Introduction
Wheat is the most abundant and widely demanded crop, owing to its economic and social value (Johnson et al., 2018). This crop is grown worldwide on more than 220 million hectares of land. It accounts for approximately 20% of a person's daily protein requirement. In recent years, global warming and climate change have dramatically affected crop yields, including wheat (Ghorbani et al., 2023a). Despite the large areas used for wheat cultivation, its productivity is significantly lower than that of rice and corn. According to forecasts, by 2050, the world's population will exceed 9.6 billion and increasing productivity by 60% will help to ensure food security (FAO, 2022).
In Kazakhstan average annual wheat production ranges from 12 to 12.5 million tons, of which only 6 million tons are used for domestic consumption, and the remainder is exported (Karatayev et.al., 2022). By 2023, Kazakhstan plans to increase the supply of high-protein wheat to the European Union while also planning to increase wheat production by 20%. According to a 2021 report, wheat stocks decreased by 4 million tons as a result of the drought in Kazakhstan. Wheat productivity is more constrained by abiotic stresses than by biotic ones. An increase in the average daily temperature, drought, and salinization are the main abiotic stresses that harm wheat productivity and quality (Kavhiza et al., 2022; Ghorbani et al., 2023b; Emamverdian et al., 2023a).
The Green Revolution resulted an improvement in wheat yield index due to a significant height reduction of the straw (Zagar and Pakina., 2014). This was facilitated by identifying the main dwarfism genes currently widespread in world breeding programs. The decrease in height resulting from the shortening of straw by reduced height genes (Rht) has long been proposed as a factor in increasing the wheat yield index. These genes cause the accumulation of DELLA proteins that inhibit growth, and, as a result, semi-dwarf plants are resistant to lodging. One of the main factors that caused an increase in wheat yield in the past was the height of plants, which systematically decreased due to the introgression of the Rht genes. Several comparative studies have shown that wheat yields decrease when plants are shortened, thereby limiting the range of plant heights for optimal yields. Consequently, the probability of a further increase in wheat yield due to an additional decrease in plant height is relatively low. Several studies have shown that most modern wheat varieties have reached their optimal height (Chandler & Harding, 2013).
Moreover, fine-tuning plant height is also essential in hybrid wheat breeding, where efficient production of hybrid seeds requires male plants to be taller than female plants. The agro technical results observed in varieties bearing the Rht-B1b or Rht-D1b allele are a 15-20% decrease in plant height and an increase in grain yield (Zhang et al., 2020). The presence of Rht-B1b or Rht-D1b individually reduced the height of the plant by 14.6%, but the combined presence of Rht-B1b and Rht-D1b reduced the height by 41%. In addition, the lines containing Rht-B1b and Rht-D1b are shorter than the genotypes containing Rht-B1a and Rht-D1a, Rht-B1b and Rht-D1b also have smaller leaves (Zhang et al., 2013). However, recent studies have shown that Rht-B1b and Rht-D1b significantly improved wheat yield potential when using nitrogen fertilizers but reduced the length of coleoptiles, the strength of seedlings, and the weight of grain (Emamverdian et al., 2023b). This leads to the need to consider such wheat indicators as grain size, the weight of 1000 grains, coleoptile length, and quality indicators in the selection of hybrids and varieties that directly affect the quantity and quality of the crop (Ukozehasi et al., 2022; Voronov et al., 2023; Zargar et al., 2023).
In breeding it is essential to consider the presence of the TaGW and TaGS genes, which determine the size of wheat grains and ensure potential yield. In Chinese wheat lines, the single nucleotide polymorphism (SNP) in the TaGW2-A1 promoter region, the most studied homolog of the A genome, is located on the 6A chromosome and encodes RING-type ubiquitin ligase E3. It is responsible for an increased mass of 1000 grains, a lower content of indolyl acetic acid (IAC), and the number of grains per ear [Identification and development of a functional marker of TaGW2 associated with grain weight in bread wheat (Triticum aestivum L.)] (Zhang et al., 2013).
TaGW2-B1 and -D1 also affect the protein content in the grain and the weight of grains and modulate the number and length of cells in the outer pericarp of developing grains. Some research claims that the TaGW2-B1 trait is stronger; however, mutants lacking B1 and D1 have more protein in their seeds. Two SNPs forming a haplotype Hap-6A-A for the TaGW6 gene, the rice ortholog GW6, regulate seed size, encode a new indole-3-acetic acid-glucose hydrolase mainly expressed in the tissues of young spikelets, and negatively regulate grain weight and width. At the same time, TaGW2-6A is associated with wider grains and a larger mass of 1000 grains. An additional haplotype, Hap-6B-1 for TaGW2-6B, has a stronger effect on grain weight than TaGW2-6A (Govta et al., 2022; Zhang et al., 2020).
The TaTGW-7A gene (TaTGW-7Ab, TaTGW-6) on chromosome 7A, encodes IAA-glucose hydrolase and increases the mass of 1000 grains and grain length (Hu et al., 2016). TaGW8B1 was positively correlated with the grain size of soft wheat. The TaGW8-B1a gene has an InDel length of 276 bp in the first intron, which is absent in the TaGW8-B1b gene. The TaGW8-B1a gene is associated with a significantly larger core width, a larger number of grains in the ear, a longer core length, a larger mass of thousands of grains, and a large number of spikelets per ear (Yan et al., 2019). The TaGS-D1 and TaGS5-3A genes are associated with the mass of 1000 grains and grain length size. The TaGS3-7A-A allele significantly increased the mass of 1000 grains. The TaGS5-3A-T haplotype, based on the SNP T/G in the sixth exon, significantly correlates with a larger grain size, a higher mass of 1000 grains, and a smaller plant height, ear length, and internode length under the ear (Ma et al., 2015). A study by Zhang et al. (2014) showed that the DNA locus of the TaGS-D1 gene has one SNP in the first intron and identified 30 SNPs, a 40 bp InDel, and a 3 bp InDel, in the second intron between genotypes with a larger and smaller mass of thousand grains (TGW) (Y. Zhang et al., 2014).
According to available data, various single-nucleotide mutations in both short-stem and coarse-grain genes can play a significant role in wheat breeding to improve productivity. Our research aims to select wheat varieties and hybrids obtained by the hybridization of Kazakhstan and foreign varieties (Australia, Africa, Russia, and China) to produce short-stemmed and coarse-grained forms based on phenotypic and genetic indicators. However, it is impossible to select hybrids under the changing harsh continental climate conditions of Kazakhstan only on the basis of phenotypic indicators. We also studied the genetic potential of varieties and hybrids using short-stemmed genes (Rht) and coarse-grained genes (TaGW, TaGS) to obtain more reliable data during selection. The purpose of this study was to evaluate the relationship of phenotypic indicators of plant height, coleoptile length, the ratio of internode length and straw diameter with such indicators as yield, the weight of 1000 seeds, and grain size with the presence in the genome of hybrids and their parents of Rht-B1b, Rht-D1b, Rht-B1a, Rht-D1a, and mass genes, and the grain size of TaGW8 and TaGS5-3A.
2. Materials and Methods
The studies were conducted between 2018 to 2022. The laboratory experiments were carried out at the Research Platform of Agricultural Biotechnology of the S. Seifullin Kazakhstan Agrotechnical Research University, Astana, Kazakhstan. Field experiments were laid out at the A.F. Khristenko Karagandinskaya Agricultural Experimental Station LLP (Central Kazakhstan) and Farm "Niva" (Northern Kazakhstan).
In previous studies we have investigated all parental forms in soil conditions (drought steppe) and in drought stress conditions in the laboratory (slowly developing drought stress and rapid dehydration) (Zotova et al., 2018). Using Amplifluor Single Nucleotide Polymorphism (SNP) technology with 19 SNP markers. SNP genotyping and 18 markers for the DREB2-type gene and encoding transcription factors TaDREB5 and TaNFYC-A7 all collection were studied and the main productive and drought tolerant varieties were selected for this study (Shavrukov et al., 2016).
Selected parenteral forms were used for hybridization. Hybridization of selected parental forms according to phenotypic indicators in drought conditions was carried out in 2018. A total of 124 parental forms and 167 hybrids were studied during the 2018-2022 years. As a perspective hybrids were choosing 20 hybrids were selected as having promising phonological characteristics during the first three generations. Semi-dwarf wheat varieties of Chinese selection (Xn03, Xn04, Xn08, Xn09, Xn10, Xn13), Australian selection (Gladius, RAC 875, Wyalkatchem, Kite), African selection (MMF-044), tall varieties of Russian selection (Saratovskaya 66, Lutestsens 141), and Kazakhstan selection were selected as parent forms (Erythrospermum 35, Tauelsizdyk 20, Astana, Akmola 2, Aktyubinka, Karabalykskaya 25, 90, Karagandinskaya 22, 25, 29, 30, 31, Shortandinskaya 2012, 2017). Hybridization was carried out by "tvel"-N. Borlaug's method of limited free pollination (Borlaug, 1980). Hybrids were the F4 generation in 2022. The hybrid nursery was laid according to the scheme of maternal, hybrid, and paternal forms, and the plot area for each sample was 1 m
2 as described earlier Each year studied collection was sowing on three agroecological points, as described above. All samples were collected from 2 m
2 area. More information about the parent forms represented in
Table 1.
2.1. Field experiment conditions
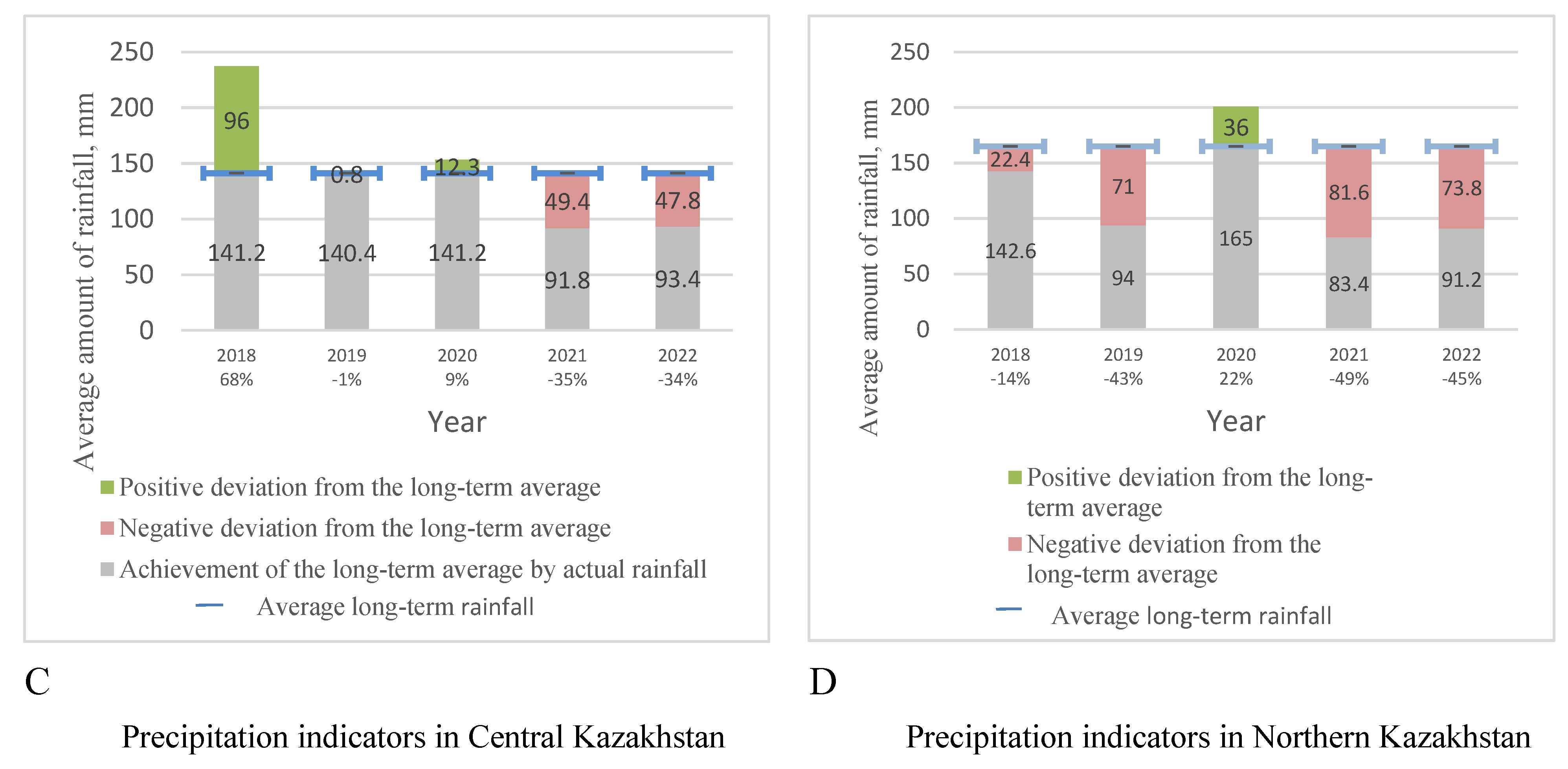
All field experiments were carried out at two agroecological points in Central and North Kazakhstan. The soil in both regions is dark chestnut and chestnut. Climatic conditions are characteristic of a sharply continental climate. The climate was arid, and in the last 5 years, climatic conditions under conditions of global warming have led to an increase in the average annual temperature and a reduction in the total amount of precipitation (Yapiyev et al., 2018). Hydrothermic index in central part of country was in 2018 – 0.72, 2019 – 0.67, 2020 – 0.63, 2021 and 2022 – 0.39. The same indicator in Northern Kazakhstan in 2018 was 0.72, in 2019 it was 0.42, in 2020 it was 0.89, and 2020 and 2022 was 0.34 and 0.39, respectively. According to Ionova et al. (2020) methodology, all years were «very drought», except 2018 and 2020 in the Northern part, which were «drought». During the period of the experiments, the climatic conditions in both points was drought or very drought. All field experiments were conducted in drought conditions without irrigation to obtain results that are characteristic of the real conditions of the regions.
2.2. Weather condition
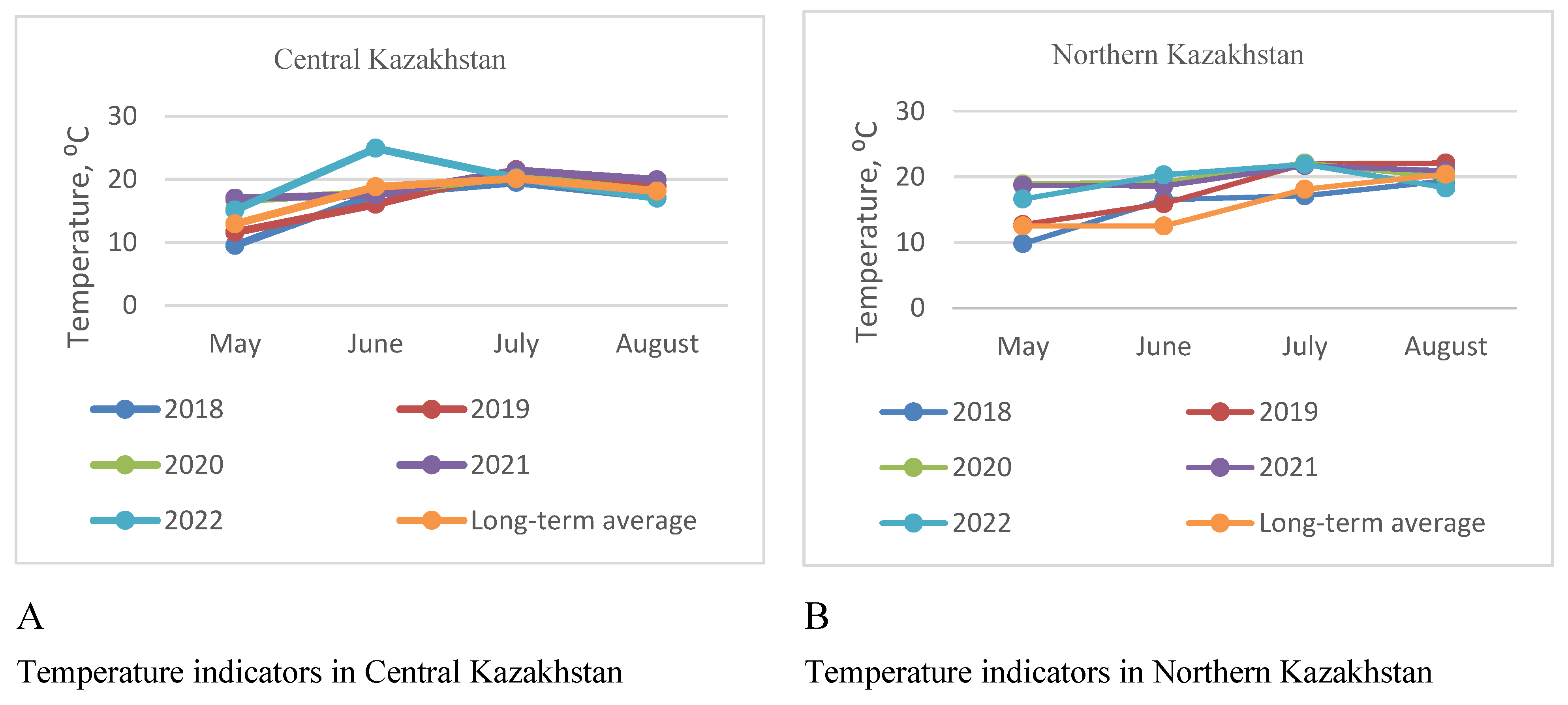
Weather conditions in Central (
Figure 1 a,c) and Northern (
Figure 1 b,d) Kazakhstan differ in average monthly temperatures and precipitation, especially during the growing season.
In both regions, over the past five years, there has been a shortage of moisture during the growing season. Over the past three years, the lack of humidity has particularly affected wheat yields. At the same time, in Central Kazakhstan, over the past five years, there has been an increase in temperature indicators compared to the long-term average and a low level of precipitation. In the country’s northern region, the average temperatures for five years are 3–5 degrees above the average annual values, and the average precipitation values are 20–26 mm less than in the central part of the country.
2.3. Phenotyping parameters of parental form and hybrids
2.3.1. Measurement of plant height
In total, 20 wheat plants were selected from the center of each plot. Then, the height of each plant from the ground to the top was measured using a ruler (accuracy 0.5 mm), and the average height of 20 plants was taken as the height of plants (PH) on this site (Tao et al., 2020).
2.3.2. Measurement of coleoptile length
The coleoptile length (CL) was measured in 10-day-old seedlings (Sidhu et al., 2020). For this, 15 seeds of the same size as each variety/hybrid were grown in a wet chamber without physical damage. Plastic trays were placed in a completely darkened box and kept in a growth chamber at a constant 22 °C. After 10 d, the average coleoptile length of 10 randomly selected seedlings was measured with an accuracy of up to a millimeter from the base of the seed to the coleoptile tip.
2.3.3. Ratio of internode diameter and length
After reaching full ripeness, each parent variety and hybrid were determined. 10 plants grown in the field were selected, and an electronic caliper (Matrix, 31611) was used to measure the outer diameter of the straw and the length of the 2nd internode. After obtaining the average, the ratio of the diameter to the length (L2/D2) of the internode was calculated as a percentage.
2.3.4. Grain length, width and number
To measure the grain length (GL) and grain width (GW), a sample of 1000 seeds was randomly selected and their GL and GW were measured with a ruler with an error (0.05 mm), the average value of each indicator was taken as the main indicator (Beral et al., 2020). The number of grains (GN) in each spike was determined as the average value of grains in the spike of 50 wheat plants after harvest and at the onset of full ripeness.
2.3.5. Weight of 1000 grains
Two samples, each containing 500 seeds, were taken from the fraction of pure seeds. They were weighed with an accuracy of up to 0.01 g. The mismatched two samples mass error was considered 3% of the average mass. If the GN coincides with the generally accepted norm, the masses of the first and second samples are summed. Thus, the result obtained indicates the 1000 grains weight (TGW). (GOST 12042-80 Seeds of Agricultural Crops. Methods for determining the mass of 1000 seeds).
2.4. Identification of genes by PCR
All genes were amplified using a VeritiPro amplifier (Applied Biosystems, USA). Primers used are listed:
Rht- B1b (5′-GGTAGGGAGGCGAGAGGCGAG-3′),
(5′-CATCCCCATGGCCATCTCGAGCTA-3′),
Rht- B1а (5′-GGTAGGGAGGCGAGAGGCGAG-3′),
(5′-CATCCCCATGGCCATCTCGAGCTG-3′),
Rht-D1b (5′-CGCGCAATTATTGGCCAGAGATAG-3′),
(5′-CCCCATGGCCATCTCGAGCTGCTA-3′),
Rht-D1a (5′-GGCAAGCAAAAGCTTCGCG-3′),
(5′-GGCCATCTCGAGCTGCAC - 3′).
TaGW8 F: CGTCATCCATTCCTTCATCG
R: GCTATATGGGTTGGTGTCGC;
TaGS5-3A-AGACATGGTGGAGCAAGAGATG
TaGS5-3A-GAACAACCTAATCCTCCTCCTGA.
The PCR reaction mixture was prepared as follows (total volume 20 µl): 1 × DreamTaq Buffer, 100 ng DNA matrix, 4 nmol dNTPs, 10 pmol of direct and reverse primers, and 1.25 U DreamTaq Hot Start DNA Polymerase (Thermo Fisher Scientific, USA). Briefly, the PCR conditions for Rht-B1b Rht-B1a alleles were, cycling program: 5 min at 95°C; seven cycles of 30 s at 95°C, 30 s at 65°C, 1 min 20 s at 72°C with a decrease in the annealing temperature by 1°C in each cycle; 30 cycles of 15 s at 94°C, 15 s at 58°C, 50 s at 72°C. Slightly different conditions were used for the Rht-D1b Rht-D1a alleles. For a total volume of 20 µl:1 × DreamTaq Buffer, 100 ng DNA matrix, 4 nmol dNTPs, 10 pmol of direct and reverse primers, 1.25 U DreamTaq Hot Start DNA Polymerase (Thermo Fisher Scientific). Cycling program: 5 min at 95°C, 42 cycles of 20 s at 95° C, 30 s at 58°C, 10 s at 72 °, and the last stage of 2 min at 72°C. PCR products were separated in a 2% agarose gel and visualized after staining with ethidium bromide using the Doc-Print imaging system (Vilber Lourmat, France) (Zhang et al., 2020).
– PCR condition for TaGW8 included the following cyclic conditions: 95°C for 5 min, 40 cycles at 95°C for 20 s, 60°C for 15 s, 72°C for 30 s, and a final extension at 72°C for 10 min. The reaction mixture (total volume 20 µl):1 × DreamTaq Buffer, 100 ng DNA matrix, 4 nmol dNTPs, 10 pmol of direct and reverse primers, 1.25 U DreamTaq Hot Start DNA Polymerase (Thermo Fisher Scientific, USA) (Yan et al., 2019). For the TaGS5-3A gene, PCR was performed in a total volume of 15 µl, with 50 ng of DNA, 10 mM of direct and reverse primers, 4 nmol DNTP, 1xDreamTaq Buffer, and 1.25 U DreamTaq Hot Start DNA Polymerase (Thermo Fisher Scientific). Amplification was carried out at 94 °C for 4 min, then 35 cycles at 94°C for 35 s, annealing for 35 s, and 72°C for elongation for 30 s, with a final elongation for 10 min (Ma et al., 2016).
2.5. Statistical analysis
Standard statistical analysis of the results was carried out using the Student's t-test (t) and p-values (p) using Microsoft Excel 2019. The p-value was calculated relative to the mother and father of the hybrid. Pearson’s correlations were used to analyze the relationships between crop components and structural indicators. The phenotypic and genetic indicators were analyzed using the SPSS version 22.0 program with a nonparametric (Man-Whitney) test.
3. Results
3.1. Phenotype analyses
3.1.1. Plant height
The PH of the parental forms was measured for five years. Depending on the weather conditions in the two zones, the difference in PH was around 10–20 cm. PH indicators in Central Kazakhstan differed sharply from those in the northern region. In previous studies, we compared the PH indicators of different parental forms and hybrids in two agroecological zones. As a result, Chinese varieties (Xn-03, Xn-04, Xn-13) had a PH of 40.32±0.04–53.28±0.08, with a decrease of 1–13 cm in the center of the country. Australian varieties were lower in Central Kazakhstan; for example, Gladius was lower by 7 cm, RAC875 by 9 cm, and Wyalkatchem by 7.71 cm. Kazakhstan varietes of breeding reacted differently to conditions in two agroecological zones; the most significant differences in PH were noted in Erythrospermum varietes 35 by 56.45 cm, Astana by 41.1 cm, Aktyubinka by 39.2 cm, Karabalykskaya 25 by 23.7 cm, Akmola 2 by 22.8 cm lower in the central part of the country. Russian varieties Saratovskaya 66 by 21.4 cm and Lutestsens 141 by 7.4 cm also showed lower results in Central Kazakhstan (Zotova et al., 2023). The PH of the hybrids obtained in the two agroecological zones did not differ significantly. On average, the height is lower in Central Kazakhstan compared to the northern region, ranging from 0.9 to 18%, and the hybrid WH 147 is 6% higher.
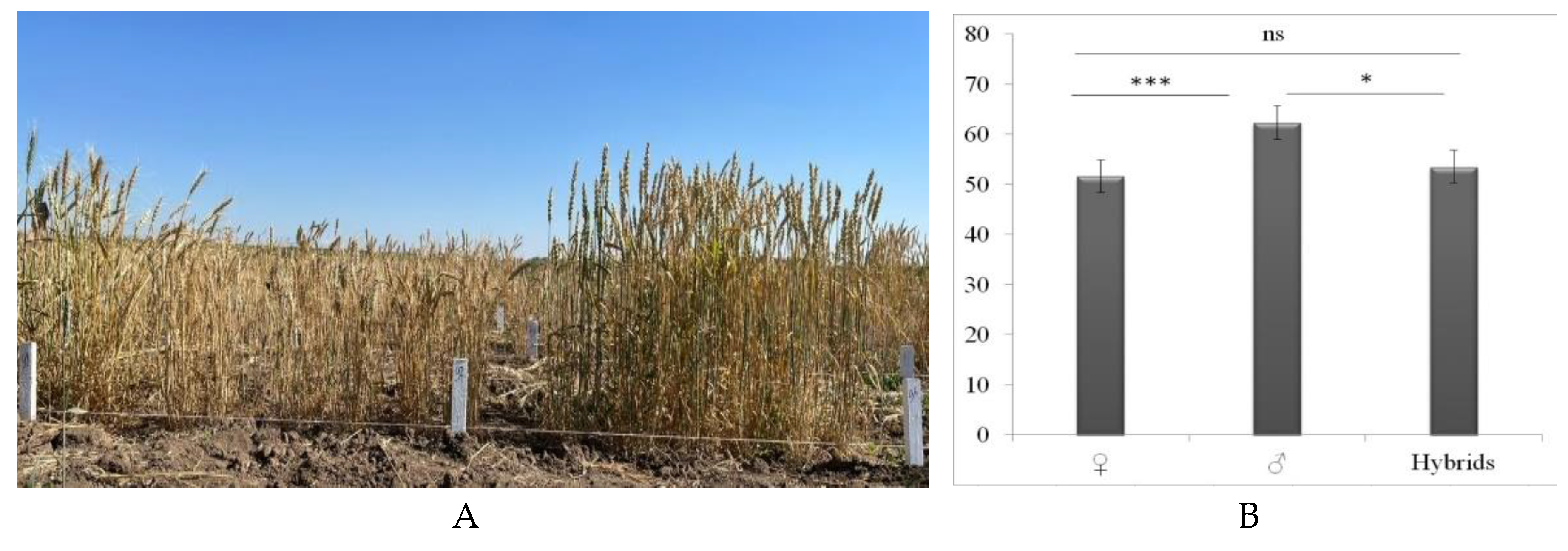
Figure 2 shows the difference in PH between tall Kazakhstan varieties and varieties of China and Kazakhstan in field average indicators of parental forms and hybrids (b), which are recognized as semi-dwarfs.
The average five-year values for the PH of maternal forms (
Figure 2) were 51.6±0.98 cm (p<0.01), paternal forms 62.3±2.5 cm (p<0.05), and the PH of hybrid forms 53.5±2.2 cm (p>0.05). Among the dwarf hybrids, WH131, WH133, WH134, WH138, WH139, WH141, WH142, WH143a, WH145, WH146, and WH147 stood out, the average PH of which ranged from 38.26±0.3–53±0.4.
The average stem diameter for the three growing seasons was 0.31 cm in rye, 0.32 cm in triticale, and 0.24 cm in wheat (Sidhu et al., 2020). These results show that the PH and grain size depend of planting year crop type. Our finding was in agreement with Zhenxian et al. (2020) results that irrigation at the tube entry stage increases the PH by 6.60–9.70%. Compared with wild-type alleles Rht-B1a and Rht-D1a, the genetic background of Rht-B1b and Rht–D1b significantly reduced PH by 14.40–15.50% and 16.90-19.10%, respectively. Rht-B1b and Rht-D1b can reduce PH by 29.10–32.50%. PH is notably negatively correlated with grain yield during irrigation, whereas PH is positively correlated with the drought coefficient (Zargar et al., 2017; Zhenxian et al., 2020). Thus, for zoned varieties adapted for a certain climatic zone, the main parameter is still the genetics of the variety.
3.1.2. Coleoptile length
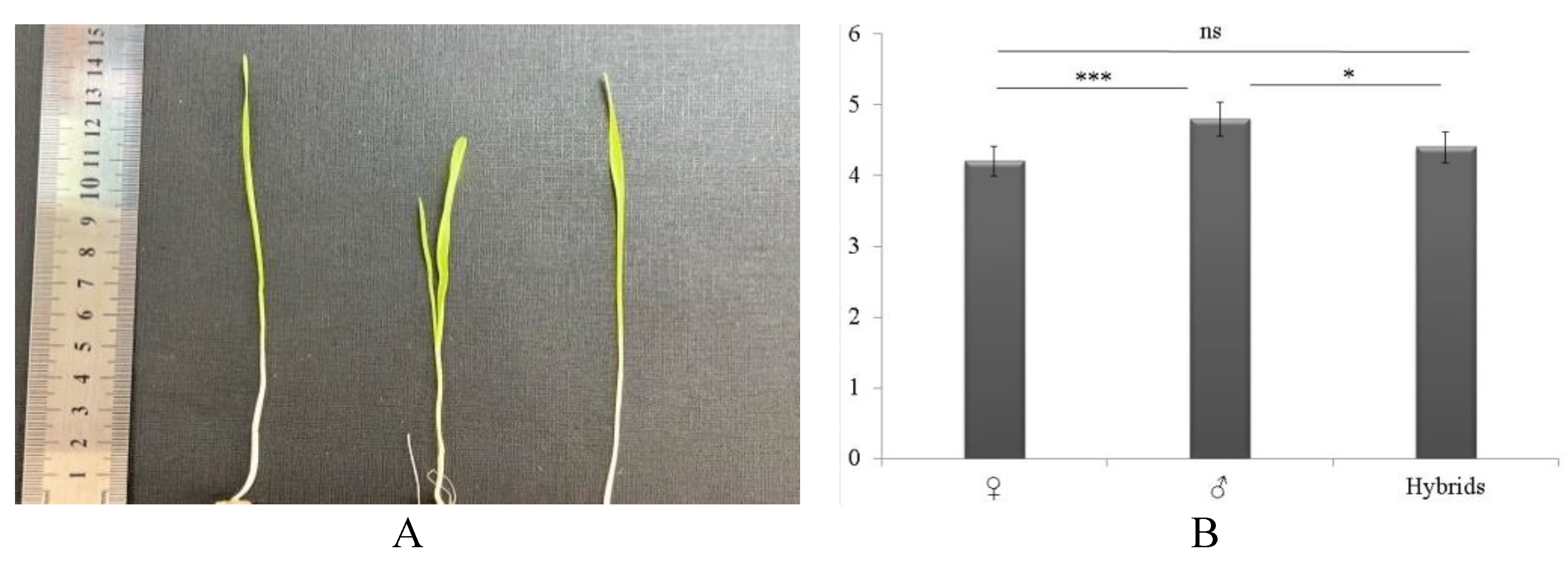
Both tall and semi-dwarf varieties were present in the samples collected. Hence, the CL (
Figure 3a) was one of the main indicators in selecting hybrids in these studies, especially given the yearly increase in drought. Thus, based on the CL in 60 samples the average value in maternal varieties was 4.1±0.15 cm (p<0.01); in paternal varieties, the CL was 4.8± 0.1 cm (p<0.05), and in hybrids, the CL was 4.4±0.15 cm (p>0.05) (
Figure 3b). Samples with a CL greater than 5 cm were identified among hybrids WH191, WH 133, and WH 134. Among the maternal varieties, the most significant were Xn-08; among the paternal varieties, Astana, Aktyubinsk, Karagandinskaya 30, Tauelsizdyk 20, Xn-09, and Lutestsens 141. Previous studies show that the CL in the arid Australian climate usually varies from 5.2 to 7.3 cm (Pumpa et al., 2013). Rebetzke et al. (2021) studied the CL in the Halberd and Scepter varieties, which were 13.2 cm and 6.5 cm, respectively, and those of Mace and Mace18 were 10.2 cm and 15.1 cm, respectively. In contrast, the long coleoptile Mace18, which contains the new dwarfism gene Rht18, took root well with deep sowing (up to 80% of the shallow depth of 40 mm). In contrast, the shorter coleoptile Mace and Scepter had a lower survival rate in deep sowing (30–40%) (Rebetzke et al., 2021). According to the scientific data and the obtained results, the CL varies from year to year and strongly depends on the climatic zone. At the same time, this indicator is decisive when choosing the depth of planting seeds.
3.1.3. Grain size
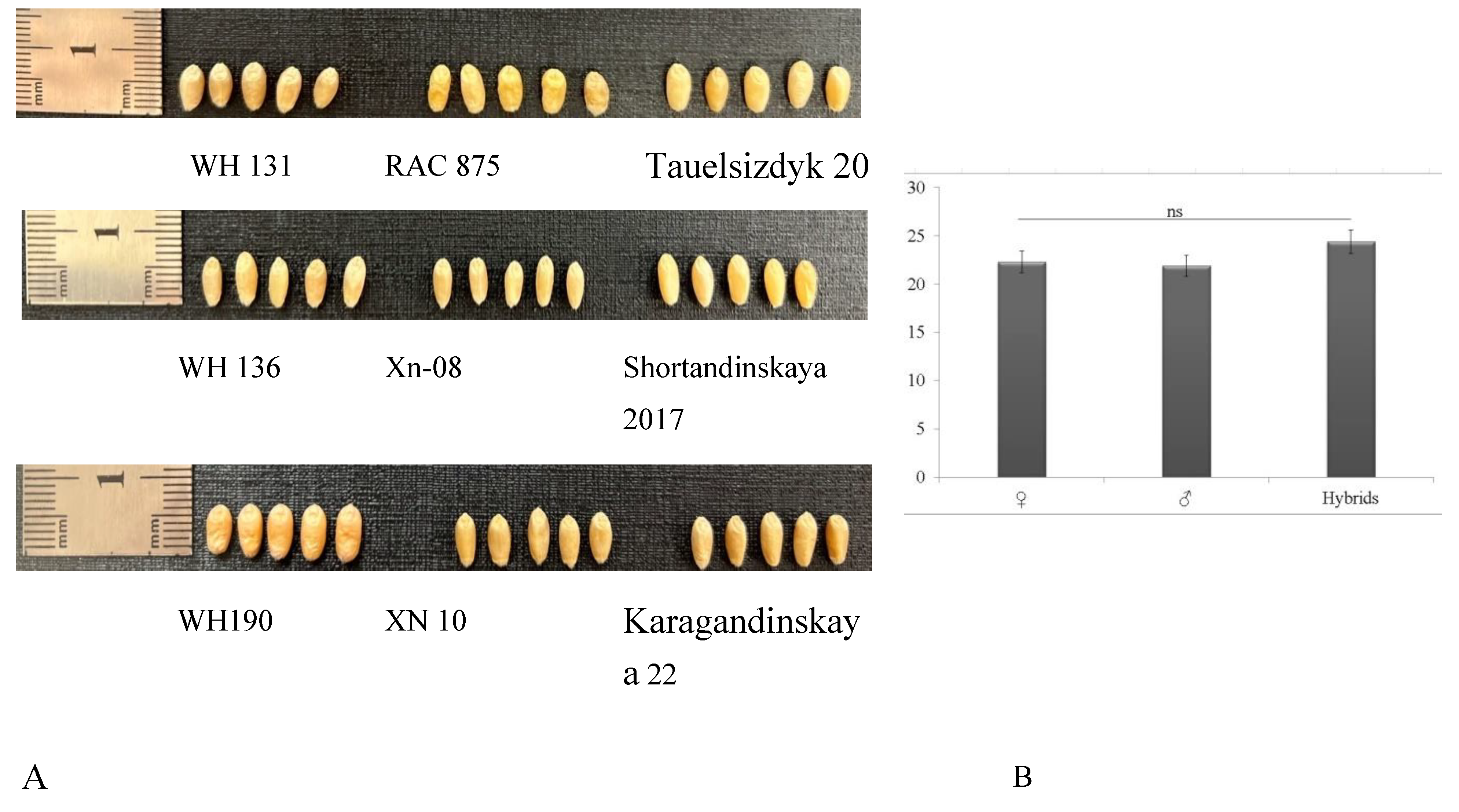
The grain size (GS) among the hybrids and parent forms significantly differed. The differences shown in
Figure 4 (a) and the average parameters of parental and hybrids forms on
Figure 4 (b). The average GS for the maternal form was 22.29 mm2, for the paternal form, 21.91 mm2, and for hybrid forms, 24.39 mm2. The highest GS were in WH137 hybrid (Xn-10 x Astana), WH131 (RAC875 x Tauelsizdyk20), WH191 (XN 08 x Karabalykskaya 90), WH193 (XN 10 x Karagandinskaya 30), WH191 (Xn-08 x Karabalykskaya 90), WH189 (Xn-08 x Karagandinskaya 29), WH190 (Xn-10 x Karagandinskaya 22) – from 27.8 to 36.4 mm2. The smallest area indicator among hybrids was observed in WH132 (Erythrospermum 35 x Xn-09) at an average level of 18 mm2. Among the parent forms, the average was 21.91–22.29 mm
2. The lowest indicator was observed in Wyalkatchem and Lutestsence 15.6 and 18.2 mm2, respectively. The maximum average values for grain area (GA)were observed in Karagandinskaya 29, MMF-044, Karabalykskaya 25, Xn-10, and Karagandinskaya 22 from 25 to 27.8 mm2. Despite minor differences in the GS in the parent forms, hybrid forms showed a higher result higher by 10.4%. At the same time, the highest indicators were in Chinese, Kazakhstan hybrids, and one hybrid RAC875x Tauelsizdyk. According to Acosta et al. (2017) results which showed that the GS of soft wheat varies from 15.41 to 18.37 mm2 (Acosta et al., 2017); in durum wheat varieties, this value is higher in the range of 17.82 to 19.6 mm2 (Beral et al., 2020).
3.1.4. Ratio of diameter and length of the second internode (JG index)
The JG index determines the resistance to lodging, which depends on the morphological parameters of the stem. A comprehensive assessment allows one to assess the available varieties and lines more accurately. For this purpose, the JG index L2/D2 is also determined, which is the ratio of the length of the second internode to its diameter (Paulikiene and Žvirdauskiene, 2023). Samples are considered stable if the ratio of the length of the lower internode to its diameter does not exceed 35%; medium–resistant, 35–45%; and lodging, more than 45%. According to the results of the experiments, in almost all samples, both parents and hybrids, were resistant to lodging. The average lodging resistance of the Saratovskaya 66 variety and the Astana variety (36.05±0.14) was the highest. The average ratio of the L2/D2 in maternal varieties was 25.6±1.0 (p<0.01), in paternal varieties, 29.9±0.9% (p<0.01), and among hybrids, it was 25.69± 0.9 (p>0.05). All samples from maternal forms and hybrids had an L2/d2 parameter below 35%; in paternal forms only, in the Astana variety, the JG index was 36.05±0.14. The varieties with the lowest index were RAC 875 (19.1±0.3), Wyalkatchem (21.9±0.3), and WH133 (13.1±0.2). Based on the results, all the samples were classified as resistant to lodging.
3.2. Correlation of phenotypic parameters
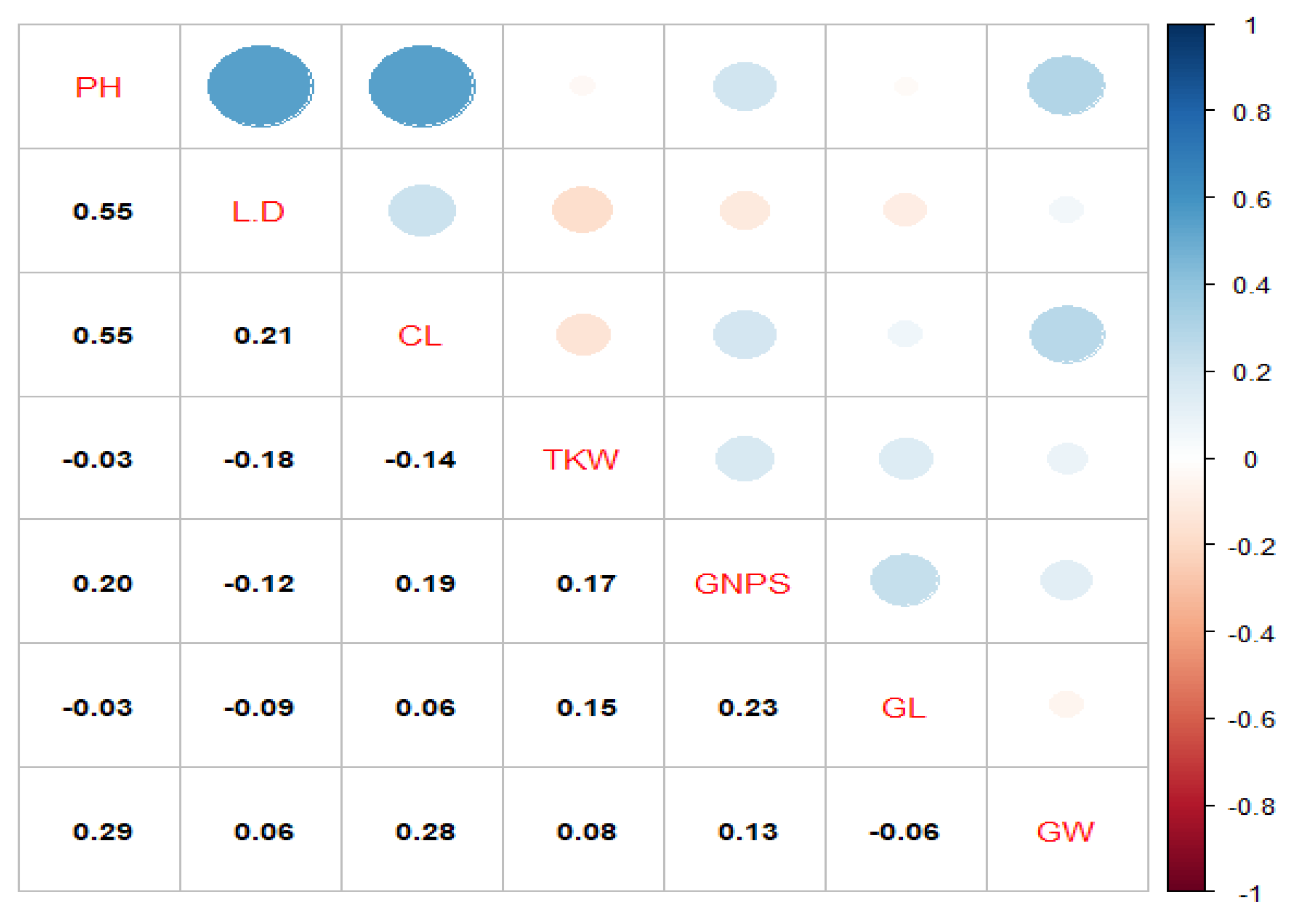
Using Pearson's correlation analysis, the following results were obtained (
Figure 5)
When correlating the indicators of short stems and productivity between varieties and hybrids, it was noted that the most interrelated phenotypic parameters were plant height/ratio of the length of the 2nd internode to its diameter (R2=0.305) and PH/CL (R2=0.3045) (p<0.001). In addition, a positive correlation was found between the PH and the CL with GW R2= 0.0751 and R2=0.0699, respectively. A positive correlation was also noted between the GN per spike and the GL (R2=0.1017). The ratio of L2/D2 had a positive correlation with the CL (R2=0.047). The TGW was positively correlated with the GN (R2=0.0307). The same indicator, CL, has a negative correlation (R2=0.0201). A significant negative correlation was noted between the L2/D2 and the TGW (R2=0.0367). There was a negative correlation between the PH and the TGW (R2=0.0024). According to the correlation analysis, the varieties of Chinese selections Xn10, Xn-08, and Xn-03, Russian selection Saratovskaya 66, and Kazakhstan selection Karagandinskaya 22, among hybrid forms WH134, WH136, WH137, WH145, WH190, and WH192, optimal for the cultivation zone, were identified according to the characteristics of short stems and coarse grains.
3.3. Genetic parameters
Samples were examined at the genetic level for the purpose of studying parental forms and hybrids, in the context of semi-dwarfism and coarseness. For this purpose, the genes of the "green revolution", Rht-B1a, Rht-B1b, Rht-D1a, and Rht-D1b, which are characteristic of almost all modern varieties, as well as the genes TaGW8-B1a, which determine coarse grain, and TaGS5-3A, which is responsible for GS, were studied.
3.3.1. Rht genes
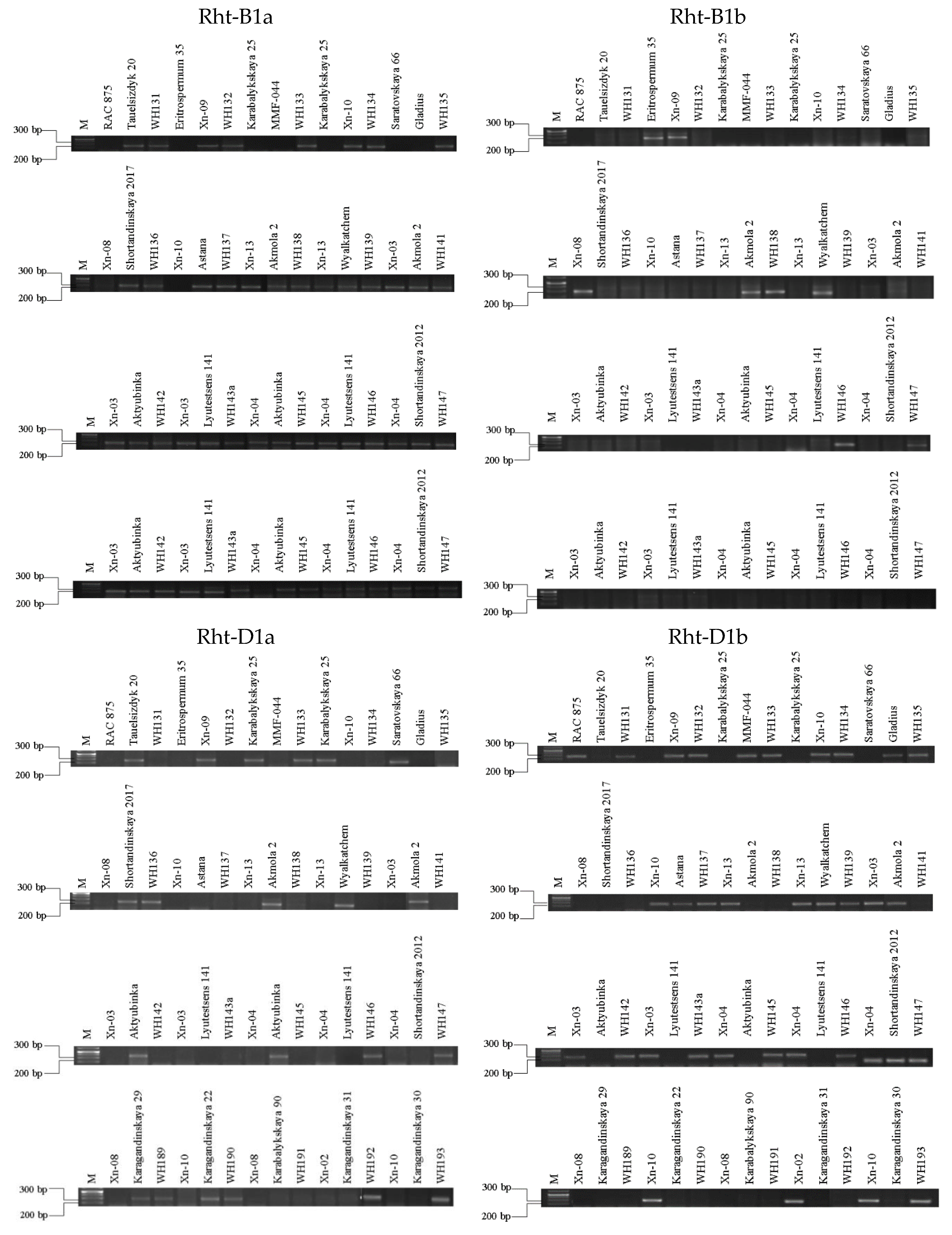
Tables S1 and S2 show the average values of all the studied indicators that determine characteristics such as short-stem and lodging resistance (PH and the L2/D2), as well as the CL, which is the main indicator that should determine the optimal PH and seed depth for the region. According to the results of genetic analysis, the presence or absence of the Rht-B1b short-stem gene does not always guarantee the absence/presence of the Rht-D1a gene, for example, in the Erithrospermum 35, Xn-08, and Akmola 2 varieties. But it gives a short-stem phenotype in association with the Rht-D1b gene (Wyalkatchem, WH 147). However, Xn-09 has 4 genes that may possibly manifest in hybrids in any generation. As 362 for the Rht-D1b gene, hybrids WH-131-135, WH-137, WH-139, WH-141-142, WH-145, WH-147, WH-193 inherited these genes from parental forms (varieties from China, Africa, Australia). The listed hybrids can be considered semi-dwarfing according to 5-year tests. The presence of a single gene with a positive effect can provide shortening of the length without a significant negative effect on the CL. Thus, the presence of the Rht-D1b gene is preferable for long-coleoptile hybrids with a shortened stem.
Thus, according to molecular genetic analysis, short-stem varieties contain at least one of the Rht-B1b and Rht-D1b genes in their genome (
Figure 6). RAC 875, MMF-044, and Gladius contained one Rht-D1b gene each. The Xn-09 variety had all four Rht gene alleles in the genome, a PH 64.74±0.87 cm, and a CL of 5.19±0.06 cm. As for Russian and Kazakhstan breeding varieties, they contained one to two Rht-B1a and Rht-D1a, genes with an average PH of 67.17 ± 0.3 cm and a CL of 4.8± 0.05 cm. The Astana, Akmola 2, and Shortandinskaya 2012 varieties had the Rht-D1b/Rht-B1b gene in the genome with a PH of 66–68 cm and a CL of 4.18-5.54 cm. The hybrids had ambiguous gene inheritance and phenotypic manifestations.
Thus, hybrids WH 131, WH 132, WH 133, WH 134, WH 135, WH 137, WH 138, WH 139, WH 141, WH 142, WH 145, and WH193 had both a positive and a negative allele in the genome; their average PH was 50.9±0.3, with a CL of 4.3±0.07. Among them, the hybrids WH 133, WH 134, and WH 137 were above 5 cm. Thus, according to the literature, the Rht-B1a Rht-D1a genes determine tall varieties, and the Rht-B1b and Rht-D1b genes are responsible for shortening the straw. In the process of crossing, parental forms were selected based on short–stem and productivity, so varieties of Chinese, Australian, and African selections acted as donors of short-stem genes, and varieties of Russian and Kazakhstan selections acted as donors of productivity. 385
3.3.2. TaGW and TaGS genes
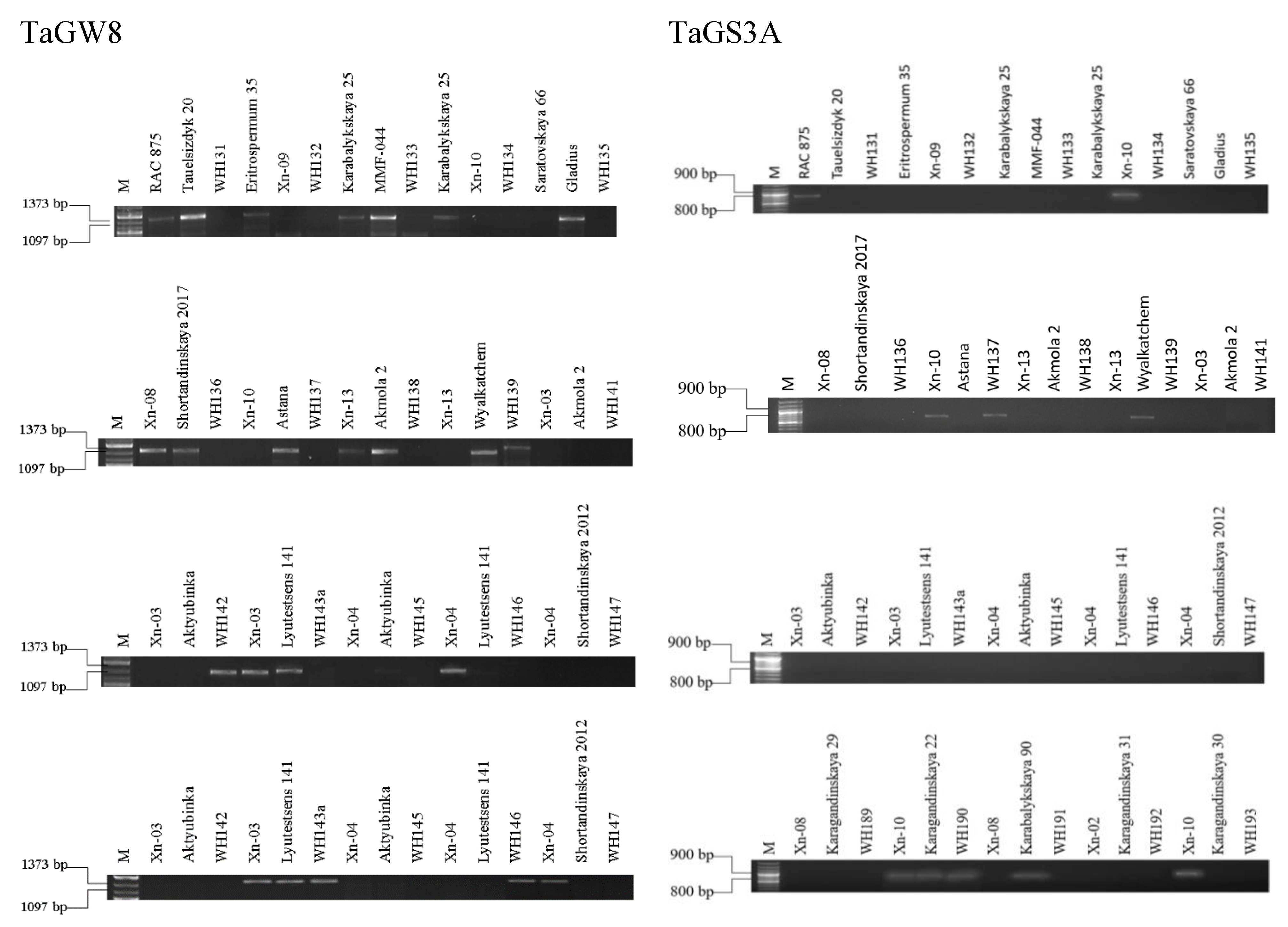
According to the productivity indicators, it is possible to distinguish the TGW, which determines the fullness and GS. Among maternal forms, the average TGW was 38.85±0.5 g (p<0.001), in paternal forms 35.5±0.6 g (p>0.05), and in hybrids, 36.5±1.03 g (p>0.05). When selecting hybrids based on the characteristics of short stems and productivity, it is essential to understand the correlation between all important parameters. Genes that determine the mass of seeds and the number of seeds in an ear phenotypically affect indicators such as the mass of 1000 seeds, grain size, and the area of one grain. Productivity analysis was carried out using the TaGW8 and TaGS3A genes, which reliably showed higher values of GW and GN (
Figure 7).
It is well documented that these two traits have a negative correlation, where an increase in GN is associated with a decrease in GW (Xie & Sparkes, 2021). However, many studies have shown a transgressive separation in yield when crossing varieties with high GN and GW (García et al., 2014; Griffiths et al., 2015). Wider but smaller seeds indicate a greater investment of resources in a smaller GN, and more numerous but smaller seeds represent a smaller investment of resources in a larger GN (Sadraset al., 2007). Studies focusing on the GN and GW in various cereals and media have determined that the GN is a highly plastic trait, whereas the GW is significantly more heritable.
The presence of both genes among the parental forms was noted in varieties RAC 875, Xn-10, Wyalkatchem, and Karagandinskaya 22. At the same time, the SNP marker on the TaGW8 gene was a significantly higher indicator of the GN per spike. Among the hybrids that inherited both genes, only WH190 was identified (XN 10 × Karagandinskaya 22), while both the hybrid and the parents had the highest TGW of 40.7–43.4 g, and the GN was 36–41 pcs. The largest GN was noted in the hybrid WH190, despite this indicator not exceeding 28 mm2 in the parents. Among the parental forms carrying positive polymorphism of the TaGW8 gene are: Tauelsizdyk 20, Erythrospermum 35, Karabalykskaya 25, Gladius, Shortandinskaya 2017, Xn-08, Xn-13, Akmola 2, Xn-03, and Aktuybinka. Of the hybrids, the presence of positive polymorphism was revealed in WH 139, WH 142, WH 145, and WH192. At the same time, the hybrid WH 139, obtained from a combination of Xn-13 and Wyalkatchem each with a 415GW of 38.76±0.23 and 33.92±0.12 and a GN of 33.8±0.5 and 22.6±0.51, respectively, had a GN of 25.2±0.37. This may indicate the regression of the trait and the possibility of its manifestation in future generations. The TaGS gene was found in the hybrid WH 137 and the Karabalykskaya 90 variety. Genes were not detected in 23 samples.
3.4. Correlations of Rht (-B1a, -B1b, D1a, D1b) semi-dwarfism genes and coarse-grained TaGW8-B1a, TaGS 5-3A
According to the Maine-Whitney correlation analysis, there is a direct dependence on the presence of short-stem genes and the phenotypic index of CL. In addition, the productivity of seeds is directly related to the presence of the TaGW8 gene and the TGW. To analyze the effects of different genetic allelic variations on agronomic traits, we used 33 parental material information to analyze the genetic effects of Rht-B1b, Rht-D1b (
Table 2), TaGS-3A, and TaGW 8 (
Table 3). Statistical analysis was performed using SPSS software version 22.0, with nonparametric tests (Mann-Whitney test). The results showed that TaGW8 significantly increased wheat grain width by 13.37%. Simultaneously, Rht-D1b significantly reduced PH and shortened CL by 51.67% and 30.45%, respectively.
Thus, in plants carrying the Rht-D1b and Rht-B1b short-stem genes, the average TGW was 35.06 g (p<0.05), while in the presence of only one Rht-B1b gene in the genome, the TGW was 38.21 g (p<0.05), while with a zero genotype, both alleles showed a value of 36.8g (p<0.05). However, in tall varieties and hybrids, the value of the TGW was lower: the zero genotypes for the Rht-D1a and Rht-B1a alleles give a TGW of 37.96 g, in the presence of the Rht-B1a allele, 36.4 g, Rht-D1a, 35.8 g, with both alleles, 37.4 g (p>0.05). However, these alleles affect the value of the GN. So, the presence of two alleles Rht-D1a and Rht-B1a gives the value of GN (24.1 pcs.), in the presence of only one allele Rht-B1a (21.5 pcs; p>0.05). When both Rht-D1b and Rht-B1b alleles are present, 18.5 of GN are produced.
4. Discussion
Hybrid selection with crossing promising varieties is a fundamental point of wheat breeding. The selection of productive wheat varieties that are adapted to the soil and climatic conditions is especially relevant in climate changing conditions and yield decreasing conditions under biotic and abiotic stresses. Climate change is not only an increase in air temperature, but also a reduction in atmospheric humidity and soil moisture (Elahi et al., 2022). Annual reduction of precipitation in Kazakhstan (Karatayev et al., 2022) led to the necessity of accelerating the selection process in the selection of productive and drought-resistant hybrids and varieties.
Despite extensive studies of yield characteristics, the main goal of grain crop breeding worldwide is to study the plasticity of genotypes while adapting to abiotic stresses. It is practically impossible to take into account all the selection parameters in the field. At the same time, the increase in yield was achieved mainly due to an increase in the number of grains in the ear while maintaining a constant mass of 1000 grains or vice versa; an increase in the mass of 1000 grains reduced the number of seeds in the ear (Tillett et al., 2022). In regions with arid climates, an increase in yield is achieved by shortening the stem, that is, by introducing Rht genes into the breeding process. However, the decrease in plant height partially affected the yield, which was especially noticeable in drought conditions. The height of wheat plants is a polygenic trait, and to date, approximately 17 genes from 21 chromosomes have been identified (Zhang et al., 2020), which does not allow for quick and versatile prediction of the qualities and characteristics of the variety.
In this study, the identification of the most drought-resistant and productive wheat varieties and hybrids was conducted in two climatic zones with arid conditions. We studied phenotypic and molecular parameters. Correlation analysis between phenotypic traits and genetic indicators confirmed necessity to choose parental forms with the presence of Rht genes and 1000 grain weight genes in the genome. A significant decreasing in plant height is achieved with the Rht- D1b gene in the genome (by 51.67%). However, this gene also affects decreasing in the coleoptile length (by 30.45%). At the same time, if the coleoptile length is at least 5 cm or longer the length of the hybrid reaches 4 cm or more (WH131, WH132, WH142, WH143a, WH193). In 40% of samples, the coleoptile length in hybrids was 4 cm or more, with the coleoptile length in both parental forms more than 4 cm and 5 cm. In 15% of hybrids, the coleoptile length was 4 cm, while the coleoptile length in one of the parent forms was 4 cm. And only in 20% of cases in hybrids the coleoptile length was 3 cm, while the coleoptile length in the parental forms was 4 cm and 5 cm or more. So, choosing parental forms with the presence of the Rht-D1b gene and with a coleoptile length more than 5 cm, in 80% of cases, hybrids will have a coleoptile length of 4 cm or more with a shorter stem.
There is ambiguous data on the reasons for the decrease in yield in short-stem varieties; it is noted that dwarf Rht alleles increase plant productivity by changing the rate of photosynthesis. Several studies have illustrated a strong correlation between genetic improvements in yield due to the introduction of semi-dwarfism genes and an increase in the rate of photosynthesis (Evans, 2013). A recent study showed that the genes of semi-dwarfism affect the leaf structure of seedlings, while semi-dwarf plants have increased stomatal density and leaf thickness with increased chlorophyll content (Nenova et al., 2014). However, it should be borne in mind that early experiments to study the effect of alleles of semi-dwarfism on photosynthesis were carried out in growing chambers, which may not reflect field conditions.
In our study, the selected parental forms and hybrids showed the dependence of dwarfism on the presence of the Rht-D1b gene. However, in the case of productivity genes, the presence of genes has less influence on the productivity of the hybrid than the characteristics of the parental forms. Thus, despite the absence of the TaGS-3A and TaGW8 genes, 65% of hybrids had a 1000 grains weight above 30 g. Hybrids WH 142, WH 145 and WH192 with the presence of one TaGW8 productivity gene in the genome showed a 1000 grain weight of 36.2 g, 40.7 g, and 45.2 g, respectively. The TaGS-3A gene was present in the genome of the hybrid WH 137, while the absence of the TaGW8 gene did not affect the 1000 grains weight (41.8 g) and the seed area of 27.8 mm2. And only in one of the WH190 hybrids, the presence of the TaGS-3A and TaGW8 genes showed a 1000 grain weight – 43.4 g and a seed area of 36.4 mm2, while both genes were present in the genome of both parents. At the same time, the plant height of the WH190 hybrid was 62.2 cm. the plant height of 60-62 cm was optimal for a 1000 grains weight over 35 g. and a seed size of more than 25 mm2.
Photosynthetic activity is associated with a higher 1000 grains weight; therefore, taller plants with a larger absorption area have a greater fullness of grains, affecting the final yield (Brown et al., 2021). Since the plants were reduced in height due to mutations in various Rht homologs, the mass of 1000 seeds also decreased, indicating that grain filling may be limited by the source of genes (Zhang et al., 2020). The increase in dry matter due to longer stems, roots, and more shoots and leaves can be considered an auxiliary product that can be a competitor in the cultivation of the main product, grain, especially in conditions of limited resources in arid lands.
In our study, the number of grains per spike in 60% of hybrids was higher than 20 pcs per spike with 1000 grains weight gene was above 30 g. The seed size in the presence of the TaGS-3A gene in the hybrid WH137 was 27.8 mm2 with a number of grains per spike 30.8 pcs and a 1000 grains weight is 42.8 g. In the hybrid WH190, the presence of the TaGS-3A gene gave a seed size value of 36.4 mm2. The maximum seed area was observed in hybrids WH189, WH190 and WH 191, 40 mm2, 36.4 m2 and 30 mm2, respectively. At the same time, the height of plants in these hybrids was 38.1 cm, 43.4 cm and 34.5 cm, respectively. Thus, the presence or absence of one of the 1000 grains weight or size of seeds genes does not guarantee a high number of seeds or weight. Although the presence of the TaGS-3A and TaGW8 genes in both parental forms give excellent indicators of height and productivity hybrids.
Gao et al. (2020) found that irrigation at the stage of entering the tube increases the height of wheat plants by 6.60–9.70%. Compared with wild-type alleles Rht-B1a and Rht-D1a, the genetic backgrounds of Rht-B1b and Rht–D1b significantly reduced plant height by 14.40–15.50% and 16.90-19.10%, respectively. Rht-B1b and Rht-D1b can reduce plant height by 29.10–32.50%. Plant height is strongly negatively correlated with grain yield during irrigation, whereas plant height is positively correlated with the drought coefficient (Zhenxian et al., 2020). At the same time, according to our research, plants with a PH of 37 cm and up to 68 cm are resistant to lodging according to the JG index, and plants with a PH of more than 60 cm have a limit index value of 33 and higher, which makes these plants more susceptible to lodging.
Yield is a complex feature, largely related to the number of productive spikes per unit area, the number of grains per ear, and the TGW. However, the GS, the spike's structure, the PH, and the signs associated with the flag leaf can also affect the yield by affecting the intensity of photosynthesis, grain filling, and movement of dry matter (Zhou et al., 2007). These traits have a higher heritability than productivity and are easier to select on small plots at the early stages of the breeding process (Faji et al., 2019; Zargar et al., 2022).
Thus, in the presence of Rht-D1b and Rht-B1b together with TaGW8 and TaGS5-3A in the parent genome, the appearance of productive hybrids increases the number of dwarf hybrids with long coleoptiles and large grains. Based on these results, we selected promising productive parent forms Karagandinskaya 22, Saratovskaya 66, Xn-10 and Xn-08 and hybrids WH134, WH136, WH137, WH145, WH190 and WH192, which showed optimal PH, with a CL and GS, which affected the increase in yield in areas with arid climate. Consequently, the study of the genetic parameters of dwarfism and productivity genes will accelerate the selection process of parental forms and hybrids and will allow obtaining stable lines based on both field results and laboratory data. The presented results show the importance of genetic selection of parental forms and hybrids, although it is important to note that further research is needed to identify a panel of genes for faster sampling.
Conclusion.Thus, in the presence of Rht-D1b and Rht-B1b together with TaGW8 and TaGS5-3A in the parent genome, the appearance of productive hybrids increases the number of dwarf hybrids with long coleoptiles and large grains. Based on these results, we selected promising productive parent forms Karagandinskaya 22, Saratovskaya 66, Xn-10 and Xn-08 and hybrids WH134, WH136, WH137, WH145, WH190 and WH192, which showed optimal PH, with a CL and GS, which affected the increase in yield in areas with an arid climate. Consequently, the study of the genetic parameters of dwarfism and productivity genes will accelerate the selection process of parental forms and hybrids and will allow obtaining stable lines based on both field results and laboratory data. The presented results show the importance of genetic selection of parental forms and hybrids.
Funding
This study was financially supported by the Ministry of Education and Science, Republic of Kazakhstan, within the framework of the Young Scientists Project No. AP13067944 «Molecular SNP-marking of bread wheat by genes TaGW, TaGS, and Rht for grain weight and lodging resistance».
Acknowledgments
We would like to express our gratitude to the staff of the Research Platform of Agricultural Biotechnology, as well as to the students of the Faculty of Agronomy of the S.Seifullin Kazakhstan Agrotechnical University. RUDN University Strategic Academic Leadership Program supported this work.
Abbreviations
PH, plant height; CL, coleoptile length; GN, grain number; GW, grain weight; TGW, thousand grains weight; L2/D2, ratio of the diameter to the length of the internode; Rht, redused height gene; SNP, single, nucleotide polymorphism; TaGS, Triticum aestivum grain size; TaGW, Triticum aestivum grain weight.
References
- Acosta, S., Patricio, C, Villaseñor Mir, H.E., García, G.A.L, Ruvalcaba, L.P., Hernández, V.A.G., Olivas, A.R. (2017). Size and number of wheat grains analyzed by digital image processing. Rev. 516 Mex. Cienc. Agríc. 8(3), 517–529. [CrossRef]
- Babkenov, A.T., Babkenova, S.A., Kairzhanov, E.K. (2019). Studying genetic resources of spring bread wheat in the environments of Northern Kazakhstan. Proceedings on applied botany, genetics and breeding. 180(4):44-47. (In Russ.). [CrossRef]
- Beral, A., Rincent, R., Gouis, J.L., Girousse, C., Allard, V. (2020). “Wheat individual grain-size variance originates from crop development and from specific genetic determinism,” PLoS One, 26;15(3), e0230689. [CrossRef]
- Bi, H., Kovalchuk, N., Langridge, P., Tricker, P.J., Lopato, S., Borisjuk, N. (2017) The impact of drought on wheat leaf cuticle properties. BMC Plant Biol. 8;17(1):85. [CrossRef]
- Borlaug, N.E. Borlaug, N.E. (1980). Wheat breeding and its impact on world food supply /Borlaug N.E. /WheatGenet. Symp. p. 36.
- Brown, M.M., Martin, J.M., Jobson, E.M., Hogg, A.C., Carr, P.M., Giroux, M.J. (2021). Evaluating the impact of Rht hypomorphic mutations in durum wheat. Crop. Sci. 62(1), 247–258. [CrossRef]
- Chandler, P.M.; Harding, C.A. ‘Overgrowth’ mutants in barley and wheat: new alleles and phenotypes of the ‘Green Revolution’ DELLA gene. J. Expt. Bot. 2013, 64, 1603–1613. Available at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3617830/. [CrossRef]
- Elahi, I., Saeed, U., Wadood, A. (2022) Effect of Climate Change on Wheat Productivity. Wheat. IntechOpen. [CrossRef]
- Ellis, H., Spielmeyer, W., Gale, R., Rebetzke, J., Richards, A. (2002). "Perfect" markers for the Rht-B1b and Rht-D1b dwarfing genes in wheat. Theor. Appl. Genet. 105(6-7):1038–1042. [CrossRef]
- Emamverdian, A., Ghorbani, A., Li, Y., Pehlivan, N., Barker, J., Ding, Y., Liu, G., Zargar, M. (2023a). Responsible mechanisms for the restriction of heavy metal toxicity in plants via the co-foliar spraying of nanoparticles. Agronomy. 13:1748. [CrossRef]
- Emamverdian, A., Ghorbani, A., Pehlivan, N., Alwahibi, M.S., Elshikh, M.S., Liu, G., Li, Y., Barker, J., Zargar, M., Chen, M. (2023b). Co-application of melatonin and zeolite boost bamboo tolerance under cadmium by enhancing antioxidant capacity, osmolyte accumulation, plant nutrient availability, and decreasing cadmium absorption. Sci. Hortic. 322:112433. [CrossRef]
- Evans, J.R. (2013). Improving photosynthesis. Plant Physiol. 62(4):1780–93. [CrossRef]
- Faji, L., Wen, W., Liu, J., Zhang, Y., Cao, S., He, Z., Rasheed, A., Guo, L., Zhang, C., Yan, J., Zhang, P., Wan, Y., Xia, X. (2019). Genetic architecture of grain yield in bread wheat based on genome-wide association studies. BMC Plant. Biol. 19 (168). [CrossRef]
- FAO (2022). Crop Prospects and Food Situation – Quarterly Global Report No. 3, 22. Rome. 20 September. [CrossRef]
- García, G.A., Serrago, R.A., González, F.G., Slafer, G.A., Reynolds, M.P., Miralles, D.J. (2014). Wheat grain number: Identification of favourable physiological traits in an elite doubled-haploid 546 population. Field Crop. Res. 168:126–134. [CrossRef]
- Ghorbani, A., Emamverdian, A., Pishkar, L., Chashmi, K.A., Salavati, J., Zargar, M., Chen, M. (2023a). Melatonin-mediated nitric oxide signaling enhances adaptation of tomato plants to aluminum stress. S. Afr. J. Bot. 162:443-450. [CrossRef]
- Ghorbani, A., Ghasemi-Omran, V.O., Chen, M. (2023b). The effect of glycine betaine on nitrogen and polyamine metabolisms, expression of glycoside-related biosynthetic enzymes, and K/Na balance of stevia under salt stress. Plants. 12:1628. [CrossRef]
- Govta N., Polda I., Sela H., Cohen Y., Beckles D.M., Korol A.B., Fahima T., Saranga Y., Krugman T. (2022). Genome-Wide Association Study in Bread Wheat Identifies Genomic Regions Associated with Grain Yield and Quality under Contrasting Water Availability. Int. J. Mol. Sci. 23(18):10575. [CrossRef]
- Griffiths, S., Wingen, L.U., Pietragalla, J., García, G., Hasan, A., Miralles, D., Calderini, D., Ankleshwaria, J.B., Waite, M.L., Simmonds J., et al. (2015). Genetic dissection of grain size and grain number trade-offs in CIMMYT wheat germplasm. PLoS ONE 10:e0118847. [CrossRef]
- Hu, M.J., Zhang, H.P., Liu, K., Cao, J.J., Wang, S.X., Jiang, H., Wu, Z.Y., Lu, J., Zhu, X.F., Xia, X.C., Sun, G.L., Ma, C.X., Chang, C. (2016). Cloning and characterization of TaTGW-7A gene associated with grain weight in wheat via SLAF-seq-BSA. Front. Plant. Sci. 20(7):1902. [CrossRef]
- Paulikiene, S., Žvirdauskiene, R. (2023). Evaluation of ˙ Hydrothermal Treatment of Winter Wheat Grain with Ozonated Water. Plants. 12, 3267. [CrossRef]
- Ionova, E., Likhovidova, V., Lobunskaya, I. (2020). Drought and hydrothermal humidity factor as one of the criteria to estimate its intensity degree (literature review). Grain Economy of Russia. P.18-22. [CrossRef]
- Johnson EN, Wang Z, Geddes CM, Coles K, Hamman B, Beres BL. (2018). pyroxasulfone is effective for management of bromus spp. In winter wheat in Western Canada. Weed Technol. 32:739–748. [CrossRef]
- Kavhiza, N.J., Zargar, M., Prikhodko, S.I., Pakina, E.N., Murtazova, K.M.-S., Nakhaev, M.R. (2022). Improving Crop Productivity and Ensuring Food Security through the Adoption of Genetically Modified Crops in Sub-Saharan Africa. Agronomy. 12: 439. [CrossRef]
- Karatayev M., Clarke M., Salnikov V., Bekseitova R., Nizamova M. (2022) Monitoring climate change, drought conditions and wheat production in Eurasia: the case study of Kazakhstan. Heliyon. 8(1). [CrossRef]
- Kurishbayev A.K., Tokbergenov I.T., Kanafin B.K., Zhengmao Zhang, Kiyan V.S. Shvidchenko V.K. (2019). Increase of productivity of spring soft wheat in the framework of exact farming system: problems, prospects. Bulletin of Science of the Kazakhstan Agrotechnical University named after S. Seifullin (interdisciplinary). - 2019. – No1 (100). - pp.107-116.
- Ma, L., Li, T., Hao, C., Wang, Y., Chen, X., Zhang, X. (2015). TaGS5-3A, a grain size gene selected during wheat improvement for larger kernel and yield. Plant. Biotechnol. J. [CrossRef]
- Nenova, V. R., Kocheva, K. V., Petrov, P. I., Georgiev, G. I., Karceva, T. V., Borner A., et al. (2014). Wheat Rht-B1 dwarfs exhibit better photosynthetic response to water deficit at seedling stage compared to the wild type. J. Agron. Crop Sci. 200:434–443. [CrossRef]
- Pumpa, J., Martin, P., McRaeand, F. (2013) Neil Coombes Coleoptile length of wheat varieties. NSW Department of Primary Industries. 1-5. https://www.dpi.nsw.gov.au/ data/assets/pdf_file/0006/459006/Coleoptile-length-of-wheat-varieties.pdf.
- Rebetzke, G., Fletcher, A., Micin, S., On-farm assessment of new long-coleoptile wheat genetics for improving grain yield with deep sowing (CSIRO Agriculture and Food) and Callum Wesley (Charlesville Ag, Southern Cross WA) Date: 09 Mar 2021.
- Sadras, V.O. (2007). Evolutionary aspects of the trade-off between seed size and number in crops. Field Crop. Res. 100:125–138. [CrossRef]
- Sidhu, J.S., Singh, D., Gill, H.S., Brar, N.K., Qiu, Y., Halder, J., Tameemi, R.A., Turnipseed, B., Sehgal, S.K. (2020). Genome-Wide Association Study uncovers novel genomic regions associated 607 with coleoptile length in hard winter wheat. Front. Genet., 10. [CrossRef]
- Shavrukov Y, Zhumalin A,Serikbay D, Botayeva M,Otemisova A, Absattarova A,Sereda G, Sereda S, Shvidchenko V,Turbekova A, Jatayev S, Lopato S,Soole K and Langridge P (2016) Expression Level of the DREB2-TypeGene, Identified with Amplifluor SNPMarkers, Correlates with Performance, and Toleranceto Dehydration in Bread WheatCultivars from Northern Kazakhstan.Front. Plant Sci. 7:1736. [CrossRef]
- Syzdykova G.T., Sereda S.G., Malitskaya N.V. (2018). Selection of spring soft wheat (Triticum Aestivum l.) varieties for the adaptability in the conditions of steppe zone of the Akmolinsk region, Kazakhstan. Sel’skokhozyaistvennaya biologiya [Agricultural Biology], 2018, V. 53, No1, pp. 103-110. [CrossRef]
- Tao, H., Feng, H., Xu, L., Miao, M., Yang, G., Yang, X., Fan, L. (2020). Estimation of the yield and plant height of winter wheat using UAV-based hyperspectral images. Sensors 24;20(4):1231. [CrossRef]
- Tillett, B.J., Hale, C.O., Martin, J.M., Giroux M.J. (2022). Genes impacting grain weight and number in wheat (Triticum aestivum L. ssp. aestivum). Plants 4,11(13):1772. [CrossRef]
- Ukozehasi, C., Ober, E.S., Griffiths, H. (2022). The other mechanisms by which the Rht genes improve the harvest index of wheat. Plants 11, 2837. [CrossRef]
- Voronov, S., Pleskachiov, Y., Shitikova, A., Zargar, M., Abdelkader, M. (2023). Diversity of the Biological and Proteinogenic Characteristics of Quinoa Genotypes as a Multi-Purpose Crop. Agronomy. 13: 279. [CrossRef]
- Xie, Q., Sparkes, D.L. (2021). Dissecting the trade-off of grain number and size in wheat. Planta. 254:3. 254, 3. [CrossRef]
- Yan, X., Zhao, L., Ren, Y., Dong, Z., Cui, D., Chen, F. (2019). Genome-wide association study revealed that the TaGW8 gene was associated with kernel size in Chinese bread wheat. Sci. Rep.9:2702. [CrossRef]
- Yadav, A.K., Carroll, A.J., Estavillo, G.M., Rebetzke, G.J., Pogson, B.J. (2019). Wheat drought tolerance in the field is predicted by amino acid responses to glasshouse-imposed drought. J Exp. Bot. 70(18):4931-4948. [CrossRef]
- Yapiyev, V., Gilman C., Kabdullayeva, T., Suleimenova, A., Shagadatova, A., Duisembay, A., Naizabekov, S., Mussurova, S., Sydykova, K., Raimkulov, I., Kabimoldayev, I., Abdrakhmanova, A., Omarkulova, S., Nurmukhambetov, D., Kudarova, A., Malgazhdar, D., Schönbach, C., Inglezakis, V. (2018) Top soil physical and chemical properties in Kazakhstan across a north-south gradient. Sci. Data. 5, 180242. [CrossRef]
- Zargar, M., Zavarykina, T., Voronov, S., Pronina, I., Bayat, M. (2022). The Recent Development in Technologies for Attaining Doubled Haploid Plants In Vivo. Agriculture. 12: 1595. [CrossRef]
- Zargar, M.; Dyussibayeva, E.; Orazov, A.; Zeinullina, A.; Zhirnova, I.; Yessenbekova, G.; Rysbekova, A. Microsatellite-Based Genetic Diversity Analysis and Population Structure of Proso Millet (Panicum miliaceum L.) in Kazakhstan. Agronomy. 13: 2514. [CrossRef]
- Zargar, M., Romanova, E., Trifonova, A., Shmelkova, E. and Kezimana, P. (2017). AFLP analysis of genetic diversity in soybean [Glycine max (L.) Merr.] cultivars Russian and foreign selection. Agronomy Research. 15 : 2217-2225. [CrossRef]
- Zargar, M., Pakina, E. (2014). Reduced rates of herbicide combined with biological components suppressing weeds in wheat fields of Moscow, Russia. Res on Crops. 15 :332-338. [CrossRef]
- Zhang, W., Li, H., Zhi, L., Su, Q., Liu, J., Ren, X., Meng, D., Zhang, N., Ji, J., Zhang, X., Li, J. (2020). Functional markers developed from TaGS3, a negative regulator of grain weight and size, for marker-assisted selection in wheat. Crop J., 8(6):943–952. [CrossRef]
- Zhang, X., Chen, J., Shi, C., Chen, J., Zheng, F., Tian, J. (2013). Function of TaGW2-6A and its effect on grain weight in wheat (Triticum aestivum L.). Euphytica 192:347–357. [CrossRef]
- Zhang, Y., Liu, J., Xia, X., He, Z. (2014) TaGS-D1, an ortholog of rice OsGS3, is associated with grain weight and grain length in common wheat. Molecular Breeding, 34(3):1097–1107. [CrossRef]
- Zhenxian, G., Wang, Y., Guoying, T., Zhao, Y., Li, C., Cao, Q., Han, R., Zhanliang, S., He, M. (2020). Plant height and its relationship with yield in wheat under different irrigation regime. Irrig. 649 Sci. 38(4):365–371. [CrossRef]
- Zhou, Y., He, Z.H., Sui, X.X., Xia, X.C., Zhang, X.K., Zhang, G.S. (2007). Genetic improvement of grain yield and associated traits in the northern China winter wheat region from 1960 to 2000. Crop Sci. (2007) 47:245–53. [CrossRef]
- Zotova L.P., Dzhataev S.А. (2019). Assessment of collective samples of spring soft wheat for drought ability in the conditions of North Kazakhstan. Bulletin of Science of the Kazakhstan Agrotechnical University named after S. Seifullin (interdisciplinary). – No1 (100). - pp.35-46. file:///C:/Users/User/Downloads/1%20(3).pdf.
- Zotova L.P., Kipshakbayeva G.A., Tleulina Z.T. (2019). Vegetatsionnyy period i urozhaynost' yarovoy myagkoy pshenitsy v usloviyakh Akmolinskoy oblasti Severnogo Kazakhstana. Proceedings of the International scientific-practical conference «Achievements and prospects of agriculture and plant growing development», dedicated to the 85 th anniversary of the Kazakhstan scientific research institute of Agriculture and Plant growing. Almalybak. – pp. 192-197. https://kazniizr.kz/wpcontent/uploads/2019/08/sbornik-konferentsii-85.pdf.
- Zotova L.P., Kipshakbayeva G.A., Dzhataev S.А. (2020). Comparative assessment of mid-late- maturing varieties of spring soft wheat in Northern Kazakhstan. 3i: intellect, idea, innovation - KRU them. A. Baitursynov, Kostanay. - No. 4. - pp. 29-36. http://rmebrk.kz/magazine/3291#.
- Zotova L, Kurishbayev A, Jatayev S, Khassanova G, Zhubatkanov A, Serikbay D, Sereda S, Sereda T, Shvidchenko V, Lopato S, Jenkins C, Soole K, Langridge P and Shavrukov Y (2018) Genes Encoding Transcription Factors TaDREB5 and TaNFYC-A7 Are Differentially Expressed in Leaves of Bread Wheat in Response to Drought, Dehydration and ABA. Front. Plant Sci. 9:1441. [CrossRef]
- Zotova, L., Kurishbayev, A., Jatayev, S., Goncharov, N.P., Shamambayeva, N., Kashapov, A., et al. (2019). The General Transcription Repressor TaDr1 Is Co-expressed With TaVrn1 and TaFT1 in Bread Wheat Under Drought. Front. Genet. 10:63. [CrossRef]
- Zotova, L.P., Sereda S.G., Dzhataev, S.A. (2017). Productivity of spring soft wheat sample in climatic conditions of Сentral Kazakhstan. Materials of Republican Scientific and Theoretical Conference "Seifullin Readings - 13: keeping traditions, creating the future”, dedicated to the 60th anniversary of the Kazakhstan Agrotechnical University named after S. Seifullin. - T.I, Part 1. - pp. 96-99. https://kazatu.edu.kz/assets/i/science/sf_13_agro_132.pdf. 13.
- Zotova, L., Sereda, T., Gadzhimuradova, A., Liang, Chen, Zhirnova, I., Ilyasova, D. (2023). Early inheritance of the short-stem and coleoptile length trait by interport hybrids of spring soft wheat in the conditions of Central and Northern Kazakhstan. Bulletin of Science of S.Seifullin KATRU. p.21- 33. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).