The central dogma of molecular biology
The discovery of RNA modifications has introduced a new layer of complexity to the ever-changing landscape of biochemistry and molecular biology. Biochemistry and molecular biology involve the study of the structure, function, and interactions between biological macromolecules. Molecular biology is based on the interplay between deoxyribonucleic acid (DNA), ribonucleic acid (RNA), and protein. These macromolecules are essential for life, with DNA serving as an information storage, RNA as an intermediary dealer of DNA’s information and a jack of all trades, and protein as a versatile, functional building block that makes a cell go. This process, where DNA is made into RNA which is made into protein, is the central dogma of Molecular Biology (Crick 1958). Despite exceptions, the central dogma continues to serve as a fundamental framework for comprehending molecular biology (Ille, Lamont and Mathews 2022). This review focuses on the chemical interactions of RNA with RNA-binding proteins, the impacts of RNA modifications on these interactions, and the connection between RNA modifications and the central dogma. Understanding how RNA interacts with protein gives insights into the processes and mechanisms responsible for gene regulation, life, and disease. A greater understanding of RNA biology will lead to new tools to investigate their roles in organisms and new therapies for human and animal diseases.
Overview of RNA and RNA modifications
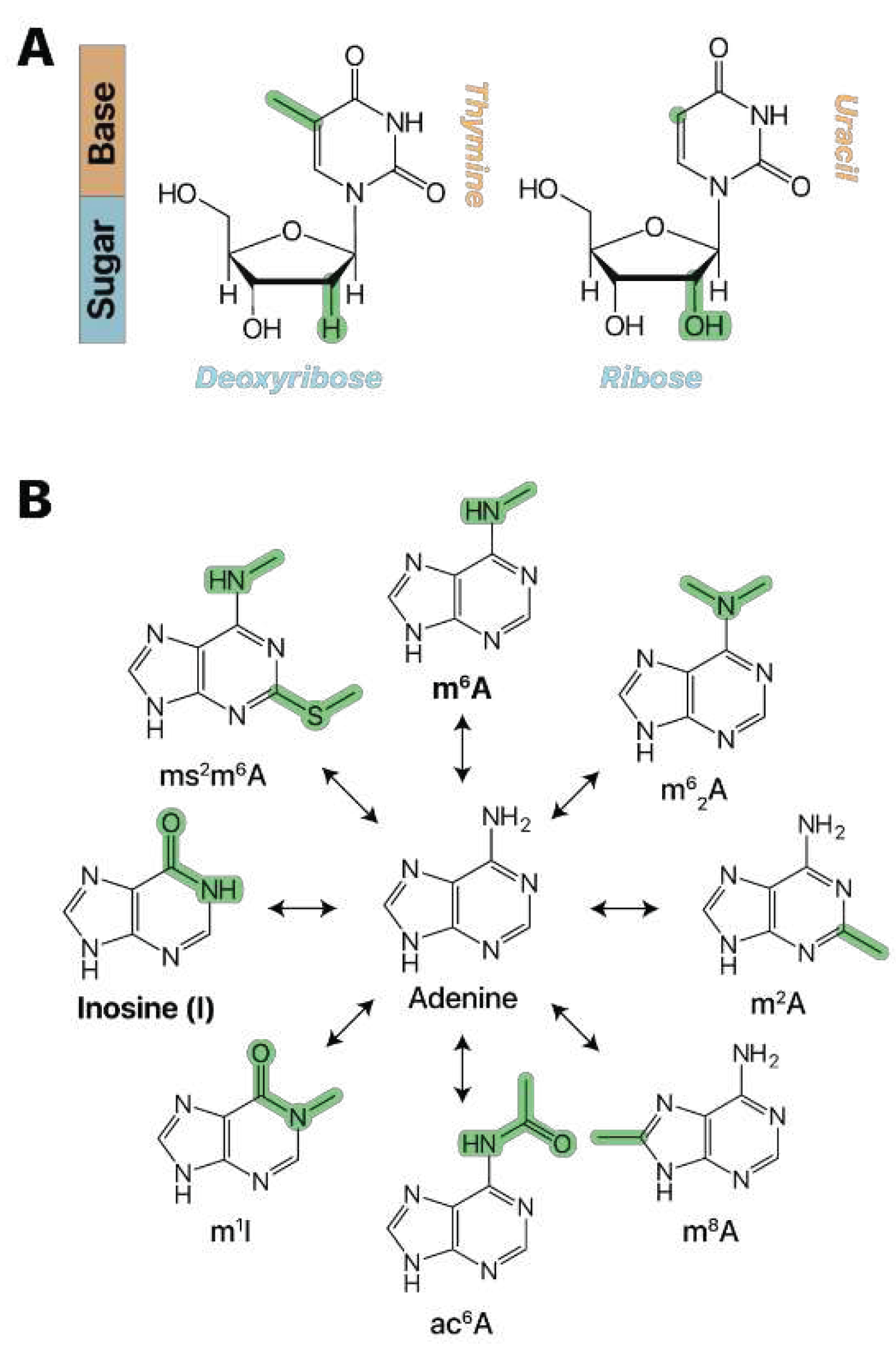
There are three key structural differences between RNA and DNA (
Minchin and Lodge 2019). First, RNA has a hydroxyl group at the 2’ position of its ribose sugar (
Figure 1A). DNA does not, thus defining its “Deoxyribose” name. Second, RNA uses adenine (A), cytosine (C), guanine (G), and uracil (U) as bases. DNA also uses A, C, G, but typically uses thymine (T) instead of U (
Figure 1A) (
Minchin and Lodge 2019), with some notable exceptions, such as the use of U instead of T in certain bacteriophage DNA (
Takahashi and Marmur 1963). DNA and RNA can form base pairs, transitioning from single-stranded nucleic acids to antiparallel double-stranded helices.. They can also form other structural assemblies, such as by folding on each other to form tertiary structures, similar to protein. However, the third difference is that RNA is typically found in cells as single-stranded, double-stranded, or in tertiary structures. DNA is primarily found as double-stranded helices, stabilizing the nucleic acid, protecting it from degradation to permit long-term storage of biological information. Thus, DNA and RNA have structural similarities, but their inherent chemical attributes enable them to be used for different purposes in biology.
RNA modifications can occur on all four bases and encompass a diverse array of chemical changes to the nitrogenous base or ribonucleoside sugar. The study of naturally occurring ribonucleoside modifications began in 1951 with the discovery of pseudouridine (Ψ) (
Cohn and Volkin 1951), an isomer of uridine where a carbon and nitrogen in the uracil ring have switched places. Since this discovery, over 140 additional modifications have been identified (
Cantara et al. 2011;
Lorenz, Lünse and Mörl 2017;
Boccaletto et al. 2022). The pace of identifying new modifications is rapidly accelerating due to the enhanced precision and accuracy provided by modern molecular biology equipment and techniques, along with the growing appreciation of RNA modifications and their involvement in a myriad of cellular pathways (
Hong et al. 2020). Other common modification examples include the addition of a hydroxymethyl group on cytidine to form 5-hydroxymethylcytidine (5hmC), as well as a variety of adenosine modifications such as N6-methyladenosine (m
6A,
Figure 1b). These chemical changes occur via specialized enzymatic pathways unique to the modification and biological context (
Fu et al. 2013;
Alseth, Dalhus and Bjoras 2014). Therefore, RNA modifications are found in differing amounts and RNA sites, depending on the organism, cell type, environment, and other factors.
The writers and erasers of m6A
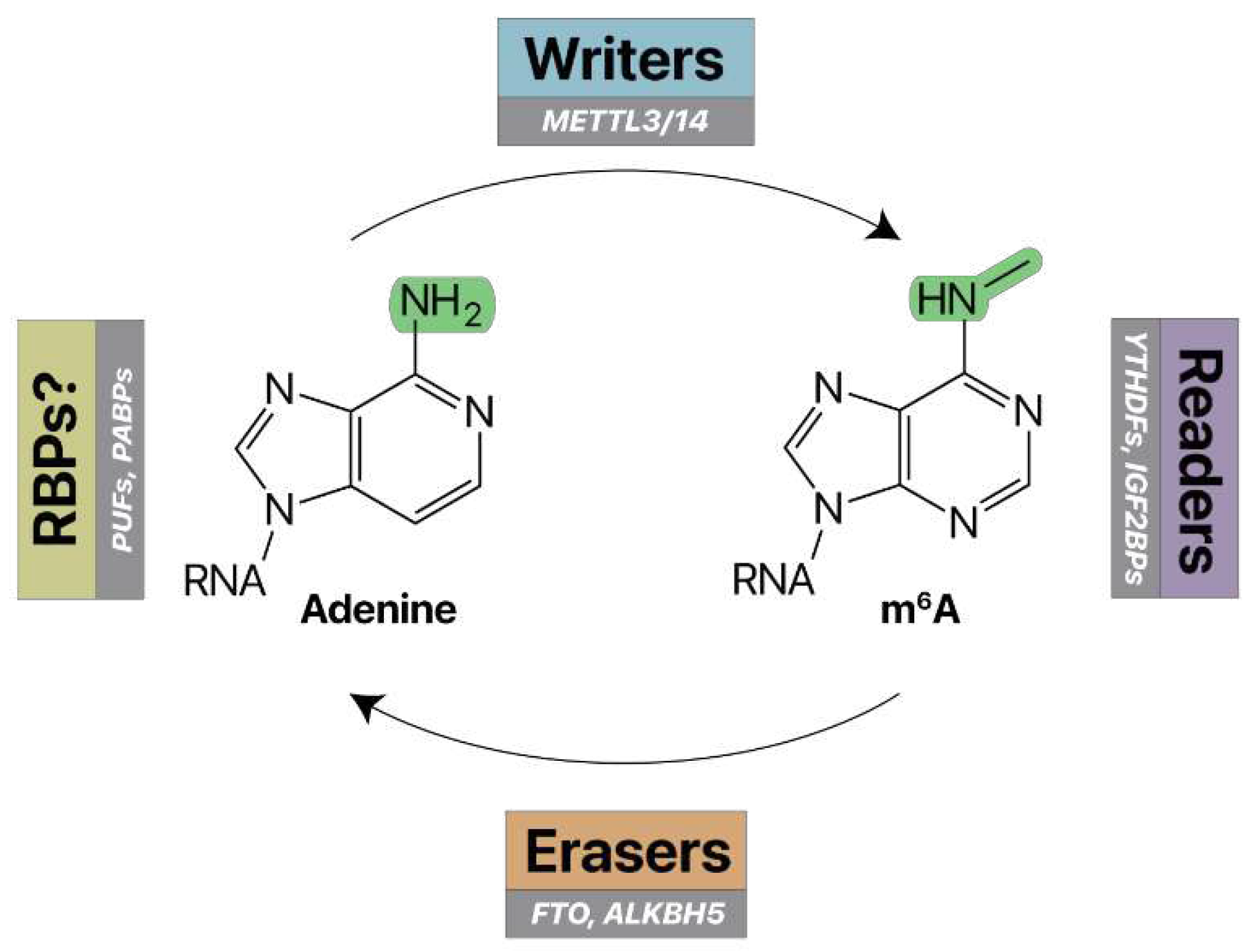
RNA modifications are managed by molecular writers and erasers (
Figure 3A) (
Patil, Pickering and Jaffrey 2018;
Shi, Wei and He 2019). Writers are enzymes that add a specific RNA modification, while erasers are enzymes that remove the modification. Writers and erasers work together to create and manage the epitranscriptome—the dynamic balance of RNA modifications within the cell. This regulation has far-reaching implications on the molecular function and expression of modified RNA targets (
Lin et al. 2016;
Oakes et al. 2017;
McCown et al. 2019;
Zhao et al. 2019).
Figure 2.
Writers, erasers, and readers of m6A. Writer enzymes (e.g. METTL3/14) add a methyl group to the nitrogen-6 position of adenosine to make N6-methyladenosine (m6A) RNA. Eraser enzymes (e.g. FTO, ALKBH5) can remove these methyl marks. Reader RNA-binding proteins (e.g. YTHDFs, IGF2BPs) specifically recognize and bind m6A RNA. Unmodified RNA may be recognized by other RNA-binding proteins (e.g. PUFs, PABPs).
Figure 2.
Writers, erasers, and readers of m6A. Writer enzymes (e.g. METTL3/14) add a methyl group to the nitrogen-6 position of adenosine to make N6-methyladenosine (m6A) RNA. Eraser enzymes (e.g. FTO, ALKBH5) can remove these methyl marks. Reader RNA-binding proteins (e.g. YTHDFs, IGF2BPs) specifically recognize and bind m6A RNA. Unmodified RNA may be recognized by other RNA-binding proteins (e.g. PUFs, PABPs).
The writers and erasers of m
6A are particularly well characterized (
Figure 3) (
Shi, Wei and He 2019;
Jiang et al. 2021) and have a significant impact on gene expression, animal development, and human disease (
Lin et al. 2016;
Yoon et al. 2017;
Choe et al. 2018;
Paris et al. 2019). As such, this review will use m
6A as a prototypical example of the chemistry, biochemistry, and biology of an RNA modification and how it interacts with proteins. Discovered in the 1970s (
Desrosiers, Friderici and Rottman 1974), m
6A is prevalent in vertebrate RNA (
Dominissini et al. 2012) and found on thousands of their messenger RNAs (mRNAs), the RNAs used to code for proteins. These mRNAs have m
6A modifications concentrated near stop codons and in their 3’ untranslated regions (
Dominissini et al. 2012;
Patil, Pickering and Jaffrey 2018). Methyltransferase complexes modify adenosine into m
6A. Although they consist of several proteins, the cores of these complexes involve methyltransferase-like (METTL) enzymes that catalyze the methylation reaction. For example, METTL3 and METTL14 assemble and can modify adenosines in mRNAs but rely on other proteins for enhanced enzymatic activity and site selection (
Liu et al. 2014;
Huang et al. 2021). METTL3 is the catalytic subunit. METTL14 and other proteins maintain the correct conformation for enzymatic activity. These other proteins can also impart preferences for specific m
6A modification sites (
Bokar et al. 1994). All methyltransferase complexes have preferences for specific RNA sequences known as motifs (
Figure 3C). The targeted RNA sequence for METTL3/METTL14 is the RRACH motif, where R = A or G, and H = A, C, or U (
Wei and Moss 1977;
Harper et al. 1990). The central A of this motif is enzymatically converted to m
6A. There are two established m
6A erasers: Alkylation B Homolog 5 (ALKBH5) and Fat Mass and Obesity-Associated protein (FTO) (
Zheng et al. 2013;
Zhao et al. 2014). These demethylases work by modifying the N
6 methyl group further to enable chemistry that can restore the base to unmodified adenosine (
Zhao et al. 2014). Both writers and erasers are associated with human disease. Overexpression of the METTL3/METTL14 m
6A writers are associated with liver, gastric, and colon cancer (
Chen et al. 2018;
Shen et al. 2020;
Zhang et al. 2020)
. The FTO m
6A eraser is associated with obesity (
Fawcett and Barroso 2010). In summary, writers and readers are the enzymatic Ying-Yang for RNA modifications like m
6A. Perturbation of this dynamic balance can lead to disease.
The readers of m6A
Readers are binding proteins that recognize specific RNA modifications. This interaction can lead to regulation of the RNA target. The best characterized readers for m6A are the YTH domain family of proteins (YTHDFs) and YTH domain-containing proteins (YTHDCs). YTHDFs and YTHDCs recognize m6A in the nucleus and cytoplasm, resulting in different biochemical functions contingent upon the specific reader protein and the cellular context (Liao, Sun and Xu 2018). For example, YTHDF2 and other YTH proteins can attract mRNA decay machinery through recruitment of the CCR4-NOT deadenylation complex (Du et al. 2016) CCR4-NOT removes the poly-A tail of mRNAs, leading to mRNA turnover. Additional functions of the YTH proteins are still being studied, but a critical aspect is that these proteins must bind to their RNA target to elicit their biochemical function (Stoilov, Rafalska and Stamm 2002; Shi, Wei and He 2019). Thus, the molecular recognition of YTH and other RNA-binding proteins depend on their interactions with target RNA.
Common RNA-Protein Interactions
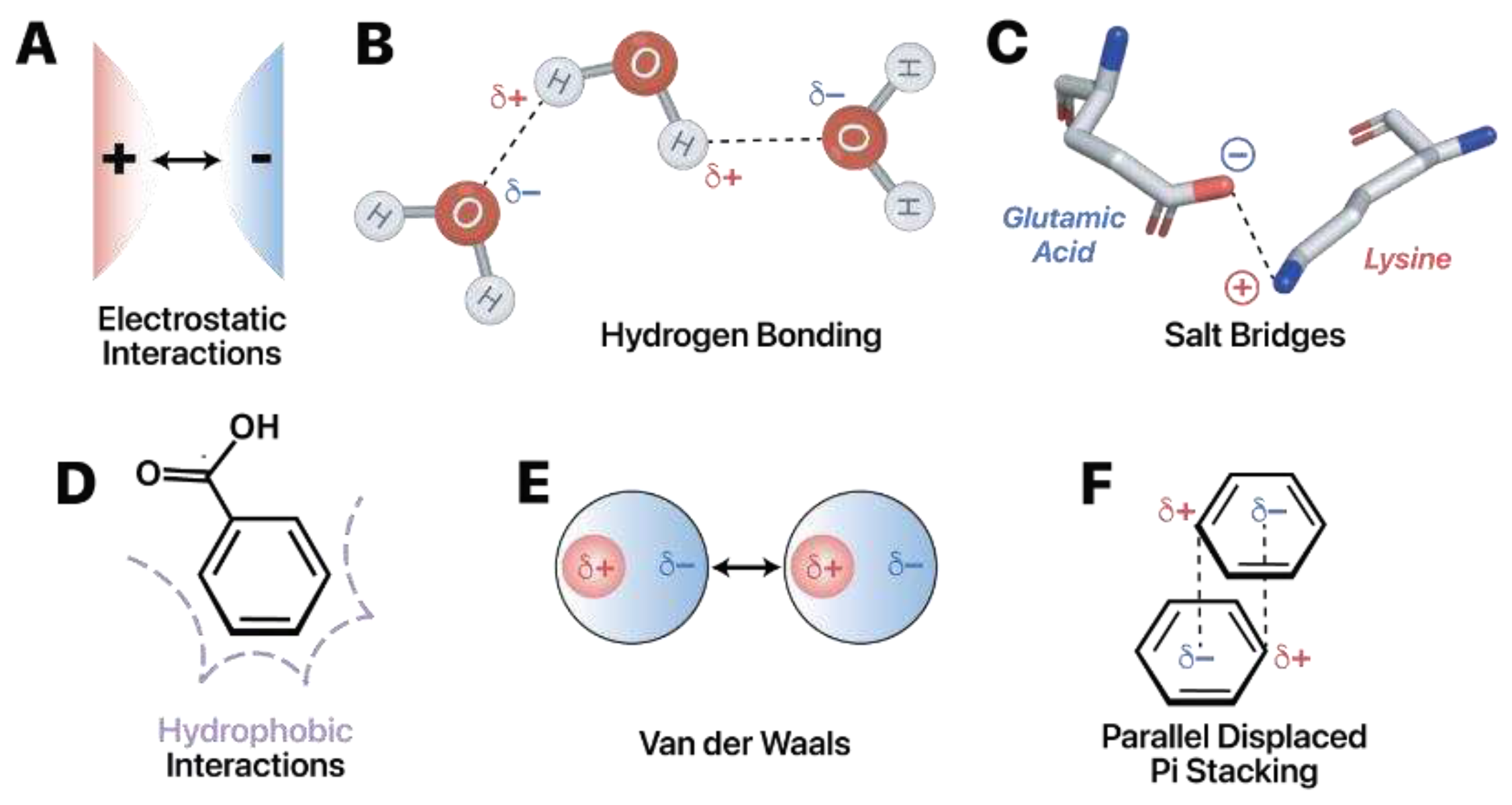
To understand how readers recognize specific RNA modifications, it is essential to have a foundation in the chemical interactions between RNA and protein. RNA-protein interactions commonly encompass: 1) electrostatic interactions, 2) hydrogen bonding, 3) salt bridges, 4) hydrophobic interactions, 5) Van der Waals interactions, and 6) pi stacking interactions (
Figure 4) (
Corley, Burns and Yeo 2020).
Electrostatic interactions arise from the attraction or repulsion between charged particles (
Figure 4A). The fundamental idea is that opposite charges attract while like charges repel. Protein amino acids have neutral, positive, or negative charges. The propensity of a particular amino acid to carry or lack a charge is governed by the chemical structure of its side chain as well as by the pH of the surrounding environment (
Zhao et al. 2014). While unmodified A, C, G, and U are almost universally neutrally charged within the cell, some RNA modifications carry a charge under physiological conditions (
Anderson, Droogmans and Grosjean 2005;
Lorenz, Lünse and Mörl 2017). Notably, the phosphate group contained in the backbone of nucleic acids carries a negative charge. Consequently, many readers have positively charged residues that nonspecifically attract nucleic acids (
Järvelin et al. 2016;
Balcerak et al. 2019).
Hydrogen bonding occurs when the partial positive charge of a hydrogen atom, bound to an electronegative atom, attracts an electronegative partner (
Figure 4B). Electronegativity denotes an atom's propensity to attract electrons. When highly electronegative atoms, like oxygen or nitrogen, are bound to hydrogen atoms, regions of partial negative charge and partial positive charge are formed, indicated as δ- or δ+, respectively. The partial positive charge occurs on the less electronegative hydrogen, in this case. Conversely, the partial negative charge occurs on the more electronegative group. When correctly oriented, these oppositely charged regions can establish attractions. Neutral hydrogen bonds at 2.4–3.0 Å distance contribute about 0.5–1.5 kcal/mol per interaction, and charged hydrogen bonds, or “salt bridges,'' within a 4.0 Å distance contribute 3.5–4.5 kcal/mol per interaction (
Figure 4C) (
Herschlag and Pinney 2018). Protein and RNA contain chemical moieties capable of hydrogen bonding, and modifications to RNA and protein frequently introduce additional groups that change this capability (
Järvelin et al. 2016;
Balcerak et al. 2019;
Hofweber and Dormann 2019). While generally weaker than covalent or ionic interactions, the collective contribution of hydrogen bonds can have considerable impact on the RNA-binding protein selectivity for a given modification. Analyses have estimated the prevalence of hydrogen bonds to the base, the ribose 2’-OH, and the RNA phosphate backbone at 36%, 24%, and 41% of RNA-protein hydrogen bonds respectively (
Treger and Westhof 2001;
Gupta and Gribskov 2011).
Hydrophobic interactions (
Figure 4D) occur as a result of molecules trying to minimize contact with the surrounding water. The interactions occur between non-polar regions at distances of 3.8–5.0 Å and contribute approximately 1–2 kcal/mol (
Dill et al. 2008;
Onofrio et al. 2014). RNA and protein have hydrophilic and hydrophobic moieties that group with like elements (
Anderson, Droogmans and Grosjean 2005;
Hofweber and Dormann 2019;
McCown et al. 2020). Hydrogen bonding drives hydrophilic interactions directly and hydrophobic interactions indirectly. Amino acids with many nonpolar carbon—carbon bonds, like leucine, isoleucine, phenylalanine, tryptophan, and others, are hydrophobic and fold together to form a “hydrophobic core.” This core may also interact with a hydrophobic moiety on RNA (
Allain 1997;
Yang 2002;
Yu et al. 2014;
Zhu et al. 2014). Up to 50% of RNA-protein interface interactions may be hydrophobic, depending on the RNA-binding protein (
Hu et al. 2018).
There are two types of Van der Waals forces: the weaker London Dispersion Forces and the stronger dipole-dipole forces (
Petrucci et al. 1997). London Dispersion Forces arise due to temporary induced dipoles—imbalances in the charge distribution surrounding molecules (
Figure 4E). Stronger Van der Waals interactions may form as a result of permanent dipoles. Hydrogen bonds exceeding a certain threshold distance, typically 3.0 Å, fall into this category. (
Allers and Shamoo 2001;
Jones et al. 2001). Both types of Van der Waals forces are weak electrostatic interactions of about 0.5–1 kcal/mol (
Corley, Burns and Yeo 2020). They largely play stabilizing roles in the binding of proteins to RNA (
Corley, Burns and Yeo 2020).
Aromatic rings aligning face-to-face (
Figure 4F) or face-to-edge results in pi stacking. These interactions typically form at distances of 2.7–4.3 Å and are relatively strong, contributing about 2–6 kcal/mol per interaction (
Wilson, Holland and Wetmore 2016). They are frequently observed in protein and RNA interactions due to the aromaticity present in RNA and many amino acids. In YTH and other RNA-binding proteins, pi stacking interactions play a crucial role in shaping the active site, effectively sandwiching the targeted base in place (
Oubridge et al. 1994;
Zhu et al. 2014).
Recognition of RNA by RNA-binding proteins
All RNA-binding proteins follow similar principles when interacting with their targets. First, they have specificity interactions that designate their sequence or secondary structure preferences. Second, they use positively charged amino acid side chains to account for the negatively charged phosphate backbone. Third, they often target the 2’ hydroxyl in RNA to differentiate from DNA.
The Pumilio and FBF protein family (PUFs) of RNA-binding proteins serve as a good example of sequence specific RNA interactors that use these three concepts. PUFs contain a conserved RNA-binding domain known as the Pumilio homology domain (PUM-HD) (
Wickens et al. 2002;
Quenault, Lithgow and Traven 2011;
Goldstrohm, Hall and McKenney 2018), of which there are many atomic-resolution crystal structures determined with and without RNA (
Wang, Zamore and Hall 2001;
Wang et al. 2002;
Lu and Hall 2011). The structure of human Pumilio 2 homology domain (hPUM2-HD) bound to RNA shows how the canonical PUM-HD has eight α-helical repeats that binds to a conserved RNA sequence, UGUANAUA, with N being A, C, G, or U (
Figure 4A) (
Gerber et al. 2006;
Morris, Mukherjee and Keene 2008;
Hafner et al. 2010). Each α-helical repeat recognizes one unpaired base via three amino acid side chains (
Campbell, Valley and Wickens 2014). Two side chains interact with an edge of the base, while the third residue forms pi stacking interactions in the plane between two bases (
Goldstrohm, Hall and McKenney 2018). Thus, in following with the first principle, amino acid side chains form a tripartite code for sequence binding specificity. PUM-HD also has arginine, lysine, and histidine side chains surrounding the RNA-binding surface, following the second principle of positively charged groups attracting negatively charged RNA
. hPUM2-HD does not have amino acids interacting with the 2’ hydroxyl groups of the RNA. Notably, this PUM-HD can bind to both RNA and DNA (
Wang et al. 2002). To summarize, the RNA-bound hPUM2-HD structure shows many of the basic characteristics observed in other RNA-binding proteins. Deviations from the basic principles, such as the lack of specificity for the 2’ hydroxyl, allow the protein to bind to a broader range of substrates.
PUM-HD recognition of adenosine at the fourth RNA position is specific and occurs almost entirely through interactions with the nucleobase (
Figure 4B). The ringed tyrosine and positively charged, flat arginine contributes favorable pi stacking interactions, while glutamine forms a hydrogen bond with the adenosine nitrogen (
Figure 4B). Uridine at the 3rd RNA position has its base similarly sandwiched between amino acid side chains, but specificity is dictated by a different set of protein residues (
Figure 4C). Uridine and adenosine are very different bases. Uridine is a pyrimidine with a single ring, while adenosine is a purine with two rings. The uracil base of uridine has two carbonyl moieties attached to its ring. In contrast, the adenine base of adenosine has an amino group attached to its ring. PUM-HD uses these moieties as chemical signatures to differentiate uridine from adenosine. The carbonyls on the uracil base form hydrogen bonds with the amide moieties of glutamine and asparagine in PUM-HD (
Figure 4C). This binding interaction is incompatible with an adenine base. At the remaining six recognition sites, PUM-HD uses specific combinations of amino acids in each α-helical repeat to target specific nucleobases (
Lu and Hall 2011). Thus, RNA-binding proteins like PUF target specific RNAs by using amino acids that account for the particular chemical signature of their desired targets.
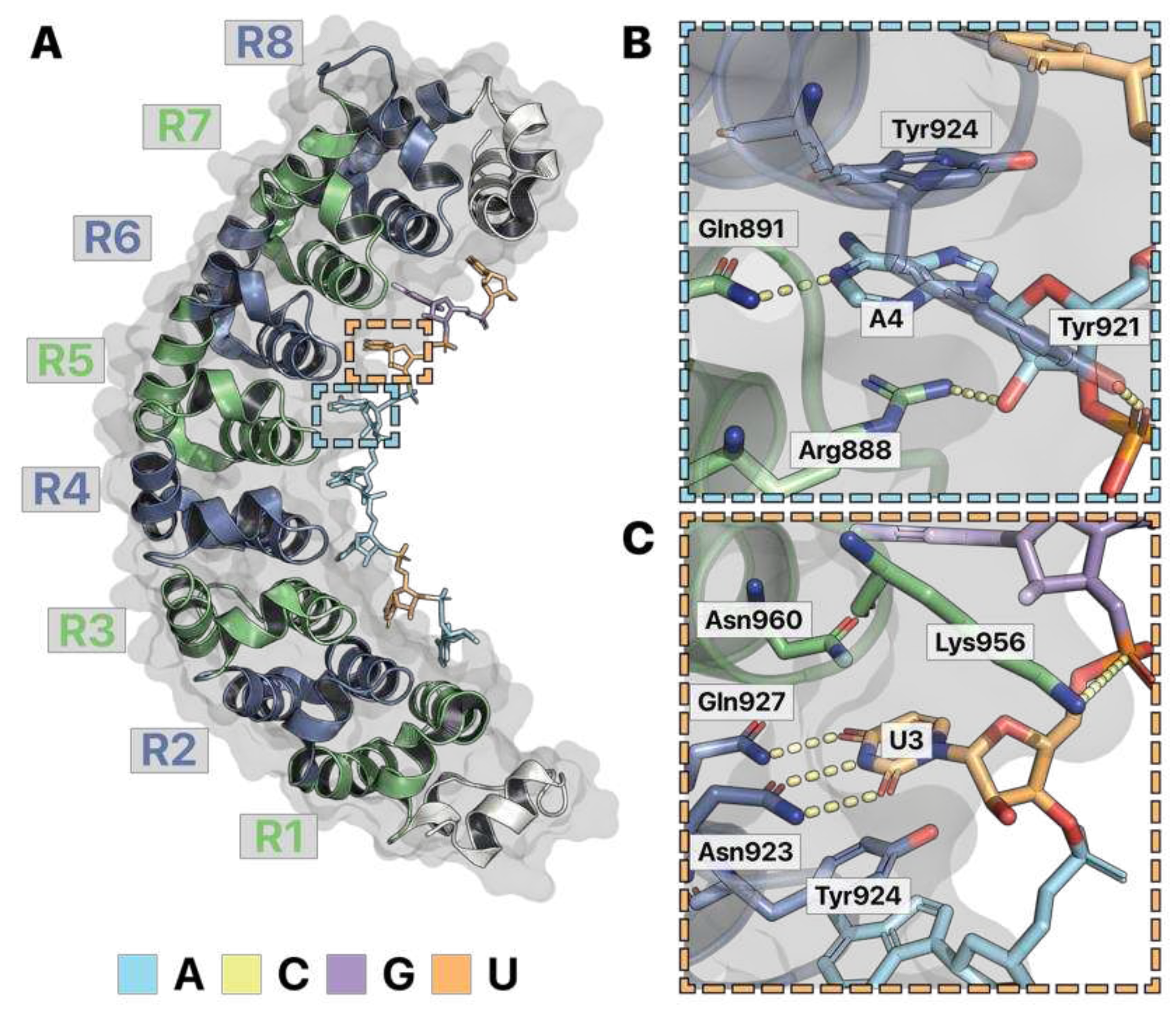
Recognition of m6A RNA by RNA-binding proteins
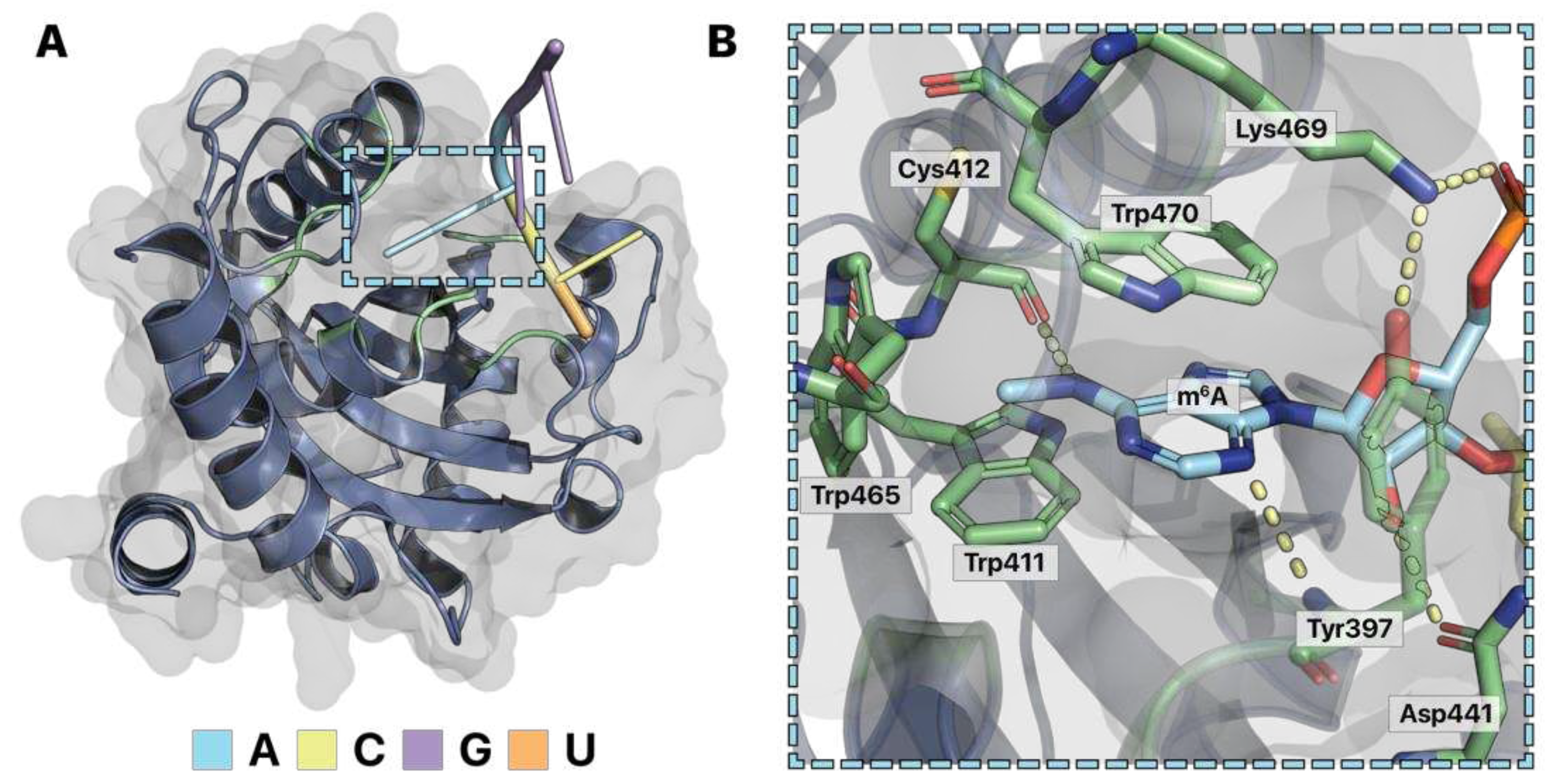
RNA-binding proteins that can bind modified RNAs like m
6A use the same binding strategies as RNA-binding proteins that target unmodified RNA. m
6A has a methyl group added to the N
6 position of adenosine (
Figure 1B). As a result, a nonpolar, bulky methyl group replaces a hydrogen, which could have formed hydrogen bonds. Similar to PUM-HD, RNA-binding proteins that target modified RNAs chemically accommodate their target to differentiate between other modified and unmodified RNAs. For example, the specificity of the YT521-B homology (YTH) domain for m
6A is explained in the atomic resolution crystal structure of YTHDF1 bound with m
6A -containing RNA (
Figure 5A) (
Xu et al. 2015). Like PUM-HD, the YTH domain has ringed amino acids that pi stack with the adenine base of m
6A. Positively charged amino acids such as lysine also form salt bridges with the RNA backbone, serving to nonspecifically attract RNA substrates. Differences are observed at the YTH specificity pocket for m
6A versus the PUM-HD pocket for unmodified adenosine. YTH forms a hydrophobic cage of three tryptophans surrounding the modification (
Figure 5B). These interactions allow the protein to differentiate m
6A from an unmodified adenosine with a hydrophilic N
6 amino group. Additionally, the backbone of the YTH peptide chain hydrogen bonds with a nitrogen of m
6A to hold the modified base in place (
Figure 5B). Lastly, an asparagine hydrogen bonds with the 2’ hydroxyl of the m
6A ribose, allowing YTH to differentiate RNA from DNA (
Figure 5B). Thus, the specificity pocket of YTH is designed to accommodate a hydrophobic chemical modification to specify m
6A and differentiate from unmodified RNA or DNA.
To summarize, the YTHDF1 and hPUM2-HD have similar strategies to target specific RNA substrates. First, both YTHDF1 and hPUM2-HD use amino acid side chains and a medley of chemical interactions to form a specificity pocket designed to accommodate the chemistry of the RNA targeted (Lu and Hall 2011; Xu et al. 2015). YTH predominantly employs hydrophobic interactions to form a pocket which accommodate the hydrophobic character of the m6A methyl group. PUM-HD utilizes hydrophilic interactions to drive its pocket specificity for the unmodified adenosine nitrogen. Second, amino acids pi stack to present the base in a proper position for the binding pocket. And third, positively charged residues on the protein's surface attract the RNA phosphate backbone to nonspecifically enhance its affinity for all RNA substrates. Other RNA-binding proteins follow the same principles that can also be appreciated in high resolution, RNA-protein structures.
New frontiers in RNA-binding proteins
The central dogma of molecular biology outlines the flow of genetic information from DNA to RNA to protein. The pivotal position of RNA, situated in between the DNA responsible for heredity and the proteins which represent functional products, renders it a key point for further research in the field of molecular biology. RNA modifications expand the RNA alphabet beyond the four standard ribonucleotides by introducing diverse alterations to their chemical structure. The modifications are created or removed by enzymes, categorized either as writers or erasers. These enzymes play a necessary role in biology for gene regulation, development, obesity, and cancer (Fawcett and Barroso 2010; Zheng et al. 2013; Lin et al. 2016; Oakes et al. 2017; Yoon et al. 2017; Choe et al. 2018; Zhou and Pang 2018; McCown et al. 2019; Paris et al. 2019; Chen and Wong 2020; Huang et al. 2022). Readers have evolved to selectively bind distinct unmodified and modified RNA. While differing in structure and sequence, these binding proteins use a conserved set of principles to recognize target RNA. The differences and similarities of RNA-binding proteins is on full display in atomic resolution RNA-protein structural models.
The study of RNA modifications is in a renaissance and undergoing exponential growth. Only a handful of modifications have been fully characterized, in part because of the lack of methods to identify their RNA targets and sites. Some methods use chemistry or RNA-binding proteins (Wang et al. 2020) to identify the sites, but these methods must be specifically tailored to each RNA modification. Universal methods to identify any type of RNA modification are challenging but also in the infant stages of development (Garalde et al. 2018; Zhang, Lu and Li 2022). Identifying RNA modification sites provides a starting point for understanding how RNA modifications affect RNA stability, folding, and function. Thus, the development of new, accurate identification methods will be key to investigate the link between currently uncharacterized RNA modifications, biology, and disease (Carlile et al. 2014; Delatte et al. 2016; Garalde et al. 2018; Khoddami et al. 2019; Acera Mateos et al. 2023). As discussed in this review, one key mechanism of m6A is the recruitment of RNA-binding proteins for RNA regulation. A safe prediction is that other RNA modifications will also recruit or prevent interactions with RNA-binding proteins as their biological mechanism. Similar to YTH, PUM-HD, and others, these RNA-binding proteins will undoubtedly follow similar strategies to recognize subtle chemical differences of modified RNA to deliver a profound impact on RNA form and function.
Funding
S.T.A. is funded by the NIH/NIGMS (R35 GM142691) and received start-up funds from the Indiana University School of Medicine and its Precision Health Initiative (PHI).
Conflict of interest statement
All authors declare that they have no conflicts of interest.
References
- Acera Mateos, P.; Zhou, Y.; Zarnack, K.; Eyras, E. Concepts and methods for transcriptome-wide prediction of chemical messenger RNA modifications with machine learning. Brief Bioinform 2023, 24. [Google Scholar] [CrossRef] [PubMed]
- Allain, F.H.T. Structural basis of the RNA-binding specificity of human U1A protein. The EMBO Journal 1997, 16, 5764–5772. [Google Scholar] [CrossRef]
- Allers, J.; Shamoo, Y. Structure-based analysis of protein-RNA interactions using the program ENTANGLE. J Mol Biol 2001, 311, 75–86. [Google Scholar] [CrossRef] [PubMed]
- Alseth, I.; Dalhus, B.; Bjoras, M. Inosine in DNA and RNA. Curr Opin Genet Dev 2014, 26, 116–123. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.T.; Droogmans, L.; Grosjean, H. Fine-tuning of RNA functions by modification and editing; Grosjean, H., Ed.; 2005; Volume 12, pp. 121–139. [Google Scholar]
- Aoki, S.T. , Lynch, T.R.; Crittenden, S.L.; Bingman, C.A.; Wickens, M.; Kimble, J. C. elegans germ granules require both assembly and localized regulators for mRNA repression. Nat Commun 2021, 12, 996. [Google Scholar] [CrossRef] [PubMed]
- Balcerak, A. Trebinska-Stryjewska, A.; Konopinski, R.; Wakula, M.; Grzybowska, E.A. RNA–protein interactions: disorder, moonlighting and junk contribute to eukaryotic complexity. Open Biology 2019, 9, 190096. [Google Scholar] [CrossRef] [PubMed]
- Boccaletto, P.; Stefaniak, F.; Ray, A.; Cappannini, A.; Mukherjee, S.; et al. MODOMICS: a database of RNA modification pathways. 2021 update. Nucleic Acids Res 2022, 50, D231–D235. [Google Scholar] [CrossRef]
- Bokar, J.A.; Rath-Shambaugh, M.E.; Ludwiczak, R.; Narayan, P.; Rottman, F. Characterization and partial purification of mRNA N6-adenosine methyltransferase from HeLa cell nuclei. Internal mRNA methylation requires a multisubunit complex. J Biol Chem 1994, 269, 17697–17704. [Google Scholar] [CrossRef]
- Campbell, Z.T.; Valley, C.T.; Wickens, M. A protein-RNA specificity code enables targeted activation of an endogenous human transcript. Nature Structural & Molecular Biology 2014, 21, 732–738. [Google Scholar]
- Cantara, W.A.; Crain, P.F.; Rozenski, J.; McCloskey, J.A.; Harris, K.A.; et al. The RNA Modification Database, RNAMDB: 2011 update. Nucleic Acids Res 2011, 39, D195–D201. [Google Scholar] [CrossRef]
- Carlile, T.M.; Rojas-Duran, M.F.; Zinshteyn, B.; Shin, H.; Bartoli, K.M.; Gilbert, W.V. Pseudouridine profiling reveals regulated mRNA pseudouridylation in yeast and human cells. Nature 2014, 515, 143–146. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Wei, L.; Law, C.T.; Tsang, F.H.C.; Shen, J.; et al. RNA N6-methyladenosine methyltransferase-like 3 promotes liver cancer progression through YTHDF2-dependent posttranscriptional silencing of SOCS2. Hepatology 2018, 67, 2254–2270. [Google Scholar] [CrossRef]
- Chen, M.; Wong, C.-M. The emerging roles of N6-methyladenosine (m6A) deregulation in liver carcinogenesis. Molecular Cancer 2020, 19. [Google Scholar] [CrossRef] [PubMed]
- Choe, J.; Lin, S.; Zhang, W.; Liu, Q.; Wang, L.; et al. mRNA circularization by METTL3–eIF3h enhances translation and promotes oncogenesis. Nature 2018, 561, 556–560. [Google Scholar] [CrossRef] [PubMed]
- Cohn, W.E.; Volkin, E. Nucleoside-5′-Phosphates from Ribonucleic Acid. Nature 1951, 167, 483–484. [Google Scholar] [CrossRef]
- Corley, M.; Burns, M.C.; Yeo, G.W. How RNA-Binding Proteins Interact with RNA: Molecules and Mechanisms. Molecular Cell 2020, 78, 9–29. [Google Scholar] [CrossRef]
- Crick, F.H. ; On protein synthesis. Symp Soc Exp Biol 1958, 12, 138–163. [Google Scholar]
- Delatte, B.; Wang, F.; Ngoc, L.V.; Collignon, E.; Bonvin, E. Transcriptome-wide distribution and function of RNA hydroxymethylcytosine. Science 2016, 351, 282–285. [Google Scholar] [CrossRef]
- Desrosiers, R.; Friderici, K.; Rottman, F. Identification of methylated nucleosides in messenger RNA from Novikoff hepatoma cells. Proceedings of the National Academy of Sciences 1974, 71, 3971–3975. [Google Scholar] [CrossRef]
- Dill, K.A.; Ozkan, S.B.; Shell, M.S.; Weikl, T.R. The Protein Folding Problem. Annual Review of Biophysics 2008, 37, 289–316. [Google Scholar] [CrossRef]
- Dominissini, D.; Moshitch-Moshkovitz, S.; Schwartz, S.; Salmon-Divon, M.; Ungar, L.; et al. Topology of the human and mouse m6A RNA methylomes revealed by m6A-seq. Nature 2012, 485, 201–206. [Google Scholar] [CrossRef] [PubMed]
- Du, H.; Zhao, Y.; He, J.; Zhang, Y.; Xi, H.; et al. YTHDF2 destabilizes m6A-containing RNA through direct recruitment of the CCR4–NOT deadenylase complex. Nature Communications 2016, 7, 12626. [Google Scholar] [CrossRef] [PubMed]
- Fawcett, K.A.; Barroso, I. The genetics of obesity: FTO leads the way. Trends Genet 2010, 26, 266–274. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Jia, G.; Pang, X.; Wang, R.N.; Wang, X.; et al. FTO-mediated formation of N6-hydroxymethyladenosine and N6-formyladenosine in mammalian RNA. Nat Commun 2013, 4, 1798. [Google Scholar] [CrossRef] [PubMed]
- Garalde, D.R.; Snell, E.A.; Jachimowicz, D.; Sipos, B.; Lloyd, J.H.; et al. Highly parallel direct RNA sequencing on an array of nanopores. Nature Methods 2018, 15, 201–206. [Google Scholar] [CrossRef] [PubMed]
- Gerber, A.P.; Luschnig, S.; Krasnow, M.A.; Brown, P.O.; Herschlag, D. Genome-wide identification of mRNAs associated with the translational regulator PUMILIO in <i>Drosophila melanogaster</i>. Proceedings of the National Academy of Sciences 2006, 103, 4487–4492. [Google Scholar]
- Goldstrohm, A.C.; Hall, T.M.T.; McKenney, K.M. Post-transcriptional Regulatory Functions of Mammalian Pumilio Proteins. Trends in Genetics 2018, 34, 972–990. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Gribskov, M. The role of RNA sequence and structure in RNA--protein interactions. J Mol Biol 2011, 409, 574–587. [Google Scholar] [CrossRef]
- Hafner, M.; Landthaler, M.; Burger, L.; Khorshid, M.; Hausser, J.; et al. Transcriptome-wide Identification of RNA-Binding Protein and MicroRNA Target Sites by PAR-CLIP. Cell 2010, 141, 129–141. [Google Scholar] [CrossRef]
- Harper, J.E.; Miceli, S.M.; Roberts, R.J.; Manley, J.L. Sequence specificity of the human mRNA N6-adenosine methylase in vitro. Nucleic Acids Research 1990, 18, 5735–5741. [Google Scholar] [CrossRef]
- Herschlag, D.; Pinney, M.M. Hydrogen Bonds: Simple after All? Biochemistry 2018, 57, 3338–3352. [Google Scholar] [CrossRef]
- Hofweber, M.; Dormann, D. Friend or foe-Post-translational modifications as regulators of phase separation and RNP granule dynamics. J Biol Chem 2019, 294, 7137–7150. [Google Scholar] [CrossRef] [PubMed]
- Hong, M.; Tao, S.; Zhang, L.; Diao, L.T.; Huang, X.; et al. RNA sequencing: new technologies and applications in cancer research. J Hematol Oncol 2020, 13, 166. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Qin, L.; Li, M.; Pu, X.; Guo, Y. A structural dissection of protein–RNA interactions based on different RNA base areas of interfaces. RSC Advances 2018, 8, 10582–10592. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Mo, J.; Liao, Z.; Chen, X.; Zhang, B. The RNA m6A writer WTAP in diseases: structure, roles, and mechanisms. Cell Death & Disease 2022, 13. [Google Scholar]
- Huang, W.; Chen, T.-Q.; Fang, K.; Zeng, Z.-C.; Ye, H.; Chen, Y.-Q. N6-methyladenosine methyltransferases: functions, regulation, and clinical potential. Journal of Hematology & Oncology 2021, 14. [Google Scholar]
- Ille, A.M.; Lamont, H.; Mathews, M.B. The Central Dogma revisited: Insights from protein synthesis, CRISPR, and beyond. WIREs RNA 2022, 13. [Google Scholar] [CrossRef]
- Järvelin, A.I.; Noerenberg, M.; Davis, I.; Castello, A. The new (dis)order in RNA regulation. Cell Communication and Signaling 2016, 14. [Google Scholar] [CrossRef]
- Jiang, X.; Liu, B.; Nie, Z.; Duan, L.; Xiong, Q.; et al. The role of m6A modification in the biological functions and diseases. Signal Transduct Target Ther 2021, 6, 74. [Google Scholar] [CrossRef]
- Jones, S.; Daley, D.T.; Luscombe, N.M.; Berman, H.M.; Thornton, J.M. Protein-RNA interactions: a structural analysis. Nucleic Acids Res 2001, 29, 943–954. [Google Scholar] [CrossRef]
- Khoddami, V.; Yerra, A.; Mosbruger, T.L.; Fleming, A.M.; Burrows, C.J.; Cairns, B.R. Transcriptome-wide profiling of multiple RNA modifications simultaneously at single-base resolution. Proceedings of the National Academy of Sciences 2019, 116, 6784–6789. [Google Scholar] [CrossRef] [PubMed]
- Liao, S.; Sun, H.; Xu, C. YTH Domain: A Family of N(6)-methyladenosine (m(6)A) Readers. Genomics Proteomics Bioinformatics 2018, 16, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Choe, J.; Du, P.; Triboulet, R.; Gregory, I.R. The m 6 A Methyltransferase METTL3 Promotes Translation in Human Cancer Cells. Molecular Cell 2016, 62, 335–345. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Yue, Y.; Han, D.; Wang, X.; Fu, Y.; et al. A METTL3–METTL14 complex mediates mammalian nuclear RNA N6-adenosine methylation. Nature Chemical Biology 2014, 10, 93–95. [Google Scholar] [CrossRef] [PubMed]
- Lorenz, C.; Lünse, C.; Mörl, M. tRNA Modifications: Impact on Structure and Thermal Adaptation. Biomolecules 2017, 7, 35. [Google Scholar] [CrossRef]
- Lu, G.; Hall, T.M.T. Alternate Modes of Cognate RNA Recognition by Human PUMILIO Proteins. Structure 2011, 19, 361–367. [Google Scholar] [CrossRef] [PubMed]
- McCown, P.J.; Ruszkowska, A.; Kunkler, C.N.; Breger, K.; Hulewicz, J.P.; et al. Naturally occurring modified ribonucleosides. WIREs RNA 2020, 11, e1595. [Google Scholar] [CrossRef] [PubMed]
- McCown, P.J.; Wang, M.C.; Jaeger, L.; Brown, J.A. Secondary Structural Model of Human MALAT1 Reveals Multiple Structure–Function Relationships. International Journal of Molecular Sciences 2019, 20, 5610. [Google Scholar] [CrossRef]
- Minchin, S.; Lodge, J. Understanding biochemistry: structure and function of nucleic acids. Essays Biochem 2019, 63, 433–456. [Google Scholar] [CrossRef]
- Morris, A.R.; Mukherjee, N.; Keene, J.D. Ribonomic Analysis of Human Pum1 Reveals cis-trans Conservation across Species despite Evolution of Diverse mRNA Target Sets. Molecular and Cellular Biology 2008, 28, 4093–4103. [Google Scholar] [CrossRef]
- Oakes, E.; Anderson, A.; Cohen-Gadol, A.; Hundley, H.A. Adenosine Deaminase That Acts on RNA 3 (ADAR3) Binding to Glutamate Receptor Subunit B Pre-mRNA Inhibits RNA Editing in Glioblastoma. Journal of Biological Chemistry 2017, 292, 4326–4335. [Google Scholar] [CrossRef] [PubMed]
- Onofrio, A.; Parisi, G.; Punzi, G.; Todisco, S.; Di Noia, M.A.; et al. Distance-dependent hydrophobic–hydrophobic contacts in protein folding simulations. Phys. Chem. Chem. Phys. 2014, 16, 18907–18917. [Google Scholar] [CrossRef] [PubMed]
- Oubridge, C.; Ito, N.; Evans, P.R.; Teo, C.H.; Nagai, K. Crystal structure at 1.92 Å resolution of the RNA-binding domain of the U1A spliceosomal protein complexed with an RNA hairpin. Nature 1994, 372, 432–438. [Google Scholar] [CrossRef]
- Paris, J.; Morgan, M.; Campos, J.; Spencer, G.J.; Shmakova, A.; et al. Targeting the RNA m6A Reader YTHDF2 Selectively Compromises Cancer Stem Cells in Acute Myeloid Leukemia. Cell Stem Cell 2019, 25, 137–148. [Google Scholar] [CrossRef] [PubMed]
- Patil, D.P.; Pickering, B.F.; Jaffrey, S.R. Reading m(6)A in the Transcriptome: m(6)A-Binding Proteins. Trends Cell Biol 2018, 28, 113–127. [Google Scholar] [CrossRef] [PubMed]
- Petrucci, R.H.; Herring, F.G.; Madura, J.D.; Bissonnette, C. General Chemistry: Principles and Modern Applications. 1997, 11e. [Google Scholar]
- Quenault, T.; Lithgow, T.; Traven, A. PUF proteins: repression, activation and mRNA localization. Trends in Cell Biology 2011, 21, 104–112. [Google Scholar] [CrossRef]
- Schrödinger, L. The PyMOL Molecular Graphics System,, pp.
- Shen, C.; Xuan, B.; Yan, T.; Ma, Y.; Xu, P.; et al. m6A-dependent glycolysis enhances colorectal cancer progression. Molecular Cancer 2020, 19. [Google Scholar] [CrossRef]
- Shi, H.; Wei, J.; He, C. Where, When, and How: Context-Dependent Functions of RNA Methylation Writers, Readers, and Erasers. Molecular Cell 2019, 74, 640–650. [Google Scholar] [CrossRef]
- Stoilov, P.; Rafalska, I.; Stamm, S. YTH: a new domain in nuclear proteins. Trends in Biochemical Sciences 2002, 27, 495–497. [Google Scholar] [CrossRef]
- Takahashi, I.; Marmur, J. Replacement of thymidylic acid by deoxyuridylic acid in the deoxyribonucleic acid of a transducing phage for Bacillus subtilis. Nature 1963, 197, 794–795. [Google Scholar] [CrossRef] [PubMed]
- Treger, M.L.; Westhof, E. Statistical analysis of atomic contacts at RNA-protein interfaces. Journal of Molecular Recognition 2001, 14, 199–214. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; McLachlan, J.; Zamore, P.D.; Hall, T.M. Modular recognition of RNA by a human pumilio-homology domain. Cell 2002, 110, 501–512. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zamore, P.D.; Hall, T.M.T. Crystal Structure of a Pumilio Homology Domain. Molecular Cell 2001, 7, 855–865. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xiao, Y.; Dong, S.; Yu, Q.; Jia, G. Antibody-free enzyme-assisted chemical approach for detection of N6-methyladenosine. Nature Chemical Biology 2020, 16, 896–903. [Google Scholar] [CrossRef] [PubMed]
- Wei, C.-M.; Moss, B. Nucleotide sequences at the N6-methyladenosine sites of HeLa cell messenger ribonucleic acid. Biochemistry 1977, 16, 1672–1676. [Google Scholar] [CrossRef] [PubMed]
- Wickens, M.; Bernstein, D.S.; Kimble, J.; Parker, R. A PUF family portrait: 3'UTR regulation as a way of life. Trends Genet 2002, 18, 150–157. [Google Scholar] [CrossRef] [PubMed]
- Wilson, K.A.; Holland, D.J.; Wetmore, S.D. Topology of RNA–protein nucleobase–amino acid π–π interactions and comparison to analogous DNA–protein π–π contacts. RNA 2016, 22, 696–708. [Google Scholar] [CrossRef]
- Xu, C.; Liu, K.; Ahmed, H.; Loppnau, P.; Schapira, M.; Min, J. Structural Basis for the Discriminative Recognition of N6-Methyladenosine RNA by the Human YT521-B Homology Domain Family of Proteins. Journal of Biological Chemistry 2015, 290, 24902–24913. [Google Scholar] [CrossRef]
- Yang, Y. Solution structure of the LicT-RNA antitermination complex: CAT clamping RAT. The EMBO Journal 2002, 21, 1987–1997. [Google Scholar] [CrossRef]
- Yoon, K.-J.; Ringeling, F.R.; Vissers, C.; Jacob, F.; Pokrass, M.; et al. Temporal Control of Mammalian Cortical Neurogenesis by m6A Methylation. Cell 2017, 171, 877–889. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.; Ye, W.; Jiang, C.; Luo, R.; Chen, H.-F. Specific Recognition Mechanism between RNA and the KH3 Domain of Nova-2 Protein. The Journal of Physical Chemistry B 2014, 118, 12426–12434. [Google Scholar] [CrossRef]
- Zhang, B.; Wu, Q.; Li, B.; Wang, D.; Wang, L.; Zhou, Y.L. m6A regulator-mediated methylation modification patterns and tumor microenvironment infiltration characterization in gastric cancer. Molecular Cancer 2020, 19. [Google Scholar] [CrossRef]
- Zhang, Y.; Lu, L.; Li, X. Detection technologies for RNA modifications. Exp Mol Med 2022, 54, 1601–1616. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Yang, Y.; Sun, B.-F.; Shi, Y.; Yang, X.; et al. FTO-dependent demethylation of N6-methyladenosine regulates mRNA splicing and is required for adipogenesis. Cell Research 2014, 24, 1403–1419. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zhao, Q.; Kaboli, P.J.; Shen, J.; Li, M.; et al. m1A Regulated Genes Modulate PI3K/AKT/mTOR and ErbB Pathways in Gastrointestinal Cancer. Transl Oncol 2019, 12, 1323–1333. [Google Scholar] [CrossRef]
- Zheng, G.; Dahl, A.J.; Niu, Y.; Fedorcsak, P.; Huang, C.-M.; et al. ALKBH5 Is a Mammalian RNA Demethylase that Impacts RNA Metabolism and Mouse Fertility. Molecular Cell 2013, 49, 18–29. [Google Scholar] [CrossRef]
- Zhou, H.-X.; Pang, X. Electrostatic Interactions in Protein Structure, Folding, Binding, and Condensation. Chemical Reviews 2018, 118, 1691–1741. [Google Scholar] [CrossRef]
- Zhu, T.; Roundtree, I.A.; Wang, P.; Wang, X.; Wang, L.; et al. Crystal structure of the YTH domain of YTHDF2 reveals mechanism for recognition of N6-methyladenosine. Cell Research 2014, 24, 1493–1496. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).