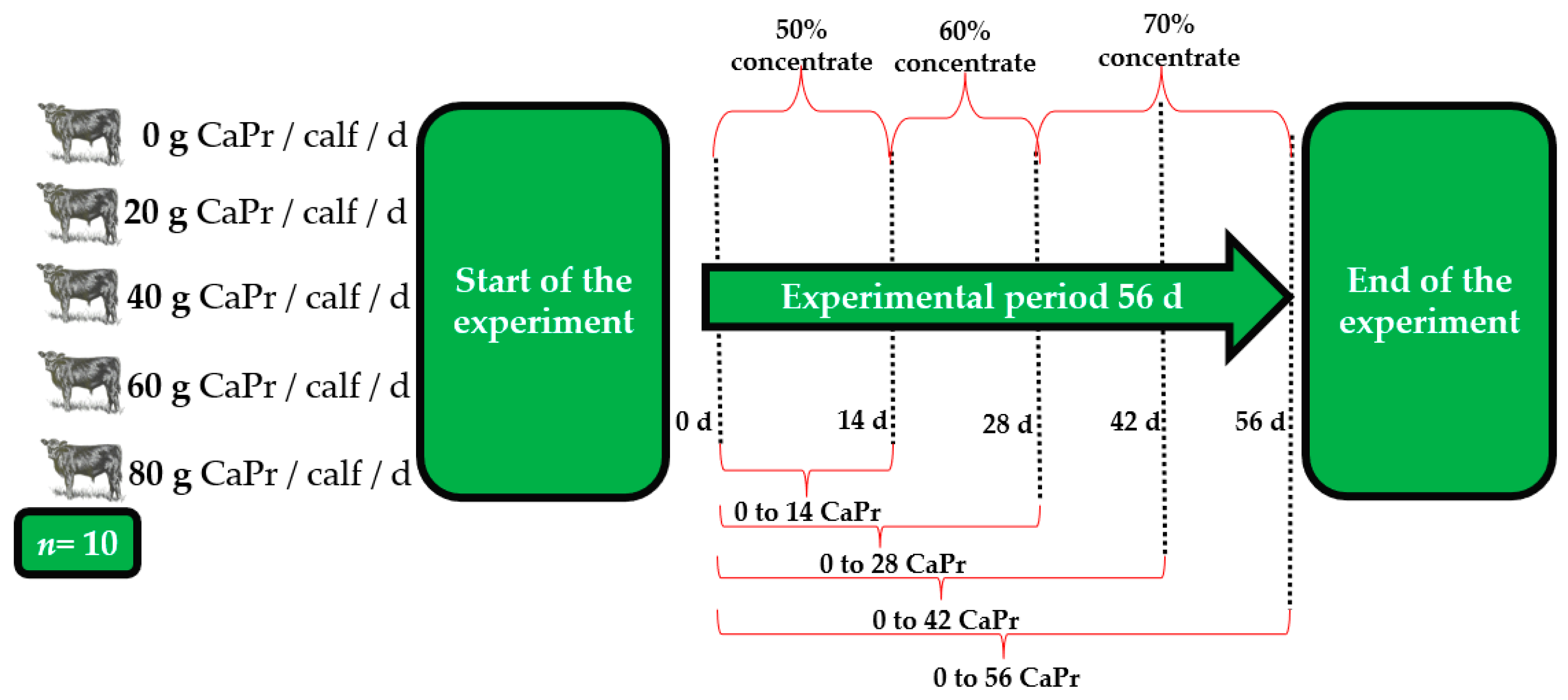
4.1. Growth Performance
The increment of energy in calf receiving diets through gluconeogenic precursors is quite limited. However, various publications concur that with receiving diets providing increasing levels of energy from concentrates, DMI, ADG and ADG:DMI ratio, significantly improve [
25,
26,
27]. Moreover, different authors with various additives agree that the most significant improvements in productive behavior are observed during the first 30 d of reception [
28,
29,
30]. On the other hand, when CaPr is supplemented in the diet and reaches the rumen, it undergoes hydrolysis at an acidic pH, resulting in the formation of Ca
2+ and propionic acid [
18]. Additionally, in the rumen: 1) The pattern of volatile fatty acids is altered [
16]; 2) Methane production decreases; 3) Digestibility of dry matter increases; 4) Fermentation efficiency improves [
15]; 5) Insulin response capacity in GLU metabolism improves [
17] and 6) Body fat reserves increase [
20]. As a cumulative result of these mechanisms, there is a promotion of energy status achieved through increased GLU synthesis via gluconeogenesis [
18]. This results in improvements in DMI, ADG, ADG:DMI ratio, and BW during the finishing phase [
19,
20].
The level of 20 g did not affect DMI at any point during the reception period. However, starting from d 28 up to d 56, there was a reduction in DMI with the increasing CaPr levels (40 to 80 g), reaching as low as 19.6% with 80 g. Similarly, when gluconeogenic precursor crude glycerin is included in the beef cattle diet at increasing proportions, DMI decreases [
31,
32,
33]. Hales et al. [
34] also described that DMI reduction in beef cattle, as crude glycerin concentration is increased, seems consistent throughout literature. A similar pattern has been observed with increasing levels of concentrate in the diets of receiving calves. There are maximal DMI levels with 60 to 72% concentrate and reductions with 90% concentrate during the first 28 d [
11,
25,
35]. In agreement, but in lambs fed finishing diets, Carrillo-Muro et al. [
19] observed reductions in DMI of 14.3% with the higher levels of 30 g CaPr, whereas with 10 g, DMI increased by 1.1% during the first 28 d [
20].
In this study, DWI was not affected when 20 g of CaPr was included, but it increased by 4.6% within the first 14 d as the inclusion level increased. In contrast, Carey et al. [
33] added crude glycerin to the drinking water of receiving calves and did not observe any effects on DWI. However, Lofgreen et al. [
25] reported that DWI was 16.6% higher at 28 d with diets containing less concentrate (20%).
Starting from day 28, there were increases of 11.8% in ADG and 4.6% in BW, with 20 g of CaPr, reaching 13.3% ADG and 4.9% BW at day 42 compared to CTL. Nevertheless, as the levels of CaPr supplementation increased, ADG decreased. In line with these findings, in lambs finished diets, Carrillo-Muro et al. [
19] observed reductions of 16.0% in ADG and 2.8% in BW with the highest levels of CaPr, ranging from 20 to 30 g. Conversely, with 10 g of CaPr during the first 28 days, they observed an increase of 26.8% in ADG and 4.7% in BW [
20]. Additionally, Lofgreen et al. [
25], reported that ADG improved after the first 7 d of calf arrival and continued to increase until d 28, especially with higher values of ENg. Similarly, Lofgreen [
27] using a diet containing 75% concentrate, detected a 6.5% increase in ADG compared to a 50% concentrate diet. In contrast, Pritchard y Méndez [
35] reported increased ADG during the 28-day reception period in calves fed diets with lower energy content compared to a diet containing 60% concentrate. However, Ladeira et al. [
32] who used various proportions of glycerin in bull diets, did not observe any effects on ADG and BW.
As for the ADG:DMI ratio, it improved by 16.7% during the first 28 d with 20 g of CaPr supplementation, but decreased with the CTL and the other levels. Similarly, Carrillo-Muro et al. [
19] in lambs finished diets observed a 5.9% improvement with the lowest level, 10 g CaPr, and a 25% improvement during the first 28 d with 10 g [
20]. In contrast, Ladeira et al. [
32] used different proportions of glycerin in bulls and found that as the level increased, the ADG:DMI ratio also increased. Fluharty and Loerch [
11] in receiving calves with increasing proportions of concentrate (70, 75, 80 and 85%), reported that this increase was more significant at d 14. Also, Lofgreen [
27] detected a 17.5% increase in the ADG:DMI ratio with 75% concentrate compared to 50% concentrate. Contrastingly, Pritchard y Méndez [
35] reported an increase in the ADG:DMI ratio during the first 28 d of the receiving period in calves fed diets with lower energy compared to a diet with 60% concentrate.
The reduced growth performance of received beef calves with higher levels of 40, 60, and 80 g CaPr can be explained by the decrease in DMI. This reduction effect on DMI is known as the hepatic oxidation theory (HOT) and was described by Allen [
36] to explain the role of the ruminant liver in signaling and controlling satiety through temporal patterns of various oxidative products, including propionic acid. Signals are transmitted from the liver to the brain through afferents in the vagus nerve and are affected by hepatic oxidation and the generation of ATP [
37,
38]. Additionally, the elevated levels of CaPr may have increased insulin secretion, leading to a reduction in DMI. Insulin reaches the brain and binds to its specific receptors on neurons, resulting in reduced DMI [
39,
40].
4.2. Enzymatic Activity
Blood measurements of enzyme activity including ALP, GGT, and AST were conducted to assess whether different levels of CaPr could impact liver or kidney functions or improve metabolism in these organs. It was observed that ALP activity decreased with the increase in the CaPr level, but the values remained within the established normal ranges (0 to 488 [
41]). Otter [
42] noted that physiologically higher ALP activity occurs in young growing cattle and is of bone origin, and increases are associated with osteoblast proliferation. Based on this, it can be inferred that received beef calves with 80 g of CaPr might reduce bone growth. As for GGT, its activity was highest with 20 g, increasing by 37.3%, slightly exceeding the reference range (6.1 to 17.4 [
41]). This could be attributed to increased liver activity in these calves [
43]. However, Ladeira et al. [
32] used different proportions of glycerin in bulls and did not observe any effects on GGT. AST activity with different levels of CaPr supplementation did not produce any effects and remained within normal ranges (48 to 100 [
44]). This aligns with Ladeira et al. [
32] in bulls and de Freitas et al. [
45] in ewes, who used different proportions of glycerin and also did not observe any effects on AST. In contrast, Silva et al. [
46] with crude glycerin in beef cattle, observed increments in AST with increasing inclusion. Carlson [
47] states that this enzyme is a nonspecific indicator of tissue damage. Muscle injury or necrosis, especially in recumbent animals, may result in marked increases in AST activities. Finally, the enzyme activity values for ALP, GGT, and AST were below the pathological range, suggesting no liver or kidney damage or improvements in the metabolism of these organs associated with CaPr supplementation in calves reception.
4.3. Serum Metabolites
The TP, which primarily includes ALB and GLO, is the main solid component of serum and serves as an indicator of an animal's nutritional status [
48]. In the current study, concentrations of TP and ALB were not affected by CaPr supplementation. Nonetheless, TP was slightly below the reference range (6.74 to 7.46 [
41]), while ALB values were within the normal range (2.7 to 4.2 [
44]). Regarding TP, this aligns with de Freitas et al. [
45] in ewes, using different proportions of crude glycerin supplementation, as they did not observe any effects on TP. Nevertheless, the concentration of GLO was slightly above the reference range (3.0 to 3.48 [
41]) with the inclusion of 40 to 80 g. Hyperproteinemia results from elevated levels of ALB, GLO, or both. Dehydration is the sole cause of hyperalbuminemia; in dehydration, both ALB and GLO levels increase. However, if there is hyperproteinemia without concurrent dehydration, it is almost always the result of hyperglobulinemia. Common causes of hyperglobulinemia include chronic antigenic stimulation and liver disease. Chronic antigenic stimulation can generally be observed in various conditions such as traumatic reticuloperitonitis, liver abscesses, or chronic pneumonia [
23]. Based on this, it can be assumed that with higher levels of 40 to 80 g CaPr, there may be chronic liver inflammation.
As for the BUN levels, they are typically used to estimate nitrogen excretion and utilization efficiency [
49]. In ruminants, BUN concentrations are influenced by various factors, including CP intake, rumen degradability, and liver and kidney function [
50]. Specifically, supplementation with 20 g reduced serum BUN levels; yet, all these values were within the normal range (10 to 25 [
51]). Carrillo-Muro et al. [
19] observed in lambs finished diets that with higher levels of 20 CaPr, BUN increased. Similarly, de Freitas et al. [
45] in ewes and Carey et al. [
33] in newly received beef calves observed increases in BUN with the highest proportions of crude glycerin. Waggoner et al. [
52] pointed out that calves with immunological issues have lower N retention, probably due to increased muscle catabolism to obtain proteins and enhance the immune response. Based on the aforementioned principles, it can be inferred that supplementation with 20 g CaPr in high-risk, newly received stocker calves promotes nitrogen utilization and reduces muscle protein catabolism. Conversely, the opposite occurs with elevated levels of 40 to 80 g or 0.
Serum creatinine (CRE) concentrations were within the normal range (1 to 2 mg/dL) reported by Kaneko et al. [
41]. This indicates that the renal glomerular filtration rate for CRE was adequate, without interference from CaPr. However, it was observed that CRE increased as the level of CaPr increased. In contrast to Ladeira et al. [
32] with bulls and de Freitas et al. [
45] in ewes, who used different levels of glycerin and did not observe effects on CRE. Otter [
42] noted that CRE can be low in emaciated cattle or those with low muscle mass or elevated in heavily muscled animals. This result suggests that a higher level of CaPr (40 to 80 g) promotes more muscle deposition than fat, which aligns with the higher values of BFT and FTR observed with 20 g.
In the current study, total bilirubin (TBIL) concentrations were not affected by CaPr supplementation and remained within the normal range (0.01 to 0.5 [
41]). TBIL is an important indicator of liver function, as it increases during severe lipidosis [
53,
54], and decreases in concentration when the liver is healthy. Therefore, based on these TBIL values, it can be concluded that different levels of CaPr do not have negative effects on liver function.
Serum lipids primarily consist of TCHO and TG. TCHO was below the range (73 to 280 [
51]), but increased as the level of CaPr increased, with only the 80 g CaPr falling within the range. No treatment effects were observed on TG, but all values were above the range (0 to 14 [
41]). The 80 g CaPr level increased TCHO levels, likely due to the increased production of propionic acid in the rumen, subsequently leading to increased TCHO production in the liver. The decrease in serum TCHO levels in this study indicates an energy deficit, while increases occur in response to the ingestion of energy-rich lipid-containing foods [
55]. As crude glycerin inclusion increased, the same conclusion was drawn by Silva et al. [
46] in beef cattle and de Freitas et al. [
45] in ewes, where they observed increases in TCHO. However, in lambs finished diets, Carrillo-Muro et al. [
19] did not observe effects with levels of 10, 20, or 30 g CaPr/lamb/d on TCHO and TG; similarly, de Freitas et al. [
45] did not observe effects with different proportions of crude glycerin inclusion. Ndlovu et al. [
56] pointed out that TCHO concentration reflects the energy metabolism in the liver, particularly lipid export in the form of very low-density lipoproteins.
The CaPr supplemented in the present study, comprising 20% Ca and 69% propionic acid, provided additional calcium beyond the nutritional requirements of the calves, which were already met by the basal diet. Consequently, it was observed that as the levels of CaPr inclusion increased, blood calcium concentration also increased, with all levels above the reference range (8.3 to 10.4 [
50]). This aligns with what Russell and Roussel [
23] mentioned, stating that hypercalcemia is fairly rare in ruminants and usually occurs as a result of the administration of Ca solutions or gels.
GLU concentrations are considered metabolic indicators of nutrient intake in beef cattle [
57]. No treatment effects were observed on GLU; however, all values were above the reference range (45 to 75 [
41]). In line with this, in lambs fed different levels of CaPr in their diets, Carrillo-Muro et al. [
19] did not observe any effects on GLU with levels of 10, 20, or 30 g CaPr. Similarly, Silva et al. [
46] in beef cattle and de Freitas et al. [
45] in ewes, did not observe effects on GLU with different proportions of crude glycerin inclusion. In contrast, Ladeira et al. [
32] used different proportions of glycerin in bulls and observed that GLU decreased as the level of inclusion increased. These GLU concentrations within the range for all treatments are related to adequate DMI intake since circulating GLU is influenced by nutrient availability [
58]. Likewise, Oosthuysen et al. [
59] reported that elevated blood GLU concentrations suggest improved energy status associated with better utilization of dietary nutrients.
The different levels of CaPr supplementation did not produce any effects or differences in electrolytes (Na
+, K
+ and Cl
-). However, the values of Na
+ and Cl
- were below the range, 132 to 152 and 97 to 111, respectively [
41]. These low values of Na
+ and Cl
- in the calves were due to the diet not completely meeting their nutritional requirements. Radostits et al. [
60] note that the most common causes of hyponatremia are the lack or inadequate level of Na
+ in the diet, and alterations in Cl
- concentration are generally associated with proportional changes in Na
+ concentration, resulting from changes in relative water balance [
47]. Another common reason for reduced Na
+ and Cl
- levels in reception calves is diarrhea, which, however, did not occur in our calves [
47]. Regarding K
+, deficiency is commonly associated with stressed cattle due to dehydration and loss of K
+ from tissues [
61].
4.4. Body Fat Reserves and Longissimus Muscle Area
Assessing lipid reserves in received calves can provide valuable insights into their nutritional status. Energy is stored in the body in the form of lipids [
7], primarily TG [
62]. When catabolized, lipids are highly efficient in energy production, yielding up to 9.4 Mcal/kg, whereas carbohydrates produce 4.2 and proteins 5.6 Mcal/kg [
63,
64]. Therefore, energy from body fat reserves can be nearly twice as much as that derived from muscles. When energy is limited in the organism, body fat reserves are the first to be depleted through adipose tissue lipolysis, releasing TG [
65]. Consequently, body fat reserves are influenced by factors such as 1) reproductive potential [
66]; 2) negative energy balance [
67]; 3) feeding level; and 4) nutrient composition [
68].
The results regarding BFT, FTR and LMA align with expectations, as there is a specific order in tissue deposition depending on the growth curve stage of young animals. Growth initially prioritizes bone and muscle, followed by fat accumulation, with higher energy or protein content in the diet stimulating fat deposition [
69,
70]. Therefore, with 20 g CaPr, LMA showed a maximum increase of 23.9% up to d 28, and thereafter, from d 42 onwards, BFT increased by 24.9%, while FTR increased by 21% up to the d 56. In accordance, Carrillo-Muro et al. [
20] observed in lamb finishing diets that, with the lowest level of 10 g CaPr, BFT increased by up to 30% by d 42 as the inclusion period increased. In contrast, Ladeira et al. [
32], who used different proportions of glycerin in bulls, did not observe effects on BFT. Regarding LMA, Carrillo-Muro et al. [
20], Martínez-Aispuro et al. [
71], Lee-Rangel et al. [
34] and Mendoza-Martínez et al. [
72], in lambs finishing diets did not observe effects on the 42 d; yet, Ladeira et al. [
32] with different proportions of glycerin in bulls observed increases with higher levels.