Submitted:
02 October 2023
Posted:
03 October 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. The IL-17 cytokines family and its receptors
3. Production and functions of the IL-17 family members
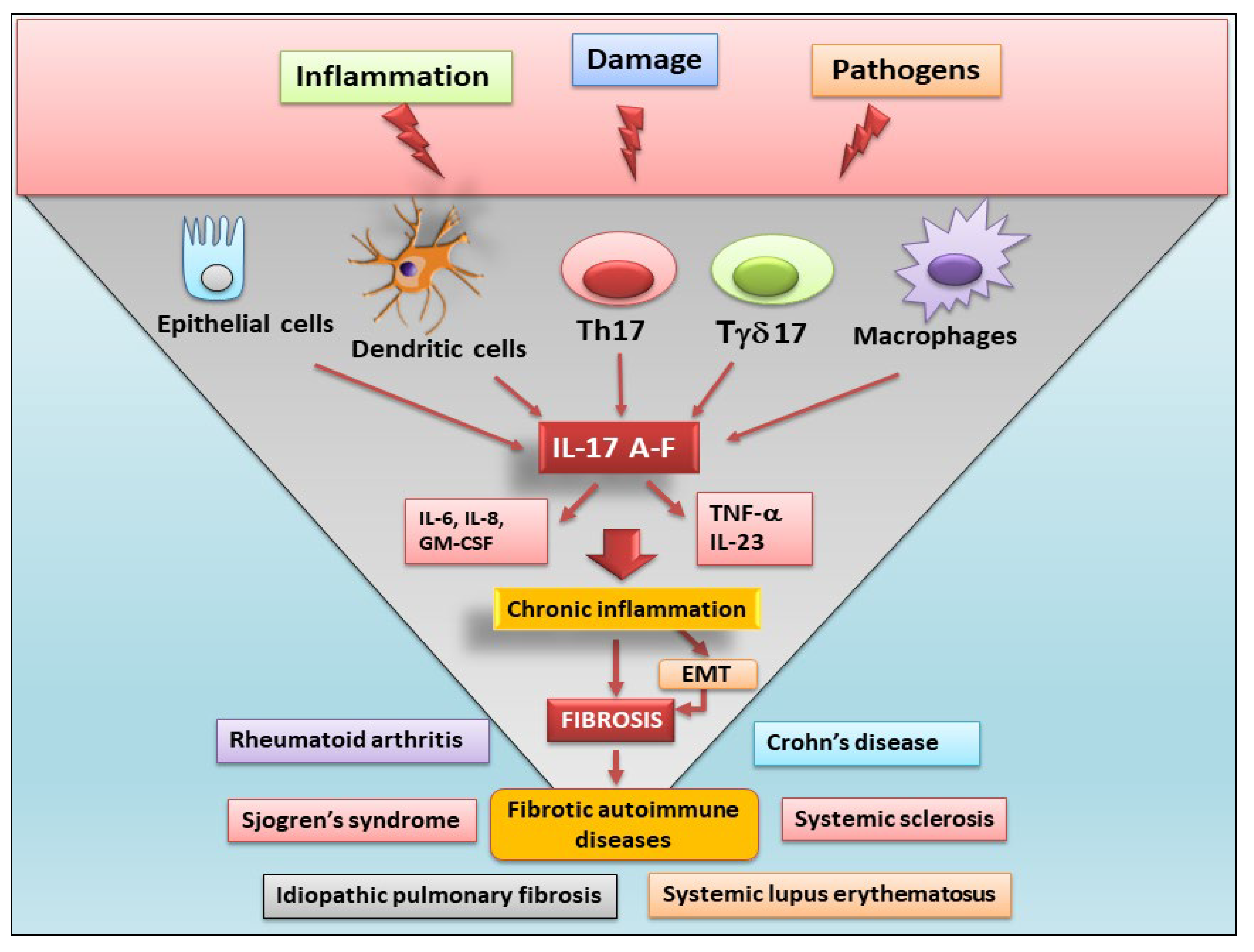
4. Role of IL-17 in fibrotic evolution
5. Fibrosis mediated by several IL-17 family members
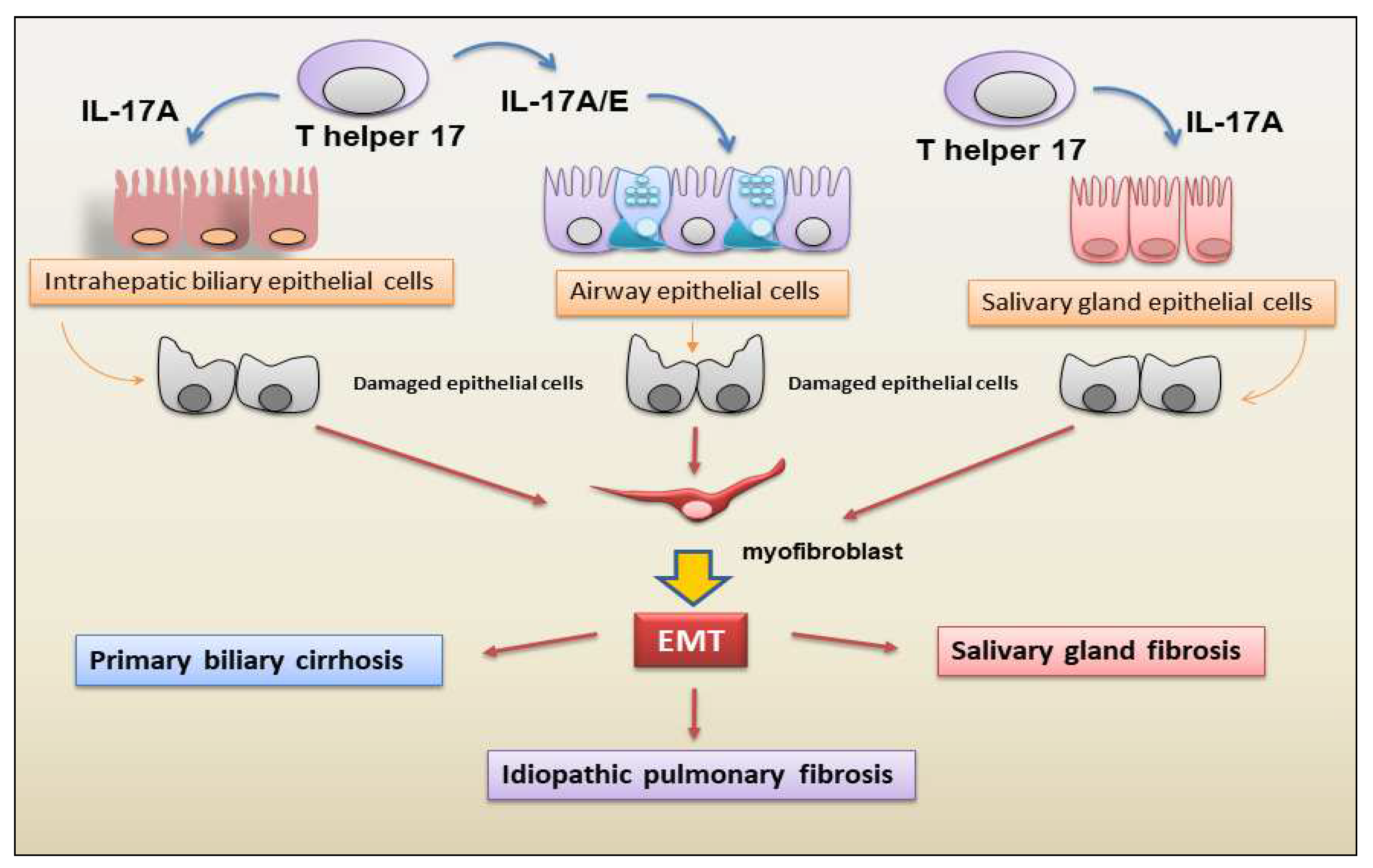
5.1. IL-17A
5.2. IL-17B
5.3. IL-17C
5.4. IL-17D
5.5. IL-17E
5.6. IL-17F
6. Epigenetic regulation of IL-17 in fibrotic diseases
6.1. Histone modification and IL-17 production
6.2. DNA demethylation in the control of IL-17 pro-fibrotic activity
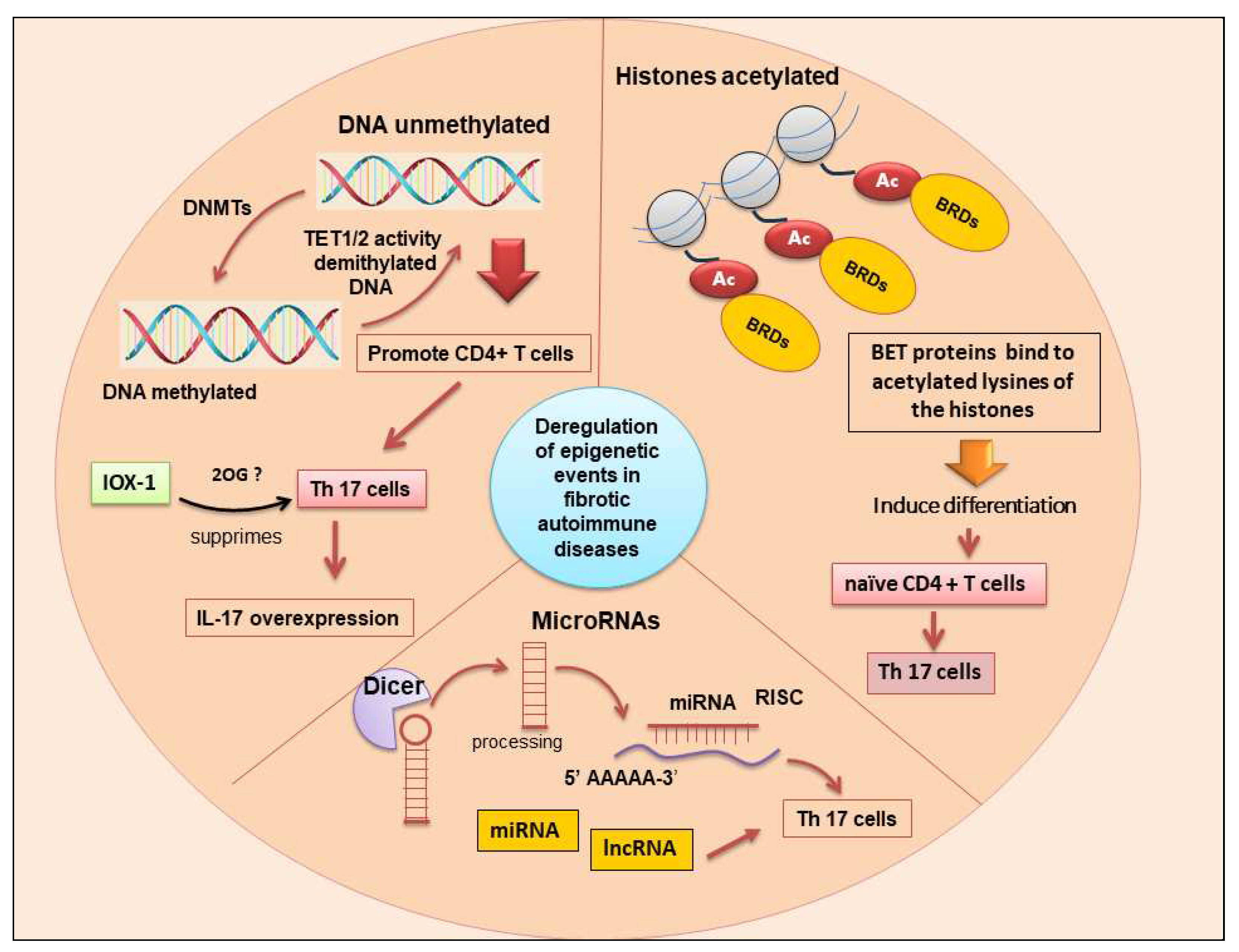
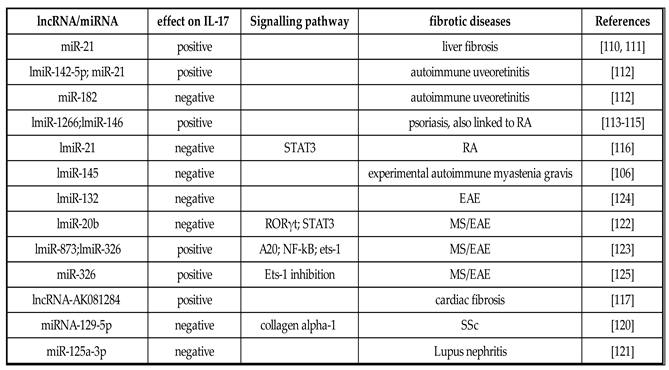
6.3. Correlations of non-coding RNA expression and IL-17 levels in fibrosis
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Distler, J.H.W.; Gyorfi, A.H.; Ramanujam, M.; Whitfield, M.L.; Konigshoff, M.; Lafyatis, R. Shared and distinct mechanisms of fibrosis. Nat. Rev. Rheumatol. 2019, 15, 705–730. [Google Scholar] [CrossRef] [PubMed]
- Mehal, W.Z.; Iredale, J.; Friedman, S.L. Scraping fibrosis: expressway to the core of fibrosis. Nat. Med. 2011, 17, 552–553. [Google Scholar] [CrossRef] [PubMed]
- Wick, G.; Grundtman, C.; Mayerl, C.; Wimpissinger, T.F.; Feichtinger,J. ; Zelger, B.; Sgonc, R.; Wolfram, D. The immunology of fibrosis. Annu. Rev. Immunol. 2013, 31, 107–135. [Google Scholar] [CrossRef]
- Enderson, N.C.; Rieder, F.; Wynn, T.A. Fibrosis: from mechanisms to medicines. Nature. 2020, 587, 555–566. [Google Scholar] [CrossRef]
- Miao, H.; Wu, X.Q.; Zhang, D.D.; Wang, Y.N.; Guo, Y.; Li, P.; Xiong, Q.; Zhao, Y.Y. Deciphering the cellular mechanisms underlying fibrosis-associated diseases and therapeutic avenues. Pharmacol. Res. 2021, 163, 105316. [Google Scholar] [CrossRef] [PubMed]
- Sisto,M. ; Ribatti, D.; Lisi, S. Organ Fibrosis and Autoimmunity: The Role of Inflammation in TGFβ-Dependent EMT. Biomolecules. 2021, 11, 310. [Google Scholar]
- Sisto, M.; Lisi, S. Towards a Unified Approach in Autoimmune Fibrotic Signalling Pathways. Int. J. Mol. Sci. 2023, 24, 9060. [Google Scholar] [CrossRef] [PubMed]
- Kany, S.; Vollrath, J.T.; Relja, B. Cytokines in Inflammatory Disease. Int. J. Mol. Sci. 2019, 20, 6008. [Google Scholar] [CrossRef]
- Kuwabara, T.; Ishikawa, F.; Kondo,M. ; Kakiuchi, T. The Role of IL-17 and Related Cytokines in Inflammatory Autoimmune Diseases. Mediators Inflamm. 2017, 2017, 3908061. [Google Scholar] [CrossRef]
- Ramani, K.; Biswas, P.S. Interleukin-17: Friend or foe in organ fibrosis. Cytokine. 2019, 120, 282–288. [Google Scholar] [CrossRef]
- Ruiz de Morales, J.M.G.; Puig, L.; Daudén, E.; Cañete, J.D.; Pablos, J.L.; Martín, A.O.; Juanatey, C.G.; Adán, A.; Montalbán, X.; Borruel, N.; Ortí, G.; Holgado-Martín, E.; García-Vidal, C.; Vizcaya-Morales, C.; Martín-Vázquez, V.; González-Gay, M.Á. Critical role of interleukin (IL)-17 in inflammatory and immune disorders: An updated review of the evidence focusing in controversies. Autoimmun. Rev. 2020, 19, 102429. [Google Scholar] [CrossRef] [PubMed]
- Kawaguchi, M.; Adachi, M.; Oda, N.; Kokubu, F.; Huang, S.K. IL-17 cytokine family. J. Allergy Clin. Immunol. 2004, 114, 1265–1273. [Google Scholar] [CrossRef] [PubMed]
- Rouvier, E.; Luciani,M. F.; Mattéi, M.G.; Denizot, F.; Golstein, P. CTLA-8, cloned from an activated T cell, bearing AU-rich messenger RNA instability sequences, and homologous to a herpesvirus saimiri gene. J. Immunol. 1993, 150, 5445–5456. [Google Scholar] [CrossRef] [PubMed]
- Yao, Z.; Painter, S.L. Fanslow, W.C.; Ulrich, D.; Macduff, B.M.; Spriggs, M.K., Armitage, R.J. Human IL-17: a novel cytokine derived from T cells. J. Immunol. 1995, 155, 5483–5486. [Google Scholar] [CrossRef] [PubMed]
- Moseley, T.A.; Haudenschild, D.R.; Rose, L.; Reddi, A.H. Interleukin-17 family and IL-17 receptors. Cytokine Growth Factor Rev. 2003, 14, 155–174. [Google Scholar] [CrossRef]
- Hymowitz, S.G.; Filvaroff, E.H.; Yin, J.P.; Lee, J.; Cai, L.; Risser, P.; Maruoka, M.; Mao, W.; Foster, J.; Kelley, R.F.; Pan, G.; Gurney, A.L.; de Vos, A.M.; Starovasnik, M.A. IL-17s adopt a cystine knot fold: Structure and activity of a novel cytokine, IL-17F, and implications for receptor binding. EMBO J. 2001, 20, 5332–5341. [Google Scholar] [CrossRef]
- Lee, J.; Ho, W.H.; Maruoka, M.; Corpuz, R.T. , Baldwin, D.T., Foster, J.S., et al. IL-17E, a novel proinflammatory ligand for the IL-17 receptor homolog IL-17Rh1. J. Biol. Chem. 2001, 276, 1660–1664. [Google Scholar] [CrossRef]
- Bie, Q.; Jin, C.; Zhang, B.; Dong, H. IL-17B: A new area of study in the IL-17 family. Mol. Immunol. 2017, 90, 50–56. [Google Scholar] [CrossRef]
- Kolls, J.K.; Lindén, A. Interleukin-17 family members and inflammation. Immunity. 2004, 21, 467–476. [Google Scholar] [CrossRef]
- Huang, X.D.; Zhang, H.; He, M.X. Comparative and evolutionary analysis of the interleukin 17 gene family in invertebrates. PLos ONE. 2015, 10, e0132802. [Google Scholar] [CrossRef]
- Yang, X.O.; Chang, S.H.; Park, H.; Nurieva, R.; Shah, B.; Acero, L.; Wang, Y.H.; Schluns, K.S.; Broaddus, R.R.; Zhu, Z.; Dong, C. Regulation of inflammatory responses by IL-17F. J. Exp. Med. 2008, 205, 1063–1075. [Google Scholar] [CrossRef] [PubMed]
- Goepfert, A.; Lehmann, S.; Blank, J.; Kolbinger, F.; Rondeau, J.M. Structural Analysis Reveals that the Cytokine IL-17F Forms a Homodimeric Complex with Receptor IL-17RC to Drive IL-17RA-Independent Signaling. Immunity. 2020, 52, 409–412. [Google Scholar] [CrossRef] [PubMed]
- Yao, Z.; Fanslow, W.C.; Seldin, M.F.; Rousseau, A.M.; Painter, S.L.; Comeau, M.R. Herpesvirus Saimiri encodes a new cytokine, IL-17, which binds to a novel cytokine receptor. Immunity. 1995, 3, 811–821. [Google Scholar] [CrossRef]
- Monin, L.; Gaffen, S.L. Interleukin 17 family cytokines: signaling mechanisms, biological activities, and therapeutic implications. Cold Spring Harb Perspect Biol. 2018, 10, a028522. [Google Scholar] [CrossRef]
- Beringer, A.; Noack, M.; Miossec, P. IL-17 in chronic inflammation: from discovery to targeting. Trends Mol. Med. 2016, 22, 230–241. [Google Scholar] [CrossRef]
- Gaffen, S.L. Structure and signalling in the IL-17 receptor family. Nat. Rev. Immunol. 2009, 9, 556–567. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Lee, H.Y.; Zhao, X.; Han, J.; Su, Y.; Sun, Q.; Shao, J.; Ge, J.; Zhao, Y.; Bai, X.; He, Y.; Wang, X.; Wang, X.; Dong, C. Interleukin-17D regulates group 3 innate lymphoid cell function through its receptor CD93. Immunity. 2021, 54, 673–686. [Google Scholar] [CrossRef]
- McGeachy, M.J.; Cua, D.J.; Gaffen, S.L. The IL-17 family of cytokines in health and disease. Immunity. 2019, 50, 892. [Google Scholar] [CrossRef]
- Pande, S.; Yang, X.; Friesel, R. Interleukin-17 receptor D (Sef) is a multi-functional regulator of cell signaling. Cell Commun. Signal. 2021, 19, 6. [Google Scholar] [CrossRef]
- Amatya, N.; Garg, A.V.; Gaffen, S. L. IL-17 Signaling: The Yin and the Yang. Trends Immunol. 2017, 38, 310–322. [Google Scholar] [CrossRef]
- Nies, J. F, Panzer, U. IL-17C/IL-17RE: Emergence of a Unique Axis in TH17 Biology. Front. Immunol. 2020, 11, 341. [Google Scholar] [CrossRef] [PubMed]
- Patel, D.D.; Kuchroo, V.K. Th17 Cell Pathway in Human Immunity: Lessons from Genetics and Therapeutic Interventions. Immunity, 2015, 43, 1040–1051. [Google Scholar] [CrossRef] [PubMed]
- Tang, M.; Lu, L.; Yu, X. Interleukin-17A Interweaves the Skeletal and Immune Systems. Front Immunol. 2021, 11, 625034. [Google Scholar] [CrossRef] [PubMed]
- Rosine, N.; Miceli-Richard, C. Innate Cells: The Alternative Source of IL-17 in Axial and Peripheral Spondyloarthritis? Front. Immunol. 2021, 11, 553742. [Google Scholar] [CrossRef]
- Mills, K.H.G. IL-17 and IL-17-producing cells in protection versus pathology. Nat. Rev. Immunol. 2023, 23, 38–44. [Google Scholar] [CrossRef]
- Lee, Y.K.; Landuyt, A.E.; Lobionda, S.; Sittipo, P.; Zhao, Q.; Maynard, C.L. TCR-independent functions of Th17 cells mediated by the synergistic actions of cytokines of the IL-12 and IL-1 families. PLoS One. 2017, 12, e0186351. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, X.; Song, X.; Xiang, C.; He, C.; Xie, Y.; Zhou, Y.; Wang, N.; Guo, G.; Zhang, W.; Li, Y.; Liu, K.; Zou, Q.; Guo, H.; Shi, Y. Interleukin 17 B regulates colonic myeloid cell infiltration in a mouse model of DSS-induced colitis. Front Immunol. 2023, 14, 1055256. [Google Scholar] [CrossRef]
- Bie, Q.; Song, H.; Chen, X.; Yang, X.; Shi, S.; Zhang, L.; Zhao, R.; Wei, L.; Zhang, B.; Xiong, H.; Zhang, B. IL-17B/IL-17RB signaling cascade contributes to self-renewal and tumorigenesis of cancer stem cells by regulating Beclin-1 ubiquitination. Oncogene. 2021, 40, 220–2216. [Google Scholar] [CrossRef]
- Bastid, J. , Dejou, C.; Docquier, A; Bonnefoy, N. The Emerging Role of the IL-17B/IL-17RB Pathway in Cancer. Front. Immunol. 2020, 11, 718. [Google Scholar] [CrossRef]
- Chung, S.H.; Ye, X.Q.; Iwakura, Y. Interleukin-17 family members in health and disease. Int. Immunol. 2021, 33, 723–729. [Google Scholar] [CrossRef]
- Ramirez-Carrozzi, V.; Sambandam, A.; Luis, E.; Lin, Z.; Jeet, S.; Lesch, J.; Hackney, J.; Kim, J.; Zhou, M.; Lai, J.; Modrusan, Z.; Sai, T.; Lee, W.; Xu, M.; Caplazi, P.; Diehl, L.; de Voss, J.; Balazs, M.; Gonzalez, L. Jr.; Singh, H.; Ouyang, W.; Pappu, R. IL-17C regulates the innate immune function of epithelial cells in an autocrine manner. Nat. Immunol. 2011, 12, 1159–1166. [Google Scholar] [CrossRef] [PubMed]
- Peng, T.; Chanthaphavong, R.S.; Sun, S.; Trigilio, J.A.; Phasouk, K.; Jin, L.; Layton, E.D.; Li, A.Z.; Correnti, C.E.; De van der Schueren, W.; Vazquez, J.; O'Day, D.R.; Glass, I.A.; Knipe, D.M.; Wald, A.; Corey, L.; Zhu, J. Keratinocytes produce IL-17c to protect peripheral nervous systems during human HSV-2 reactivation. J. Exp. Med. 2017, 214, 2315–2329. [Google Scholar] [CrossRef] [PubMed]
- Saddawi-Konefka, R.; Seelige, R.; Gross, E.T.; Levy, E.; Searles, S.C.; Washington, A. Jr. Nrf2 Induces IL-17D to Mediate Tumor and Virus Surveillance. Cell Rep. 2016, 16, 2348–58. [Google Scholar] [CrossRef] [PubMed]
- Seelige, R.; Washington, A. Jr.; Bui, J.D. The ancient cytokine IL-17D is regulated by Nrf2 and mediates tumor and virus surveillance. Cytokine. 2017, 91, 10–12. [Google Scholar] [CrossRef] [PubMed]
- Starnes, T.; Broxmeyer, H.E.; Robertson, M.J.; Hromas, R. Cutting edge: IL-17D, a novel member of the IL-17 family, stimulates cytokine production and inhibits hemopoiesis. J. Immunol. 2002, 169, 642–646. [Google Scholar] [CrossRef]
- Kleinschek, M.A. , Owyang, A.M., Joyce-Shaikh, B., Langrish, C.L., Chen, Y., Gorman, D.M.; Blumenschein, W.M.; McClanahan, T.; Brombacher, F.; Hurst, S.D.; Kastelein, R.A.;Cua, D.J.IL-25 regulates Th17 function in autoimmune inflammation. J. Exp. Med. 2007, 204, 161–170. [Google Scholar] [CrossRef]
- Owyang, A.M.; Zaph, C.; Wilson, E.H.; Guild, K.J.; McClanahan, T.; Miller, H.R. Interleukin 25 regulates type 2 cytokine-dependent immunity and limits chronic inflammation in the gastrointestinal tract. J. Exp. Med. 2006, 203, 843–849. [Google Scholar] [CrossRef]
- Huang, Q.; Chu, S.; Yin, X.; Yu, X.; Kang, C.; Li, X.; Qiu, Y. Interleukin-17A-Induced Epithelial-Mesenchymal Transition of Human Intrahepatic Biliary Epithelial Cells: Implications for Primary Biliary Cirrhosis. Tohoku J. Exp. Med. 2016, 240, 269–275. [Google Scholar] [CrossRef]
- Gaffen, S.L.; Jain, R.; Garg, A.V.; Cua, D.J. The IL-23-IL-17 immune axis: from mechanisms to therapeutic testing. Nat. Rev. Immunol. 2014, 14, 585–600. [Google Scholar] [CrossRef]
- Amara, S.; Lopez, K.; Banan, B.; Brown, S.K.; Whalen, M.; Myles, E.; Ivy, M.T.; Johnson, T.; Schey, K.L.; Tiriveedhi, V. Synergistic effect of pro-inflammatory TNFα and IL-17 in periostin mediated collagen deposition: potential role in liver fibrosis. Mol. Immunol. 2015, 64, 26–35. [Google Scholar] [CrossRef]
- Tan, Z.; Qian, X.; Jiang, R.; Liu, Q.; Wang, Y.; Chen, C.; Wang, X.; Ryffel, B.; Sun, B. IL-17A plays a critical role in the pathogenesis of liver fibrosis through hepatic stellate cell activation. J. Immunol. 2013, 191, 1835–1844. [Google Scholar] [CrossRef]
- Jiang, G.; Liu, C.T.; Zhang, W.D. IL-17A and GDF15 are able to induce epithelial-mesenchymal transition of lung epithelial cells in response to cigarette smoke. Exp. Ther. Med. 2018, 16, 12–20. [Google Scholar] [CrossRef]
- Sisto, M.; Lorusso, L.; Tamma, R.; Ingravallo, G.; Ribatti, D.; Lisi, S. Interleukin-17 and -22 synergy linking inflammation and EMT-dependent fibrosis in Sjögren's syndrome. Clin. Exp. Immunol. 2019, 198, 261–272. [Google Scholar] [CrossRef] [PubMed]
- Wilson, M.S.; Madala, S.K.; Ramalingam, T.R.; Gochuico, B.R.; Rosas, I.O.; Cheever, A.W.; et al. Bleomycin and IL-1β-mediated pulmonary fibrosis is IL-17A dependent. J. Exp. Med. 2010, 207, 535–552. [Google Scholar] [CrossRef]
- Liu, Y.; Zhu, H.; Su, Z.; Sun, C.; Yin, J.; Yuan, H.; Sandoghchian, S.; Jiao, Z.; Wang, S.; Xu, H. IL-17 contributes to cardiac fibrosis following experimental autoimmune myocarditis by a PKCβ/Erk1/2/NF-κB-dependent signaling pathway. Int. Immunol. 2012, 24, 605–612. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.F.; Yuan, J.; Liao, M.Y.; Xia, N.; Tang, T.T.; Li, J.J.; Jiao, J.; Dong, W.Y.; Nie, S.F.; Zhu, Z.F.; Zhang, W.C.; Lv, B.J.; Xiao, H.; Wang, Q.; Tu, X.; Liao, Y.H.; Shi, G.P.; Cheng, X. IL-17A promotes ventricular remodeling after myocardial infarction. J. Mol. Med. 2014, 92, 1105–1116. [Google Scholar] [CrossRef] [PubMed]
- Gasse, P.; Riteau, N.; Vacher, R.; Michel, M.L.; Fautrel, A.; di Padova, F.; Fick, L.; Charron, S.; Lagente, V.; Eberl, G.; Le Bert, M.; Quesniaux, V.F.; Huaux, F.; Leite-de-Moraes, M.; Ryffel, B.; Couillin, I. IL-1 and IL-23 mediate early IL-17A production in pulmonary inflammation leading to late fibrosis. PLoS One. 2011, 6, e23185. [Google Scholar] [CrossRef]
- Todd, N.W.; Luzina, I.G.; Atamas, S.P. Molecular and cellular mechanisms of pulmonary fibrosis. Fibrogenesis Tissue Repair. 2012, 5, 11. [Google Scholar] [CrossRef]
- Gurczynski, S.J.; Moore, B.B. IL-17 in the lung: the good, the bad, and the ugly. Am. J. Physiol. Lung Cell Mol. Physiol. 2018, 314, L6–L16. [Google Scholar] [CrossRef]
- Nie, Y.J.; Wu, S.H.; Xuan, Y.H.; Yan, G. Role of IL-17 family cytokines in the progression of IPF from inflammation to fibrosis. Mil. Med. Res. 2022, 9, 21. [Google Scholar] [CrossRef]
- Sønder, S.U.; Saret, S.; Tang, W.; Sturdevant, D.E.; Porcella, S.F.; Siebenlist, U. IL-17-induced NF-kappaB activation via CIKS/Act1: physiologic significance and signaling mechanisms. J. Biol. Chem. 2011, 286, 12881–12890. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Zepp, J.; Li, X. Function of Act1 in IL-17 family signaling and autoimmunity. Adv. Exp. Med. Biol. 2012, 946, 223–235. [Google Scholar] [PubMed]
- Reynolds, J.M.; Lee, Y.H.; Shi, Y.; Wang, X.; Angkasekwinai, P.; Nallaparaju, K.C. ; Interleukin-17B Antagonizes Interleukin-25-Mediated Mucosal Inflammation. Immunity. 2015, 42, 692–603. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Chen, J.; Huang, A.; Stinson, J.; Heldens, S.; Foster, J.; Dowd, P.; Gurney, A.L.; Wood, W.I. Cloning and characterization of IL-17B and IL-17C, two new members of the IL-17 cytokine family. Proc. Natl. Acad. Sci. U S A. 2000, 97, 773–778. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Ullrich, S.J.; Zhang, J.; Connolly, K.; Grzegorzewski, K.J.; Barber, M.C.; et al. A novel cytokine receptor-ligand pair. Identification, molecular characterization, and in vivo immunomodulatory activity. J. Biol. Chem. 2000, 275, 19167–19176. [Google Scholar] [CrossRef] [PubMed]
- Morrow, K.N.; Coopersmith, C.M.; Ford, M.L. IL-17, IL-27, and IL-33: A Novel Axis Linked to Immunological Dysfunction During Sepsis. Front. Immunol. 2019, 10, 1982. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Chen, X.; Wang, J.; Lou, Q.; Lou, Y.; Li, L.; Wang, H.; Chen, J.; Wu, M.; Song, X.; Qian, Y. Dysregulated Lung Commensal Bacteria Drive Interleukin-17B Production to Promote Pulmonary Fibrosis through Their Outer Membrane Vesicles. Immunity. 2019, 50, 692–706. [Google Scholar] [CrossRef]
- Krohn, S.; Nies, J.F.; Kapffer, S.; Schmidt, T.; Riedel, J.H.; Kaffke, A.; Peters, A.; Borchers, A.; Steinmetz, O.M.; Krebs, C.F.; Turner, J.E.; Brix, S.R.; Paust, H.J.; Stahl, R.A.K.; Panzer, U. IL-17C/IL-17 Receptor E Signaling in CD4+ T Cells Promotes TH17 Cell-Driven Glomerular Inflammation. J. Am. Soc. Nephrol. 2018, 29, 1210–1222. [Google Scholar] [CrossRef]
- Vandeghinste, N.; Klattig, J.; Jagerschmidt, C.; Lavazais, S.; Marsais, F.; Haas, J.D.; Auberval, M.; Lauffer, F.; Moran, T.; Ongenaert, M.; Van Balen, M.; Dupont, S.; Lepescheux, L.; Garcia, T.; Härtle, S.; Eyerich, K.; Fallon, P.G.; Brys, R.; Steidl, S. Neutralization of IL-17C Reduces Skin Inflammation in Mouse Models of Psoriasis and Atopic Dermatitis. J. Invest. Dermatol. 2018, 138, 1555–1563. [Google Scholar] [CrossRef]
- Chang, S.H.; Reynolds, J.M.; Pappu, B.P.; Chen, G.; Martinez, G.J.; Dong, C. Interleukin-17C promotes Th17 cell responses and autoimmune disease via interleukin-17 receptor E. Immunity. 2011, 35, 611–621. [Google Scholar] [CrossRef]
- Conti, H.R.; Whibley, N.; Coleman, B.M.; Garg, A.V.; Jaycox, J.R.; Gaffen, S.L. Signaling through IL-17C/IL-17RE is dispensable for immunity to systemic, oral and cutaneous candidiasis. PLoS ONE. 2015, 10, e0122807. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Zhang, X.; Yang, C.; Wang, S.; Shen, H. Essential role of IL-17 in acute exacerbation of pulmonary fibrosis induced by non-typeable Haemophilus influenzae. Theranostics. 2022, 12, 5125–5137. [Google Scholar] [CrossRef]
- Vella, G.; Ritzmann, F.; Wolf, L.; Kamyschnikov, A.; Stodden, H.; Herr, C.; Slevogt, H.; Bals, R.; Beisswenger, C. IL-17C contributes to NTHi-induced inflammation and lung damage in experimental COPD and is present in sputum during acute exacerbations. PLoS One. 2021, 16, e0243484. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Sun, S.; Liu, D. IL-17D: A Less Studied Cytokine of IL-17 Family. Int Arch Allergy Immunol. 2020, 181, 618–623. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Dong, C. IL-25 in allergic inflammation. Immunol. Rev. 2017, 278, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Xu, X. .; Luo, S.; Li, B.; Dai, H.; Zhang, J. IL-25 contributes to lung fibrosis by directly acting on alveolar epithelial cells and fibroblasts. Exp. Biol. Med. 2019, 244, 770–780. [Google Scholar] [CrossRef]
- Hams, E.; Armstrong, M.E.; Barlow, J.L.; Saunders, S.P.; Schwartz, C.; Cooke, G.; Fahy, R.J.; Crotty, T.B.; Hirani, N.; Flynn, R.J.; Voehringer, D.; McKenzie, A.N.; Donnelly, S.C.; Fallon, P.G. IL-25 and type 2 innate lymphoid cells induce pulmonary fibrosis. Proc. Natl. Acad. Sci U S A. 2014, 111, 367–372. [Google Scholar] [CrossRef]
- Yang, X.O.; Chang, S.H.; Park, H.; Nurieva, R. , Shah, B.; Acero, L, et al. Regulation of inflammatory responses by IL-17F. J. Exp. Med 2008, 205, 1063–1075. [Google Scholar] [CrossRef]
- Hot, A.; Miossec, P. Effects of interleukin (IL)-17A and IL-17F in human rheumatoid arthritis synoviocytes. Ann. Rheum. Dis. 2011, 70, 727–732. [Google Scholar] [CrossRef]
- Ritzmann, F.; Lunding, L.P.; Bals, R.; Wegmann, M.; Beisswenger, C. IL-17 Cytokines and Chronic Lung Diseases. Cells. 2022, 11, 2132. [Google Scholar] [CrossRef]
- Bettelli, E.; Carrier, Y.; Gao, W.; Korn, T.; Strom, T.B.; Oukka, M.; Weiner, H.L.; Kuchroo, V.K. Reciprocal developmental pathways for the generation of pathogenic effector Th17 cells and regulatory T cells. Nature 2006, 441, 235–238. [Google Scholar] [CrossRef] [PubMed]
- Raphael, I.; Joern, R.R.; Forsthuber, T.G. Memory CD4+ T Cells in Immunity and Autoimmune Diseases. Cells. 2020, 9, 531. [Google Scholar] [CrossRef]
- Allen, R.J.; Porte, J.; Braybrooke, R.; Flores, C.; Fingerlin, T.E.; Oldham, J.M.; Guillen-Guio, B.; Ma, S.F.; Okamoto, T.; John, A.E.; et al. Genetic variants associated with susceptibility to idiopathic pulmonary fibrosis in people of European ancestry: a genome-wide association study. Lancet Respir Med. 2017, 5, 869–880. [Google Scholar] [CrossRef] [PubMed]
- Dagneaux, L.; Owen, A.R.; Bettencourt, J.W.; Barlow, J.D.; Amadio, P.C.; Kocher, J.P.; Morrey, M.E; Sanchez-Sotelo, J.; Berry, D.J.; van Wijnen, A.J.; Abdel, M.P. Human Fibrosis: Is There Evidence for a Genetic Predisposition in Musculoskeletal Tissues? J. Arthroplasty. 2020, 35, 3343–3352. [Google Scholar] [CrossRef]
- Liu, Y. , Wen, D., Ho, C. et al. Epigenetics as a versatile regulator of fibrosis. J. Transl. Med. 2023, 21, 164. [Google Scholar] [CrossRef]
- Wei, G.; Wei, L.; Zhu, J.; Zang, C.; Hu-Li, J.; Yao, Z.; Cui, K.; Kanno, Y.; Roh, T.Y.; Watford, W.T.; Schones, D.E.; Peng, W.; Sun, H.W.; Paul, W.E.; O'Shea, J.J.; Zhao, K. Global mapping of H3K4me3 and H3K27me3 reveals specificity and plasticity in lineage fate determination of differentiating CD4+ T cells. Immunity. 2009, 30, 155–167. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.P.; Tsai, Y.G.; Lin, T.Y.; Wu, M.J.; Lin, C.Y. The attenuation of renal fibrosis by histone deacetylase inhibitors is associated with the plasticity of FOXP3+IL-17+ T cells. BMC Nephrol. 2017 18, 225. [CrossRef]
- Seto, E. , Yoshida, M. Erasers of histone acetylation: the histone deacetylase enzymes. Cold Spring Harb. Perspect Biol. 2014, 6, a018713. [Google Scholar] [CrossRef]
- Felisbino, M.B.; McKinsey, T.A. Epigenetics in cardiac fibrosis. JACC Basic Transl. Sci. 2018, 3, 704–15. [Google Scholar] [CrossRef]
- Barcena-Varela, M.; Colyn, L.; Fernandez-Barrena, M.G. Epigenetic mechanisms in hepatic stellate cell activation during liver fibrosis and carcinogenesis. Int. J. Mol. Sci. 2019, 20, 2507. [Google Scholar] [CrossRef]
- Goschl, L.; Preglej, T.; Boucheron, N.; et al. Histone deacetylase 1 (HDAC1): a key player of T cell-mediated arthritis. J. Autoimmun. 2020, 108. [Google Scholar] [CrossRef]
- Regna, N.L.; Chafin, C.B.; Hammond, S.E.; Puthiyaveetil, A.G.; Caudell, D.L.; Reilly, C.M. Class I and II histone deacetylase inhibition by ITF2357 reduces SLE pathogenesis in vivo. Clin. Immunol. 2014, 151, 29–42. [Google Scholar] [CrossRef] [PubMed]
- Ichiyama, K.; Chen, T.; Wang, X.; Yan X, Kim BS, Tanaka S, Ndiaye-Lobry D, Deng Y, Zou Y, Zheng P, Tian Q, Aifantis I, Wei L, Dong C. The methylcytosine dioxygenase Tet2 promotes DNA demethylation and activation of cytokine gene expression in T cells. Immunity. 2015, 42, 613–626. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Zou, J.; Wang, M.; Ding, X.; Chepelev, I.; Zhou, X.; Zhao, W.; Wei, G.; Cui, J.; Zhao, K.; Wang, H.Y.; Wang, R.F. Critical role of histone demethylase Jmjd3 in the regulation of CD4+ T-cell differentiation. Nat. Commun. 2014, 5, 5780. [Google Scholar] [CrossRef] [PubMed]
- Takada, I. DGCR14 induces Il17a gene expression through the RORgamma/BAZ1B/RSKS2 complex. Mol. Cell Biol. 2015, 35, 344–355. [Google Scholar] [CrossRef]
- Bandukwala, H.S.; Gagnon, J.; Togher, S.; et al. Selective inhibition of CD4+ T-cell cytokine production and autoimmunity by BET protein and c-Myc inhibitors. Proc. Natl. Acad. Sci. U S A. 2012, 109, 14532–14537. [Google Scholar] [CrossRef]
- Cribbs, A.P.; Terlecki-Zaniewicz, S.; Philpott, M.; Baardman, J.; Ahern, D.; Lindow, M.; Obad, S.; Oerum, H.; Sampey, B.; Mander, P.K.; Penn, H.; Wordsworth, P.; Bowness, P.; de Winther, M.; Prinjha, R.K.; Feldmann, M.; Oppermann, U. Histone H3K27me3 demethylases regulate human Th17 cell development and effector functions by impacting on metabolism. Proc. Natl. Acad. Sci. U S A. 2020, 117, 6056–6066. [Google Scholar] [CrossRef]
- Mele, D.A.; Salmeron, A.; Ghosh, S.; Huang, H.R.; Bryant, B.M.; Lora, J.M. BET bromodomain inhibition suppresses TH17-mediated pathology. J. Exp. Med. 2013, 210, 2181–2190. [Google Scholar] [CrossRef]
- Klein, K. Bromodomain protein inhibition: a novel therapeutic strategy in rheumatic diseases. RMD Open. 2018, 4, e000744. [Google Scholar] [CrossRef]
- Chen, K.; Campfield, B.T.; Wenzel, S.E.; McAleer, J.P.; Kreindler, J.L.; Kurland, G.; Gopal. R.; Wang, T.; Chen, W., Eddens, T., Quinn, K.M.; Myerburg, M.M.; Horne, W.T.; Lora, J.M.; Albrecht, B.K.; Pilewski, J.M.; Kolls, J.K. Antiinflammatory effects of bromodomain and extraterminal domain inhibition in cystic fibrosis lung inflammation. JCI Insight. 2016, 1, e87168. [Google Scholar] [CrossRef]
- Fragoulis, G.E.; Siebert, S.; McInnes, I.B. Therapeutic targeting of IL-17 and IL-23 cytokines in immune-mediated diseases. Annu. Rev. Med. 2016, 67, 337–353. [Google Scholar] [CrossRef]
- Pastor, W.A.; Aravind, L.; Rao, A. TETonic shift: biological roles of TET proteins in DNA demethylation and transcription. Nat. Rev. Mol. Cell Biol. 2013, 14, 341–356. [Google Scholar] [CrossRef]
- Hu, X.; Zou, Y.; Copland, D.A.; Schewitz-Bowers, L.P.; Li, Y.; Lait, P.J.P.; Stimpson, M.; Zhang, Z.; Guo, S.; Liang, J.; Chen, T.; Li, J.J.; Yuan, S.; Li, S.; Zhou, P.; Liu, Y.; Dick, A.D.; Wen, X.; Lee, R.W.J.; Wei, L. Epigenetic drug screen identified IOX1 as an inhibitor of Th17-mediated inflammation through targeting TET2. E Bio Medicine. 2022, 86, 104333. [Google Scholar] [CrossRef] [PubMed]
- Antar, S.A.; Ashour, N.A.; Marawan, M.E.; Al-Karmalawy, A.A. Fibrosis: Types, Effects, Markers, Mechanisms for Disease Progression, and Its Relation with Oxidative Stress, Immunity, and Inflammation. Int. J. Mol. Sci. 2023, 24, 4004. [Google Scholar] [CrossRef] [PubMed]
- Zapletal, D.; Kubicek, K.; Svoboda, P.; Stefl, R. Dicer structure and function: conserved and evolving features. EMBO Rep. 2023, 24, e57215. [Google Scholar] [CrossRef] [PubMed]
- Wang, B. Base Composition Characteristics of Mammalian miRNAs. J. Nucleic Acids. 2013, 2013, 951570. [Google Scholar] [CrossRef]
- Ghafouri-Fard, S. .; Abak, A.; Talebi, S.F.; Shoorei, H.; Branicki, W.; Taheri, M.; Akbari Dilmaghani, N. Role of miRNA and lncRNAs in organ fibrosis and aging. Biomed Pharmacother. 2021, 143, 112132. [Google Scholar]
- Quinn, J.J.; Chang, H.Y. Unique features of long non-coding RNA biogenesis and function. Nat. Rev. Genet. 2016, 17, 47–62. [Google Scholar] [CrossRef]
- Mattick, J.S.; Amaral, P.P.; Carninci, P. et al. Long non-coding RNAs: definitions, functions, challenges and recommendations. Nat. Rev. Mol. Cell Biol. 2023, 24, 430–447. [Google Scholar] [CrossRef]
- Li, Q.Y.; Gong, T.; Huang, Y.K.; Kang, L.; Warner, C.A.; Xie, H.; Chen, L.M.; Duan, X.Q. Role of noncoding RNAs in liver fibrosis. World J. Gastroenterol. 2023, 29, 1446–1459. [Google Scholar] [CrossRef]
- Yao, S.X.; Zhang, G.S.; Cao, H.X.; Song, G.; Li, Z.T.; Zhang, W.T. Correlation between microRNA-21 and expression of Th17 and Treg cells in microenvironment of rats with hepatocellular carcinoma. Asian Pac. J. Trop. Med. 2015 8, 762–765. [CrossRef]
- Ishida, W.; Fukuda, K.; Sakamoto, S.; Koyama, N.; Koyanagi,A. ; Yagita, H.; Fukushima, A. Regulation of experimental autoimmune uveoretinitis by anti-delta-like ligand 4 monoclonal antibody. Invest. Ophthalmol.Vis.Sci. 2011, 52, 8224–30. [Google Scholar] [CrossRef] [PubMed]
- Ichihara, A.; Jinnin, M.; Oyama, R.; Yamane, K.; Fujisawa, A.; Sakai, K.; Masuguchi, S.; Fukushima, S.; Maruo, K.; Ihn, H. Increased serum levels of miR-1266 in patients with psoriasis vulgaris. Eur. J. Dermatol. 2012, 22, 68–71. [Google Scholar] [CrossRef] [PubMed]
- Xia, P.; Fang, X.; Zhang, Z.H.; Huang, Q.; Yan, K.X.; Kang, K.F.; Han, L.; Zheng, Z.Z. Dysregulation of miRNA146a versus IRAK1 induces IL-17 persistence in the psoriatic skin lesions. Immunol. Lett. 2012, 148, 151–162. [Google Scholar] [CrossRef]
- Niimoto, T.; Nakasa, T.; Ishikawa, M. , Okuhara, A.; Izumi, B.; Deie, M.; Suzuki, O.; Adachi, N.; Ochi, M. MicroRNA-146a expresses in interleukin-17 producing T cells in rheumatoid arthritis patients. BMC Musculoskelet. Disord. 2010, 11, 209. [Google Scholar] [CrossRef]
- Dong, L.; Wang, X.; Tan, J.; Li, H.; Qian, W.; Chen, J.; Chen, Q.; Wang, J.; Xu, W.; Tao, C.; Wang, S. Decreased expression of microRNA-21 correlates with the imbalance of Th17 and Treg cells in patients with rheumatoid arthritis. J. Cell Mol. Med. 2014, 18, 2213–2224. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, Y.Y.; Li, T.T.; Wang, J.; Jiang, Y.; Zhao, Y.; Jin, X.X.; Xue, G.L.; Yang, Y.; Zhang, X.F.; Sun, Y.Y.; Zhang, Z.R.; Gao, X.; Du, Z.M.; Lu, Y.J.; Yang, B.F.; Pan, Z.W. Ablation of interleukin-17 alleviated cardiac interstitial fibrosis and improved cardiac function via inhibiting long non-coding RNA-AK081284 in diabetic mice. J. Mol. Cell Cardiol. 2018, 115, 64–72. [Google Scholar] [CrossRef] [PubMed]
- Castro-Villegas, C.; Pérez-Sánchez, C.; Escudero, A.; Filipescu, I.; Verdu, M.; Ruiz-Limón, P.; Aguirre, M.A.; Jiménez-Gomez, Y.; Font, P.; Rodriguez-Ariza, A.; Peinado, J.R.; Collantes-Estévez, E.; González-Conejero, R.; Martinez, C.; Barbarroja, N.; López-Pedrera, C. Circulating miRNAs as potential biomarkers of therapy effectiveness in rheumatoid arthritis patients treated with anti-TNFα. Arthritis Res. Ther. 2015, 17, 49. [Google Scholar] [CrossRef]
- O'Reilly, S.; Hügle, T.; van Laar, J.M. T cells in systemic sclerosis: a reappraisal. Rheumatology (Oxford). 2012, 51, 1540–1549. [Google Scholar] [CrossRef]
- Nakashima, T.; Jinnin, M.; Yamane, K.; Honda, N.; Kajihara, I.; Makino, T.; Masuguchi, S.; Fukushima, S.; Okamoto, Y.; Hasegawa, M.; Fujimoto, M.; Ihn, H. Impaired IL-17 signaling pathway contributes to the increased collagen expression in scleroderma fibroblasts. J. Immunol. 2012, 188, 3573–3583. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, X.; Deng, Y. miR-125a-3p decreases levels of interlukin-17 and suppresses renal fibrosis via down-regulating TGF-β1 in systemic lupus erythematosus mediated Lupus nephritic mice. Am. J. Transl. Res. 2019, 11, 1843–1853. [Google Scholar] [PubMed]
- Zhu, E.; Wang, X.; Zheng, B.; Wang, Q.; Hao, J.; Chen, S.; Zhao, Q.; Zhao, L.; Wu, Z.; Yin, Z. miR-20b suppresses Th17 differentiation and the pathogenesis of experimental autoimmune encephalomyelitis by targeting RORγt and STAT3. J. Immunol. 2014, 192, 5599–5609. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; He, F.; Pang, R.; Zhao, D.; Qiu, W.; Shan, K.; Zhang, J.; Lu, Y.; Li, Y.; Wang, Y. Interleukin-17 (IL-17)-induced microRNA 873 (miR-873) contributes to the pathogenesis of experimental autoimmune encephalomyelitis by targeting A20 ubiquitin-editing enzyme. J. Biol. Chem. 2014, 289, 28971–28986. [Google Scholar] [CrossRef]
- Hanieh, H.; Alzahrani, A. MicroRNA-132 suppresses autoimmune encephalomyelitis by inducing cholinergic anti-inflammation: a new Ahr-based exploration. Eur. J. Immunol. 2013, 43, 2771–2782. [Google Scholar] [PubMed]
- Du, C.; Liu, C.; Kang, J.; Zhao, G.; Ye, Z.; Huang, S.; Li, Z.; Wu, Z.; Pei, G. MicroRNA miR-326 regulates TH-17 differentiation and is associated with the pathogenesis of multiple sclerosis. Nat. Immunol. 2009, 10, 1252–1259. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).





