Submitted:
03 October 2023
Posted:
04 October 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Epidemiology of Persistent OD Induced by COVID-19
3. Role of Host Factors in OD
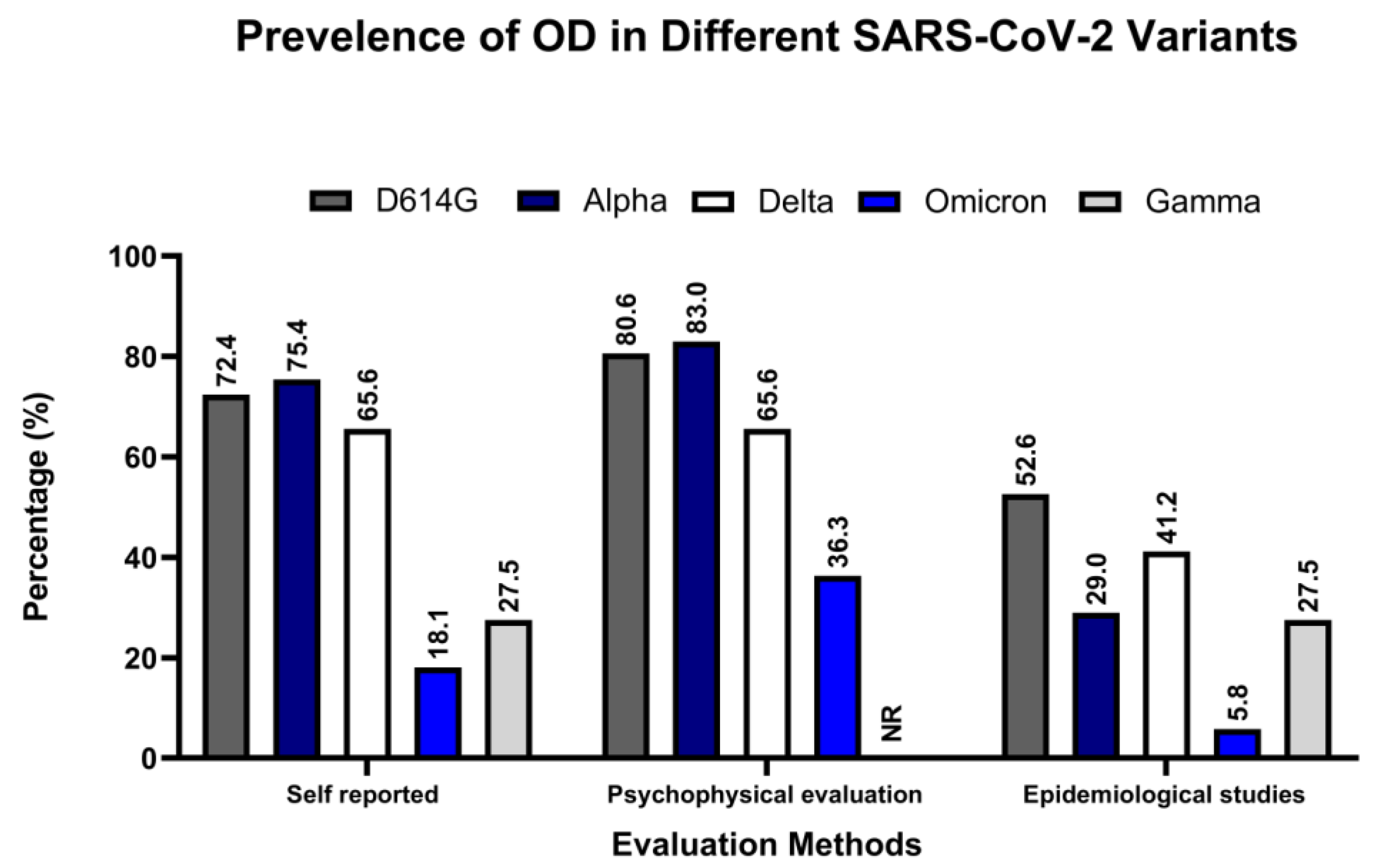
4. Association of Different Variants of SARS-CoV-2 with OD
5. Risk Factors of OD
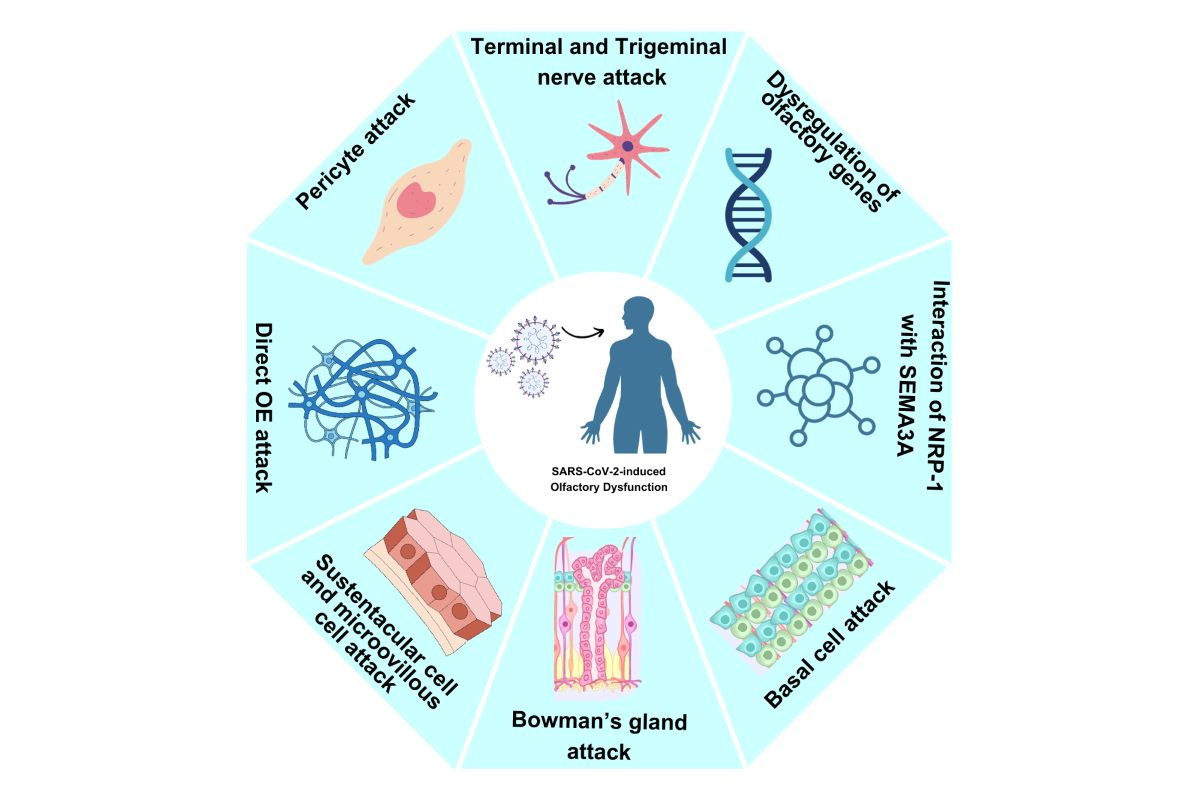
6. Pathophysiological Mechanisms of Actions of OD
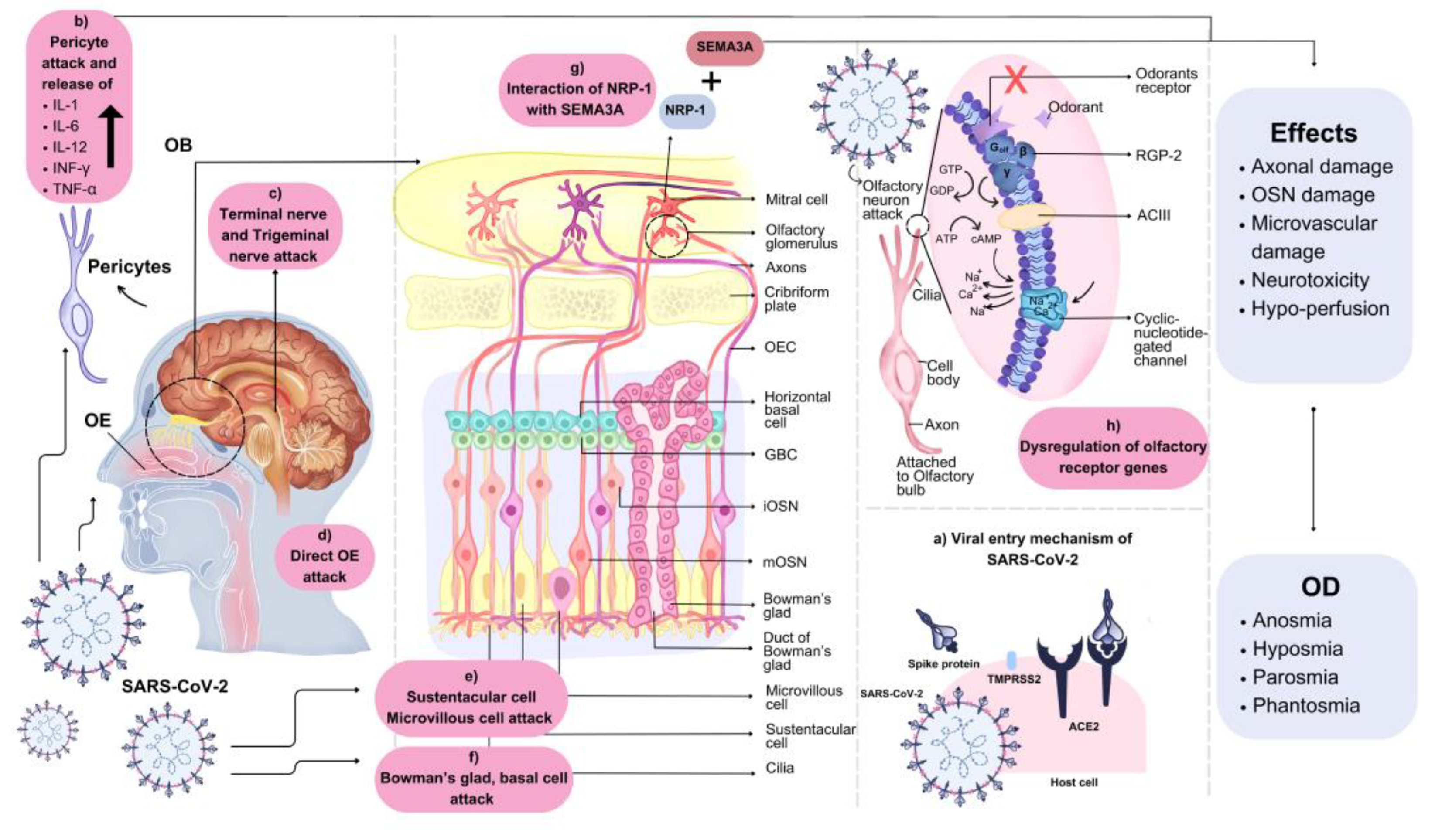
6.1. Central Nervous System (CNS) Entrance
6.2. OB and Sustentacular Cell Damage
6.3. Neural Routes Inflammation
6.4. Non-Neuronal Cells Damage
6.5. Decreased OB Volume
6.6. Deregulation of Olfactory Receptor Genes
6.7. Drug-Induced OD
7. Current Therapeutic Options
8. Limitations and Knowledge Gaps
9. Future Perspectives and Conclusions
Authors Contribution
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability
Conflicts of Interest
References
- Pan, A., et al., Association of Public Health Interventions With the Epidemiology of the COVID-19 Outbreak in Wuhan, China. JAMA, 2020. 323(19): p. 1915-1923. [CrossRef]
- Kim, H., Outbreak of novel coronavirus (COVID-19): What is the role of radiologists? European Radiology, 2020. 30(6): p. 3266-3267. [CrossRef]
- Brito, D.T.M., et al., The possible benefits of vitamin D in COVID-19. Nutrition, 2021. 91-92: p. 111356. [CrossRef]
- Parker, A.M., et al., Addressing the post-acute sequelae of SARS-CoV-2 infection: a multidisciplinary model of care. The Lancet Respiratory Medicine, 2021. 9(11): p. 1328-1341. [CrossRef]
- Huang, C., et al., 6-month consequences of COVID-19 in patients discharged from hospital: a cohort study. Lancet, 2021. 397(10270): p. 220-232. [CrossRef]
- Fernández-de-las-Peñas, C., et al., Defining Post-COVID Symptoms (Post-Acute COVID, Long COVID, Persistent Post-COVID): An Integrative Classification. International Journal of Environmental Research and Public Health, 2021. 18(5): p. 2621.
- Tan, B.K.J., et al., Prognosis and persistence of smell and taste dysfunction in patients with covid-19: meta-analysis with parametric cure modelling of recovery curves. Bmj, 2022. 378: p. e069503. [CrossRef]
- Patel, Z.M., et al., International consensus statement on allergy and rhinology: Olfaction. International Forum of Allergy & Rhinology, 2022. 12(4): p. 327-680. [CrossRef]
- Said, M., et al., Clinical factors associated with lower health scores in COVID-19-related persistent olfactory dysfunction. Int Forum Allergy Rhinol, 2022. 12(10): p. 1242-1253. [CrossRef]
- Burges Watson, D.L., et al., Altered smell and taste: Anosmia, parosmia and the impact of long Covid-19. PLoS One, 2021. 16(9): p. e0256998. [CrossRef]
- Koyama, S., E. Mori, and R. Ueha, Insight into the mechanisms of olfactory dysfunction by COVID-19. Auris Nasus Larynx, 2022. p. [CrossRef]
- Koyama, S., R. Ueha, and K. Kondo, Loss of Smell and Taste in Patients With Suspected COVID-19: Analyses of Patients' Reports on Social Media. J Med Internet Res, 2021. 23(4): p. e26459. [CrossRef]
- Parma, V., et al., More Than Smell-COVID-19 Is Associated With Severe Impairment of Smell, Taste, and Chemesthesis. Chem Senses, 2020. 45(7): p. 609-622. [CrossRef]
- Koyama, S., et al., Possible Use of Phytochemicals for Recovery from COVID-19-Induced Anosmia and Ageusia. Int J Mol Sci, 2021. 22(16): p. [CrossRef]
- Lechien, J.R., et al., Olfactory and gustatory dysfunctions as a clinical presentation of mild-to-moderate forms of the coronavirus disease (COVID-19): a multicenter European study. European Archives of Oto-Rhino-Laryngology, 2020. 277(8): p. 2251-2261. [CrossRef]
- Parma, V., et al., More Than Smell—COVID-19 Is Associated With Severe Impairment of Smell, Taste, and Chemesthesis. Chemical Senses, 2020. 45(7): p. 609-622. [CrossRef]
- Bagheri, S., et al., Coincidence of COVID-19 epidemic and olfactory dysfunction outbreak. Med J Islam Repub. Iran, 2020.
- Hopkins, C., P. Surda, and N. Kumar, Presentation of new onset anosmia during the COVID-19 pandemic. Rhinology, 2020. 58(3): p. 295-298. [CrossRef]
- Gane, S.B., C. Kelly, and C. Hopkins, Isolated sudden onset anosmia in COVID-19 infection. A novel syndrome? Rhinology, 2020. 58(3): p. 299-301. [CrossRef]
- Lechien, J.R., et al., Objective olfactory testing in patients presenting with sudden onset olfactory dysfunction as the first manifestation of confirmed COVID-19 infection. medRxiv, 2020. p. 2020.04.15.20066472. [CrossRef]
- Menni, C., et al., Loss of smell and taste in combination with other symptoms is a strong predictor of COVID-19 infection. MedRxiv, 2020. p. 2020.04. 05.20048421.
- Mao, L., et al., Neurologic Manifestations of Hospitalized Patients With Coronavirus Disease 2019 in Wuhan, China. JAMA Neurol, 2020. 77(6): p. 683-690. [CrossRef]
- Lechien, J.R., et al., Olfactory and gustatory dysfunctions as a clinical presentation of mild-to-moderate forms of the coronavirus disease (COVID-19): a multicenter European study. Eur Arch Otorhinolaryngol, 2020. 277(8): p. 2251-2261. [CrossRef]
- Haldrup, M., M.I. Johansen, and A.W. Fjaeldstad, Lugte-og smagstab som primære symptom på COVID-19. Ugeskr Læger, 2020. 182: p. V04200205.
- Hannum, M.E., et al., Objective Sensory Testing Methods Reveal a Higher Prevalence of Olfactory Loss in COVID-19–Positive Patients Compared to Subjective Methods: A Systematic Review and Meta-Analysis. Chemical Senses, 2020. 45(9): p. 865-874. [CrossRef]
- Chiesa-Estomba, C.M., et al., Patterns of smell recovery in 751 patients affected by the COVID-19 outbreak. Eur J Neurol, 2020. 27(11): p. 2318-2321. [CrossRef]
- Gerkin, R.C., et al., Recent Smell Loss Is the Best Predictor of COVID-19 Among Individuals With Recent Respiratory Symptoms. Chem Senses, 2021. 46: p. [CrossRef]
- Blomberg, B., et al., Long COVID in a prospective cohort of home-isolated patients. Nature Medicine, 2021. 27(9): p. 1607-1613. [CrossRef]
- Klein, H., et al., Onset, duration and unresolved symptoms, including smell and taste changes, in mild COVID-19 infection: a cohort study in Israeli patients. Clin Microbiol Infect, 2021. 27(5): p. 769-74. [CrossRef]
- von Bartheld, C.S., M.M. Hagen, and R. Butowt, Prevalence of Chemosensory Dysfunction in COVID-19 Patients: A Systematic Review and Meta-analysis Reveals Significant Ethnic Differences. ACS Chem Neurosci, 2020. 11(19): p. 2944-2961. [CrossRef]
- Moein, S.T., et al., Smell dysfunction: a biomarker for COVID-19. Int Forum Allergy Rhinol, 2020. 10(8): p. 944-950. [CrossRef]
- Butowt, R., K. Bilinska, and C.S. von Bartheld, Olfactory dysfunction in COVID-19: new insights into the underlying mechanisms. Trends Neurosci, 2023. 46(1): p. 75-90. [CrossRef]
- Chee, J., et al., Pathophysiology of SARS-CoV-2 Infection of Nasal Respiratory and Olfactory Epithelia and Its Clinical Impact. Curr Allergy Asthma Rep, 2023. 23(2): p. 121-131. [CrossRef]
- Karimian, A., M. Behjati, and M. Karimian, Molecular mechanisms involved in anosmia induced by SARS-CoV-2, with a focus on the transmembrane serine protease TMPRSS2. Arch Virol, 2022. 167(10): p. 1931-1946. [CrossRef]
- Beltrán-Corbellini, Á., et al., Acute-onset smell and taste disorders in the context of COVID-19: a pilot multicentre polymerase chain reaction based case-control study. Eur J Neurol, 2020. 27(9): p. 1738-1741. [CrossRef]
- Tong, J.Y., et al., The Prevalence of Olfactory and Gustatory Dysfunction in COVID-19 Patients: A Systematic Review and Meta-analysis. Otolaryngol Head Neck Surg, 2020. 163(1): p. 3-11. [CrossRef]
- Ibekwe, T.S., A.J. Fasunla, and A.E. Orimadegun, Systematic Review and Meta-analysis of Smell and Taste Disorders in COVID-19. OTO Open, 2020. 4(3): p. 2473974x20957975. [CrossRef]
- Liang, Y., et al., Neurosensory dysfunction: A diagnostic marker of early COVID-19. Int J Infect Dis, 2020. 98: p. 347-352. [CrossRef]
- Butowt, R., K. Bilińska, and C. von Bartheld, Why Does the Omicron Variant Largely Spare Olfactory Function? Implications for the Pathogenesis of Anosmia in Coronavirus Disease 2019. J Infect Dis, 2022. 226(8): p. 1304-1308. [CrossRef]
- Klimek, L., et al., Olfactory dysfunction is more severe in wild-type SARS-CoV-2 infection than in the Delta variant (B.1.617.2). World Allergy Organ J, 2022. 15(6): p. 100653. [CrossRef]
- Vaira, L.A., et al., Olfactory and gustatory function impairment in COVID-19 patients: Italian objective multicenter-study. Head Neck, 2020. 42(7): p. 1560-1569. [CrossRef]
- Andrews, P.J., et al., Olfactory and taste dysfunction among mild-to-moderate symptomatic COVID-19 positive health care workers: An international survey. Laryngoscope Investig Otolaryngol, 2020. 5(6): p. 1019-1028. [CrossRef]
- Lechien, J.R., et al., Prevalence and 6-month recovery of olfactory dysfunction: a multicentre study of 1363 COVID-19 patients. J Intern Med, 2021. 290(2): p. 451-461. [CrossRef]
- Speth, M.M., et al., Olfactory Dysfunction and Sinonasal Symptomatology in COVID-19: Prevalence, Severity, Timing, and Associated Characteristics. Otolaryngol Head Neck Surg, 2020. 163(1): p. 114-120. [CrossRef]
- Whitcroft, K.L. and T. Hummel, Olfactory Dysfunction in COVID-19: Diagnosis and Management. Jama, 2020. 323(24): p. 2512-2514. [CrossRef]
- Hintschich, C.A., et al., Persisting olfactory dysfunction in post-COVID-19 is associated with gustatory impairment: Results from chemosensitive testing eight months after the acute infection. PLoS One, 2022. 17(3): p. e0265686. [CrossRef]
- Bussière, N., et al., Chemosensory Dysfunctions Induced by COVID-19 Can Persist up to 7 Months: A Study of Over 700 Healthcare Workers. Chem Senses, 2021. 46: p. [CrossRef]
- Petrocelli, M., et al., Six-month smell and taste recovery rates in coronavirus disease 2019 patients: a prospective psychophysical study. J Laryngol Otol, 2021. 135(5): p. 436-441. [CrossRef]
- Tan, B.K.J., et al., Prognosis and persistence of smell and taste dysfunction in patients with covid-19: meta-analysis with parametric cure modelling of recovery curves. BMJ, 2022. 378: p. e069503. [CrossRef]
- Oliveira-Pinto, A.V., et al., Sexual Dimorphism in the Human Olfactory Bulb: Females Have More Neurons and Glial Cells than Males. PLOS ONE, 2014. 9(11): p. e111733. [CrossRef]
- Brand, G. and J.L. Millot, Sex differences in human olfaction: between evidence and enigma. Q J Exp Psychol B, 2001. 54(3): p. 259-70. [CrossRef]
- Sorokowski, P., et al., Sex Differences in Human Olfaction: A Meta-Analysis. Front Psychol, 2019. 10: p. 242. [CrossRef]
- Oleszkiewicz, A., et al., Updated Sniffin' Sticks normative data based on an extended sample of 9139 subjects. Eur Arch Otorhinolaryngol, 2019. 276(3): p. 719-728. [CrossRef]
- Larsson, M., M. Lövdén, and L.-G. Nilsson, Sex differences in recollective experience for olfactory and verbal information. Acta Psychologica, 2003. 112(1): p. 89-103. [CrossRef]
- Doty, R.L. and E.L. Cameron, Sex differences and reproductive hormone influences on human odor perception. Physiol Behav, 2009. 97(2): p. 213-28. [CrossRef]
- Schriever, V.A., et al., Size of nostril opening as a measure of intranasal volume. Physiol Behav, 2013. 110-111: p. 3-5. [CrossRef]
- Verbeurgt, C., et al., Profiling of olfactory receptor gene expression in whole human olfactory mucosa. PLoS One, 2014. 9(5): p. e96333. [CrossRef]
- Pereira, N.L., et al., COVID-19: Understanding Inter-Individual Variability and Implications for Precision Medicine. Mayo Clin Proc, 2021. 96(2): p. 446-463. [CrossRef]
- Kim, J.W., et al., Regional and Chronological Variation of Chemosensory Dysfunction in COVID-19: a Meta-Analysis. J Korean Med Sci, 2021. 36(4): p. e40. [CrossRef]
- Kemmelmeier, M. and W.A. Jami, Mask Wearing as Cultural Behavior: An Investigation Across 45 U.S. States During the COVID-19 Pandemic. Front Psychol, 2021. 12: p. 648692. [CrossRef]
- Hintschich, C.A., et al., Prevalence of acute olfactory dysfunction differs between variants of SARS-CoV-2—results from chemosensitive testing in wild type, VOC alpha (B.1.1.7) and VOC delta (B.1617.2). European Archives of Oto-Rhino-Laryngology, 2022. 279(11): p. 5445-5447. [CrossRef]
- Hintschich, C.A., et al., Prevalence of acute olfactory dysfunction differs between variants of SARS-CoV-2-results from chemosensitive testing in wild type, VOC alpha (B.1.1.7) and VOC delta (B.1617.2). Eur Arch Otorhinolaryngol, 2022. 279(11): p. 5445-5447. [CrossRef]
- Vaira, L.A., et al., Prevalence of olfactory dysfunction in D614G, alpha, delta and omicron waves: a psychophysical case-control study. Rhinology journal, 2022. 0(0): p. 0-0. [CrossRef]
- Cardoso, C.C., et al., Olfactory Dysfunction in Patients With Mild COVID-19 During Gamma, Delta, and Omicron Waves in Rio de Janeiro, Brazil. Jama, 2022. 328(6): p. 582-583. [CrossRef]
- Menni, C., et al., Symptom prevalence, duration, and risk of hospital admission in individuals infected with SARS-CoV-2 during periods of omicron and delta variant dominance: a prospective observational study from the ZOE COVID Study. Lancet, 2022. 399(10335): p. 1618-1624. [CrossRef]
- Laracy, J.C., et al., Comparison of coronavirus disease 2019 (COVID-19) symptoms at diagnosis among healthcare personnel before and after the emergence of the omicron variant. Infect Control Hosp Epidemiol, 2022. p. 1-3. [CrossRef]
- Nalbandian, A., et al., Post-acute COVID-19 syndrome. Nat Med, 2021. 27(4): p. 601-615. [CrossRef]
- Groff, D., et al., Short-term and Long-term Rates of Postacute Sequelae of SARS-CoV-2 Infection: A Systematic Review. JAMA Netw Open, 2021. 4(10): p. e2128568. [CrossRef]
- Reis, D., et al., Long-term prevalence of taste and olfactory dysfunction in COVID-19 patients: A cross-sectional study. Oral Dis, 2022. 28 Suppl 2: p. 2516-2521. [CrossRef]
- Cristillo, V., et al., Age and subtle cognitive impairment are associated with long-term olfactory dysfunction after COVID-19 infection. J Am Geriatr Soc, 2021. 69(10): p. 2778-2780. [CrossRef]
- Wang, J., et al., Prevalence of taste and smell dysfunction in mild and asymptomatic COVID-19 patients during Omicron prevalent period in Shanghai, China: a cross-sectional survey study. BMJ Open, 2023. 13(3): p. e067065. [CrossRef]
- Johnson, B.J., et al., Patient factors associated with COVID-19 loss of taste or smell patient factors in smell/taste loss COVID-19. Laryngoscope Investig Otolaryngol, 2022. 7(6): p. 1688-1694. [CrossRef]
- Shiue, I., Adult taste and smell disorders after heart, neurological, respiratory and liver problems: US NHANES, 2011-2012. Int J Cardiol, 2015. 179: p. 46-8. [CrossRef]
- Healey, Q., et al., Symptoms and signs of long COVID: A rapid review and meta-analysis. J Glob Health, 2022. 12: p. 05014. [CrossRef]
- Güney, B., et al., Changes in olfactory bulbus volume and olfactory sulcus depth in the chronic period after COVID-19 infection. Acta Oto-Laryngologica, 2021. 141(8): p. 786-790. [CrossRef]
- Tan, C.J.-W., et al., Neuroradiological Basis of COVID-19 Olfactory Dysfunction: A Systematic Review and Meta-Analysis. The Laryngoscope, 2022. 132(6): p. 1260-1274. [CrossRef]
- Hoang, M.P., et al., Self-reported olfactory and gustatory dysfunction and psychophysical testing in screening for COVID-19: A systematic review and meta-analysis. International Forum of Allergy & Rhinology, 2022. 12(5): p. 744-756. [CrossRef]
- Pires, Í.d.A.T., et al., Intensive Olfactory Training in Post-COVID-19 Patients: A Multicenter Randomized Clinical Trial. American Journal of Rhinology & Allergy, 2022. 36(6): p. 780-787. [CrossRef]
- de Melo, E.G.M., et al., Association between chemosensory dysfunctions and inflammatory biomarkers in patients with SARS-CoV-2 infection: a systematic review and meta-analysis. Inflammopharmacology, 2022. 30(6): p. 2079-2087. [CrossRef]
- Rashid, R.A., A. Zgair, and R.M. Al-Ani, Effect of nasal corticosteroid in the treatment of anosmia due to COVID-19: A randomised double-blind placebo-controlled study. American Journal of Otolaryngology, 2021. 42(5): p. 103033. [CrossRef]
- García-Meléndez, D.D., et al., Persistent olfactory dysfunction in mild COVID-19 patients: A descriptive study of the characteristics and association with other symptoms. Medicina Clínica, 2022. p. [CrossRef]
- Pendolino, A.L., et al., A multicenter real-life study to determine the efficacy of corticosteroids and olfactory training in improving persistent COVID-19-related olfactory dysfunction. Laryngoscope Investigative Otolaryngology, 2023. 8(1): p. 46-54. [CrossRef]
- Speth, M.M., et al., Olfactory Dysfunction and Sinonasal Symptomatology in COVID-19: Prevalence, Severity, Timing, and Associated Characteristics. Otolaryngology–Head and Neck Surgery, 2020. 163(1): p. 114-120. [CrossRef]
- Kim, J.-W., et al., Regional and Chronological Variation of Chemosensory Dysfunction in COVID-19: a Meta-Analysis. J Korean Med Sci, 2021. 36(4): p.
- Tong, J.Y., et al., The Prevalence of Olfactory and Gustatory Dysfunction in COVID-19 Patients: A Systematic Review and Meta-analysis. Otolaryngology–Head and Neck Surgery, 2020. 163(1): p. 3-11. [CrossRef]
- Hajikhani, B., et al., Olfactory and gustatory dysfunction in COVID-19 patients: A meta-analysis study. Physiol Rep, 2020. 8(18): p. e14578. [CrossRef]
- Hoffmann, M., et al., SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell, 2020. 181(2): p. 271-280.e8. [CrossRef]
- Lechien, J.R., et al., ACE2 & TMPRSS2 Expressions in Head & Neck Tissues: A Systematic Review. Head Neck Pathol, 2021. 15(1): p. 225-235. [CrossRef]
- Sungnak, W., et al., SARS-CoV-2 entry factors are highly expressed in nasal epithelial cells together with innate immune genes. Nat Med, 2020. 26(5): p. 681-687. [CrossRef]
- Glass, W.G., et al., Mechanisms of host defense following severe acute respiratory syndrome-coronavirus (SARS-CoV) pulmonary infection of mice. J Immunol, 2004. 173(6): p. 4030-9. [CrossRef]
- Li, K., et al., Middle East Respiratory Syndrome Coronavirus Causes Multiple Organ Damage and Lethal Disease in Mice Transgenic for Human Dipeptidyl Peptidase 4. J Infect Dis, 2016. 213(5): p. 712-22. [CrossRef]
- Talbot, P.J., et al., Neurotropism of human coronavirus 229E. Adv Exp Med Biol, 1993. 342: p. 339-46. [CrossRef]
- Cecchini, M.P., et al., Persistent chemosensory dysfunction in a young patient with mild COVID-19 with partial recovery 15 months after the onset. Neurol Sci, 2022. 43(1): p. 99-104. [CrossRef]
- Li, Y.C., W.Z. Bai, and T. Hashikawa, The neuroinvasive potential of SARS-CoV2 may play a role in the respiratory failure of COVID-19 patients. J Med Virol, 2020. 92(6): p. 552-555. [CrossRef]
- Lima, M., et al., Unraveling the Possible Routes of SARS-COV-2 Invasion into the Central Nervous System. Curr Treat Options Neurol, 2020. 22(11): p. 37. [CrossRef]
- Dubé, M., et al., Axonal Transport Enables Neuron-to-Neuron Propagation of Human Coronavirus OC43. J Virol, 2018. 92(17): p. [CrossRef]
- Cantuti-Castelvetri, L., et al., Neuropilin-1 facilitates SARS-CoV-2 cell entry and infectivity. Science, 2020. 370(6518): p. 856-860. [CrossRef]
- Bilinska, K., et al., Expression of the SARS-CoV-2 Entry Proteins, ACE2 and TMPRSS2, in Cells of the Olfactory Epithelium: Identification of Cell Types and Trends with Age. ACS Chem Neurosci, 2020. 11(11): p. 1555-1562. [CrossRef]
- Chen, M., et al., Elevated ACE-2 expression in the olfactory neuroepithelium: implications for anosmia and upper respiratory SARS-CoV-2 entry and replication. Eur Respir J, 2020. 56(3): p. [CrossRef]
- Zhou, Z., et al., Understanding the neurotropic characteristics of SARS-CoV-2: from neurological manifestations of COVID-19 to potential neurotropic mechanisms. J Neurol, 2020. 267(8): p. 2179-2184. [CrossRef]
- Wang, L.H. and S.M. Strittmatter, A family of rat CRMP genes is differentially expressed in the nervous system. J Neurosci, 1996. 16(19): p. 6197-207. [CrossRef]
- Reza, J.N., I. Gavazzi, and J. Cohen, Neuropilin-1 is expressed on adult mammalian dorsal root ganglion neurons and mediates semaphorin3a/collapsin-1-induced growth cone collapse by small diameter sensory afferents. Mol Cell Neurosci, 1999. 14(4-5): p. 317-26. [CrossRef]
- Kirschenbaum, D., et al., Inflammatory olfactory neuropathy in two patients with COVID-19. Lancet, 2020. 396(10245): p. 166. [CrossRef]
- Toor, S.M., et al., T-cell responses and therapies against SARS-CoV-2 infection. Immunology, 2021. 162(1): p. 30-43. [CrossRef]
- Cazzolla, A.P., et al., Taste and Smell Disorders in COVID-19 Patients: Role of Interleukin-6. ACS Chemical Neuroscience, 2020. 11(17): p. 2774-2781. [CrossRef]
- Mastrangelo, A., M. Bonato, and P. Cinque, Smell and taste disorders in COVID-19: From pathogenesis to clinical features and outcomes. Neuroscience Letters, 2021. 748: p. 135694. [CrossRef]
- Cooper, K.W., et al., COVID-19 and the Chemical Senses: Supporting Players Take Center Stage. Neuron, 2020. 107(2): p. 219-233. [CrossRef]
- Wei, G., et al., Olfactory Dysfunction in Patients With Coronavirus Disease 2019: A Review. Frontiers in Neurology, 2022. 12: p. [CrossRef]
- Xydakis, M.S., et al., Post-viral effects of COVID-19 in the olfactory system and their implications. The Lancet Neurology, 2021. 20(9): p. 753-761. [CrossRef]
- Khan, S. and J. Gomes, Neuropathogenesis of SARS-CoV-2 infection. Elife, 2020. 9: p. [CrossRef]
- Kishimoto-Urata, M., et al., Prolonged and extended impacts of SARS-CoV-2 on the olfactory neurocircuit. Sci Rep, 2022. 12(1): p. 5728. [CrossRef]
- Käufer, C., et al., Microgliosis and neuronal proteinopathy in brain persist beyond viral clearance in SARS-CoV-2 hamster model. eBioMedicine, 2022. 79: p. [CrossRef]
- Khan, M., et al., Visualizing in deceased COVID-19 patients how SARS-CoV-2 attacks the respiratory and olfactory mucosae but spares the olfactory bulb. Cell, 2021. 184(24): p. 5932-5949.e15. [CrossRef]
- Brann, D.H., et al., Non-neuronal expression of SARS-CoV-2 entry genes in the olfactory system suggests mechanisms underlying COVID-19-associated anosmia. Science Advances, 2020. 6(31): p. eabc5801. [CrossRef]
- Chen, M., et al., Evolution of nasal and olfactory infection characteristics of SARS-CoV-2 variants. bioRxiv, 2022. p. 2022.04.12.487379. [CrossRef]
- Hou, Y.J., et al., SARS-CoV-2 Reverse Genetics Reveals a Variable Infection Gradient in the Respiratory Tract. Cell, 2020. 182(2): p. 429-446.e14. [CrossRef]
- Zou, L., et al., SARS-CoV-2 Viral Load in Upper Respiratory Specimens of Infected Patients. New England Journal of Medicine, 2020. 382(12): p. 1177-1179. [CrossRef]
- Liao, B., et al., Long-term Consequences of COVID-19: Chemosensory Disorders. Curr Allergy Asthma Rep, 2023. 23(2): p. 111-119. [CrossRef]
- Kay, L.M., COVID-19 and olfactory dysfunction: a looming wave of dementia? Journal of Neurophysiology, 2022. 128(2): p. 436-444. [CrossRef]
- Glezer, I., et al., Viral infection and smell loss: The case of COVID-19. Journal of Neurochemistry, 2021. 157(4): p. 930-943. [CrossRef]
- Araújo, L., V. Arata, and R.G. Figueiredo, Olfactory Disorders in Post-Acute COVID-19 Syndrome. Sinusitis, 2021. 5(2): p. 116-122.
- de Melo, G.D., et al., COVID-19–related anosmia is associated with viral persistence and inflammation in human olfactory epithelium and brain infection in hamsters. Science Translational Medicine, 2021. 13(596): p. eabf8396. [CrossRef]
- Ylikoski, J., M. Markkanen, and A. Mäkitie, Pathophysiology of the COVID-19 – entry to the CNS through the nose. Acta Oto-Laryngologica, 2020. 140(10): p. 886-889. [CrossRef]
- Park, G.C., et al., ACE2 and TMPRSS2 immunolocalization and oral manifestations of COVID-19. Oral Diseases, 2022. 28(S2): p. 2456-2464. [CrossRef]
- Bilinska, K., C.S. von Bartheld, and R. Butowt, Expression of the ACE2 Virus Entry Protein in the Nervus Terminalis Reveals the Potential for an Alternative Route to Brain Infection in COVID-19. Frontiers in Cellular Neuroscience, 2021. 15: p. [CrossRef]
- Ramakrishnan, S., et al., Acquisition of spontaneous electrical activity during embryonic development of gonadotropin-releasing hormone-3 neurons located in the terminal nerve of transgenic zebrafish (Danio rerio). Gen Comp Endocrinol, 2010. 168(3): p. 401-7. [CrossRef]
- Meinhardt, J., et al., Olfactory transmucosal SARS-CoV-2 invasion as a port of central nervous system entry in individuals with COVID-19. Nature Neuroscience, 2021. 24(2): p. 168-175. [CrossRef]
- Brann, D.H., et al., Non-neuronal expression of SARS-CoV-2 entry genes in the olfactory system suggests mechanisms underlying COVID-19-associated anosmia. Sci Adv, 2020. 6(31): p. [CrossRef]
- Ye, Q., et al., SARS-CoV-2 infection in the mouse olfactory system. Cell Discov, 2021. 7(1): p. 49. [CrossRef]
- Frosolini, A., et al., Magnetic Resonance Imaging Confirmed Olfactory Bulb Reduction in Long COVID-19: Literature Review and Case Series. Brain Sciences, 2022. 12(4): p. 430.
- Güney, B., et al., Changes in olfactory bulbus volume and olfactory sulcus depth in the chronic period after COVID-19 infection. Acta Otolaryngol, 2021. 141(8): p. 786-790. [CrossRef]
- Yildirim, D., et al., A Comparative Olfactory MRI, DTI and fMRI Study of COVID-19 Related Anosmia and Post Viral Olfactory Dysfunction. Acad Radiol, 2022. 29(1): p. 31-41. [CrossRef]
- Sinnarajah, S., et al., RGS2 regulates signal transduction in olfactory neurons by attenuating activation of adenylyl cyclase III. Nature, 2001. 409(6823): p. 1051-1055. [CrossRef]
- Zazhytska, M., et al., Non-cell-autonomous disruption of nuclear architecture as a potential cause of COVID-19-induced anosmia. Cell, 2022. 185(6): p. 1052-1064.e12. [CrossRef]
- Avnat, E., et al., Elevated Expression of RGS2 May Underlie Reduced Olfaction in COVID-19 Patients. Journal of Personalized Medicine, 2022. 12(9): p. 1396.
- Jones, D.T. and R.R. Reed, G<sub>olf</sub>: an Olfactory Neuron Specific-G Protein Involved in Odorant Signal Transduction. Science, 1989. 244(4906): p. 790-795. [CrossRef]
- Ebrahimi, F.A.W. and A. Chess, Olfactory G proteins: Simple and complex signal transduction. Current Biology, 1998. 8(12): p. R431-R433. [CrossRef]
- Tabatabaeizadeh, S.A., Zinc supplementation and COVID-19 mortality: a meta-analysis. Eur J Med Res, 2022. 27(1): p. 70. [CrossRef]
- Debbaneh, P., et al., Drug-induced olfactory and gustatory dysfunction: Analysis of FDA adverse events reporting system. Auris Nasus Larynx, 2023. p. [CrossRef]
- Hummel, T., et al., Position paper on olfactory dysfunction. Rhinol Suppl, 2017. 54(26): p. 1-30. [CrossRef]
- Wang, L., L. Chen, and T. Jacob, Evidence for peripheral plasticity in human odour response. J Physiol, 2004. 554(Pt 1): p. 236-44. [CrossRef]
- Whitcroft, K.L. and T. Hummel, Clinical Diagnosis and Current Management Strategies for Olfactory Dysfunction: A Review. JAMA Otolaryngol Head Neck Surg, 2019. 145(9): p. 846-853. [CrossRef]
- Konstantinidis, I., et al., Use of olfactory training in post-traumatic and postinfectious olfactory dysfunction. Laryngoscope, 2013. 123(12): p. E85-90. [CrossRef]
- Everts, P., et al. Platelet-Rich Plasma: New Performance Understandings and Therapeutic Considerations in 2020. International Journal of Molecular Sciences, 2020. 21,. [CrossRef]
- Farrag, T.Y., et al., Effect of Platelet Rich Plasma and Fibrin Sealant on Facial Nerve Regeneration in a Rat Model. The Laryngoscope, 2007. 117(1): p. 157-165. [CrossRef]
- Yan, C.H., et al., Use of platelet-rich plasma for COVID-19–related olfactory loss: a randomized controlled trial. International Forum of Allergy & Rhinology. n/a(n/a): p. [CrossRef]
- Seo, B.S., et al., Treatment of postviral olfactory loss with glucocorticoids, Ginkgo biloba, and mometasone nasal spray. Arch Otolaryngol Head Neck Surg, 2009. 135(10): p. 1000-4. [CrossRef]
- Schriever, V.A., et al., Treatment of smell loss with systemic methylprednisolone. Rhinology, 2012. 50(3): p. 284-9. [CrossRef]
- Kim, D.H., et al., Prognosis of Olfactory Dysfunction according to Etiology and Timing of Treatment. Otolaryngol Head Neck Surg, 2017. 156(2): p. 371-377. [CrossRef]
- Aiyegbusi, O.L., et al., Symptoms, complications and management of long COVID: a review. J R Soc Med, 2021. 114(9): p. 428-442. [CrossRef]
- Di Stadio, A., et al., Ultramicronized Palmitoylethanolamide and Luteolin Supplement Combined with Olfactory Training to Treat Post-COVID-19 Olfactory Impairment: A Multi-Center Double-Blinded Randomized Placebo- Controlled Clinical Trial. Curr Neuropharmacol, 2022. 20(10): p. 2001-2012. [CrossRef]
- D'Ascanio, L., et al., Randomized clinical trial "olfactory dysfunction after COVID-19: olfactory rehabilitation therapy vs. intervention treatment with Palmitoylethanolamide and Luteolin": preliminary results. Eur Rev Med Pharmacol Sci, 2021. 25(11): p. 4156-4162. [CrossRef]
- De Luca, P., et al., Effect of Ultra-Micronized Palmitoylethanolamide and Luteolin on Olfaction and Memory in Patients with Long COVID: Results of a Longitudinal Study. Cells, 2022. 11(16): p. [CrossRef]
- Kern, R.C., et al., Treatment of olfactory dysfunction, II: studies with minocycline. Laryngoscope, 2004. 114(12): p. 2200-4. [CrossRef]
- Henkin, R.I., I. Velicu, and L. Schmidt, An open-label controlled trial of theophylline for treatment of patients with hyposmia. Am J Med Sci, 2009. 337(6): p. 396-406. [CrossRef]
- Rezaeian, A., Effect of Intranasal Insulin on Olfactory Recovery in Patients with Hyposmia: A Randomized Clinical Trial. Otolaryngol Head Neck Surg, 2018. 158(6): p. 1134-1139. [CrossRef]
- Quint, C., et al., The quinoxaline derivative caroverine in the treatment of sensorineural smell disorders: a proof-of-concept study. Acta Otolaryngol, 2002. 122(8): p. 877-81.
- Whitcroft, K.L., et al., Intranasal sodium citrate solution improves olfaction in post-viral hyposmia. Rhinology, 2016. 54(4): p. 368-374. [CrossRef]
- Hummel, T., S. Heilmann, and K.B. Hüttenbriuk, Lipoic acid in the treatment of smell dysfunction following viral infection of the upper respiratory tract. Laryngoscope, 2002. 112(11): p. 2076-80. [CrossRef]
- Ogawa, T., et al., Recovery over time and prognostic factors in treated patients with post-infectious olfactory dysfunction: a retrospective study. Annals of Otology, Rhinology & Laryngology, 2020. 129(10): p. 977-982.
- Reden, J., et al., Olfactory function in patients with postinfectious and posttraumatic smell disorders before and after treatment with vitamin A: a double-blind, placebo-controlled, randomized clinical trial. The Laryngoscope, 2012. 122(9): p. 1906-1909.
- Bhutani, D.L., et al., Resolution of COVID-19 induced anosmia following treatment with ST266. Otolaryngol Case Rep, 2022. 25: p. 100475. [CrossRef]
- Tsetsos, N., K. Markou, and I. Konstantinidis, Effect of monoclonal antibodies on olfactory dysfunction caused by chronic rhinosinusitis with nasal polyps: a systematic review and meta-analysis. Int Forum Allergy Rhinol, 2020. 10(7): p. 893-900. [CrossRef]
- Wood, D.A., A. Aleem, and D. Davis, Providing Access To Monoclonal Antibody Treatment Of Coronavirus (COVID-19) Patients In Rural And Underserved Areas, in StatPearls. 2023, StatPearls Publishing.
- Khan, R.S., et al., Intranasal delivery of a novel amnion cell secretome prevents neuronal damage and preserves function in a mouse multiple sclerosis model. Scientific Reports, 2017. 7(1): p. 1-12.
- Grinblat, G.A., et al., RGC neuroprotection following optic nerve trauma mediated by intranasal delivery of amnion cell secretome. Investigative Ophthalmology & Visual Science, 2018. 59(6): p. 2470-2477.
- Tsetsos, N., K. Markou, and I. Konstantinidis, Effect of monoclonal antibodies on olfactory dysfunction caused by chronic rhinosinusitis with nasal polyps: a systematic review and meta-analysis. International Forum of Allergy & Rhinology, 2020. 10(7): p. 893-900. [CrossRef]
- Karamali, K., M. Elliott, and C. Hopkins, COVID-19 related olfactory dysfunction. Curr Opin Otolaryngol Head Neck Surg, 2022. 30(1): p. 19-25. [CrossRef]
- Yong, S.J., Long COVID or post-COVID-19 syndrome: putative pathophysiology, risk factors, and treatments. Infect Dis (Lond), 2021. 53(10): p. 737-754. [CrossRef]
- Hopkins, C., L.A. Vaira, and G. De Riu, Self-reported olfactory loss in COVID-19: is it really a favorable prognostic factor? Int Forum Allergy Rhinol, 2020. 10(7): p. 926. [CrossRef]
- Chee, J., et al., Pathophysiology of SARS-CoV-2 Infection of Nasal Respiratory and Olfactory Epithelia and Its Clinical Impact. Current Allergy and Asthma Reports, 2023. 23(2): p. 121-131. [CrossRef]
- Neuland, C., et al., Health-related and specific olfaction-related quality of life in patients with chronic functional anosmia or severe hyposmia. Laryngoscope, 2011. 121(4): p. 867-72. [CrossRef]
- Kohli, P., et al., The Association Between Olfaction and Depression: A Systematic Review. Chem Senses, 2016. 41(6): p. 479-86. [CrossRef]
- Mattes, R.D., et al., Dietary evaluation of patients with smell and/or taste disorders. Am J Clin Nutr, 1990. 51(2): p. 233-40. [CrossRef]
- Aschenbrenner, K., et al., The influence of olfactory loss on dietary behaviors. Laryngoscope, 2008. 118(1): p. 135-44. [CrossRef]
- Attems, J., L. Walker, and K.A. Jellinger, Olfactory bulb involvement in neurodegenerative diseases. Acta Neuropathologica, 2014. 127(4): p. 459-475. [CrossRef]
- Wilson, R.S., et al., Lewy Bodies and Olfactory Dysfunction in Old Age. Chemical Senses, 2011. 36(4): p. 367-373. [CrossRef]
- Yoo, H.S., et al., Olfactory dysfunction in Alzheimer's disease– and Lewy body–related cognitive impairment. Alzheimer's & Dementia, 2018. 14(10): p. 1243-1252. [CrossRef]
- Kay, L.M., COVID-19 and olfactory dysfunction: a looming wave of dementia? J Neurophysiol, 2022. 128(2): p. 436-444. [CrossRef]
- Takeda, A., et al., Olfactory dysfunction and dementia in Parkinson's disease. J Parkinsons Dis, 2014. 4(2): p. 181-7. [CrossRef]
| Ref. | Study design andregion | Number of Participants | Mean participantage (Year) | OD prevalence | Recovery duration | Common Symptoms |
|---|---|---|---|---|---|---|
| [49] | Systematic Review, Meta-Analysis; Europe, America, Asia | 3699 | 30.0–55.8 | 3–11% | 30–180 days | Cough, fatigue, rhinorrhea, sore throat, muscle, and joint pains. |
| [75] | Meta-analysis; Turkey | 41 | 40.27 ± 14.5 | 45% | 10–12 months | NR |
| [74] | Systematic reviews and meta-analysis; Europe, Asia, North America, and Australia. | 10643 | 35–64 | 17% | >12 weeks | Fatigue, dyspnoea, myalgia, cough. |
| [76] | Systematic Review and Meta-Analysis. | 16-91 | 34.3– 45.4 | 63% | 2 months | Nasal obstruction, mucosal congestion. |
| [77] | Cohort, Case-control; North America, Europe, South America, Australia, and Africa, Asia. | 42902 | 28–67 | 43.9% | NR | Headache and rhinorrhea. |
| [78] | A Multicenter Randomized Clinical Trial; Curitiba, Londrina, and Brazil. | 80 | 36.7 ± 10.3 | 82.5% | 1–2 weeks | Headache and nausea. |
| [79] | Systematic reviews and meta-analysis; Turkey, The United Kingdom, Morocco, China, Spain, Italy, and the United States | 3218 | NR | 26.9% | NR | Fever, dry cough, headache, dyspnoea, myalgia or arthralgia, fatigue, diarrhea, and vomiting. |
| [80] | Randomized double-blind placebo-controlled study; Iraq. | 276 | 29 | NR | 1–4 weeks | Nasal obstruction, rhinorrhea, sneezing, and facial pain. |
| [81] | Observational, descriptive, and single-center study. | 86 | 37.2 | 70.9% | 2 weeks | Brain fog. |
| [82] | A multicenter real-life cohort study; London, United Kingdom, Padua, Italy. | 44 | 40.5 | 81.8% | 6–12 months | Nasal Obstruction and rhinorrhoea. |
| [83] | Prospective, cross-sectional; Aarau. | 103 | 46.8 | 61.2% | NR | Nasal obstruction, cough, mucus production, rhinorrhea. |
| [25] | A systematic review and meta-analysis. | 7,178 | NR | 98.3% | >2 weeks | Nasal obstruction, postnasal drip, or runny nose. |
| [84] | Meta-Analysis; Asia, Europe, Middle East East, Latin America, North America and Africa. | 13,527 | 44– 52.7 | 51.4% | NR | NR |
| [85] | Systematic Review and Meta-analysis; North America, Europe, and Asia. |
1627 | 36.9– 61.6 | 52.73% | NR | Nasal congestion, Peripheral nervous system complications, taste impairment. |
| [86] | Meta-analysis; France, Italy, China, Iran, Singapore, USA, Germany. |
1,354 | 34 –65 | 61.3% | NR | Fever, myalgia, chills, dyspnea, sore throat, cough. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





