Submitted:
04 October 2023
Posted:
06 October 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
3. Results
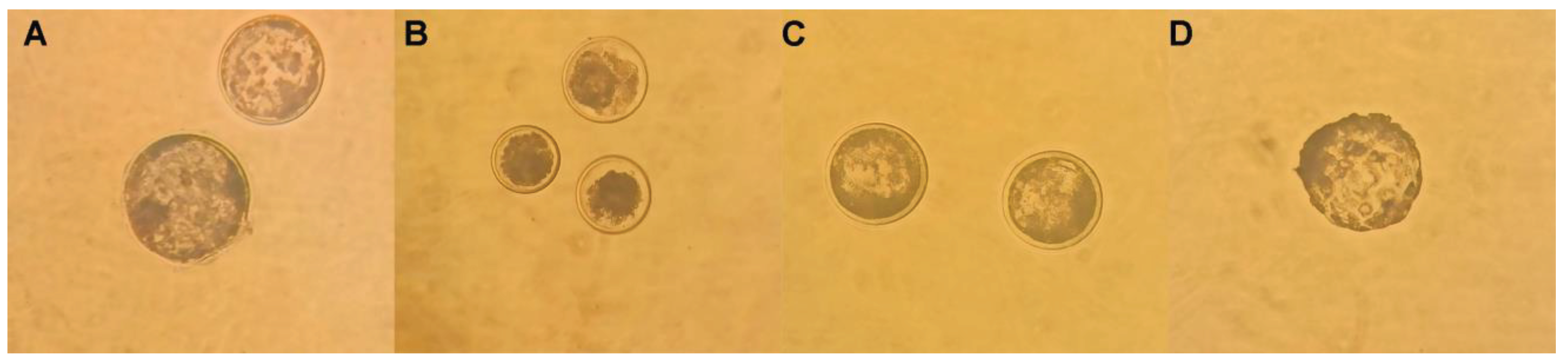
3.1. Oocyte collection
3.2. In vitro maturation (IVM) of oocytes, in vitro fertilization (IVF) and in vitro development (IVD) of embryos
3.3. Vitrification and devitrification of embryos
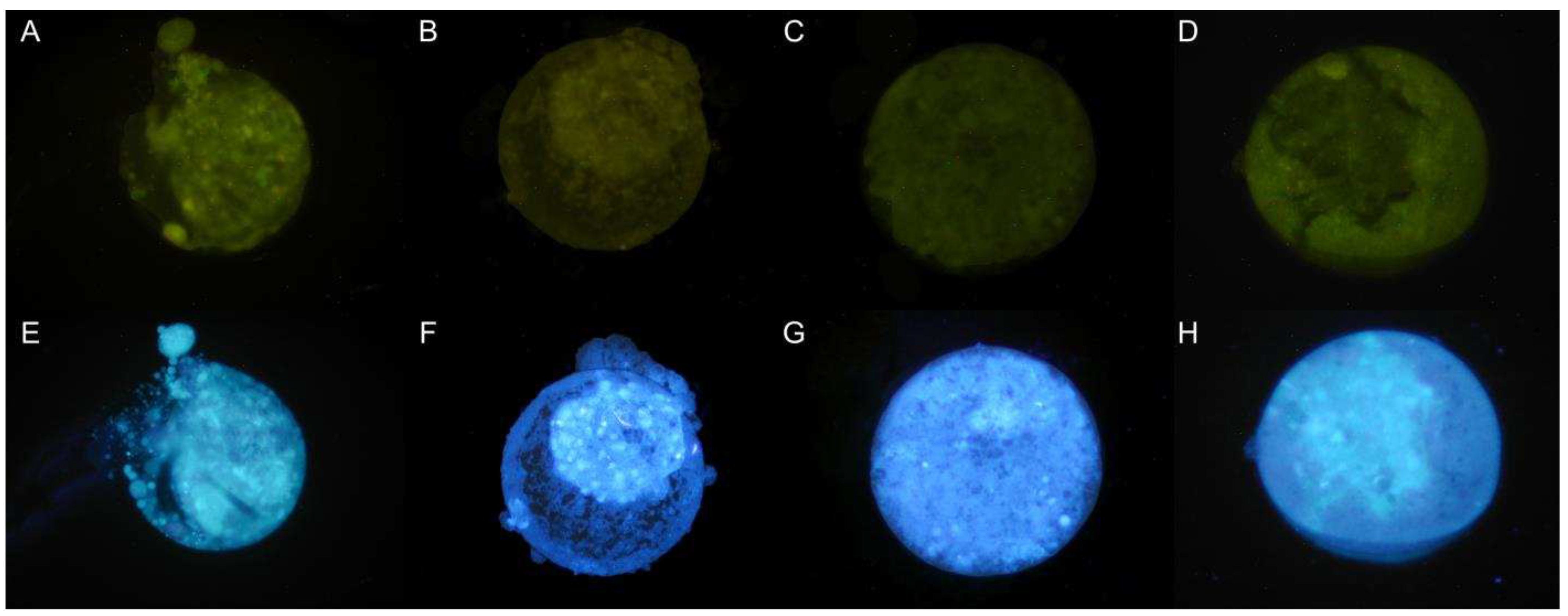
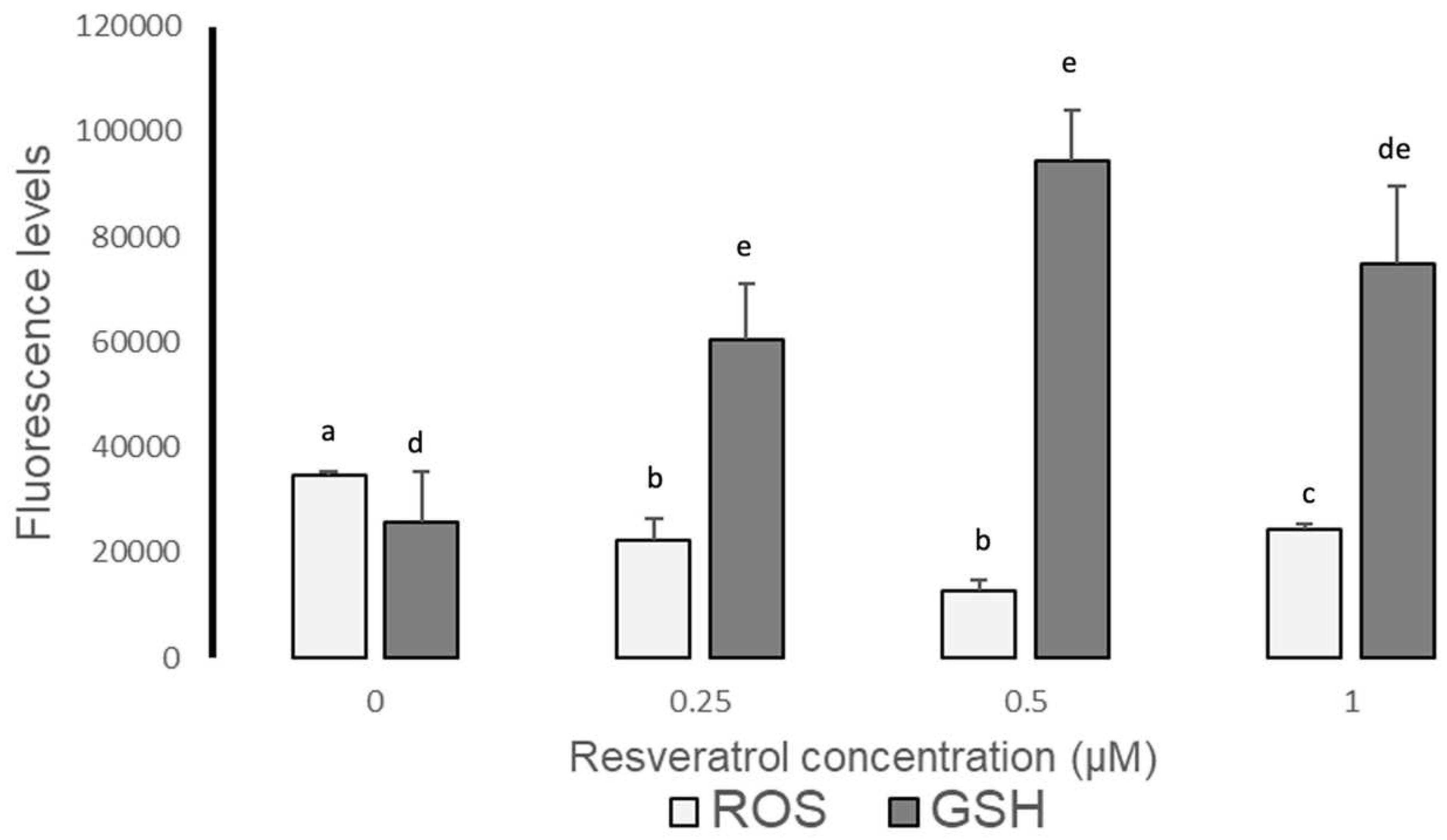
3.4. ROS and GSH levels in devitrified embryos
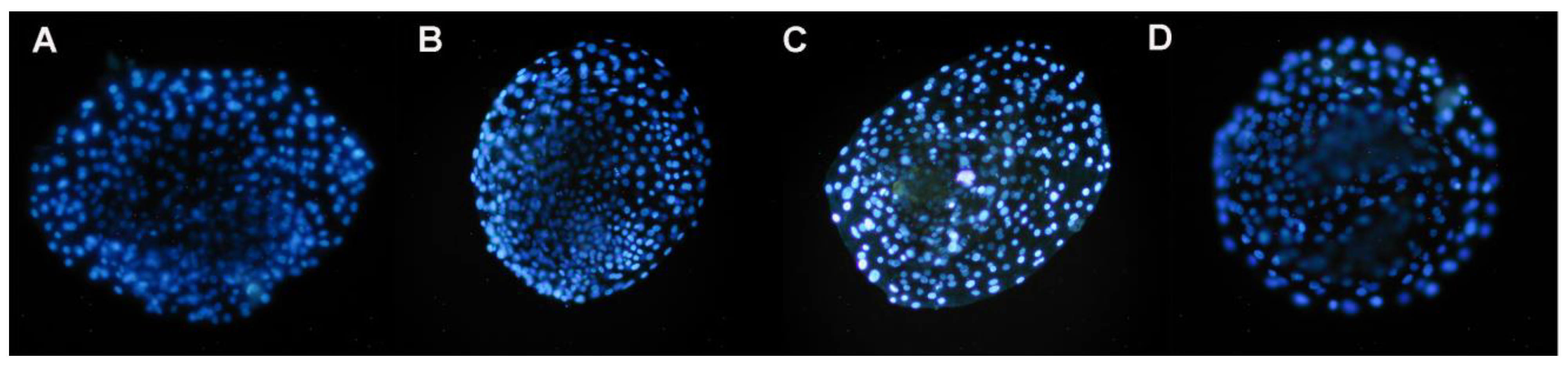
3.5. DAPI staining of nuclei
3.5.1. Presence of nuclei in early blastocysts prior to vitrification
3.5.2. Presence of nuclei in late blastocysts after devitrification
4. Discussion
5. Conclusions
6. Patents
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Parra-Cortés R. I., Valderrama-Rodas M. & Martínez-Correal G. (2021) Situación actual y perspectivas de la ganadería de bovinos Criollos en América Latina. Archivos Latinoamericanos de Producción Animal, 2(3-4). [CrossRef]
- Rodríguez Suástegui J. L., Romo García S. D., Casas Hernández E., Hernández Pichardo J. E. (2017). Desarrollo de mórulas de ovino en medio simple o secuencial: Relación entre evaluación morfológica y viabilidad embrionaria. Revista de Salud Animal, 39(1), 9-18.
- Martínez Ibarra J.L., Espinoza Mendoza E.A., Rangel Santos R., Ambriz-García D.A., Navarro-Maldonado M.C. (2019). Effect of resveratrol on the in vitro maturation of ovine (Ovis aries) oocytes and the subsequent development of handmade cloned embryos. Veterinaria México, 5(4). [CrossRef]
- Mukherjee, A., Malik, H., Saha, A. P., Dubey, A., Singhal, D. K., Boateng, S., Saugandhika, S., Kumar, S., De, S., Guha, S. K., & Malakar, D. (2014). Resveratrol treatment during goat oocytes maturation enhances developmental competence of parthenogenetic and hand-made cloned blastocysts by modulating intracellular glutathione level and embryonic gene expression. Journal of Assisted Reproduction and Genetics, 31(2), 229–239. [CrossRef]
- Zabihi, A., Shabankareh, H. K., Hajarian, H., & Foroutanifar, S. (2021). In vitro maturation medium supplementation with resveratrol improves cumulus cell expansion and developmental competence of Sanjabi sheep oocytes. Livestock Science, 243. [CrossRef]
- Madrid Gaviria, S., López Herrera, A., Urrego, R., Restrepo Betancur, G., & Echeverri Zuluaga, J. J. (2019). Effect of resveratrol on vitrified in vitro produced bovine embryos: Recovering the initial quality. Cryobiology, 89, 42–50. [CrossRef]
- Kwak, S. S., Cheong, S. A., Jeon, Y., Lee, E., Choi, K. C., Jeung, E. B., & Hyun, S. H. (2012). The effects of resveratrol on porcine oocyte in vitro maturation and subsequent embryonic development after parthenogenetic activation and in vitro fertilization. Theriogenology, 78(1), 86–101. [CrossRef]
- Abdul Rahman, N.-S., Mohamed Noor Khan, N.-A., Eshak, Z., Sarbandi, M.-S., Mohammad Kamal, A.-A., Abd Malek, M., Abdullah, F., Abdullah, M.A. & Othman, F. (2022). Exogenous L-Glutathione improves vitrification outcomes in murine preimplantation embryos. Antioxidants, 11, 2100: 1-21. https:// doi.org/10.3390/antiox11112100.
- García-Martínez, T., Vendrell-Flotats, M., Martínez-Rodero, I., Ordóñez-León, E. A., Álvarez-Rodríguez, M., López-Béjar, M., Yeste, M. & Mogas, T. (2020). Glutathione Ethyl Ester Protects In Vitro-Maturing Bovine Oocytes against Oxidative Stress Induced by Subsequent Vitrification/Warming. International Journal of Molecular Sciences, 21, 7547: 1-22. [CrossRef]
- Xiang, D., Jia, B., Zhang, B., Liang, J., Hong, Q., Wei, H., & Wu, G. (2022). Astaxanthin supplementation improves the subsequent developmental competence of vitrified porcine zygotes. Frontiers in Veterinary Science, 9:871289. [CrossRef]
- Giraldo G., J. J. (2011). Efecto del crioprotector dimetilformamida sobre la viabilidad de embriones bovinos producidos in vitro. Tesis Maestría. Universidad Nacional de Colombia, 96 pages. [CrossRef]
- Asociación para el Estudio de la Biología de la Reproducción (ASEBIR). (2015). Criterios ASEBIR de valoración morfológica de oocitos, embriones tempranos y blastocistos humanos. Cuadernos de Embriología Clínica. Madrid, 3er Edición: 9–75.
- Filipiak Y., Larocca C., Martínez M. (2017). Comportamiento del Semen Bovino Sexado Congelado-Descongelado en Fertilización in vitro (FIV) Capacitado Mediante BO en dos Concentraciones versus Percoll. International Journal of Morphology, 35(4), 1337-1341.
- Salgado-Cruz E., y Lopera-Vásquez R. (2020). Aspectos esenciales sobre las técnicas de fertilización in vitro en bovinos”. Revista de Investigaciones Veterinarias del Perú, 31(3), e17138. [CrossRef]
- Bhat, M. H., Sharma, V., Khan, F. A., Naykoo, N. A., Yaqoob, S. H., Vajta, G., Khan, H. M., Fazili, M. R., Ganai, N. A., & Shah, R. A. (2015). Open pulled straw vitrification and slow freezing of sheep IVF embryos using different cryoprotectants. Reproduction, Fertility, and Development, 27(8), 1175–1180. [CrossRef]
- Vajta G. (2000). Vitrification of the oocytes and embryos of domestic animals. Animal Reproduction Science, 60-61, 357–364. [CrossRef]
- Sosa F. & Hansen P. J. (2019) Protocol for embryo vitrification using open pulled straws. Procedures for In Vitro Production of Bovine Embryos - University of Florida.
- González Mendoza, D. F. (2020). Efecto del protocolo de vitrificación y sistemas de empaque sobre la tasa de supervivencia de embriones ovinos obtenidos in vivo. Tesis Maestría. Universidad Nacional de Colombia.
- Vazquez-Avendaño, J. R., Hernández-Martínez, S., Hernández-Pichardo, J. E., Rivera-Rebolledo, J. A., Ambríz-García, D. A., & Navarro-Maldonado, M. del C. (2017). Efecto del uso de medio secuencial humano en la producción de blastocistos de hembra Ovis canadensis mexicana por clonación manual. Acta Zoológica Mexicana (n.s.), 33(2), 328–338. [CrossRef]
- Guerin, P., El Mouatassim, S. & Menezo, Y. (2001). Oxidative stress y protección contra especies reactivas de oxígeno en el embrión preimplantacional y su entorno. Human Reproduction Update, 7: 175–189.
- Méndez, M. S., Argudo, D. E., Soria, M. E., Galarza, L. R., & Perea, F. P. (2020). Efecto de la adición de melatonina en el medio de maduración y/o vitrificación de ovocitos sobre la producción in vitro de embriones bovinos. Revista de Investigaciones Veterinarias del Perú, 31(1), e17557. Epub 31 de marzo de 2020. [CrossRef]
- Miclea I., Pacala N., Hettig A., Zahan M. & Miclea V. (2012). Las com binaciones de alfa tocoferol y ácido ascórbico influyen en la maduración de los ovocitos de oveja. Artículos Científicos Ciencias Animales y Biotecnologías, 45: 310–313.
- Sollecito, N.V., Pereira, E. C. M., Grázia, J. G. V., Neves, B. P., Couto, B. V. R., Andrade, V. B., Miranda, M. S., Silva, J. K. R. & Borges, A. M. (2019). La actividad antioxidante del extracto oleoso obtenido de Lippia origanoides mejora la calidad de los embriones bovinos producidos in vitro. Arquivo Brasileiro de Medicina Veterinária e Zootecnia, 71: 723–731.
- Brykczynska, U., Hisano, M., Erkek, S., Ramos, L., Oakeley, E. J., Roloff, T. C.; et al. (2010). Repressive and active histone methylation mark distinct promoters in human and mouse spermatozoa. Nature Structural and Molecular Biology. 17(6): 679–687.
- De Castro, L. S., De Assis, P. M., Siquiera, A. F. P., Hamilton, T. R. S., Mendes, C. M., Losano, J. D. A.; et al. (2015). Sperm oxidative stress is detrimental to embryo development: A dose dependent study model and a new and more sensitive oxidative status evaluation. Oxidative Medicine and Cellular Longevity. 2016: 1–12. [CrossRef]
- Selivanov, V. A., Votyakova, T. V., Pivtoraiko, V. N., Zeak, J., Sukhomlin, T., Trucco, M.; et al. (2011). Reactive oxygen species production by forward and reverse electron fluxes in the mitochondrial respiratory chain. Beard DA, editor. PLoS Computational Biology, 31, 7(3): e1001115.
- Yakes, F. M. & Van Houten, B. (1997). Mitochondrial DNA damage is more extensive and persists longer than nuclear DNA damage in human cells following oxidative stress. Proceedings of the National Academy of Sciences of the United States of America, 21, 94(2): 514–519.
- Finkel, T, & Holbrook, N. J. (2000). Oxidants, oxidative stress and the biology of ageing. Nature; 9, 408(6809): 239–247.
- Chinnery, P. F., Elliott, H. R., Hudson, G., Samuels, D. C. & Relton, C. L. (2012). Epigenetics, epidemiology and mitochondrial DNA diseases. International Journal of Epidemiology 41(1): 177–187.
- Han, Y. & Chen, J. Z. (2013). Oxidative stress induces mitochondrial DNA damage and cytotoxicity through independent mechanisms in human cancer cells. Biomedical Research International, ID 825065, 8 pages. [CrossRef]
- Guo, C., Sun, L., Chen, X. & Zhang, D. (2013). Oxidative stress, mitochondrial damage and neurodegenerative diseases. Neural Regeneration Research, 8(21): 2003–2014.
- Kirkinezos, I. G. & Moraes, C. T. (2001). Reactive oxygen species and mitochondrial diseases. Seminars in Cell and Developmental Biology, 12(6): 449–457.
- Combelles, C. M., Gupta, S., Agarwal, A. (2009). ¿Podría el estrés oxidativo influir en la maduración in vitro de los ovocitos? Reproductive Biomed Online, 18: 864–880.
- Asiye, I. S., Ozen, B. O., Alper, B. (2018). Maduración in vitro de ovocitos bovinos: Efectos beneficiosos de la cisteamina. Journal of Dairy, Veterinary & Animal Research, 7: 64–65.
- Ileana, M. N. P., Andrea, H., Marius., Z., Vasile, M. (2012). Alfa-tocoferol y las combinaciones de ácido ascórbico influyen en la maduración de los ovocitos de oveja. Animal Science Biotechnology, 45: 310–313.
- Sunderam, S., Kissin, D. M., Crawford, S. B., Folger, S. G., Jamieson, D. J., Warner, L. & Barfield, W. D. (2015). Vigilancia de la tecnología reproductiva asistida. Estados Unidos, 2012. Informe semanal de morbilidad y mortalidad: Resúmenes de vigilancia 64: 1–29.
- Lees, J. G., Gardner, D. K., Harvey, A. J. (2017). Pluripotent stem cell metabolism and mitochondria: Beyond ATP. Stem Cells International, 2017, ID 2874283, 17 pages . [CrossRef]
- Vargas Reyes, J. N. (2013). Efecto de las técnicas de congelación lenta y vitrificación con etilenglicol sobre la calidad poscriopreservación de embriones bovinos producidos in vitro. https://ciencia.lasalle.edu.co/maest_ciencias_veterinarias/10.
- Youngs, C. R. (2011) Cryopreservation of Preimplantation Embryos of Cattle, Sheep, and Goats. Biology. [CrossRef]
- Giaretta, E., Spinaci, M., Bucci, D., Tamanini, C. & Galeati, G. (2013). Effects of resveratrol on vitrified porcine oocytes. Oxidative Medicine and Cellular Longevity, 2013, ID 920257: 7 pages.
- Salzano, A., Albero, G., Zullo, G., Neglia, G., Abdel-Wahab, A., Bifulco, G., Zicarelli, L., & Gasparrini, B. (2014). Effect of resveratrol supplementation during culture on the quality and cryotolerance of bovine in vitro produced embryos. Animal Reproduction Science, 151(3-4), 91–96. [CrossRef]
- Battin, E. E., & Brumaghim, J. L. (2009). Antioxidant activity of sulfur and selenium: A review of reactive oxygen species scavenging, glutathione peroxidase, and metal-binding antioxidant mechanisms. Cell Biochemistry and Biophysics, 55(1), 1–23. [CrossRef]
- De Matos, D. G., Gasparrini, B., Pasqualini, S. R., & Thompson, J. G. (2002). Effect of glutathione synthesis stimulation during in vitro maturation of ovine oocytes on embryo development and intracellular peroxide content. Theriogenology, 57(5), 1443–1451. [CrossRef]
- Zuelke, K. A., Jeffay, S. C., Zucker, R. M. & Perreault, S. D. (2003). Glutathione (GSH) concentrations vary with the cell cycle in maturing hamster oocytes, zygotes, and pre-implantation stage embryos. Molecular Reproduction and Development, 64(1): 106–112.
- De Matos, D. G., Furnus, C. C., Moses, D. F., Martinez, A. G., & Matkovic, M. (1996). Stimulation of glutathione synthesis of in vitro matured bovine oocytes and its effect on embryo development and freezability. Molecular Reproduction and Development, 45(4), 451–457. [CrossRef]
- De Matos, D. G., Furnus, C. C., & Moses, D. F. (1997). Glutathione synthesis during in vitro maturation of bovine oocytes: Role of cumulus cells. Biology of Reproduction, 57(6), 1420–1425. [CrossRef]
- Hernández Martínez S., Hernández Pichardo J.E., Vazquez Avendaño J.R., Ambríz García D.A. & Navarro Maldonado M.C. (2020) Developmental dynamics of cloned Mexican bighorn sheep embryos using morphological quality standards. Veterinary Medicine and Science, 6: 382–392. [CrossRef]
- Lorenzo-Torres, A., Rangel-Santos, R., Ruíz-Flores, A. & Ambríz-García, D. (2022). In vitro embryo production from ewes at different physiological stages. Journal of Veterinary Sciences, 23(6): e87. [CrossRef]
- Agata Anzalone, D., Palazzese, L., Czernik, M., Sabatucci, A., Valbonetti, L., Capra, E., & Loi, P. (2021) Controlled spermatozoa–oocyte interaction improves embryo quality in sheep. Scientifc Reports, 11: 22629. [CrossRef]
- Mastrorocco, A., Cacopardo, L.; Lamanna, D., Temerario, L., Brunetti, G., Carluccio, A., Robbe, D. & Dell’Aquila, M. E. (2021). Bioengineering approaches to improve in vitro performance of prepubertal lamb oocytes. Cells, 10: 1458. [CrossRef]
- Nadri, T., Towhidi, A., Zeinoaldini, S., Riazi, G., Sharafi, M., Zhandi, M., Kastelic, J., & Gholami, D. (2022). Supplementation of freezing medium with encapsulated or free glutathione during cryopreservation of bull sperm. Reproduction in Domestic Animals = Zuchthygiene, 57(5), 515–523. [CrossRef]
| RESVERATROL | 0 µM | 0.25 µM | 0.5 µM | 1 µM |
|---|---|---|---|---|
| IVM | 75 ± 6.5a | 74 ± 8.5a | 81 ± 6.1b | 81 ± 6.5b |
| CLEAVAGE (IVD) | 63.1 ± 5.4a | 60 ± 4.6a | 68.6 ± 4.7b | 69.2 ± 4.7b |
| EARLY BLASTOCYSTS | 8.4 ± 3.4a | 13.5 ± 4.1a | 16.2 ± 6a | 16.8 ± 6.5a |
| LATE BLASTOCYSTS | 30.2 ± 3.9a | 25.6 ± 3.1a | 30.2 ± 3.5a | 31.4 ± 4.5a |
| Resveratrol | ||||
|---|---|---|---|---|
| Late Blastocysts | 0 µM | 0.25 µM | 0.5 µM | 1 µM |
| Mean ± SE | 58 ± 20.9a | 41 ± 23.6a | 59 ± 7.3a | 64 ± 13.8a |
| Variables | Concentración de resveratrol | |||
|---|---|---|---|---|
| Fluorescence Intensity Units | 0 µM | 0.25 µM | 0.5 µM | 1 µM |
| ROS (Mean ± SE) | 34.722 ± 768.4a | 22.229 ± 4,314.2bc | 12.929 ± 1,789.3b | 24.447 ± 1,104.8c |
| GSH (Mean ± SE) | 25.733 ± 9,637a | 60.478 ± 10,586b | 94.446 ± 9,589b | 74.759 ± 14,800ab |
| Variable | Resveratrol concentration | |||
|---|---|---|---|---|
| Number of nuclei | 0 µM | 0.25 µM | 0.5 µM | 1 µM |
| Mean ± SE | 267±28.8a | 260±33.2ac | 185±11.7bc | 354±21.5d |
| Variable | Resveratrol concentration | |||
|---|---|---|---|---|
| Number of nuclei | 0 µM | 0.25 µM | 0.5 µM | 1 µM |
| Mean ± SD | 109±4.3a | 90±12.3a | 147±21b | 150±19.4b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





