Submitted:
03 October 2023
Posted:
04 October 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and methods
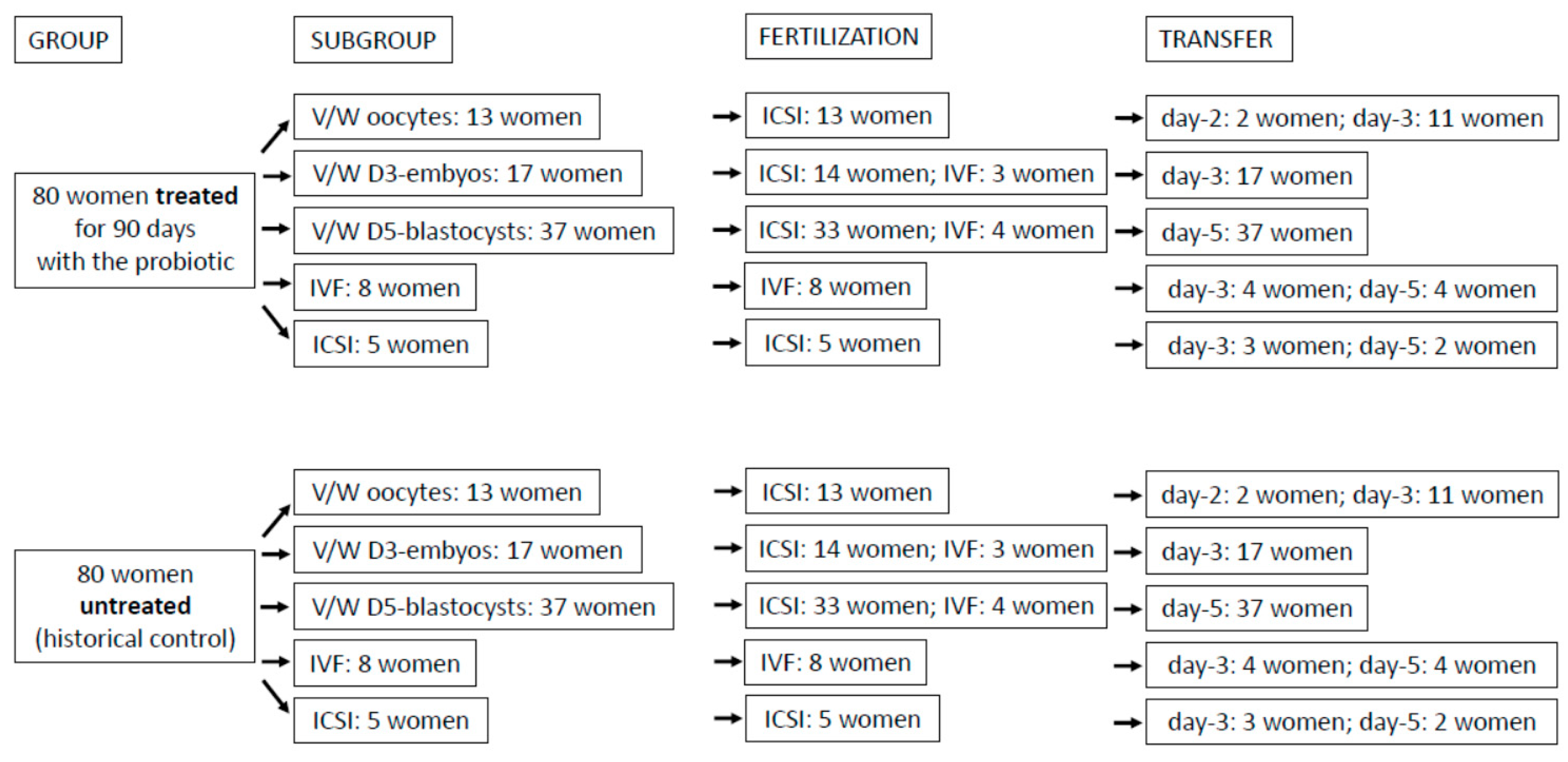
2.1. The study
2.2. Embryo culture and vitrification/warming protocol
2.3. Probiotic product
2.4. Statistical analysis
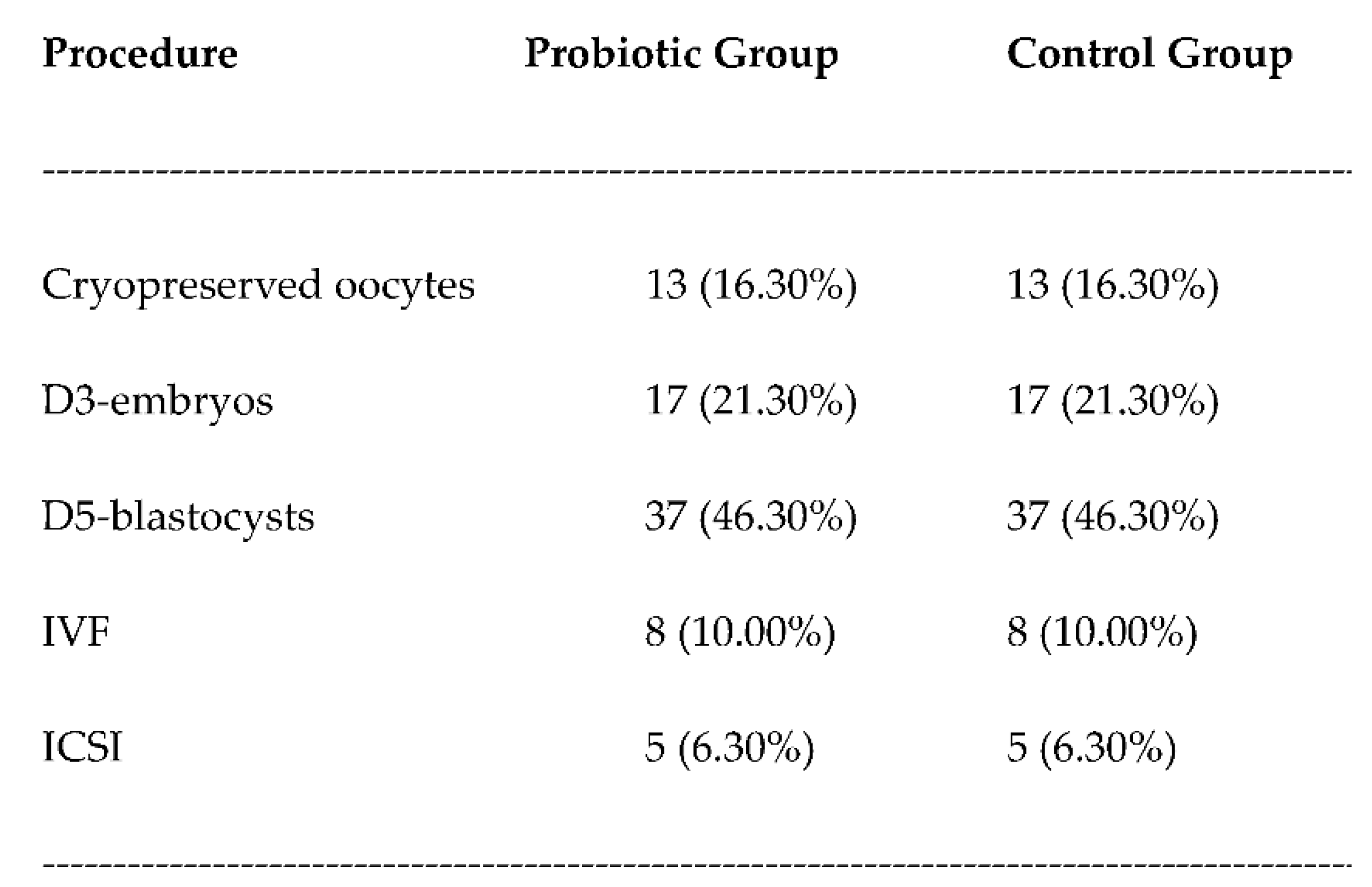
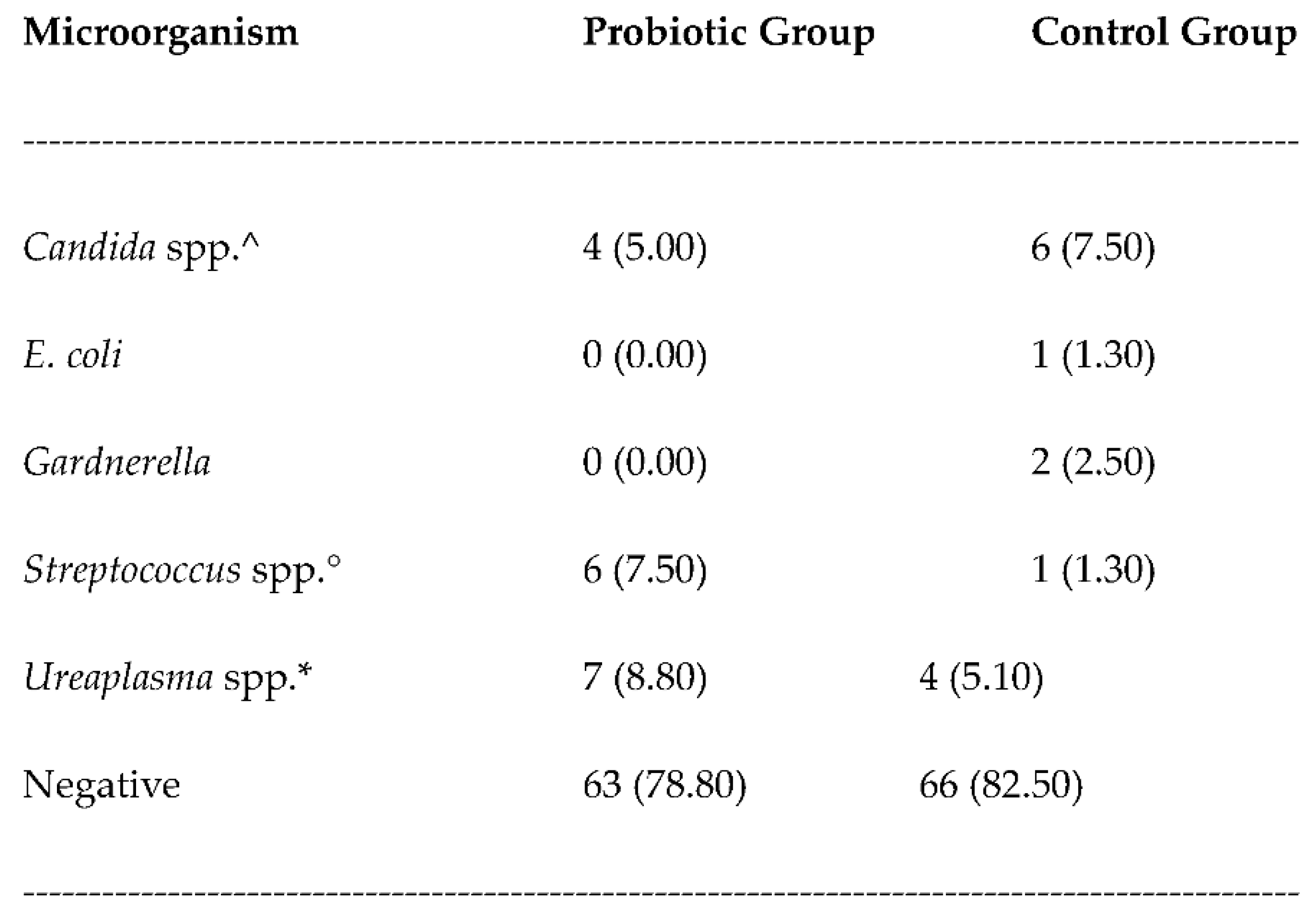
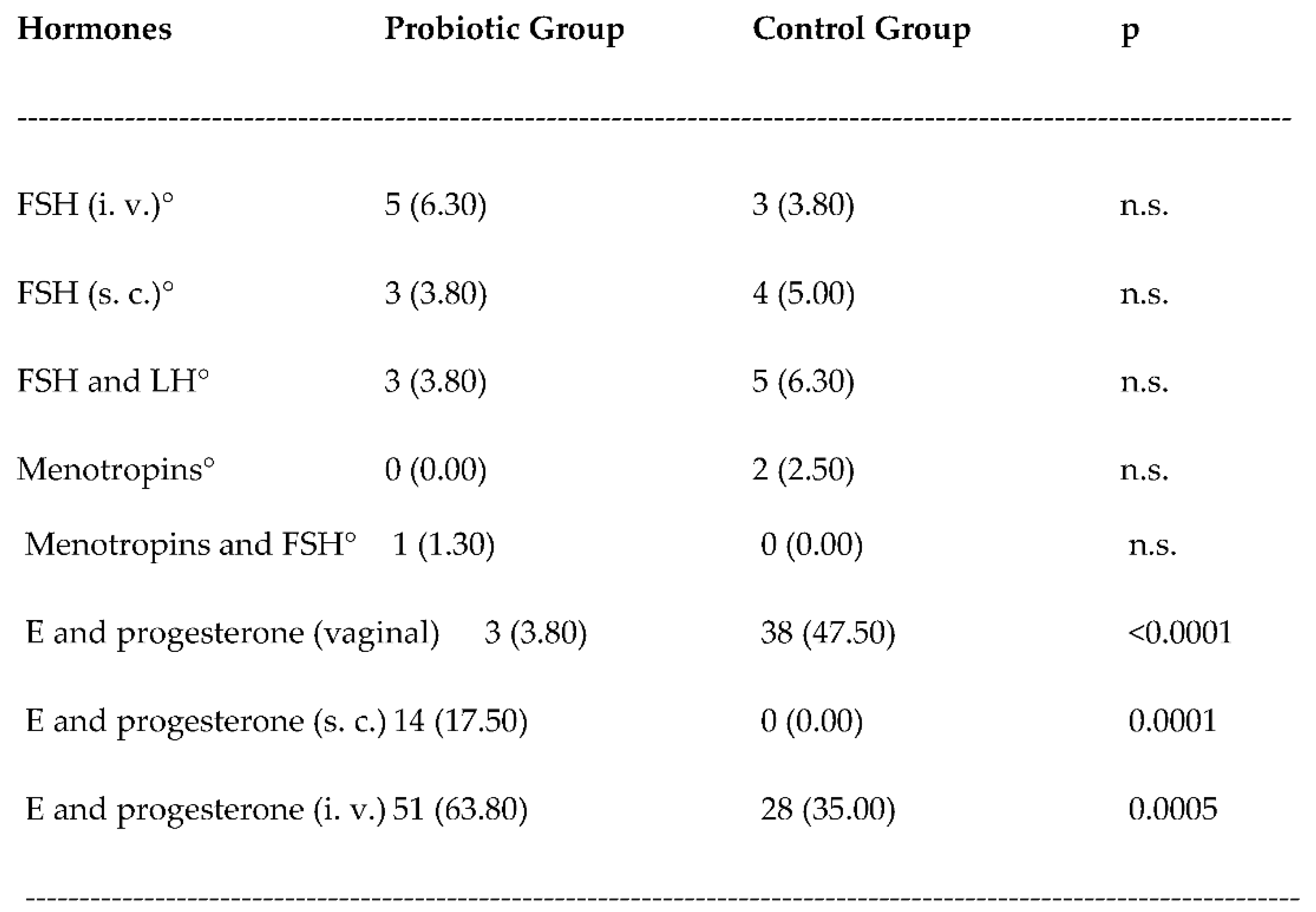
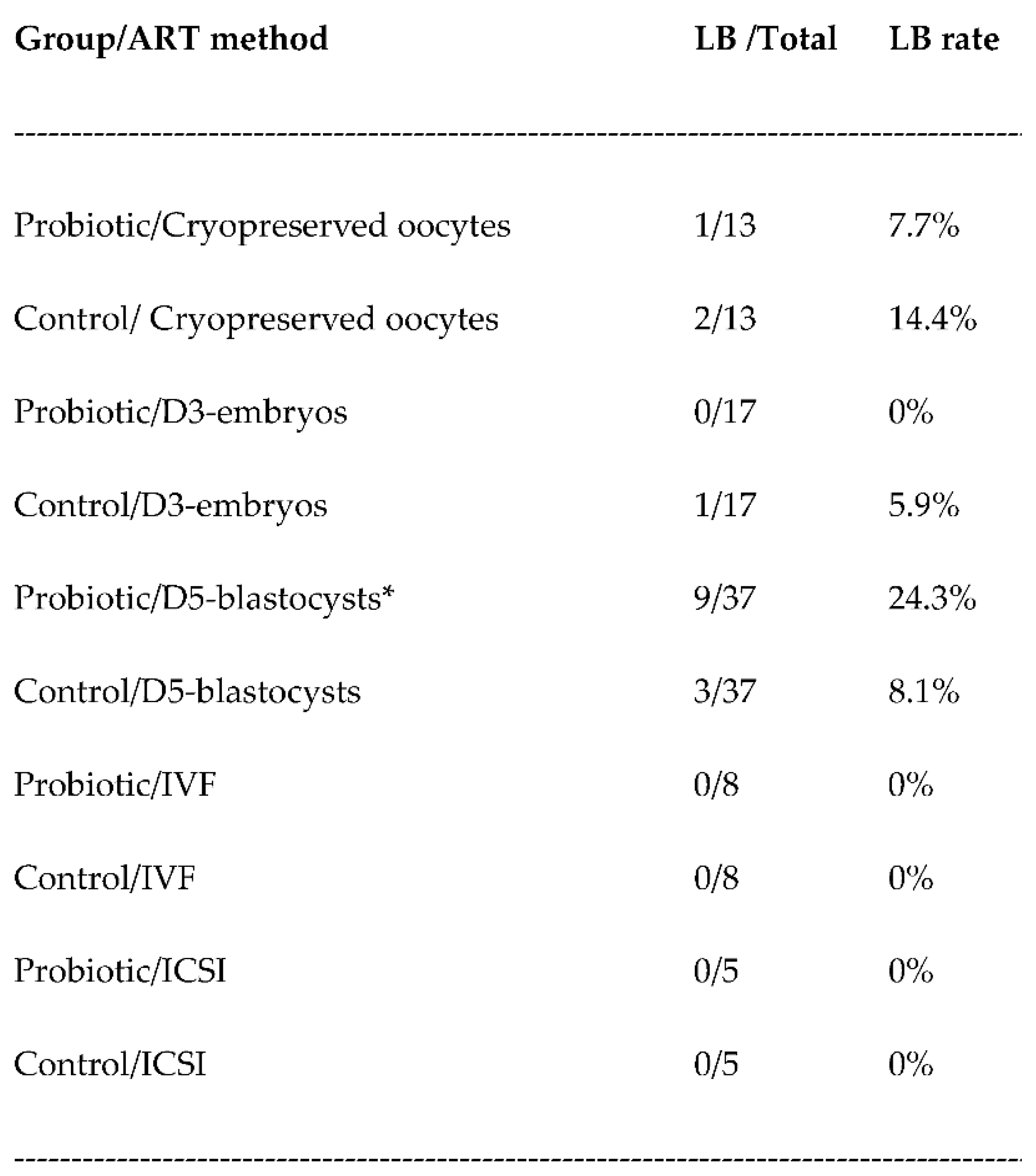
3. Results
3.1. Safety
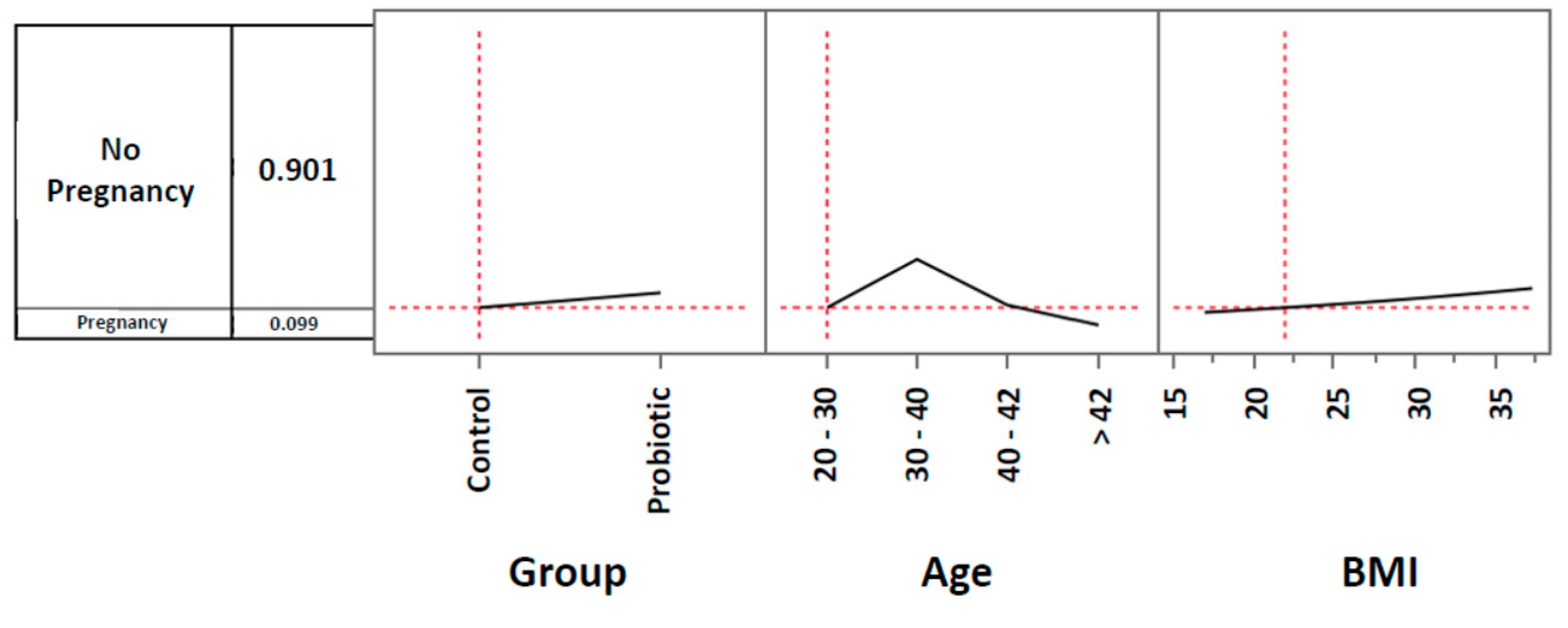
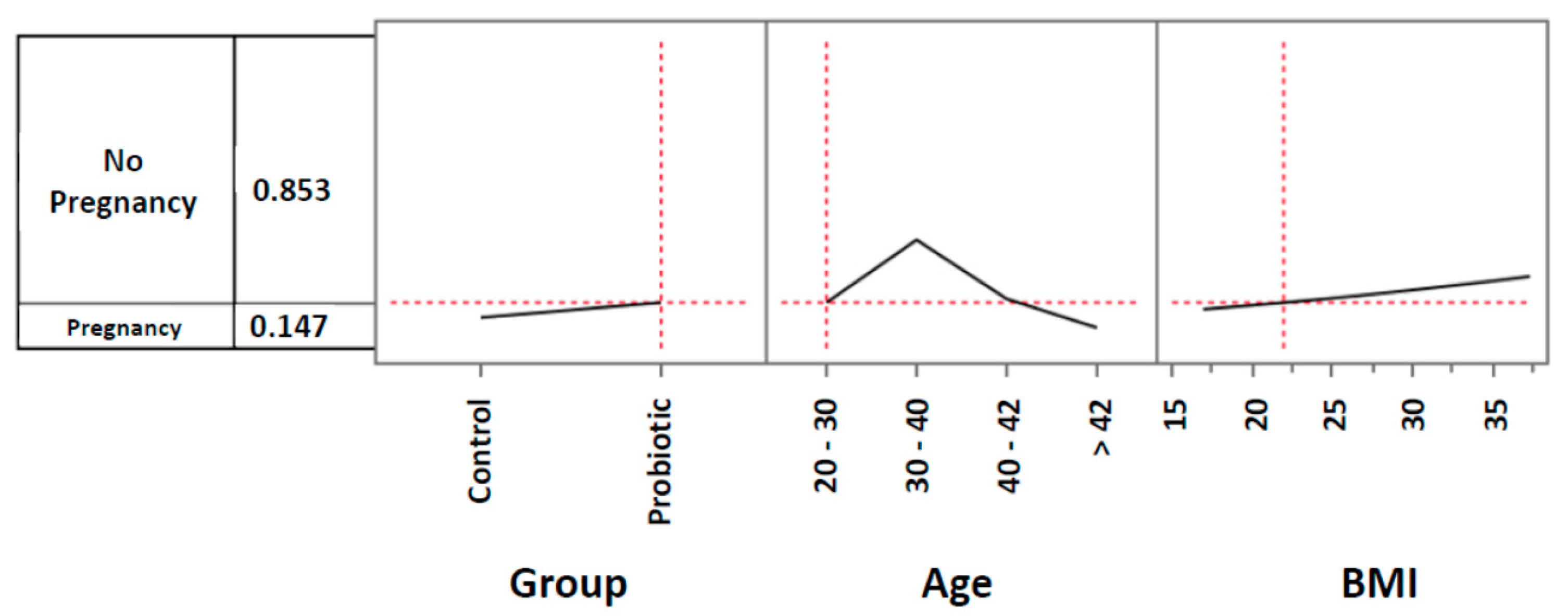
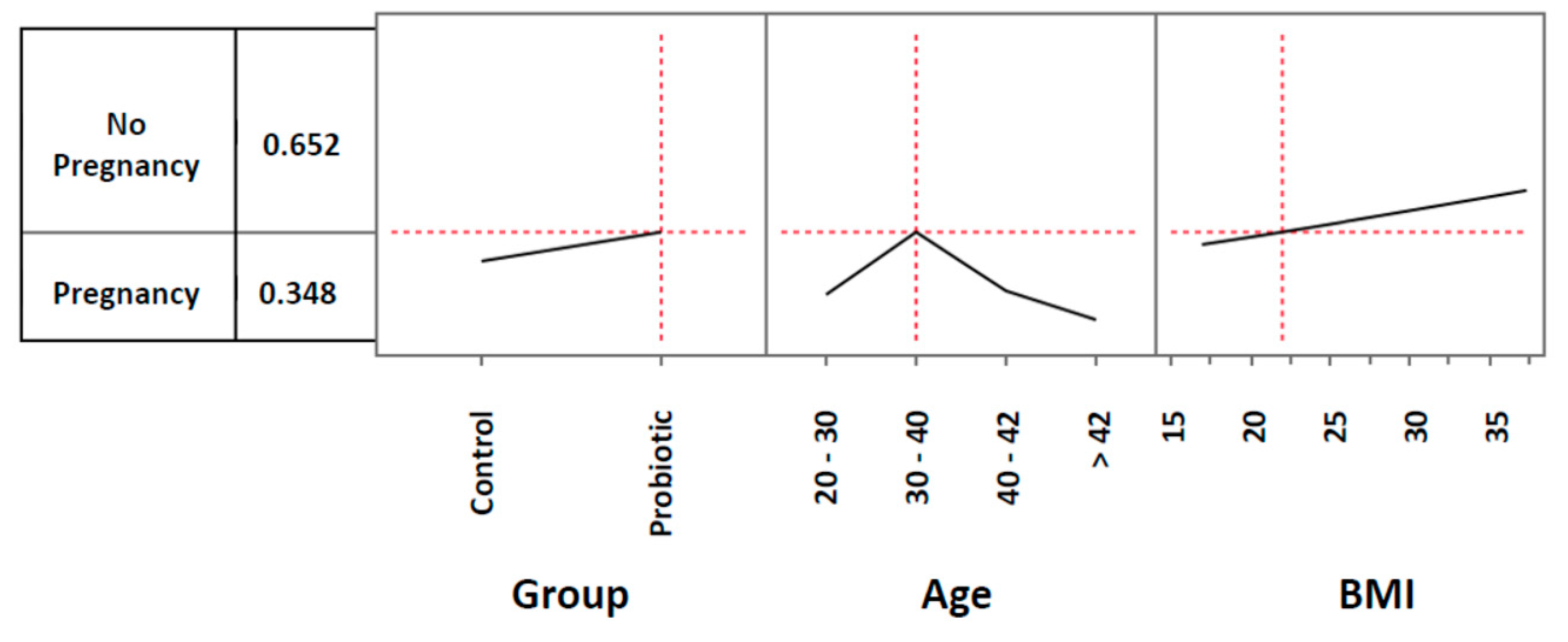
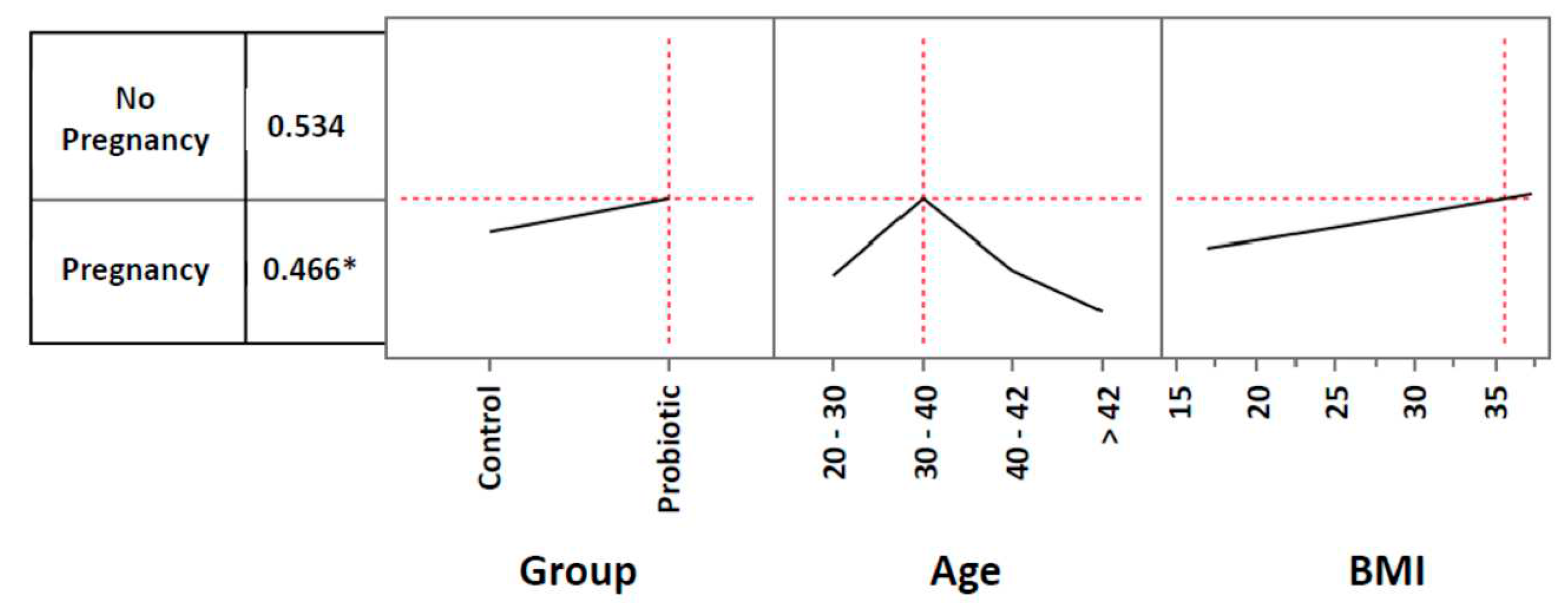
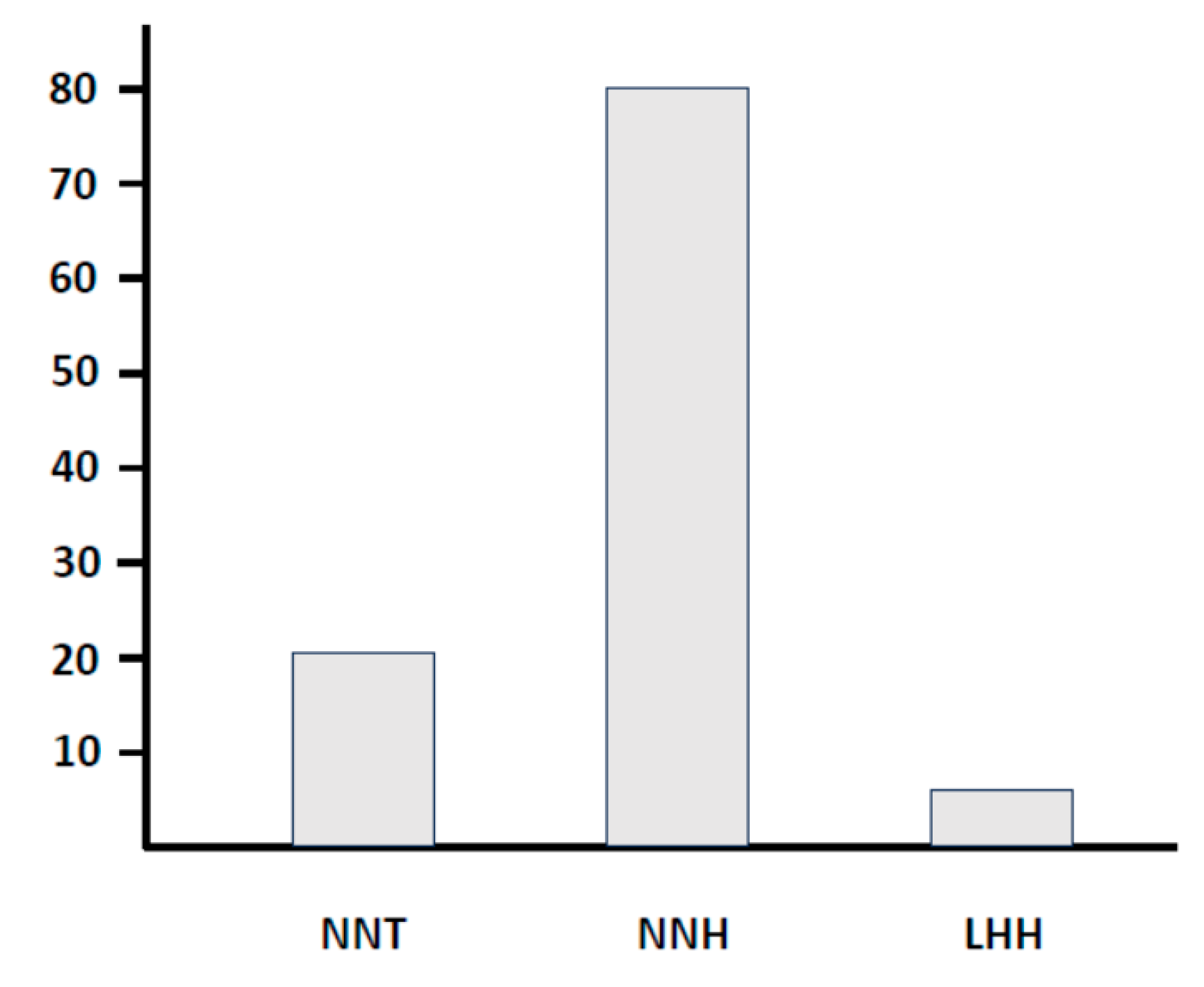
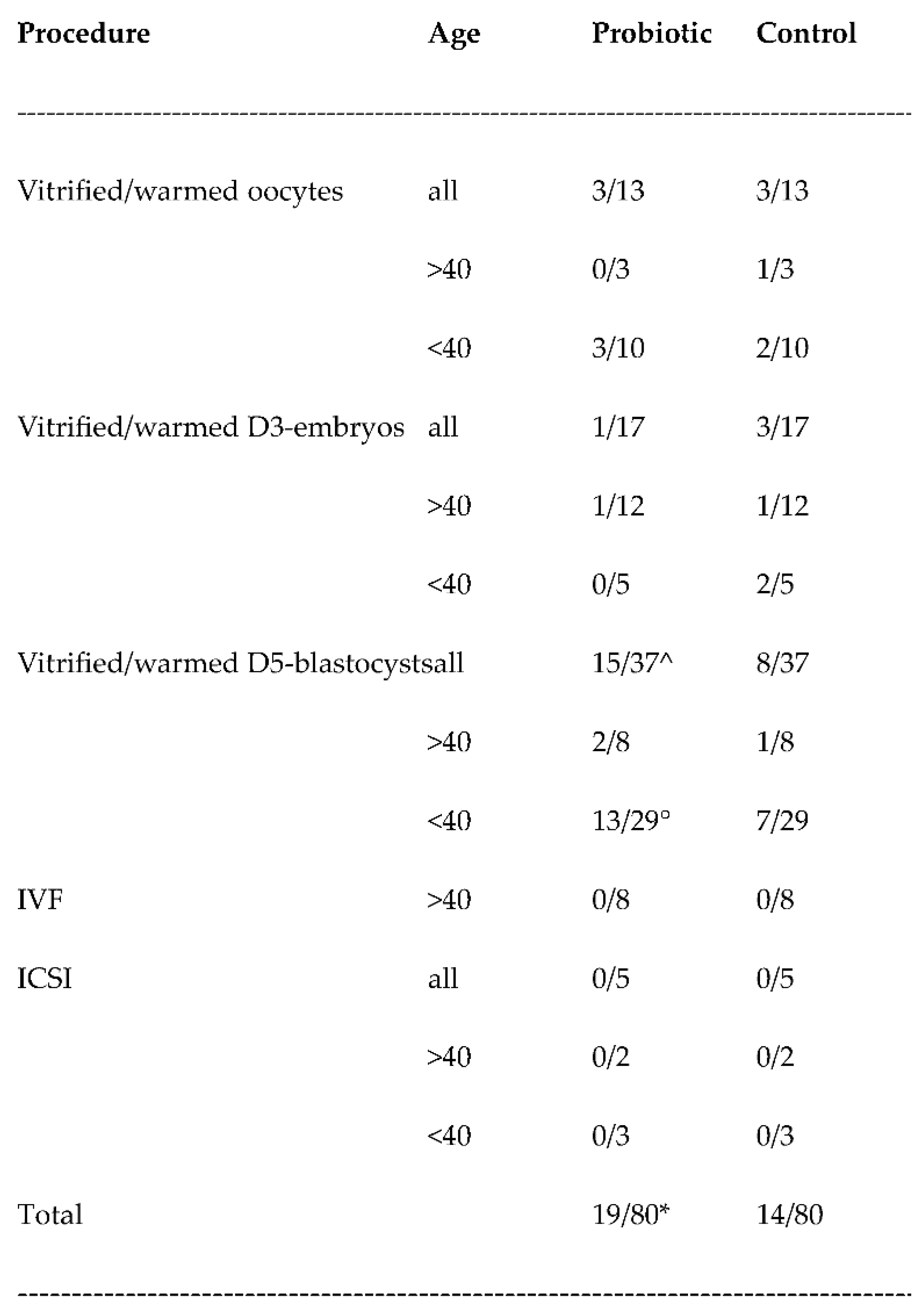
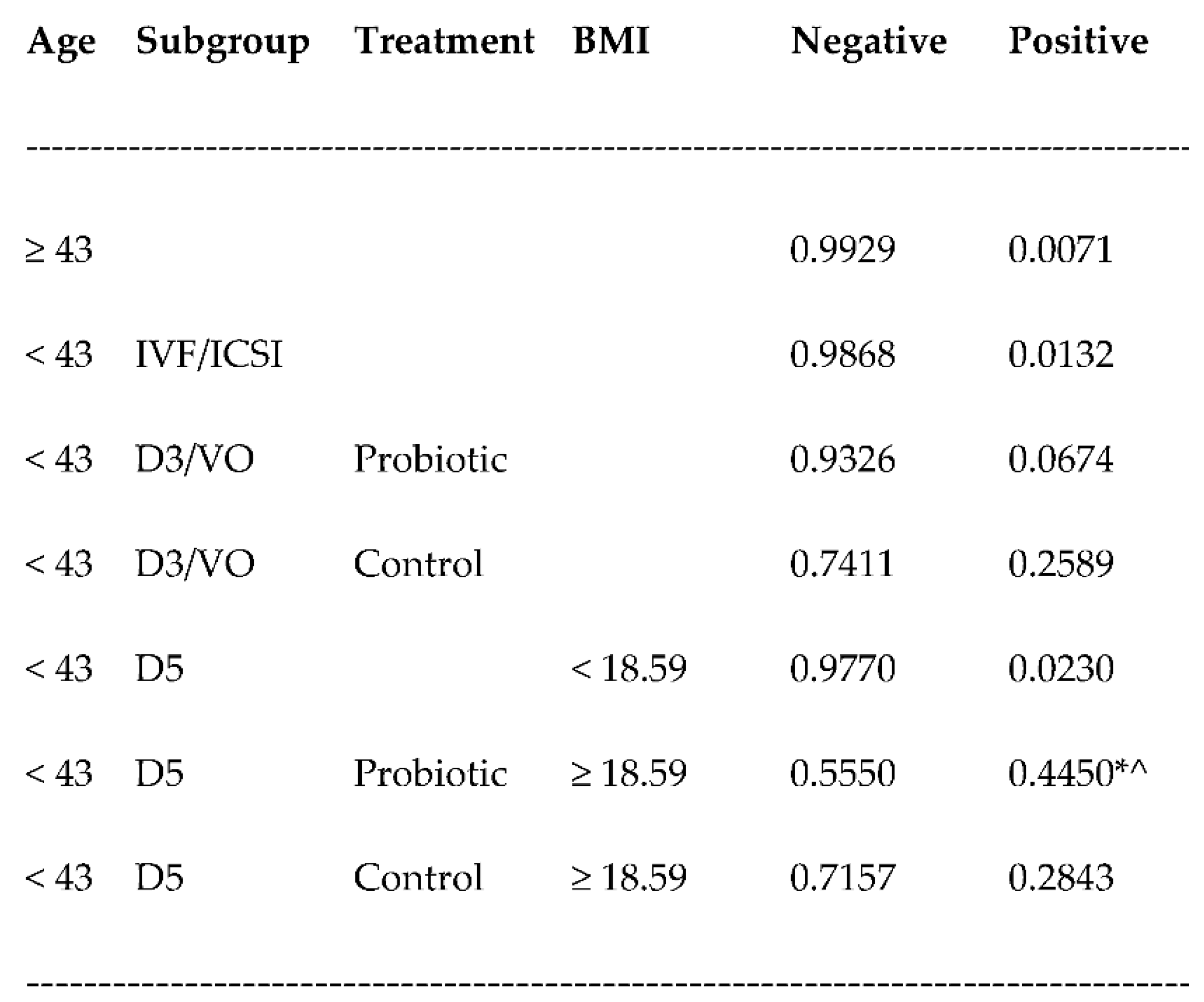
3.2. Pregnancy rates
3.3. Live birth rates
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Buhl Borgstrøm MB, Ahrendt Bjerregaard A, Olsen SF, Gabrielsen A, Humaidan P, Kesmodel US. Food & Fertility Study: study protocol for a Danish multicentre prospective cohort study investigating the association between food intake and semen quality, pregnancy and birth outcomes in infertile women and men. BMJ Open. 2023, 13, e068354. [Google Scholar]
- Boivin J, Bunting L, Collins JA, Nygren KG. International estimates of infertility prevalence and treatment-seeking: potential need and demand for infertility medical care. Hum Reprod. 2007, 22, 1506–1512. [Google Scholar] [CrossRef] [PubMed]
- Ombelet W, Cooke I, Dyer S, Serour G, Devroey P. Infertility and the provision of infertility medical services in developing countries. Hum Reprod Update. 2008, 14, 605–621. [Google Scholar] [CrossRef] [PubMed]
- Pinborg, A. Short- and long-term outcomes in children born after assisted reproductive technology. BJOG. 2019, 126, 145–148. [Google Scholar] [CrossRef]
- Becker G, Castrillo M, Jackson R, Nachtigall RD. Infertility among low-income Latinos. Fertil Steril. 2006, 85, 882–887. [Google Scholar] [CrossRef]
- Hull MG, Cahill DJ. Female infertility. Endocrinol Metab Clin North Am. 1998, 27, 851–876. [Google Scholar] [CrossRef]
- Mori R, Hayakawa T, Hirayama M, Ozawa F, Yoshihara H, Goto S, et al. Cervicovaginal microbiome in patients with recurrent pregnancy loss. J Reprod Immunol. 2023, 157, 103944. [Google Scholar] [CrossRef]
- Ravel J, Gajer P, Abdo Z, Schneider GM, Koenig SS, McCulle SL, et al. Vaginal microbiome of reproductive-age women. Proc Natl Acad Sci U S A. 2011, 108 (Suppl. 1), 4680–4687. [Google Scholar] [CrossRef]
- Greenbaum S, Greenbaum G, Moran-Gilad J, Weintraub AY. Ecological dynamics of the vaginal microbiome in relation to health and disease. Am J Obstet Gynecol. 2019, 220, 324–335. [Google Scholar] [CrossRef]
- Hong X, Ma J, Yin J, Fang S, Geng J, Zhao H, et al. The association between vaginal microbiota and female infertility: a systematic review and meta-analysis. Arch Gynecol Obstet. 2020, 302, 569–578. [Google Scholar] [CrossRef]
- Hong X, Zhao J, Yin J, Zhao F, Wang W, Ding X, et al. The association between the pre-pregnancy vaginal microbiome and time-to-pregnancy: a Chinese pregnancy-planning cohort study. BMC Med. 2022, 20, 246. [Google Scholar]
- Hoeger KM, Dokras A, Piltonen T. Update on PCOS: Consequences, Challenges, and Guiding Treatment. J Clin Endocrinol Metab. 2021, 106, e1071–e1083. [Google Scholar] [CrossRef] [PubMed]
- Silvestris E, de Pergola G, Rosania R, Loverro G. Obesity as disruptor of the female fertility. Reprod Biol Endocrinol. 2019, 16, 22. [Google Scholar]
- Gu Y, Zhou G, Zhou F, Li Y, Wu Q, He H, et al. Gut and Vaginal Microbiomes in PCOS: Implications for Women’s Health. Front Endocrinol (Lausanne). 2022, 13, 808508. [Google Scholar] [CrossRef] [PubMed]
- Hong X, Qin P, Huang K, Ding X, Ma J, Xuan Y, et al. Association between polycystic ovary syndrome and the vaginal microbiome: A case-control study. Clin Endocrinol (Oxf). 2020, 93, 52–60. [Google Scholar] [CrossRef]
- Oh HY, Seo SS, Kong JS, Lee JK, Kim MK. Association between Obesity and Cervical Microflora Dominated by Lactobacillus iners in Korean Women. J Clin Microbiol. 2015, 53, 3304–3309. [Google Scholar] [CrossRef]
- Singer M, Borg M, Ouburg S, Morré SA. The relation of the vaginal microbiota to early pregnancy development during in vitro fertilization treatment-A meta-analysis. J Gynecol Obstet Hum Reprod. 2019, 48, 223–229. [Google Scholar] [CrossRef]
- Vergaro P, Tiscornia G, Barragán M, García D, Rodriguez A, Santaló J, et al. Vaginal microbiota profile at the time of embryo transfer does not affect live birth rate in IVF cycles with donated oocytes. Reprod Biomed Online. 2019, 38, 883–891. [Google Scholar] [CrossRef]
- Fu M, Zhang X, Liang Y, Lin S, Qian W, Fan S. Alterations in Vaginal Microbiota and Associated Metabolome in Women with Recurrent Implantation Failure. mBio. 2020, 11, e03242–19. [Google Scholar]
- Amato V, Papaleo E, Pasciuta R, Viganò P, Ferrarese R, Clementi N, et al. Differential Composition of Vaginal Microbiome, but Not of Seminal Microbiome, Is Associated With Successful Intrauterine Insemination in Couples With Idiopathic Infertility: A Prospective Observational Study. Open Forum Infect Dis. 2019, 7, ofz525. [Google Scholar]
- Kennedy KM, de Goffau MC, Perez-Muñoz ME, Arrieta MC, Bäckhed F, Bork P, et al. Questioning the fetal microbiome illustrates pitfalls of low-biomass microbial studies. Nature. 2023, 613, 639–649. [Google Scholar] [CrossRef] [PubMed]
- Moreno I, Simon C. Deciphering the effect of reproductive tract microbiota on human reproduction. Reprod Med Biol. 2018, 18, 40–50. [Google Scholar]
- Moreno I, Garcia-Grau I, Perez-Villaroya D, Gonzalez-Monfort M, Bahçeci M, Barrionuevo MJ, et al. Endometrial microbiota composition is associated with reproductive outcome in infertile patients. Microbiome. 2022, 10, 1. [Google Scholar]
- Toson B, Simon C, Moreno I. The Endometrial Microbiome and Its Impact on Human Conception. Int J Mol Sci. 2022, 23, 485. [Google Scholar] [CrossRef] [PubMed]
- Peuranpää P, Holster T, Saqib S, Kalliala I, Tiitinen A, Salonen A, et al. Female reproductive tract microbiota and recurrent pregnancy loss: a nested case-control study. Reprod Biomed Online. 2022, 45, 1021–1031. [Google Scholar] [CrossRef]
- Jepsen IE, Saxtorph MH, Englund ALM, Petersen KB, Wissing MLM, Hviid TVF, et al. Probiotic treatment with specific lactobacilli does not improve an unfavorable vaginal microbiota prior to fertility treatment-A randomized, double-blinded, placebo-controlled trial. Front Endocrinol (Lausanne). 2022, 13, 1057022. [Google Scholar] [CrossRef]
- Haahr T, Freiesleben NC, Pinborg A, Nielsen HS, Hartvig V, Mikkelsen AL, et al. Effect of clindamycin and a live biotherapeutic on the reproductive outcomes of IVF patients with abnormal vaginal microbiota: protocol for a double-blind, placebo-controlled multicentre trial. BMJ Open. 2020, 10, e035866. [Google Scholar] [CrossRef]
- Bertuccioli A, Cardinali M, Zonzini G, Cazzaniga M, Di Pierro F. Lactobacillus crispatus M247: Characteristics of a Precision Probiotic Instrument for Gynecological and Urinary Well-Being. Microbiology Research. 2022, 13, 963–971. [Google Scholar] [CrossRef]
- Fan J, Wang M, Wang C, Cao Y. Advances in human chorionic gonadotropin detection technologies: a review. Bioanalysis. 2017, 9, 1509–1529. [Google Scholar] [CrossRef]
- Di Pierro F, Polzonetti V, Patrone V, Morelli L. Microbiological Assessment of the Quality of Some Commercial Products Marketed as Lactobacillus crispatus-Containing Probiotic Dietary Supplements. Microorganisms. 2019, 7, 524. [Google Scholar] [CrossRef]
- Altman, DG. Confidence intervals for the number needed to treat. BMJ. 1998, 317, 1309–1312. [Google Scholar] [CrossRef] [PubMed]
- Friedman, JH. Number needed to treat (NNT); number needed to harm (NNH). Med Health R I. 2012, 95, 2–3. [Google Scholar] [PubMed]
- Andrade, C. Likelihood of Being Helped or Harmed as a Measure of Clinical Outcomes in Psychopharmacology. J Clin Psychiatry. 2017, 78, e73–e75. [Google Scholar] [CrossRef]
- Deyhoul N, Mohamaddoost T, Hosseini M. Infertility-related risk factors: a systematic review. Int J Women’s Health Reprod Sci. 2017, 5, 24–29. [Google Scholar] [CrossRef]
- Haller-Kikkatalo K, Salumets A, Uibo R. Review on autoimmune reactions in female infertility: antibodies to follicle stimulating hormone. Clin Dev Immunol. 2012, 2012, 762541. [Google Scholar]
- Ravel J, Moreno I, Simón C. Bacterial vaginosis and its association with infertility, endometritis, and pelvic inflammatory disease. Am J Obstet Gynecol. 2021, 224, 251–257. [Google Scholar] [CrossRef]
- Farquhar C, Marjoribanks J. Assisted reproductive technology: an overview of Cochrane Reviews. Cochrane Database Syst Rev. 2018, 8, CD010537. [Google Scholar]
- Korrovits P, Lapp E, Mändar R. Couple-related factors of ART outcome. Clin Exp Obstet Gynecol. 2016, 43, 747–750. [Google Scholar] [CrossRef]
- Koort K, Sõsa K, Türk S, Lapp E, Talving E, Karits P, et al. Lactobacillus crispatus-dominated vaginal microbiome and Acinetobacter-dominated seminal microbiome support beneficial ART outcome. Acta Obstet Gynecol Scand. 2023, 102, 921–934. [Google Scholar] [CrossRef]
- Di Pierro F, Bertuccioli A, Cazzaniga M, Zerbinati N, Guasti L. A clinical report highlighting some factors influencing successful vaginal colonization with probiotic Lactobacillus crispatus. A clinical report highlighting some factors influencing successful vaginal colonization with probiotic Lactobacillus crispatus. Minerva Medica 2023, 114. [Google Scholar]
- Di Pierro F, Criscuolo AA, Dei Giudici A, Senatori R, Sesti F, Ciotti M, Piccione E. Oral administration of Lactobacillus crispatus M247 to papillomavirus-infected women: results of a preliminary, uncontrolled, open trial. Minerva Obstet Gynecol. 2021, 73, 621–631. [Google Scholar]
- Dellino M, Cascardi E, Laganà AS, Di Vagno G, Malvasi A, Zaccaro R, et al. Lactobacillus crispatus M247 oral administration: Is it really an effective strategy in the management of papillomavirus-infected women? Infect Agent Cancer. 2022, 17, 53.
- Di Pierro F, Bertuccioli A, Cattivelli D, Soldi S, Elli M. Lactobacillus crispatus M247: A possible tool to counteract CST IV. Nutrafoods. 2018, 17, 169–172.
- Lev-Sagie A, Goldman-Wohl D, Cohen Y, Dori-Bachash M, Leshem A, Mor U, et al. Vaginal microbiome transplantation in women with intractable bacterial vaginosis. Nat Med. 2019, 25, 1500–1504. [Google Scholar] [CrossRef] [PubMed]
- Wrønding T, Vomstein K, Bosma EF, Mortensen B, Westh H, Heintz JE, et al. Antibiotic-free vaginal microbiota transplant with donor engraftment, dysbiosis resolution and live birth after recurrent pregnancy loss: a proof-of-concept case study. eClinicalMedicine. 2023, 2023, 102070. [Google Scholar]
- Chadchan SB, Singh V, Kommagani R. Female reproductive dysfunctions and the gut microbiota. J Mol Endocrinol. 2022, 69, R81–R94. [Google Scholar] [CrossRef]
- Jiang I, Yong PJ, Allaire C, Bedaiwy MA. Intricate Connections between the Microbiota and Endometriosis. Int J Mol Sci. 2021, 22, 5644. [Google Scholar] [CrossRef]
- Salliss ME, Farland LV, Mahnert ND, Herbst-Kralovetz MM. The role of gut and genital microbiota and the estrobolome in endometriosis, infertility and chronic pelvic pain. Hum Reprod Update. 2021, 28, 92–131. [Google Scholar] [CrossRef]
- Voltan S, Martines D, Elli M, Brun P, Longo S, Porzionato A, et al. Lactobacillus crispatus M247-derived H2O2 acts as a signal transducing molecule activating peroxisome proliferator activated receptor-gamma in the intestinal mucosa. Gastroenterology. 2008, 135, 1216–1227. [Google Scholar] [CrossRef]
- Castagliuolo I, Galeazzi F, Ferrari S, Elli M, Brun P, Cavaggioni A, et al. Beneficial effect of auto-aggregating Lactobacillus crispatus on experimentally induced colitis in mice. FEMS Immunol Med Microbiol. 2005, 43, 197–204. [Google Scholar] [CrossRef]
- Voltan S, Castagliuolo I, Elli M, Longo S, Brun P, D’Incà R, et al. Aggregating phenotype in Lactobacillus crispatus determines intestinal colonization and TLR2 and TLR4 modulation in murine colonic mucosa. Clin Vaccine Immunol. 2007, 14, 1138–1148. [Google Scholar] [CrossRef] [PubMed]
- Pantazis A, Clark SJ. A parsimonious characterization of change in global age-specific and total fertility rates. PLoS One. 2018, 13, e0190574. [Google Scholar]
- Liu K, Case A, Reproductive Endocrinology And Infertility Committee. Advanced reproductive age and fertility. J Obstet Gynaecol Can. 2011, 33, 1165–1175. [Google Scholar] [CrossRef] [PubMed]
- Cimadomo D, Fabozzi G, Vaiarelli A, Ubaldi N, Ubaldi FM, Rienzi L. Impact of Maternal Age on Oocyte and Embryo Competence. Front Endocrinol (Lausanne). 2018, 9, 327. [Google Scholar] [CrossRef] [PubMed]
- Curtiss N, Balachandran A, Krska L, Peppiatt-Wildman C, Wildman S, Duckett J. Age, menopausal status and the bladder microbiome. Eur J Obstet Gynecol Reprod Biol. 2018, 228, 126–129. [Google Scholar] [CrossRef]
- Satpathy HK, Fleming A, Frey D, Barsoom M, Satpathy C, Khandalavala J. Maternal obesity and pregnancy. Postgrad Med. 2008, 120, E01–E09. [Google Scholar]
- Sermondade N, Huberlant S, Bourhis-Lefebvre V, Arbo E, Gallot V, Colombani M, et al. Female obesity is negatively associated with live birth rate following IVF: a systematic review and meta-analysis. Hum Reprod Update. 2019, 25, 439–451. [Google Scholar] [CrossRef]
- Fabozzi G, Cimadomo D, Allori M, Vaiarelli A, Colamaria S, Argento C, et al. Maternal body mass index associates with blastocyst euploidy and live birth rates: the tip of an iceberg? Reprod Biomed Online. 2021, 43, 645–654. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





