Submitted:
02 October 2023
Posted:
03 October 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
3. Results
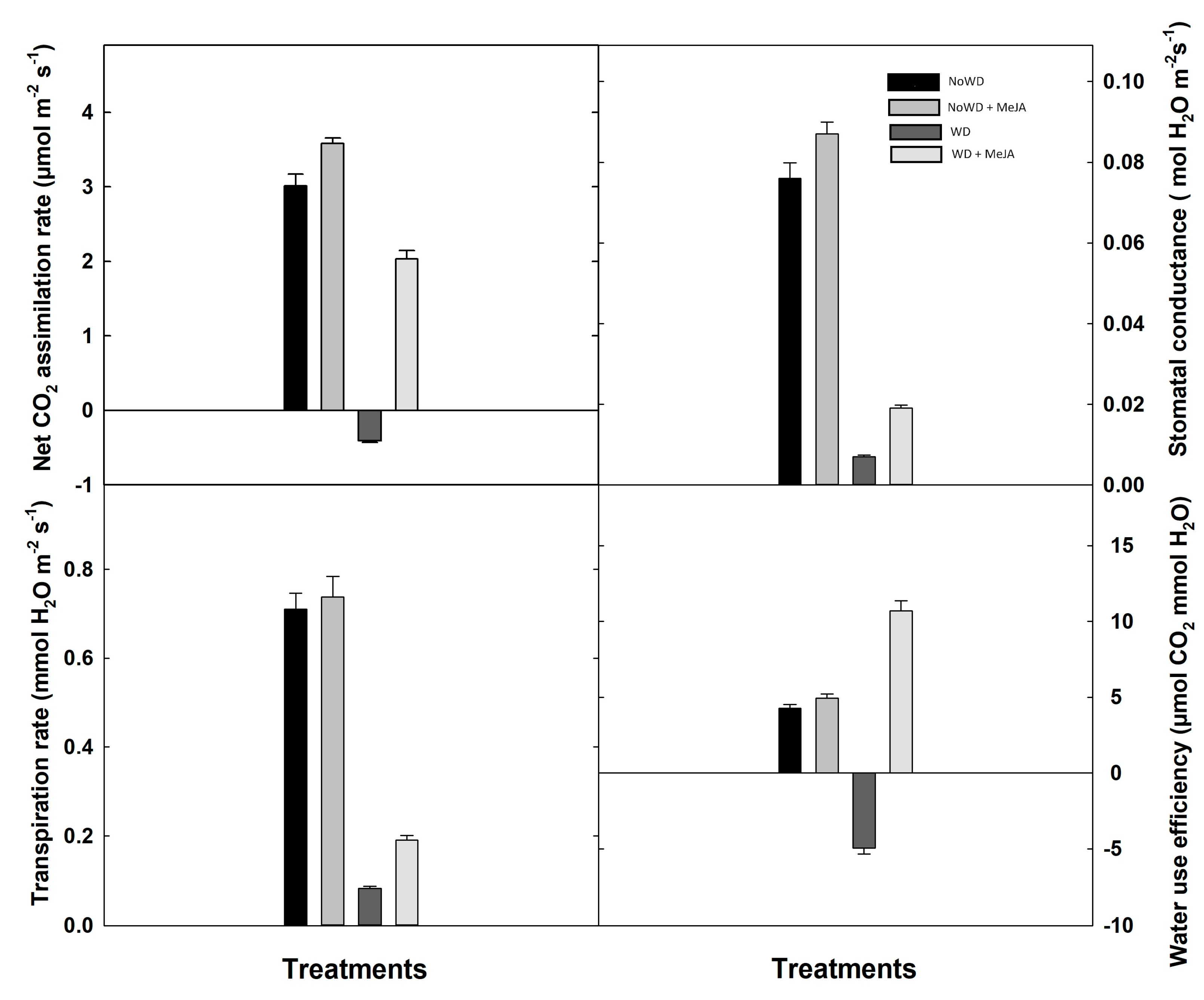
3.1. Physiological Parameters
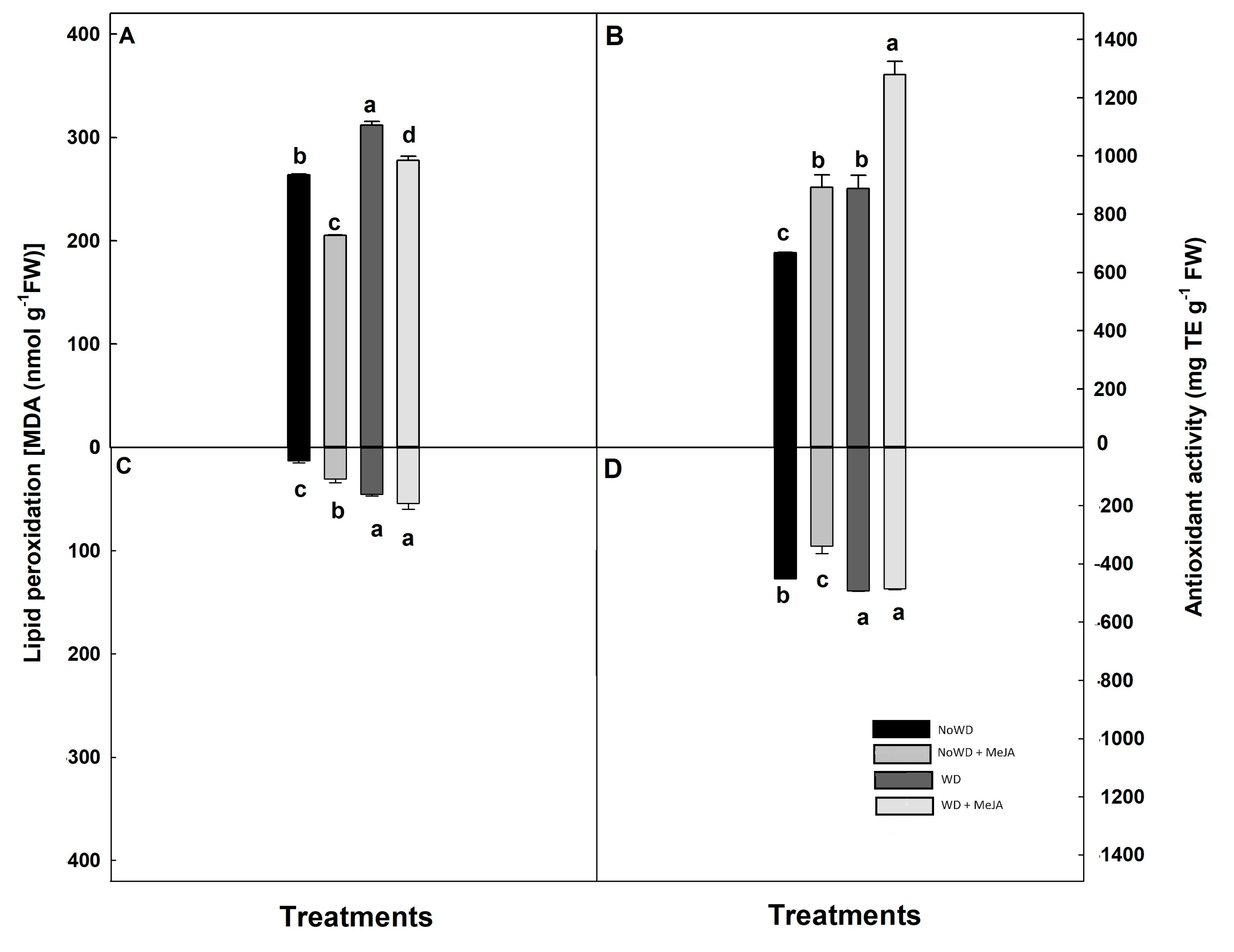
3.2. Biochemical Determinations
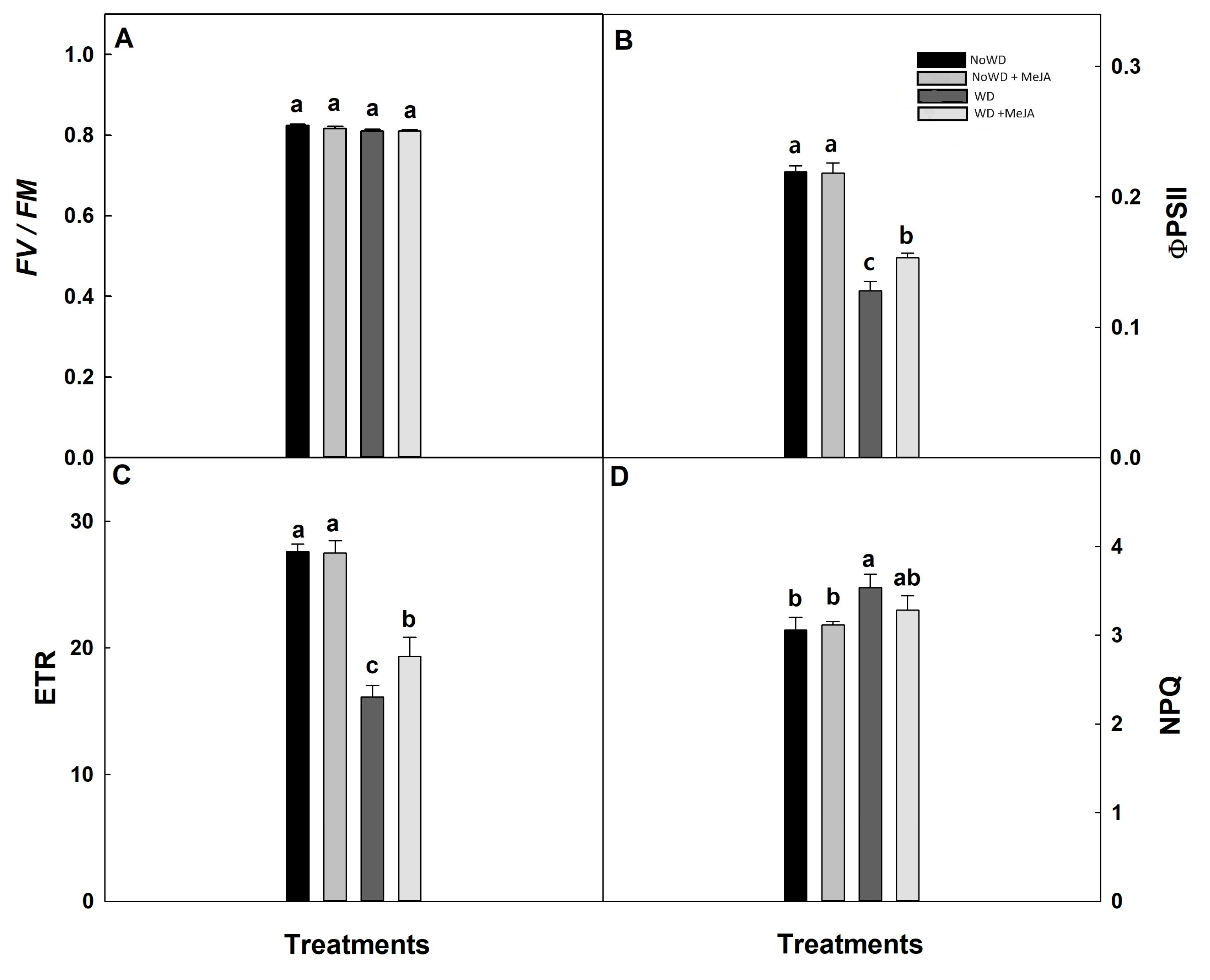
3.3. Photosynthetic Performance
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fekete, B.M. State of the world's water resources. In Climate Vulnerability: Understanding and Addressing Threats to Essential Resources; Pielke Sr., R.A., Ed.; Elsevier Inc.,: Amsterdam, 2013; pp. 11–23. [Google Scholar]
- Westmacott, J.R.; Burn, D.H. Climate change effects on the hydrologic regime within the Churchill-Nelson River Basin. J. Hydrol. 1997, 202, 263–279. [Google Scholar] [CrossRef]
- Wolf, A.T. Criteria for equitable allocations: The heart of international water conflict. Nat. Resour. Forum. 1999, 23, 3–30. [Google Scholar] [CrossRef]
- Boyer, J. Plant productivity and environment. Science 1982, 218, 443. [Google Scholar] [CrossRef]
- Rosa, L.; Chiarelli, D.D.; Rulli, M.C.; Dell’Angelo, J.; D’Odorico, P. Global agricultural economic water scarcity. Sci. Adv. 2020, 6, 1–10. [Google Scholar] [CrossRef]
- Urban, L.; Aarrouf, J.; Bidel, L.P.R. Assessing the effects of water deficit on photosynthesis using parameters derived from measurements of leaf gas exchange and of chlorophyll a fluorescence. Front. Plant Sci. 2017, 8, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Hasanuzzaman, M.; Borhannuddin Bhuyan, M.H.M.; Parvin, K.; Bhuiyan, T.F.; Anee, T.I.; Nahar, K.; Hossen, Md.S.; Zulfiqar, F.; Alam, Md.M.; Fujita, M. Regulation of ROS metabolism in plants under environmental stress: A review of recent experimental evidence. Int. J. Mol. Sci. 2020, 21, 8695. [Google Scholar] [CrossRef]
- Mansoor, S.; Wani, O.A.; Lone, J.K.; Manhas, S.; Kour, N.; Alam, P.; Ahmad, A.; Ahmad, P. Reactive oxygen species in plants: From source to sink. Antioxidants 2022, 11, 225. [Google Scholar] [CrossRef]
- Singh, R.; Parihara, P.; Singha, S.; Mishra, R.K.; Singh, V.P.; Prasad, S.M. Reactive oxygen species signaling and stomatal movement: Current updates and future perspectives. Redox Biol. 2017, 11, 213–218. [Google Scholar] [CrossRef]
- Keles, Y.; Oncel, I. Response of antioxidative defence system to temperature and water stress combinations in wheat seedlings. Plant Sci. 2002, 163, 783–790. [Google Scholar] [CrossRef]
- Luna, C.M.; Pastori, G.M.; Driscoll, S.; Groten, K.; Bernard, S.; Foyer, C.H. Drought controls on H2O2 accumulation, catalase (CAT) activity and CAT gene expression in wheat. J Exp Bot. 2005, 56, 417. [Google Scholar] [CrossRef]
- Fahad, S.; Bajwa, A.A.; Nazir, U.; Anjum, S.A.; Farooq, A.; Zohaib, A.; Sadia, S.; Nasim, W.; Adkins, S.; Saud, S.; Ihsan, M.Z.; Alharby, H.; Wu, C.; Wang, D.; Huang, J. Crop Production under drought and heat stress: Plant responses and management options. Front. Plant Sci. 2017, 8, 1147. [Google Scholar] [CrossRef] [PubMed]
- Hasanuzzaman, M.; Borhannuddin Bhuyan, M.H.M.; Zulfiqar, F.; Raza, A.; Mohsin, S.M.; Al Mahmud, J.; Fujita, M.; Fotopoulos, V. Reactive oxygen species and antioxidant defense in plants under abiotic stress: Revisiting the crucial role of a universal defense regulator. Antioxidants 2020, 9, 681. [Google Scholar] [CrossRef]
- Iqbal, S.; Wang, X.; Mubeen, I.; Kamran, M.; Kanwal, I.; Díaz, G.A.; Abbas, A.; Parveen, A.; Atiq, M.N.; Alshaya, H.; Zin El-Abedin, T.K.; Fahad, S. Phytohormones trigger drought tolerance in crop plants: Outlook and future perspectives. Front. Plant Sci. 2022, 12, 799318. [Google Scholar] [CrossRef] [PubMed]
- Kwak, J.M.; Nguyen, V.; Schroeder, J.I. The role of reactive oxygen species in hormonal responses1. Plant Physiol. 2006, 141, 323–329. [Google Scholar] [CrossRef] [PubMed]
- Verma, V.; Ravindran, P.; Kumar, P.P. 2016. Plant hormone-mediated regulation of stress responses. BMC Plant Biol. 2016, 16, 86. [Google Scholar] [CrossRef] [PubMed]
- Reyes-Díaz, M.; Ulloa-Inostroza, E.M.; Gonzalez-Villagra, J.; Ivanov, A.G.; Kurepin, L.V. Phytohormonal responses to soil acidity in plants. In Plant hormones under challenging environmental factors; Ahammed, G.J., Yu, J.-Q., Eds.; Springer-Verlag: Berlin Heidelberg; New York, 2016; pp. 133–155. [Google Scholar]
- Ulloa-Inostroza, E.M.; Alberdi, M.; Meriño-Gergichevich, C.; Reyes-Díaz, M. Low doses of exogenous methyl jasmonate applied simultaneously with toxic aluminum improve the antioxidant performance of Vaccinium corymbosum. Plant Soil 2017, 412, 81–96. [Google Scholar] [CrossRef]
- Reyes-Díaz, M.; Inostroza-Blancheteau, C.; Millaleo, R.; Cruces, E.; Wulff-Zottele, C.; Alberdi, M.; Mora, M.L. Long-term aluminum exposure effects on physiological and biochemical features of highbush blueberry cultivars. J. Am. Soc. Hortic. Sci. 2010, 135, 1–11. [Google Scholar] [CrossRef]
- Inostroza-Blancheteau, C.; Reyes-Diaz, M.; Aquea, F.; Nunes-Nesi, A.; Alberdi, M.; Arce-Johnson, P. Biochemical and molecular changes in response to aluminium-stress in highbush blueberry (Vaccinium corymbosum L). Plant Physiol. Bioch. 2011, 49, 1005–1012. [Google Scholar] [CrossRef]
- Larcher, W. Physiological plant ecology. Ecophysiology and stress physiology of functional groups, 4th edition; Springer-Verlag: Berlin Heidelberg; New York, 2003; 504p. [Google Scholar]
- Du, Z.; Bramlage, W.J. Modified thiobarbituric acid assay for measuring lipidoxidation in sugar-rich plant tissue extracts. J. Agr. Food Chem. 1992, 40, 1556–1570. [Google Scholar] [CrossRef]
- Chinnici, F.; Bendini, A.; Gaiani, Y.; Riponi, C. Radical scavenging activities of peels and pulps from cv. Golden delicious apples as related to their phenolic composition. J. Agr. Food Chem. 2004, 52, 4684–4689. [Google Scholar] [CrossRef]
- Slinkard, K.; Singleton, V.A. Total phenol analysis: automation and comparison with manual methods. Am. J. Enol. Vitic. 1977, 28, 29–55. [Google Scholar] [CrossRef]
- Cheng, G.W.; Breen, P.J. Activity of phenylalanine ammonia-lyase (PAL) and concentrations of anthocyanins and phenolics in developing strawberry fruit. J. Am. Soc. Hort. Sci. 1991, 116, 865–869. [Google Scholar] [CrossRef]
- Castrejón, A.D.R.; Eichholz, I.; Rohn, S.; Kroh, L.W.; Huyskens-Keil, S. Phenolic profile and antioxidant activity of highbush blueberry (Vaccinium corymbosum L.) during fruit maturation and ripening. Food Chem. 2008, 109, 564–572. [Google Scholar] [CrossRef]
- Ribera, A.E.; Reyes-Díaz, M.; Alberdi, M.; Zuñiga, G.E.; Mora, M.L. Antioxidant compounds in skin and pulp of fruits change among genotypes and maturity stages in highbush blueberry (Vaccinium corymbosum L.) grown in southern Chile. J. Soil. Sci. Plant Nutr. 2010, 10, 509–536. [Google Scholar] [CrossRef]
- Reyes-Díaz, M.; Alberdi, M.; Mora, M.L. Short-term aluminum stress differentially affects the photochemical efficiency of photosystem II in highbush blueberry genotypes. J. Am. Soc. Hortic. Sci. 2009, 134, 1–8. [Google Scholar] [CrossRef]
- Maxwell, K.; Johnson, G. Chlorophyll fluorescence. A practical guide. J. Exp. Bot. 2000, 51, 659–668. [Google Scholar] [CrossRef] [PubMed]
- Locke, A.M.; Ort, D.R. Leaf hydraulic conductance declines in coordination with photosynthesis, transpiration and leaf water status as soybean leaves age regardless of soil moisture. J. Exp. Bot. 2014, 65(22), 6617–6627. [Google Scholar] [CrossRef]
- Garcia-Plazaola, J.I.; Becerril, J.M. A rapid HPLC method to measure liphophilic antioxidant in stressed plants: simultaneous determination of carotenoids and tocopherols. Phytocheml. Anal. 1999, 10, 307–313. [Google Scholar] [CrossRef]
- Li, C.; Wang, P.; Menzies, N.W.; Lombi, E.; Kopittke, P.M. Effects of methyl jasmonate on plant growth and leaf properties. J. Plant Nutr. Soil Sci. 2018, 181(3), 1–10. [Google Scholar] [CrossRef]
- Ribera-Fonseca, A.; Jiménez, D.; Leal, P.; Riquelme, I.; Roa, J.C.; Alberdi, M.; Peek, R.M.; Reyes-Díaz, M. The anti-proliferative and anti-invasive effect of leaf extracts of blueberry plants treated with methyl jasmonate on human gastric cancer in vitro is related to their antioxidant properties. Antioxidants 2020, 9, 45. [Google Scholar] [CrossRef]
- Balboa, K.; Ballesteros, G.I.; Molina-Montenegro, M.A. Integration of physiological and molecular traits would help to improve the insights of drought resistance in highbush blueberry cultivars. Plants 2020, 9, 1457. [Google Scholar] [CrossRef] [PubMed]
- Améglio, T.; Roux, L.; Perrier, M.C. Water relations of highbush blueberry under drought conditions. Acta Hortic. 2000, 537, 273–278. [Google Scholar] [CrossRef]
- Molnar, S.; Clapa, D.; Viorel, M. Response of the five highbush blueberry cultivars to in vitro induced drought stress by polyethylene glycol. Agronomy 2022, 12(3), 732. [Google Scholar] [CrossRef]
- Wang, S.Y. Methyl jasmonate reduces water stress in strawberry. Plant Growth Regul. 1999, 18, 127–134. [Google Scholar] [CrossRef]
- Ma, C.; Wang, Z.Q.; Zhang, L.T.; Sun, M.M.; Lin, T.B. Photosynthetic responses of wheat (Triticum aestivum L.) to combined effects of drought and exogenous methyl jasmonate. Photosynthetica 2014, 52, 377–385. [Google Scholar] [CrossRef]
- Ðurić, M.; Subotić, A.; Prokić, L.; Trifunović-Momčilov, M.; Milošević, S. Alterations in physiological, biochemical, and molecular responses of Impatiens walleriana to drought by methyl jasmonate foliar application. Genes 2023, 14, 1072. [Google Scholar] [CrossRef]
- Su, Y.; Huang, Y.; Dong, X.; Wang, R.; Tang, M.; Cai, J.; Chen, J.; Zhang, X.; Nie, G. Exogenous methyl jasmonate improves heat tolerance of perennial ryegrass through alteration of osmotic adjustment, antioxidant defense, and expression of jasmonic acid-responsive genes. Front. Plant Sci. 2021, 12, 664519. [Google Scholar] [CrossRef]
| Treatments | FC (%) |
MeJA (mM) |
RLA [Mean ± SE (cm2/g)] |
RWC [Mean ± SE (%)] |
|---|---|---|---|---|
| NoWD | 80 | 0 | 197.6 ± 3.3 a | 63.3 ± 3.4 ab |
| NoWD + MeJA | 80 | 10 | 197.7 ± 10.9 a | 69.3 ± 3.0 a |
| WD | 20 | 0 | 132.0 ± 8.1 c | 54.2 ± 3.5 c |
| WD + MeJA | 20 | 10 | 150.9 ± 7.3 b | 61.4 ± 2.1 b |
| Treatments | FC (%) | MeJA (µM) | Total Phenols [Chlorogenic Acid eq (μg g−1 FW)] | Total Anthocyanins (mg Cyanidin 3-O-Glycoside/g−1 FW)] | Delphinidin (mg/g−1 PF) | Cyanidin (mg/g−1 PF) | Petunidin (µg/g−1 PF) | Peonidin (µg/g−1 PF) |
|---|---|---|---|---|---|---|---|---|
| NoWD | 80 | 0 | 1433.7 ± 33.2 a | 1.0 ± 0.0 b | 50.4 ± 3.1 a | 3.2 ± 0.0 b | 146.5 ± 6.1 a | 77.0 ± 2.7 b |
| NoWD + MeJA | 80 | 10 | 1524.1 ± 154.5 a | 1.5 ± 0.0 a | 41.3 ± 0.1 b | 3.5 ± 0.0 a | 142.9 ± 5.0 a | 92.3 ± 4.2 a |
| WD | 20 | 0 | 1053.8 ± 47.9 b | 0.2 ± 0.0 c | 22.1 ± 2.0 d | 1.3 ± 0.1 c | 99.9 ± 7.0 b | 48.9 ± 5.9 c |
| WD + MeJA | 20 | 10 | 1345.9 ± 123.9 a | 0.9 ± 0.1 b | 31.6 ± 1.8 c | 1.5 ± 0.0 c | 144.9 ± 4.8 a | 52.4 ± 3.4 c |
| FC (%) | MeJA (µM) | Chlorophyll a+b | Chlorophyll a/b | Beta Carotene | Lutein | Violaxanthin | Antheraxanthin | Zeaxanthin | Neoxanthin | |
|---|---|---|---|---|---|---|---|---|---|---|
| NoWD | 80 | 0 | 1751.3 ± 126.6 a | 2.6 ± 0.2 ab | 110.2 ± 5.9 a | 188.1 ± 10.2 a | 81.4 ± 6.0 a | 27.3 ± 1.5 b | 70.3 ± 3.0 b | 87.9 ± 5.9 a |
| NoWD + MeJA | 80 | 10 | 1101.3 ± 60.7 c | 2.7 ± 0.0 ab | 63.7 ± 3.8 b | 116.7 ± 3.6 b | 66.4 ± 1.5 ab | 16.8 ± 1.3 c | 59.3 ± 1.4 b | 47.4 ± 1.6 c |
| WD | 20 | 0 | 1409.5 ± 49.9 b | 2.6 ± 0.0 b | 72.8 ± 4.9 b | 139.7 ± 9.3 b | 39.8 ± 2.9 c | 42.8 ± 0.7 a | 83.8 ± 9.5 b | 62.1 ± 3.5 b |
| WD + MeJA | 20 | 10 | 1461.2 ± 31.6 b | 2.8 ± 0.1 a | 82.7 ± 4.5 b | 149.5 ± 8.4 b | 52.5 ± 5.4 bc | 36.7 ± 0.5 a | 127.4 ± 13.7 a | 66.1 ± 1.7 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).





