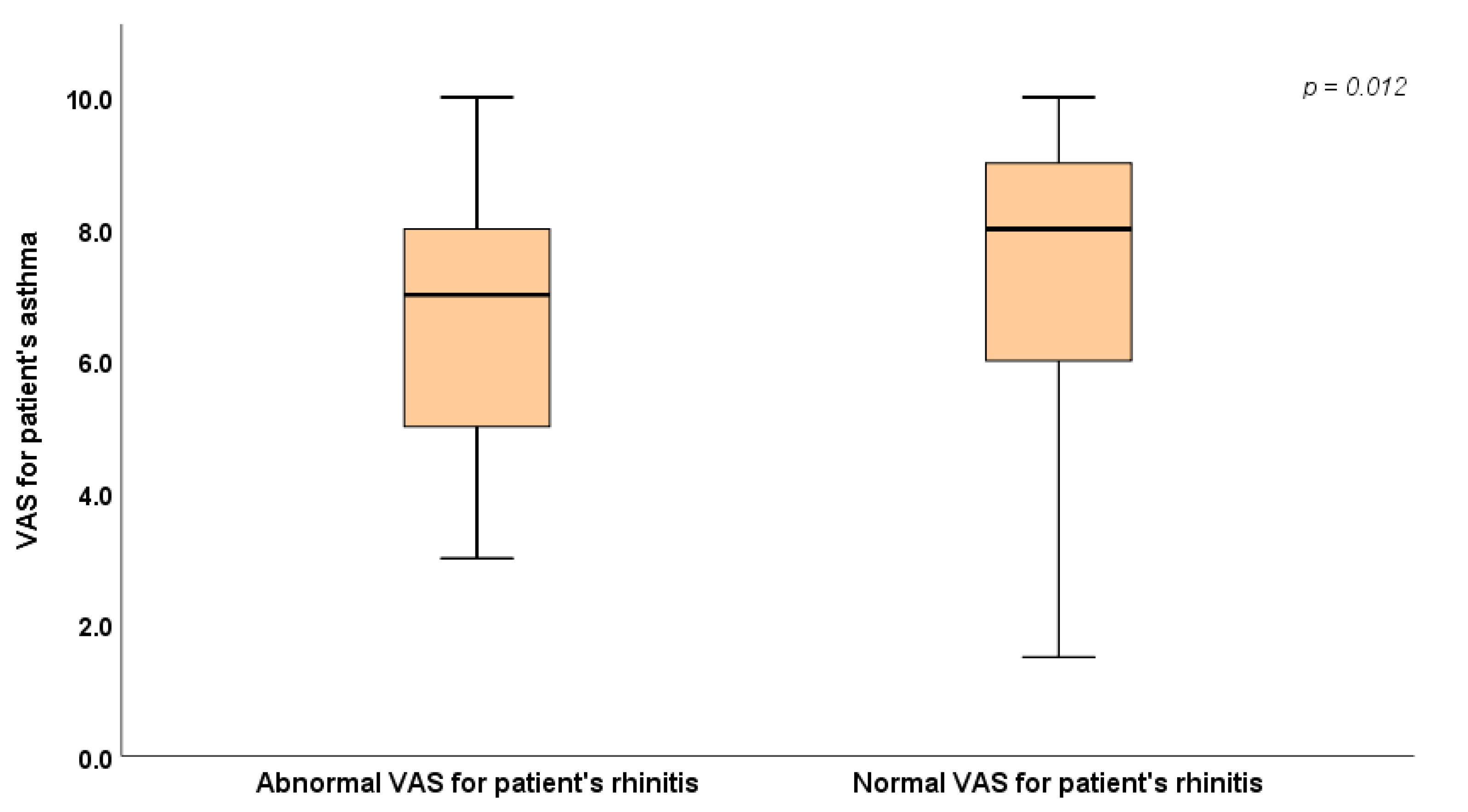
| VAS for patient's asthma (subgroup of patients with asthma) | 7.0 (5.0 - 8.0) | 8.0 (6.0 - 9.0) | 0.012* | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





