1. Introduction
Frontotemporal dementia (FTD) is the second most common presenile dementia, with an annual prevalence and incidence of 0.01-4.6 and 0.0-0.3 per 1000 people, respectively [
1]. However, because the clinical symptoms of FTD, such as alterations in brain structures and content of protein aggregates, are diverse and similar to those of other dementias (Alzheimer’s and Parkinson’s diseases) [
2], FTD has been relatively poorly investigated. Moreover, the psychiatric symptoms associated with FTD, such as interpersonal conduct and personal regulation, sometimes lead to misdiagnoses of depression or bipolar disorder [
3]. Although brain imaging methods, including magnetic resonance imaging, can identify variants of frontotemporal dementia, they are expensive and restricted to early-stage diagnosis. Hence, further investigations of inexpensive and objective markers of FTD are required.
Quantitative electroencephalography (qEEG) is an inexpensive, noninvasive, and convenient tool for assessing alterations in neuronal activity after dementia. qEEG enables the objective evaluation of neurological abnormalities in cognitive function by manifesting the degree of synchronization or desynchronization of connected neurons. Functional connectivity (FC) and spectral power of electroencephalography (EEG) are mainly used to distinguish patients with different types of dementia from healthy controls (CTLs) [
4]. However, with respect to FTD, qEEG studies attempting to distinguish FTD from healthy controls have hitherto been unsuccessful or flawed. First, spectral power approaches have yielded contradictory results. Lower global-averaged alpha, beta, and spectral ratios between slow and fast rhythms were observed in patients with FTD compared with those in normal CTLs [
5]. However, another study using similar global field power analyses identified only a decreased alpha in FTD [
6].
In regional analyses, no significance in alpha and spectral ratios, whereas increased frontal theta and delta power, increased parietal theta, and decreased temporal and parietal beta power were observed in patients with FTD compared with those in healthy participants used as CTLs [
7]. In contrast, using the same regional analyses, no significant changes were detected in these indicators for Pick’s disease, a variant of FTD [
8]. Using standardized low-resolution electromagnetic tomography (sLORETA) revealed only lower frontal and temporal alpha power in patients with FTD compared with those in normal CTLs [
6]. However, using the same sLORETA and spectral power methods, another study observed increased theta power over all regional lobes in FTD [
9]. Particularly, for spectral power approaches, despite analyses using the same method (e.g., sLORETA, global field power, and spectral power of regional electrodes), each study resulted in different significance in different frequency bands in different lobes. Such an inconsistency may result from the inappropriateness of previous spectral power indices in accurately capturing abnormalities in FTD.
Despite their excellent ability to obtain phase information combined with EEG amplitudes, FC analyses are highly inconsistent. Several EEG FC studies have failed to produce consistent results regarding depression [
10]. Such inconsistencies in FC were also observed in empirical studies on FTD. Synchronization likelihood, mean clustering coefficient (local connectivity), characteristic path length (global connectivity), and global minimum spanning tree measures analyses found no difference in FC between patients with FTD and CTLs [
11,
12,
13]. However, an increase in the degree of correlation in the lower alpha band was observed in patients with FTD [
12]. In contrast, no significant difference in the alpha and beta bands in the phase lag index was observed between patients with FTD and CTLs; instead, an increased phase lag index in the delta band was observed [
13]. In addition, hypoconnectivity in the mid- and long-range frontotemporal networks in the alpha and beta bands was observed by Weighted Symbolic Mutual Information in the behavioral variant of FTD [
14]. Determining whether these opposing evaluations of FTD result from state dependency, methodological problems, or the use of slightly different indexes is difficult, exposing scientists to the possibility of interpretational pitfalls unless advanced statistical evaluation or meta-analysis is conducted [
15]. Additionally, FC is sometimes a weak parameter for determining causal brain interactions [
16]. These studies indicated that FC may not be appropriate for diagnosing FTD.
Therefore, new EEG markers, as an alternative to the previously inconsistent qEEG methods, should be examined for distinguishing patients with FTD from healthy CTLs. This new qEEG index should be related to the neurological symptoms and pathology of FTD because patients with FTD show EEG and neuropathological patterns that are distinctive from those of patients with Parkinson’s or Alzheimer’s disease [
17,
18,
19,
20,
21]. The association between the frontal and temporal lobes appears to be a plausible and reasonable indicator for the diagnosis of FTD as severe frontotemporal degeneration is a distinctive pathological hallmark of FTD. Impaired interactions in the frontotemporal network in patients with FTD represent different interactions between the frequency bands of each lobe [
21,
22]. Therefore, the relationships between the temporal and frontal frequency bands may be promising indicators of FTD.
In addition, the spectral ratios between frequency bands can be used to successfully evaluate cognition and detect dementia, including Alzheimer’s disease, Parkinson’s disease, and Lewy body dementia [
23,
24,
25,
26,
27,
28]. However, the EEG spectral ratios of frontal frequency power/temporal frequency power have not yet been investigated. The current study aimed to introduce a new, accurate, and consistent qEEG marker that can be used to diagnose FTD and other neurological abnormalities. We hypothesized that the spectral power ratio between the frontal and temporal lobes would consistently and successfully detect FTD. The study focused on the spectral power ratio between the frontal and temporal lobes as an efficient and effective qEEG marker for detecting FTD. The effects of different parameters in the measurement of spectral power were compared to determine the consistency of this index.
2. Materials and Methods
2.1. Participants
Preprocessed datasets from OpenNeuro were used [
29,
30,
31]. In total, 23 patients with FTD and 29 healthy CTLs at the Second Department of Neurology, AHEPA General Hospital, Thessaloniki, Greece were included in the dataset. The initial diagnosis of patients with FTD was performed according to the criteria provided by the Diagnostic and Statistical Manual of Mental Disorders, 3rd ed., revised (DSM-IIIR, DSM IV, ICD-10) and the National Institute of Neurological, Communicative Disorders and Stroke—Alzheimer’s Disease and Related Disorders Association (NINCDS—ADRDA) [
29,
30,
31]. The cognitive state of each participant was assessed using the International Mini-Mental State Examination (scale: 0–30). Lower Mini-Mental State Examination scores indicated cognitive impairment. The disease duration was measured in months (first quartile, 24 months; second quartile, 25 months; third quartile, 28.5 months).
This study was approved by the Public Institutional Review Board Designated by the Ministry of Health and Welfare (P01-202304-01-023) and was conducted according to the tenets of the Declaration of Helsinki. All participants provided written informed consent.
2.2. EEG acquisition and preprocessing
Each participant was subjected to an eye-closed resting-state EEG at the Second Department of Neurology of the AHEPA General Hospital of Thessaloniki, Greece. A Nihon Kohden EEG 2100 device with a 10//20 system and sampling frequency of 500 Hz was used. The two electrodes on the mastoids were used as references. Each recording lasted over 10 min. Each EEG dataset was preprocessed from an original study (31). A 0.5–45 Hz filter was applied. After being rereferenced to A1-A2, the raw data were cleaned with the ASR plugin in EEGLAB [
32,
33]. An independent component analysis was conducted to distinguish and remove artifactual brain components in the eyes and muscles. Finally, all data were average referenced.
2.3. Spectral analysis
The absolute average spectral power of each frequency band of delta (1–4 Hz), theta (4–8 Hz), alpha (8–13 Hz), beta (13–32 Hz), and gamma (32–45 Hz) was calculated using the discrete fast Fourier transform (FFT) at a 2-s FFT window length and 10 steps per Hz. Spectral power was separated into frontal (electrodes Fp1, Fp2, F3, F4, F7, F8, and Fz) and temporal (electrodes T3, T4, T5, and T6) power. Frontal and temporal power in each frequency band was calculated, and frontal/temporal power ratios for every combination of each frequency band were calculated (frontal frequency power/temporal frequency power). For the consistency test, 2s/5 and 1s/10 steps were additionally applied for the FFT window length/step size per Hz to measure the same spectral power. The 2s/10 step (FFT window length/step size per Hz) represented 1000/5000/500 samples (window/FFT/overlapping length), whereas the 2s/5 step represented 1000/2500/500 samples. The 1s/10 step represented 500/5000/250 samples. In addition, because average reference might be invalid for low-density electrodes, infinity reference (EEGLAB plugin REST) was applied to compare results at 2s/10s [
34,
35].
2.4. Statistical analysis
Clinicodemographic patient characteristics were assessed using rank-sum and chi-square tests as data were nonnormally distributed. The Mann-Whitney U test was used to determine the difference in the frontal/temporal brain ratio between patients with FTD and CTL. Only the variables significantly different between the two groups were included in statistical analyses. Receiver operating characteristic (ROC) and precision-recall (PR) curve analyses were performed to determine the diagnostic accuracy of each spectral power ratio in the frontal and temporal lobes. The area under the curve (AUC) of the ROC and PR was compared using the Delong’s and logit method, respectively [
36,
37,
38]. With a standardized prevalence of 50 %, the Youden index was used to determine specificity, sensitivity, cutoff point, positive predictive value, and negative predictive value [
39,
40,
41]. Bootstrapping with 5000 iterations and 978 seeds was applied to measure the confidence interval of the maximum Youden Index and the optimal cutoff point. Multiple logistic regression with a stepwise method (enter variables if probability <0.05 and remove if >0.1) was applied with covariates of age and sex to determine the discrimination ability of lobar spectral power and F/T spectral power ratios. The same statistical analyses were applied to the spectral power using different FFT parameters (2s/5s and 1s/10s). The Statistical Package for the Social Sciences version 25.0 (IBM Corp., Armonk, NY, USA) and MedCalc® Statistical Software version 20.218 (MedCalc Software Ltd., Ostend, Belgium;
https://www.medcalc.org; 2023) were used for all statistical analyses.
3. Results
We did not detect any significant differences in age (p = 0.068) and sex (p = 0.930) between groups. However, the FTD group showed lower Mini-Mental State Examination scores (p < 0.001) compared with the CTL group. The clinicodemographic characteristics of the patients are presented in
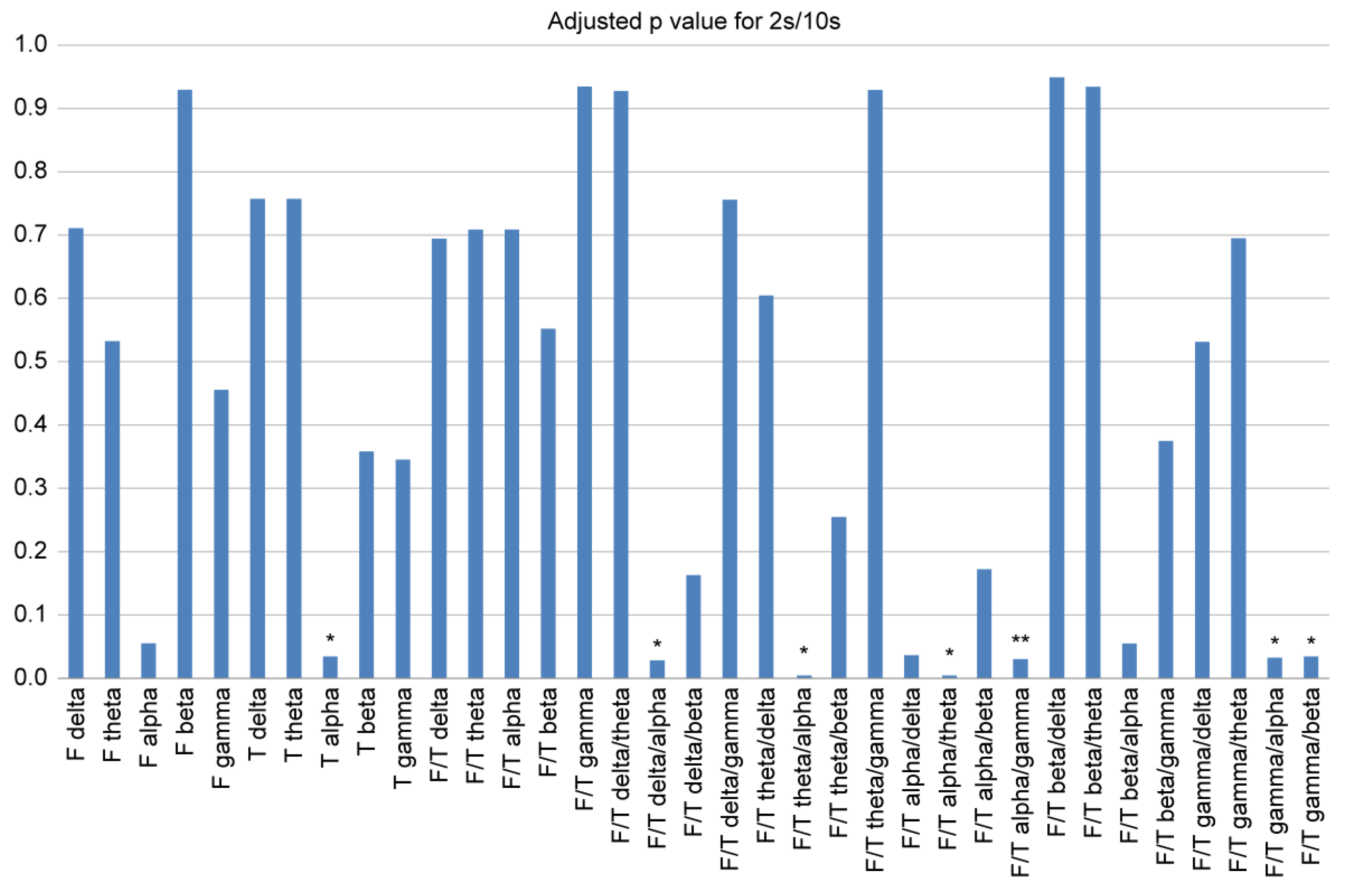
Table 1. For a condensed and effective comparison, we performed the Mann-Whitney U test to select consistently significant indices of the spectral power ratio between the frontal and temporal lobes (
Figure 1). Regarding individual regional power, we observed that the FTD group showed significantly smaller temporal alpha powers than those in the CTL group (p < 0.05). Whereas, in the case of the frontal/temporal (F/T) spectral power ratio, the FTD group showed larger frontal/temporal (F/T) delta/alpha (p < 0.05), theta/alpha (p < 0.05), gamma/alpha (p < 0.05), and gamma/beta (p < 0.05) ratios compared with those in the CTL group. We observed smaller F/T spectral power ratios for F/T alpha/theta (p < 0.05) and F/T alpha/gamma (p < 0.01) during FTD. Significance tests for each FFT parameter and referencing method are presented in
Figure 1 and Supplementary
Figures S1–S3. We found that with different referencing methods and different FFT parameters, only F/T theta/alpha and F/T gamma/alpha were consistently higher in the FTD group than in the CTL group. Meanwhile, we noticed that the F/T alpha/theta and F/T alpha/gamma were consistently smaller in the FTD group compared with that in the CTL group.
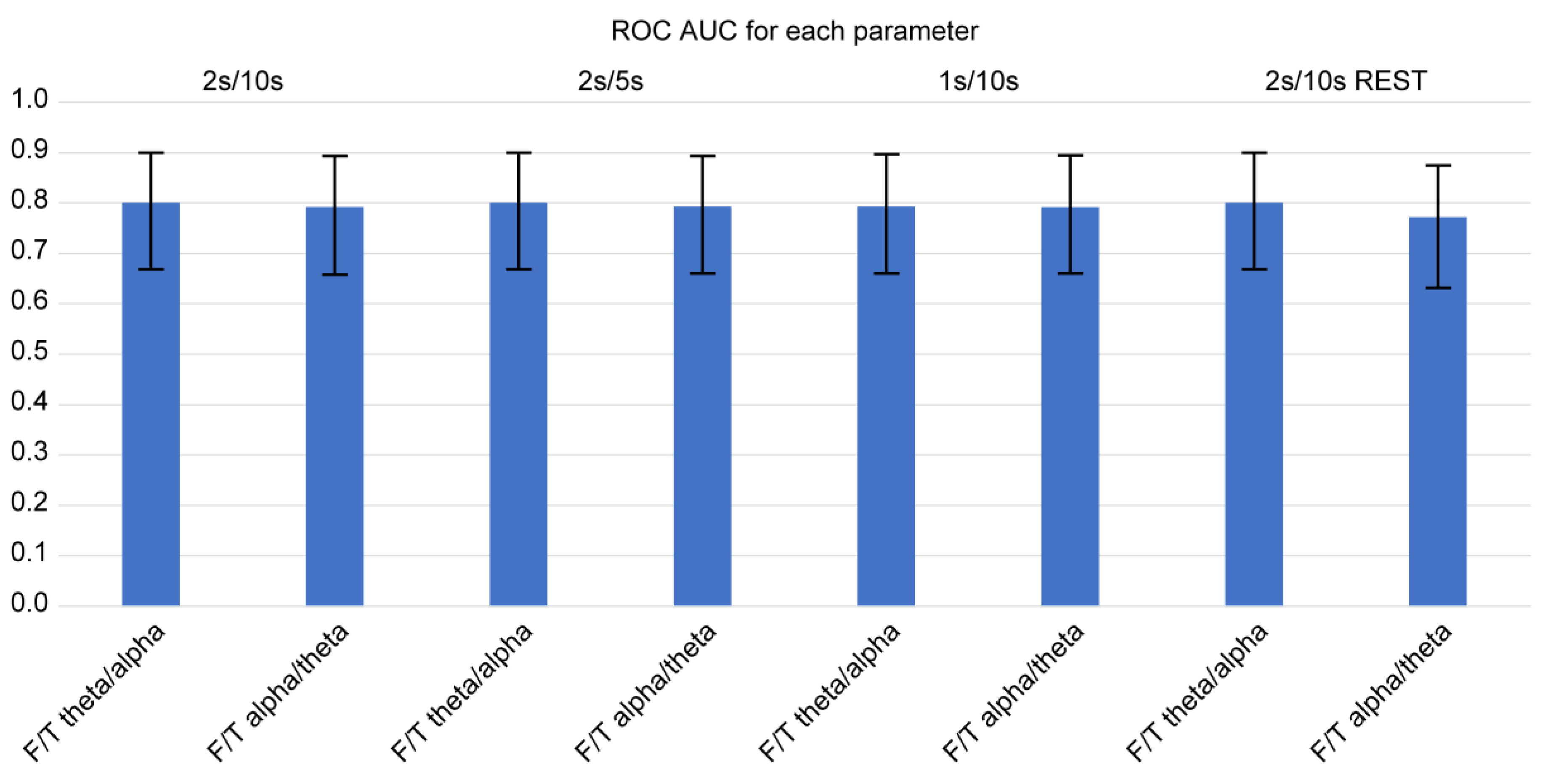
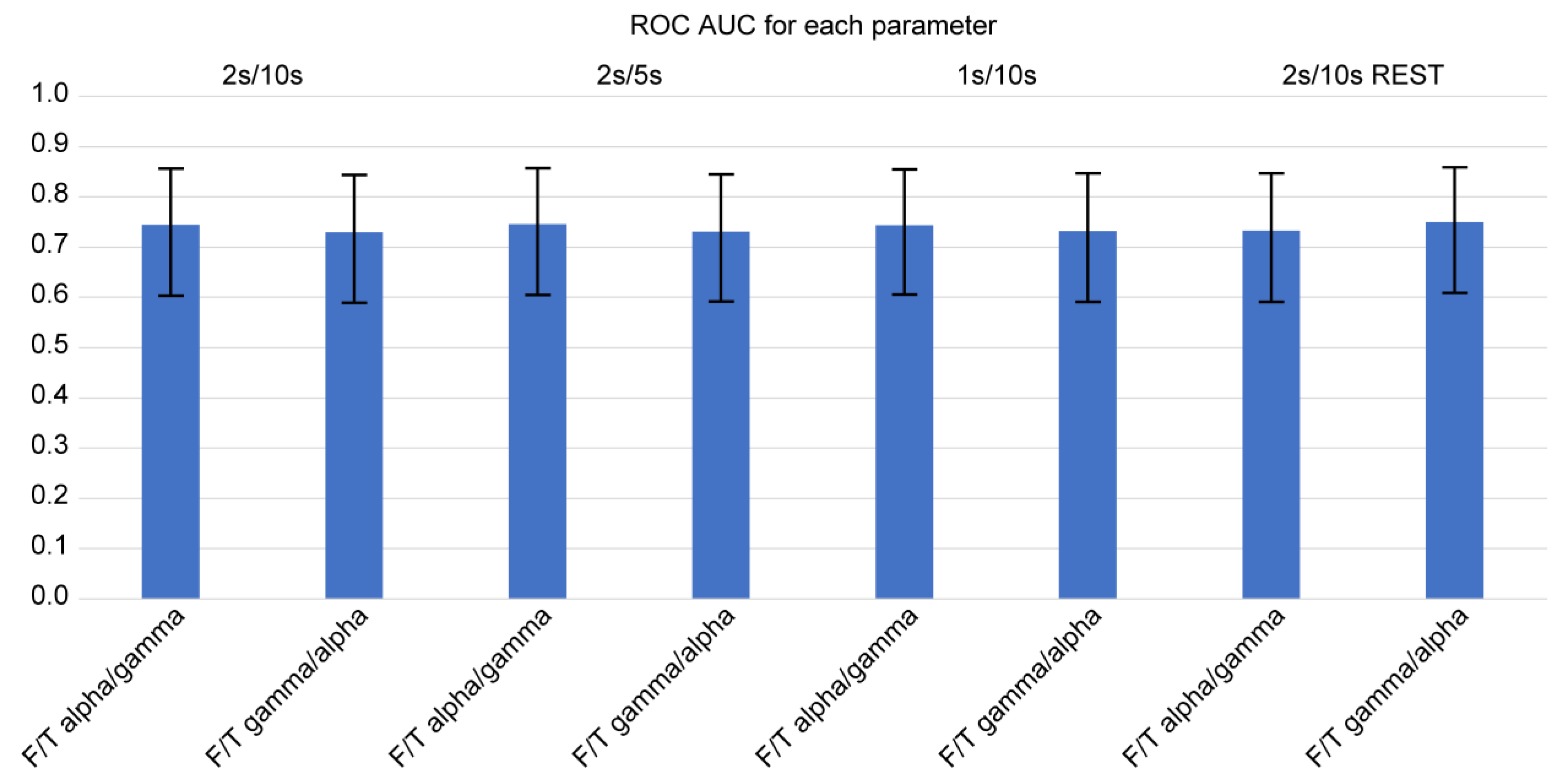
Using Delong’s method for diagnostic sensitivity and specificity, we calculated the AUC of ROC curves among the four consistently significant F/T spectral power ratios for determining the occurrence of FTD (
Figure 2 and
Figure 3). Of note, we examined the spectral power ratios that showed significant differences between the FTD and CTL groups for consistency. We determined that the AUCs of all indices (F/T theta/alpha, F/T alpha/theta, F/T alpha/gamma, F/T gamma/alpha) were over 0.7. The F/T theta/alpha power had the highest AUC score compared with other F/T power ratios for every parameter. However, we did not observe any significant difference in the AUC among the indices. The F/T theta/alpha had the highest discrimination score at 2s/10s with REST (AUC: 0.811 ± 0.113), which was only 2 % greater than its lowest score at 1s/10s (AUC: 0.795 ± 0.125). The F/T alpha/theta had the highest discrimination score at 2s/10s, 2s/5s, and 1s/10s (AUC: 0.793 ± 0.133), which was only 3 % greater than its lowest score at 2s/10s with REST (AUC: 0.772 ± 0.121). Interestingly, we found that neither varying parameters nor referencing methods changed the AUCs of all four power ratios. We also did not observe any significant differences in AUCs between different parameters.
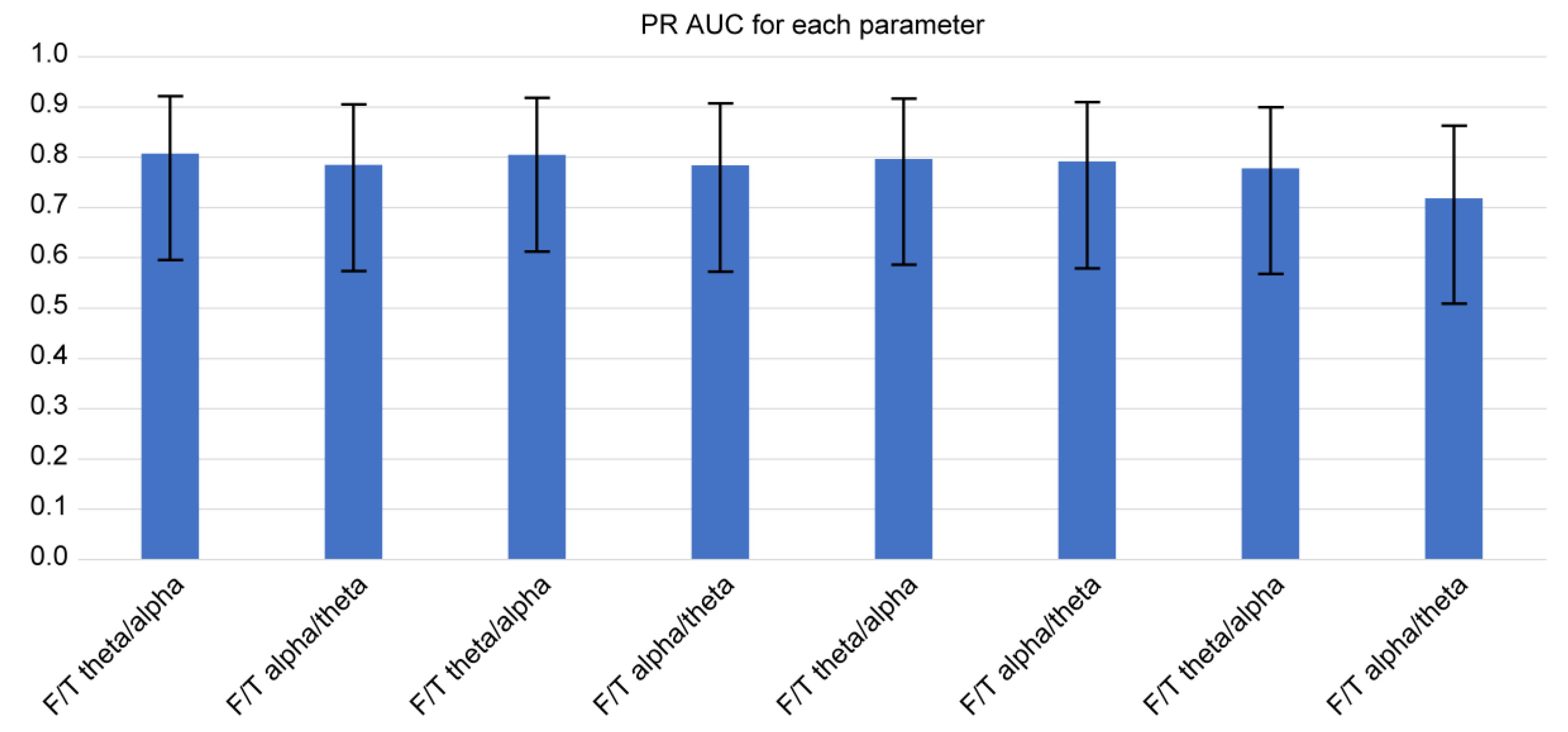
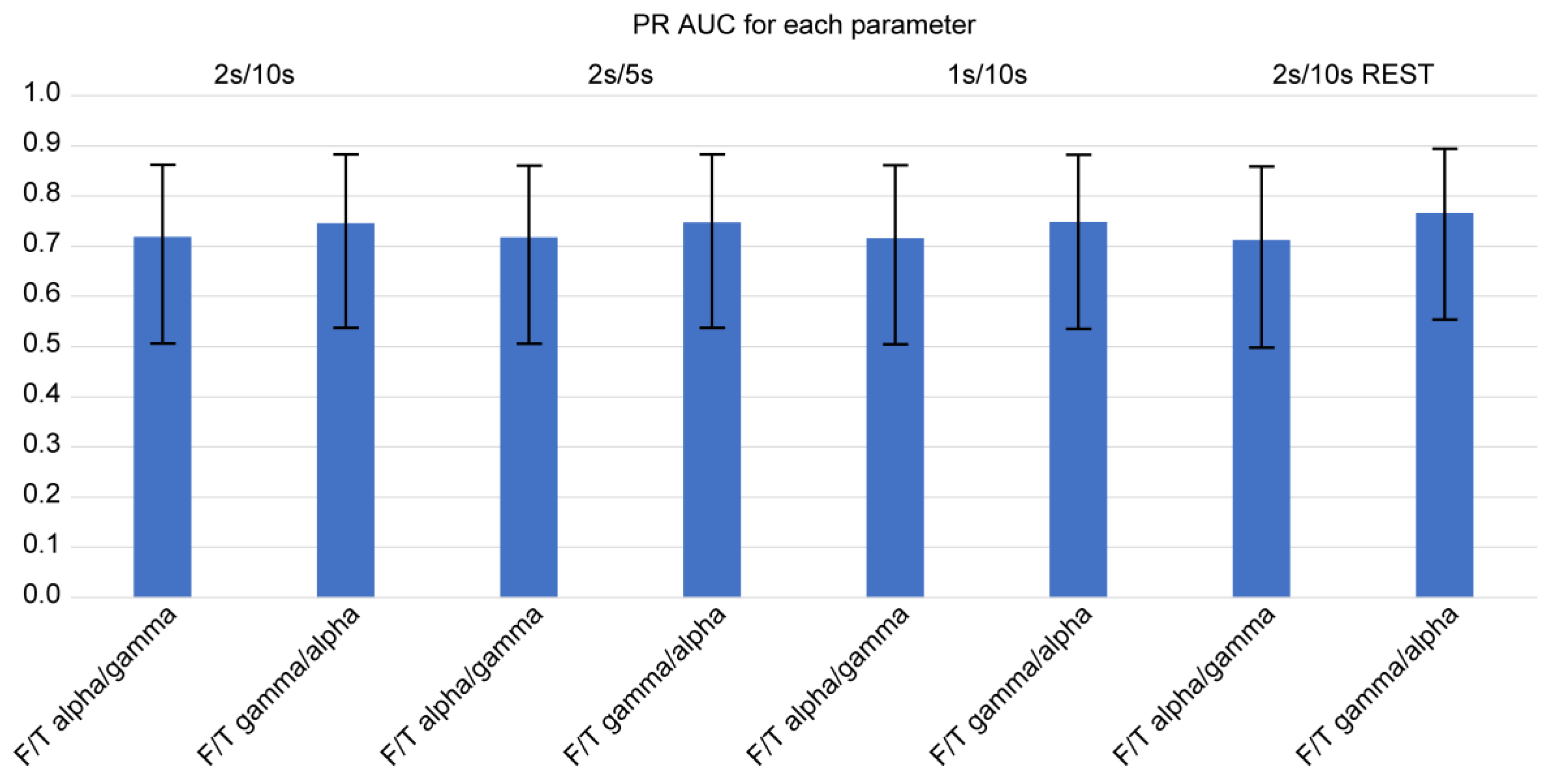
Using the logit and nonlinear interpolation method, we calculated the AUC of PR curves of the two consistently significant F/T spectral power ratios for diagnostic sensitivity and positive predictive value in the determination of the presence of FTD (
Figure 4 and
Figure 5). We further examined the spectral power ratios that showed significant differences between the FTD and CTL groups for consistency. We found that the F/T theta/alpha power had the highest AUC (0.816 ± 0.160) at 2s/10s with REST, which was only 2 % greater than its lowest score at 1s/10s (AUC: 0.799 ± 0.164). The F/T alpha/theta power had the highest AUC (0.791 ± 0.166) at 1s/10s, which was 3 % greater than its lowest score at 2s/10s with REST (AUC: 0.767 ± 0.170). Similarly, we determined that neither varying parameters nor referencing methods changed the AUCs of all four power ratios. However, we did not observe any significant differences in the AUCs between different parameters and among all four spectral power ratios.
The indicators of diagnostic accuracy, including sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) at the optimal cutoff point using the Youden index, are presented in
Table 2 and
Table 3. For standardization, we used a prevalence of 50 % to estimate the predictive values. At 2s/10s, we did not detect any significant difference in the cutoff point, sensitivity, specificity, maximum Youden index, PPV, and NPV between the two spectral power ratios, as indicated by an overlapping 95 % confidence interval. The diagnostic accuracy values for each parameter at the optimal cutoff point by the Youden index are also presented in
Table 2 and
Table 3. We further examined the spectral power ratios that showed significant differences between the FTD and CTL groups for consistency. For every FFT and referencing method, no significant differences were observed in the cutoff point, sensitivity, specificity, maximum Youden index, PPV, and NPV among the spectral power ratios, as indicated by an overlapping 95 % confidence interval.
We applied a multiple logistic regression model with stepwise variable selection to determine whether fluctuations in each F/T spectral power ratio led to a higher probability of developing FTD (
Table 4). We found that at 2s/10s, only the F/T theta/alpha power ratio was recognized as a significant independent variable affecting the probability of FTD. In the model, an increase in the F/T theta/alpha power ratio significantly increased the likelihood of developing FTD (odds ratio = 132.538, 95 % CI: 3.896–4508.770). The results of multiple logistic regression analysis using stepwise variable selection for each parameter are shown in
Table 4 for consistency. We observed that at 2s/10s, 2s/5s, 1s/10s, and 2s/10s with REST, the F/T theta/alpha power ratio was consistently a significant independent variable affecting the probability of developing FTD. In all models, an increase in the F/T theta/alpha power ratio significantly increased the likelihood of FTD (odds ratio >1).
4. Discussion
Previous attempts to diagnose FTD using qEEG have reported conflicting findings. In this study, we found that F/T spectral power ratios have more accurate and consistent diagnostic power for detecting FTD than individual regional power in a certain lobe (e.g., frontal alpha and temporal alpha). For every FFT parameter and referencing method used in the current study, F/T theta/alpha, F/T alpha/theta, F/T alpha/gamma, and F/T gamma/alpha were consistently altered in the FTD group. No such consistency was observed when the individual regional powers of the FTD and CTL groups were compared. These F/T power ratios also consistently showed a mean AUC of over 0.7, indicating good accuracy [
42]. Moreover, the AUCs of the ROC and PR curves were maintained, although the FFT parameters were modified.
The sensitivity, specificity, Youden index, PPV, and NPV at the optimal cutoff point from the maximum Youden index were also maintained despite the variations in FFT parameters and referencing methods. This result implied that compared with individual frequency power in a certain lobe (e.g., frontal alpha, temporal theta, and temporal alpha), the spectral power ratio between specific frequency bands is more consistent in distinguishing between patients with FTD and CTLs, regardless of which parameters are used for signal processing. Particularly, although different FFT parameters usually lead to different comparison results owing to their effects on the trade-off between time and frequency resolution, some F/T spectral ratios are likely to be resistant to such parameter fluctuations, at least for FTD. This further supported their high usefulness and validity for the diagnosis of FTD. Importantly, this simple approach can lay the foundation for the implementation of several easier applications for FTD diagnosis. Combining the results from analyses using different FFT parameters and referencing methods, the current study demonstrated that F/T theta/alpha, F/T alpha/theta, F/T alpha/gamma, and F/T gamma/alpha exhibited consistently high AUCs, showing a consistently high causal association with the likelihood of developing FTD. Hence, among F/T spectral power ratios, F/T theta/alpha, F/T alpha/theta, F/T alpha/gamma, and F/T gamma/alpha could be the most effective diagnostic markers for FTD.
Patients with FTD experience severe frontotemporal degeneration. However, this does not imply that all frequency bands oscillating in the frontotemporal region are impaired. Each crossfrequency interaction is a unique representative of a distinctive neuronal network in which different frequency bands represent different groups of synchronized or desynchronized neurons [
22]. The current study used average spectral power to express distinctive neuronal networks that are altered by frontotemporal degeneration as a relationship between certain frontal frequency powers and certain temporal frequency powers. Alterations in F/T theta/alpha and F/T alpha/theta may indicate impairments in the network relating theta and alpha oscillations due to frontotemporal degeneration, which could be related to changes in the temporal, frontal, and subcortical functional network in the insula, anterior cingulate cortex, temporal lobe, medial frontal lobe, and orbitofrontal cortex [
21,
43,
44,
45,
46]. For example, a dramatic increase in F/T theta/alpha may be attributed to both the increase in frontal theta and decrease in temporal alpha. Increased frontal theta may also be associated with the frontal midline theta rhythm that is negatively associated with sympathetic activation, which is frequently impaired, resulting in bradycardia in FTD, unlike in Alzheimer’s disease and other types of dementia [
47,
48]. Decreased temporal alpha may be affected by abnormal thalamic activity and related reduced glucose metabolism in FTD [
49,
50,
51,
52,
53]. Meanwhile, alterations in F/T alpha/gamma and F/T gamma/alpha may imply typical impairments of frontal executive functions and the hippocampus in patients with FTD, both of which are highly associated with gamma oscillations [
54,
55,
56]. However, interpreting the differences in F/T spectral power ratios remains a challenge. First, precisely associating certain frequency powers with specific regions of the brain is difficult. Further simultaneous EEG and functional magnetic resonance imaging analyses or LORETA are likely to solve these spatial resolution problems. Second, the lack of phase information in F/T spectral power ratios indicates that F/T spectral power ratios may represent simultaneous alterations in various neuronal networks [
22]. This time-ignored feature makes it impossible to associate F/T spectral power ratios with specific functional networks (e.g., the default mode network) because many neuronal networks and their consequences are mixed and inseparable. However, compared with functional connectivity and other phase-coupled analyses, space-based connectivity is less susceptible to state dependency, possibly leading to more consistent and distinguishable differences between patients with FTD and healthy CTLs [
10,
57]. Third, a paucity of sample sizes and variability in FFT parameters might have led to an over- or underestimation of the results of the current study. Several variants of FTD, different disease durations, and different cognitive states among patients with FTD may affect the F/T spectral power ratios. In addition, the three different variations of the FFT parameters used here may be insufficient for testing the consistency of these analyses.
Fourth, although the diagnostic accuracy was not altered after the FFT parameters were modified, slight differences (not statistically different) were detected in the AUCs of ROC and PR curves between average and infinity reference methods. Because different referencing methods have different advantages and limitations, further analyses are needed for determining their clinical usefulness. Fifth, further investigations are needed to distinguish the F/T spectral power ratios of FTD from those of Alzheimer’s and other dementias. Neurologically distinctive abnormalities in FTD might hint the means by which we can determine whether altered F/T spectral power ratios are essentially overlapping features among dementias or caused by different sources. Finally, representative accuracy indicators, such as sensitivity, PPV, and AUC of the ROC curve, do not represent diagnostic accuracy in real tests. The optimal cut-off points in the current study were determined using the Youden index. However, the Youden index method assumes equal weights on sensitivity and specificity, ignores the costs of decisions, and pursues only the maximized sum of sensitivity and specificity, which clearly is not the case in the real world. In addition, disease prevalence was standardized at 50 % to estimate the PPV and NPV; whereas the actual prevalence was much lower. Nevertheless, these representative values could be used for simpler and easier comparison with other diagnostic markers because the Youden index provides a general overview of diagnostic accuracy, and the actual disease prevalence, or pretest probability, is usually not fixed.
5. Conclusions
In conclusion, despite the remaining challenges, the spectral power ratios between two lobes may be worth investigating further as a new qEEG parameter for screening and diagnosing FTD or possibly other dementias, such as Alzheimer’s and Parkinson’s diseases. In particular, diseases related to specific lobes may have distinctive spectral ratios between the frequency bands of abnormal lobes. The current study provided important baseline evidence for future studies on the EEG diagnosis of neurological abnormalities.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org. Supplementary Figure S1. Differences in spectral power ratio, expressed as adjusted p value, between FTD and CTL at 2s/5 step for FFT window length/step size per Hz. One and two asterisks represent p < 0.05 and p < 0.01, respectively; Supplementary Figure S2. Differences in spectral power ratio between FTD and CTL, expressed as adjusted p value, at 1s/10 step for FFT window length/step size per Hz. One and two asterisks represent p < 0.05 and p < 0.01, respectively; Supplementary Figure S3. Differences in spectral power ratio between FTD and CTL, expressed as p value, with infinity reference and at 2s/10 step for FFT window length/step size per Hz. One and two asterisks represent p < 0.05 and p < 0.01, respectively.
Author Contributions
Conceptualization, J.C.; methodology, J.C.; software, J.C.; validation, J.C., C.C.; formal analysis, J.C.; investigation, J.C.; resources, J.C.; data curation, J.C.; writing—original draft preparation, J.C.; writing—review and editing, C.C.; visualization, J.C.; supervision, C.C.; project administration, J.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
This study was approved by the Public Institutional Review Board Designated by the Ministry of Health and Welfare (P01-202304-01-023) and was conducted according to the tenets of the Declaration of Helsinki.
Informed Consent Statement
All participants provided written informed consent.
Data Availability Statement
Conflicts of Interest
The authors declare no conflict of interest.
References
- Hogan, D.B.; Jetté, N.; Fiest, K.M.; Roberts, J.I.; Pearson, D.; Smith, E.E.; Roach, P.; Kirk, A.; Pringsheim, T.; Maxwell, C.J. The prevalence and incidence of frontotemporal dementia: a systematic review. Can. J. Neurol. Sci. 2016, 43 (Suppl. 1), S96–S109. [Google Scholar] [CrossRef]
- Gorno-Tempini, M.L.; Hillis, A.E.; Weintraub, S.; Kertesz, A.; Mendez, M.; Cappa, S.F.; Ogar, J.M.; Rohrer, J.D.; Black, S.; Boeve, B.F.; et al. Classification of primary progressive aphasia and its variants. Neurology 2011, 76, 1006–1014. [Google Scholar] [CrossRef] [PubMed]
- Woolley, J.D.; Khan, B.K.; Murthy, N.K.; Miller, B.L.; Rankin, K.P. The diagnostic challenge of psychiatric symptoms in neurodegenerative disease: rates of and risk factors for prior psychiatric diagnosis in patients with early neurodegenerative disease J. Clin. Psychiatry 2011, 72, 126–133. [Google Scholar] [CrossRef]
- Nardone, R.; Sebastianelli, L.; Versace, V.; Saltuari, L.; Lochner, P.; Frey, V.; Golaszewski, S.; Brigo, F.; Trinka, E.; Höller, Y. ; Usefulness of EEG techniques in distinguishing frontotemporal dementia from alzheimer's disease and other dementias. Dis. Markers 2018, 2018, 6581490. [Google Scholar] [CrossRef]
- Lindau, M.; Jelic, V.; Johansson, S.E.; Andersen, C.; Wahlund, L.O.; Almkvist, O. Quantitative EEG abnormalities and cognitive dysfunctions in frontotemporal dementia and Alzheimer's disease, Dement. Geriatr. Cogn. Disord. 2003, 15, 106–114. [Google Scholar] [CrossRef]
- Nishida, K.; Yoshimura, M.; Isotani,T. ; Yoshida, T.; Kitaura, Y.; Saito, A.; Mii, H.; Kato, M.; Makekita, M.Y.; Suwa, A.; et al; Differences in quantitative EEG between frontotemporal dementia and Alzheimer's disease as revealed by LORETA. Clin. Neurophysiol. 2011, 122, 1718–1725. [Google Scholar] [CrossRef]
- Yener, G.G.; Leuchter, A.F.; Jenden, D.; Read, S.L.; Cummings, J.L.; Miller, B.L. Quantitative EEG in frontotemporal dementia. Clin. Electroencephalogr. 1996, 27, 61–68. [Google Scholar] [CrossRef]
- Stigsby, B.; Jóhannesson, G.; Ingvar, D.H. ; Regional EEG analysis and regional cerebral blood flow in Alzheimer's and Pick's diseases. Electroencephalogr. Clin. Neurophysiol. 1981, 51, 537–547. [Google Scholar] [CrossRef] [PubMed]
- Caso, F.; Cursi, M.; Magnani, G.; Fanelli, G.; Falautano, M.; Comi, G.; Leocani, L.; Minicucci, F. Quantitative EEG and LORETA: valuable tools in discerning FTD from AD. Neurobiol. Aging 2012, 33, 2343–2356. [Google Scholar] [CrossRef] [PubMed]
- Newson, J.J.; Thiagarajan, T.C. EEG frequency bands in psychiatric disorders: a review of resting state studies. Front. Hum. Neurosci. 2018, 12, 521. [Google Scholar] [CrossRef]
- Pijnenburg, Y.A.L; Strijers, R.L.M.; Made, Y.V.; van der Flier, W.M.; Scheltens, P.; Stam, C.J. Investigation of resting-state EEG functional connectivity in frontotemporal lobar degeneration. Clin. Neurophysiol. 2008, 119, 1732–1738. [Google Scholar] [CrossRef] [PubMed]
- de Haan, W.; Pijnenburg, Y.A.; Strijers, R.L.; van der Made, Y.; van der Flier, W.M.; Scheltens, P.; Stam, C.J. Functional neural network analysis in frontotemporal dementia and Alzheimer's disease using EEG and graph theory. BMC Neurosci. 2009, 10, 101. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Gouw, A.A.; Hillebrand, A.; Tijms, B.M.; Stam, C.J.; van Straaten, E.C.; Pijnenburg, Y.A. Different functional connectivity and network topology in behavioral variant of frontotemporal dementia and Alzheimer's disease: an EEG study. Neurobiol. Aging 2006, 42, 150–162. [Google Scholar] [CrossRef]
- Dottori, M.; Sedeño, L.; Martorell Caro, M.; Alifano, F.; Hesse, E.; Mikulan, E.; García, A.M.; Ruiz-Tagle, A.; Lillo, P.; Slachevsky, A.; et al. Towards affordable biomarkers of frontotemporal dementia: A classification study via network's information sharing. Sci. Rep. 2017, 7, 3822. [Google Scholar] [CrossRef] [PubMed]
- Bastos, A.M.; Schoffelen, J.M. A tutorial review of functional connectivity analysis methods and their interpretational pitfalls. Front. Syst. Neurosci. 2015, 9, 175. [Google Scholar]
- Vink, J.J.T.; Klooster, D.C.W.; Ozdemir, R.A.; Westover, M.B.; Pascual-Leone, A.; Shafi, M.M. EEG Functional Connectivity is a Weak Predictor of Causal Brain Interactions. Brain Topogr. 2020, 33, 221–237. [Google Scholar] [CrossRef] [PubMed]
- Passant, U.; Rosén, I.; Gustafson, L.; Englund, E. The heterogeneity of frontotemporal dementia with regard to initial symptoms, qEEG and neuropathology. Int. J Geriatric Psych. 2005, 20, 983–988. [Google Scholar] [CrossRef] [PubMed]
- Julin, P.; Wahlund, L.O.; Basun, H.; Persson, A.; Måre, K.; Rudberg, U. Clinical diagnosis of frontal lobe dementia and Alzheimer’s disease: relation to cerebral perfusion, brain atrophy and electroencephalography. Dementia 1995, 6, 142–147. [Google Scholar] [CrossRef]
- Johannesson, G.; Hagberg, B.; Gustafson, L.; Ingvar, D.H. EEG and cognitive impairment in presenile dementia. Acta Neurol. Scand. 1979, 59, 225–240. [Google Scholar] [CrossRef]
- Rosén, I.; Gustafson, L.; Risberg, J. Multichannel EEG frequency analysis and somatosensory-evoked potentials in patients with different types of organic dementia. Dementia 1993, 4, 43–49. [Google Scholar] [CrossRef]
- Olney, N.T.; Spina, S.; Miller, B.L. Frontotemporal Dementia. Neurol. Clin. 2017, 35, 339–374. [Google Scholar] [CrossRef] [PubMed]
- Thammasan, N.; Miyakoshi, M. Cross-frequency power-power coupling analysis: a useful cross-frequency measure to classify ICA-decomposed EEG. Sensors 2020, 20, 7040. [Google Scholar] [CrossRef]
- Wen, T.Y.; Aris, S.M. Electroencephalogram (EEG) stress analysis on alpha/beta ratio and theta/beta ratio. Indones. J. Electr. 2020, 17, 175–182. [Google Scholar]
- Schmidt, M.T.; Kanda, P.A.; Basile, L.F.; da Silva Lopes, H.F.; Baratho, R.; Demario, J.L.; Jorge, M.S.; Nardi, A.E.; Machado, S.; Ianof, J.N.; et al. Index of alpha/theta ratio of the electroencephalogram: a new marker for Alzheimer's disease. Front. Aging Neurosci. 2013, 5, 60. [Google Scholar] [CrossRef] [PubMed]
- Özbek, Y.; Fide, E.; Yener, G.G. Resting-state EEG alpha/theta power ratio discriminates early-onset Alzheimer's disease from healthy controls. Clin. Neurophysiol. 2021, 132, 2019–2031. [Google Scholar] [CrossRef] [PubMed]
- Baik, K.; Jung, J.H.; Jeong, S.H.; Chung, S.J.; Yoo, H.S.; Lee, P.H.; Sohn, Y.H.; Kang, S.W.; Ye, B.S. Implication of EEG theta/alpha and theta/beta ratio in Alzheimer's and Lewy body disease. Sci. Rep. 2022, 12, 18706. [Google Scholar] [CrossRef] [PubMed]
- Jaramillo-Jimenez, A.; Suarez-Revelo, J.X.; Ochoa-Gomez, J.F.; Carmona Arroyave, J.A.; Bocanegra, Y.; Lopera, F.; Buriticá, O.; Pineda-Salazar, D.A.; Moreno Gómez, L.; Tobón Quintero, C.A.; et al. Resting-state EEG alpha/theta ratio related to neuropsychological test performance in Parkinson's Disease. Clin. Neurophysiol. 2021, 132, 756–764. [Google Scholar] [CrossRef] [PubMed]
- Zawiślak-Fornagiel, K.; Ledwoń, D.; Bugdol, M.; Romaniszyn-Kania, P.; Małecki, A.; Gorzkowska, A.; Mitas, A.W. The increase of theta power and decrease of alpha/theta ratio as a manifestation of cognitive impairment in parkinson's disease. J. Clin. Med. 2023, 12, 1569. [Google Scholar] [CrossRef] [PubMed]
- Miltiadous, A.; Tzimourta, K.D.; Afrantou, T.; Ioannidis, P.; Grigoriadis, N.; Tsalikakis, D.G.; Angelidis, P.; Markos, G. A dataset of 88 EEG recordings from: Alzheimer's disease, Frontotemporal dementia and Healthy subjects; OpenNeuro, 2023. [Google Scholar] [CrossRef]
- Miltiadous, A.; Tzimourta, K.D.; Giannakeas, N.; Tsipouras, M.G.; Afrantou, T.; Ioannidis, P.; Tzallas, A.T. Alzheimer's disease and frontotemporal dementia: a robust classification method of EEG signals and a comparison of validation methods. Diagnostics 2021, 11, 1437. [Google Scholar] [CrossRef]
- Miltiadous, A.; Tzimourta, K.D.; Afrantou, T.; Ioannidis, P.; Grigoriadis, N.; Tsalikakis, D.G.; Angelidis, P.; Tsipouras, M.G.; Glavas, E.; Giannakeas, N.; et al. A dataset of 88 EEG recordings. In Alzheimer's Disease, Frontotemporal Dementia and Healthy Subjects; OpenNeuro, 2023; Available online: https://openneuro.org/datasets/ds004504/versions/1.0.2/file-display/README.
- Delorme, A.; Makeig, S. EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J. Neurosci. Methods. 2004, 134, 9–21. [Google Scholar] [CrossRef]
- Miyakoshi, M.; Kothe, C. clean_rawdata. version 2.1. 2019, https://github.com/sccn/clean_rawdata/wiki.
- Dong, L.; Li, F.; Liu, Q.; Wen, X.; Lai, Y.; Xu, P.; Yao, D. MATLAB Toolboxes for Reference Electrode Standardization Technique (REST) of Scalp EEG. Front. Neurosci. 2017, 11, 601. [Google Scholar] [CrossRef] [PubMed]
- Yao, D.; Qin, Y.; Hu, S.; Dong, L.; Bringas Vega, M.L.; Valdés Sosa, P.A. Which Reference Should We Use for EEG and ERP practice? Brain Topogr. 2019, 32, 530–549. [Google Scholar] [CrossRef] [PubMed]
- DeLong, E.R.; DeLong, D.M.; Clarke-Pearson, D.L. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics 1988, 44, 837–845. [Google Scholar] [CrossRef] [PubMed]
- Boyd, K.; Eng, K.H.; Page, C.D. Area under the Precision-Recall Curve: Point Estimates and Confidence Intervals. Adv. Inf. Systems Eng. 2013, 451–466. [Google Scholar]
- Davis, J.; Goadrich, M. The relationship between Precision-Recall and ROC curves, in: Proceedings of the 23rd International Conference on Machine Learning. ACM Press Association for Computing Machinery, New York, 2006; pp. 233–240.
- Youden, W.J. Index for rating diagnostic tests. Cancer 1950, 3, 32–35. [Google Scholar] [CrossRef] [PubMed]
- Trevethan, R. Sensitivity, specificity, and predictive values: Foundations, pliabilities, and pitfalls in research and practice. Front. Public Health 2017, 5, 307. [Google Scholar] [CrossRef] [PubMed]
- Heston, T.F. Standardizing predictive values in diagnostic imaging research. J. Magn. Reson. Imaging 2011, 33, 505, author reply 506-7. [Google Scholar] [CrossRef]
- Simundic, A.M. Diagnostic accuracy-part 1: basic concepts: sensitivity and specificity, ROC analysis, STARD statement. Point Care 2012, 11, 6–8. [Google Scholar] [CrossRef]
- García-Cordero, I.; Sedeño, L.; Fraiman, D.; Craiem, D.; de la Fuente, L.A.; Salamone, P.; Serrano, C.; Sposato, L.; Manes, F.; Ibañez, A. Stroke and Neurodegeneration Induce Different Connectivity Aberrations in the Insula. Stroke 2015, 46, 2673–2677. [Google Scholar] [CrossRef]
- Seeley, W.W.; Crawford, R.; Rascovsky, K.; Kramer, J.H.; Weiner, M.; Miller, B.L.; Gorno-Tempini, M.L. Frontal paralimbic network atrophy in very mild behavioral variant frontotemporal dementia. Arch. Neurol. 2008, 65, 249–255. [Google Scholar] [CrossRef]
- Melloni, M.; Billeke, P.; Baez, S.; Hesse, E.; de la Fuente, L.; Forno, G.; Birba, A.; García-Cordero, I.; Serrano, C.; Plastino, A.; et al. Your perspective and my benefit: multiple lesion models of self-other integration strategies during social bargaining. Brain 2016, 139, 3022–3040. [Google Scholar] [CrossRef]
- Agosta, F.; Sala, S.; Valsasina, P.; Meani, A.; Canu, E.; Magnani, G.; Cappa, S.F.; Scola, E.; Quatto, P.; Horsfield, M.A.; et al. Brain network connectivity assessed using graph theory in frontotemporal dementia. Neurology 2013, 81, 134–343. [Google Scholar] [CrossRef]
- Kubota, Y.; Sato, W.; Toichi, M.; Murai, T.; Okada, T.; Hayashi, A.; Sengoku, A. Frontal midline theta rhythm is correlated with cardiac autonomic activities during the performance of an attention demanding meditation procedure. Brain Res. Cogn. Brain Res. 2001, 11, 281–287. [Google Scholar] [CrossRef] [PubMed]
- Robles Bayón, A.; Gude Sampedro, F.; Torregrosa Quesada, J.M. Bradycardia in frontotemporal dementia. Neurología 2014, 29, 76–85. [Google Scholar] [CrossRef] [PubMed]
- Schreckenberger, M.; Lange-Asschenfeldt, C.; Lochmann, M.; Mann, K.; Siessmeier, T.; Buchholz, H.G.; Bartenstein, P.; Gründer, G. The thalamus as the generator and modulator of EEG alpha rhythm: a combined PET/EEG study with lorazepam challenge in humans. NeuroImage 2004, 22, 637–644. [Google Scholar] [CrossRef] [PubMed]
- Garrett, L.R.; Niccoli, T. Frontotemporal dementia and glucose metabolism. Front. Neurosci. 2022, 16, 812222. [Google Scholar] [CrossRef] [PubMed]
- Goldman, R.I.; Stern, J.M.; Engel, J.; Cohen, M.S. Simultaneous EEG and fMRI of the alpha rhythm. Neuroreport. 2002, 13, 2487–2492. [Google Scholar] [CrossRef] [PubMed]
- Hughes, S.W.; Crunelli, V. Thalamic mechanisms of EEG alpha rhythms and their pathological implications, Neuroscientist 2005, 11, 357–372. 11.
- Bocchetta, M.; Gordon, E.; Cardoso, M.J.; Modat, M.; Ourselin, S.; Warren, J.D.; Rohrer, J.D. Thalamic atrophy in frontotemporal dementia - Not just a C9orf72 problem. NeuroImage Clin. 2018, 18, 675–681. [Google Scholar] [CrossRef]
- Fries, P.; Reynolds, J.H.; Rorie, A.E.; Desimone, R. Modulation of oscillatory neuronal synchronization by selective visual attention. Science 2001, 291, 1560–1563. [Google Scholar] [CrossRef] [PubMed]
- Laakso, M.P.; Frisoni, G.B.; Könönen, M.; Mikkonen, M.; Beltramello, A.; Geroldi, C.; Bianchetti, A.; Trabucchi, M.; Soininen, H.; Aronen, H.J. Hippocampus and entorhinal cortex in frontotemporal dementia and Alzheimer's disease: a morphometric MRI study. Biol. Psychiatry 2000, 47, 1056–1063. [Google Scholar] [CrossRef]
- Ribary, U.; Ioannides, A.A.; Singh, K.D.; Hasson, R.; Bolton, J.P.; Lado, F.; Mogilner, A.; Llinás, R. Magnetic field tomography of coherent thalamocortical 40-Hz oscillations in humans. Proc Natl Acad Sci U S A 1991, 88, 11037–11041. [Google Scholar] [CrossRef] [PubMed]
- Stopford, C.L.; Thompson, J.C.; Neary, D.; Richardson, A.M.; Snowden, J.S. Working memory, attention, and executive function in Alzheimer's disease and frontotemporal dementia. Cortex 2012, 48, 429–446. [Google Scholar] [CrossRef] [PubMed]
- Helm, K.; Viol, K.; Weiger, T.M.; Tass, P.A.; Grefkes, C.; Del Monte, D.; Schiepek, G. Neuronal connectivity in major depressive disorder: a systematic review. Neuropsychiat. Dis. Treat. 2018, 14, 2715–2737. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).