1. Introduction
Thyroid surgery has been for years one of the most common elective procedures performed in general surgery. More than 30,000 thyroid surgeries are performed annually in Poland, with 12-15% of the procedures due to thyroid cancer [
1], 45,000 in France, 60,000 in Germany; more than 4,000 in Austria [
2]. Nowadays, thyroid surgery is safe with a mortality rate approaching 0%, although until the late 19th century it was a procedure with a mortality risk up to 40%. Hence, these surgeries were banned among others by the French Academy of Medicine, and prominent American surgeon David Gross warned against performing them [
3,
4,
5]. The greatest progress in thyroid surgery was made at the turn of the 20th century with the achievements of the magnificent seven of thyroid surgery: Billorth, Kocher, Halsted, Mayo, Chile, Danhill, and Lahey. The introduction of modern anesthesia, the principles of antiseptics, new surgical instruments, and the principles of safe excision of the thyroid gland reduced the mortality rate after thyroid surgery to 0.5% [
6] and resulted in an increasing number of thyroid surgeries performed. Despite the progress that has been made, these surgeries are still fraught with the risk of complications such as hemorrhage requiring reoperation, vocal fold paralysis, and hypoparathyroidism which significantly impair quality of life. Hence, all efforts in recent decades have focused on minimizing the percentage of their occurrence. Will the so-called "myth of the 1% in thyroid surgery" [
7], regarding the number of complications after thyroid surgery, be achieved in the 21st century? The last 25 years in thyroid surgery seem to have been groundbreaking with the introduction of new techniques in thyroid surgery. Undoubted milestones have been the implementation and standardization of laryngeal nerve monitoring techniques [
8,
9,
10], techniques to facilitate intraoperative identification of the parathyroid glands [
11,
12] and minimally invasive techniques [
13] as robotic surgery [
14] or transoral endoscopic thyroidectomy vestibular approach – TOETVA- thyroid surgery without a surgical scar [
15].
The indications for thyroid surgery remain a separate issue. In the past three decades, the number of thyroid surgeries for thyroid cancer has tripled [
16], and further projections indicate that the number will continue to rise. Are we facing an epidemic of thyroid cancer or is the increase in thyroidectomies due to the widespread availability of thyroid ultrasound and the ease of fine needle aspiration biopsy? [
17,
18]. Also, modern treatment options for Graves disease and other inflammatory and non-neoplastic thyroid diseases are changing the indications for surgery. The demonstration of the safety of radioiodine therapy in benign thyroid conditions and the possibility of thyrostatic treatment provides an alternative to surgical management [
19,
20]. The last two decades in thyroid surgery have seen rapid development of ablative techniques in particular for benign thyroid lesions, which also has the potential to change the indications for thyroid surgery [
21].
What has changed in thyroid surgery in the last quarter century? What are the trends in thyroid surgery? Have new technologies minimized the complication rate? Where is endocrine surgery heading for at the beginning of the 21st century?
The purpose of this study was to analyze 25 years of experience in thyroid surgery at a center specializing in the surgical treatment of thyroid disorders in terms of patient demographics, indications for surgical treatment, the type of thyroid surgery performed, and postoperative complications. The impact of recent advances on changes in endocrine surgery was evaluated.
2. Materials and Methods
On February 28, 2019 and on May 13, 2020, the consent of the Bioethics Committee of the Medical University of Wroclaw to conduct the study was obtained (KB- 156/2019; KB-280/2020).
We analyzed 25 years of experience in endocrine surgery at the Department of General, Gastroenterological and Endocrine Surgery at the Medical University of Wroclaw; as of 2018 - new name: Department of General, Minimally Invasive and Endocrine Surgery. The observation period was from 1996 to 2020, with a total of 3748 patients (7285 RLNs at risk of injury). Uniform, well-archived material was collected in 1996-2003 (first observation period, 8 years) and in 2011-2015, 2018-2020 (second observation period, 8 years). In the first period, medical records of 2707 patients undergoing thyroid surgery ( 5414 RLNs at risk of injury) were collected; the 2nd period included 1041 patients (1871 RLNs at risk). Demographics of patients treated in both groups are shown in
Table 1.
The research study planned to compare period I (1996-2003) vs. period II ( 2011-2015, 2018-2020) to answer the question: how has thyroid surgery changed over the past 25 years with a special attention on:
I- Clinical diagnosis and indications for surgical treatment.
II- Type of thyroid surgeries performed.
III- Post-operative complications: vocal fold paralysis, post-operative hypoparathyroidism, bleeding after thyroid surgery
IV- Secondary operations on the thyroid gland
All patients qualified for thyroid surgery were euthyroid (TSH and fT4 were determined preoperatively), had thyroid ultrasound and biopsy. In addition, standard tests were performed in preparation for surgery: blood morphology, biochemical blood tests, blood type, chest X-ray, electrocardiogram.
Surgical treatment: all thyroid surgeries were performed at a single endocrine surgery center, involving two generations of surgeons, the so-called junior and senior surgeons; all surgeries were performed in a classical manner with a typical Kocher collar incision neck opening; no minimally invasive-laparoscopic surgeries were performed. Different approaches were used over the years to identify the RLN - from lack of identification, through visual identification, to neuromonitoring, which was first introduced in 2011. RLN monitoring was carried out according to the recommendations of the International Neural Monitoring Study Group [
9] employing a NIM-3.0 nerve monitor (Medtronic, Jacksonville, USA) and an intermittent IONM technique. A monopolar stimulating probe was used for nerve stimulation with a current amplitude of 1 mA (range 0.5-1.5 mA) and 3- Hz pulses of 200 ms each for 1-2 s.
Vocal fold paralysis: in Period I, only 771 (28.48%) of patients had an ENT examination before thyroid surgery (indirect videoscopy); in Period II all patients underwent an ENT examination preoperatively (indirect videoscopy, videolaryngoscopy). ENT examination after thyroid surgery was performed among those patients in group I who presented phonation disorders in the immediate period after thyroid surgery; ENT examination after thyroid surgery was not a standard procedure. In period II, all patients who qualified for the study had an ENT examination by 7 days after thyroid surgery. The number of vocal fold paralysis was calculated both per patient and per number of RLNs at risk of injury. Transient vocal fold palsy was defined as resolving up to 12 months after thyroid surgery. Permanent paralysis was defined as paralysis persisting more than 12 months after surgery. The total number of paralysis was defined as the sum of all paralysis: transient and permanent, assessed in the immediate period after thyroid surgery. Patients with preoperative vocal fold paralysis were excluded from the study.
Postoperative hypoparathyroidism: in the period I- years 1996-2003 PTH, Ca, phosphorus testing was determined only in patients with symptomatic tetany, while in 2011-2015, 2018-2020 all patients had PTH, Ca, phosphorus levels determined before as well as after thyroid surgery. Hypoparathyroidism in the immediate postoperative period has been evaluated; the collected material did not allow of hypoparathyroidism differentiation into temporary and permanent.
Postoperative bleeding: patients who required reoperation due to a hematoma in the surgical site during the immediate postoperative period.
Demographic data, clinical data from medical records, surgical treatment and complications were entered into an Excel database.
2.1. Statistical Analysis
Statistical analysis was based on data collected from N= 3748 patients, which constituted the study sample from the general population. The variables analyzed were both nominal, including dichotomous and quotient (quantitative). The choice of statistical techniques used was dictated by the nature of the random variables being compared with each other.
To characterize the quantitative variables, basic descriptive statistics were calculated for them: mean value, standard deviation, and 95% confidence intervals (95% ± CI) for mean value and standard deviation. Normality of the distributions of the quantitative variables was assessed with the Shapiro-Willk W-test, and homogeneity of variance was assessed with the tests: Levene and Brown-Forsyth assuming a significance level of α=0.05. For variables on nominal scales, including dichotomous ones, tables of counts were determined containing absolute raw counts and the percentage and cumulative contribution of each category to the nominal variable.
Pearson's non-parametric Chi test2 was used to assess the statistical significance of correlations between variables on nominal scales.
In assessing the statistical correlation between dichotomous and quotient variables, two types of statistical analyses were used, depending on the result of the Shapiro-Willka W-test: the parametric Student's t-test for independent samples in the case of variables with a normal distribution, or the non-parametric Mann-Whitney U-test in situations where the variables being compared did not meet this assumption.
A significance level of α=0.05 was assumed in all statistical analyses performed. Statistical analyses were carried out using the computer program STATISTICA PL® version 13.3 with the add-on - Set Plus version 3.0.
3. Results
I. Clinical diagnoses, indications for surgical treatment
Table 2. shows the clinical diagnoses of patients operated on between 1996-2003 (period I) vs. 2011-2015, 2018-2020 (period II). Indications for thyroid surgery included nodular goiter, toxic nodular goiter, Graves disease (GD), inflammatory goiter, thyroid cancer and changed at the level of statistical significance ( p<0.00001) over the observed years. In 1996-2003, operations due to inflammatory goiter accounted for 2.51%, in 2011-2015 and 2018-2020, they were completely eliminated. The percentage of patients treated for thyroid cancer increased almost threefold at the level of statistical significance (3.73% vs. 10.9%), the percentage of patients with papillary carcinoma in 2nd period increased almost fourfold (2.25% vs. 8.84%) and was at the level of statistical significance. No statistically significant differences were observed in the incidence of other thyroid cancers (p<0.05).
Of the 101 patients operated on for thyroid cancer in the 1st period, 97 (96%) had thyroid cancer in primary goiter, and 4 (4%) had thyroid cancer in recurrent goiter. In contrast, in the 2nd period, out of 105 patients with thyroid cancer, 87 (82.86%) were observed to have cancer in primary goiter and 18 (17.14%) in recurrent goiter.
The mean volume of goiter in the first period was greater, at 51.26 ml vs. 43.35 ml in the second interval, although the differences were not statistically significant (p >0.05). Retrosternal goiter was significantly more common (p=0.0068) in the earlier years than in the later period, although tracheal constriction or displacement was more frequently observed in the later years (29.66% vs. 38.42%, p <0.00001).
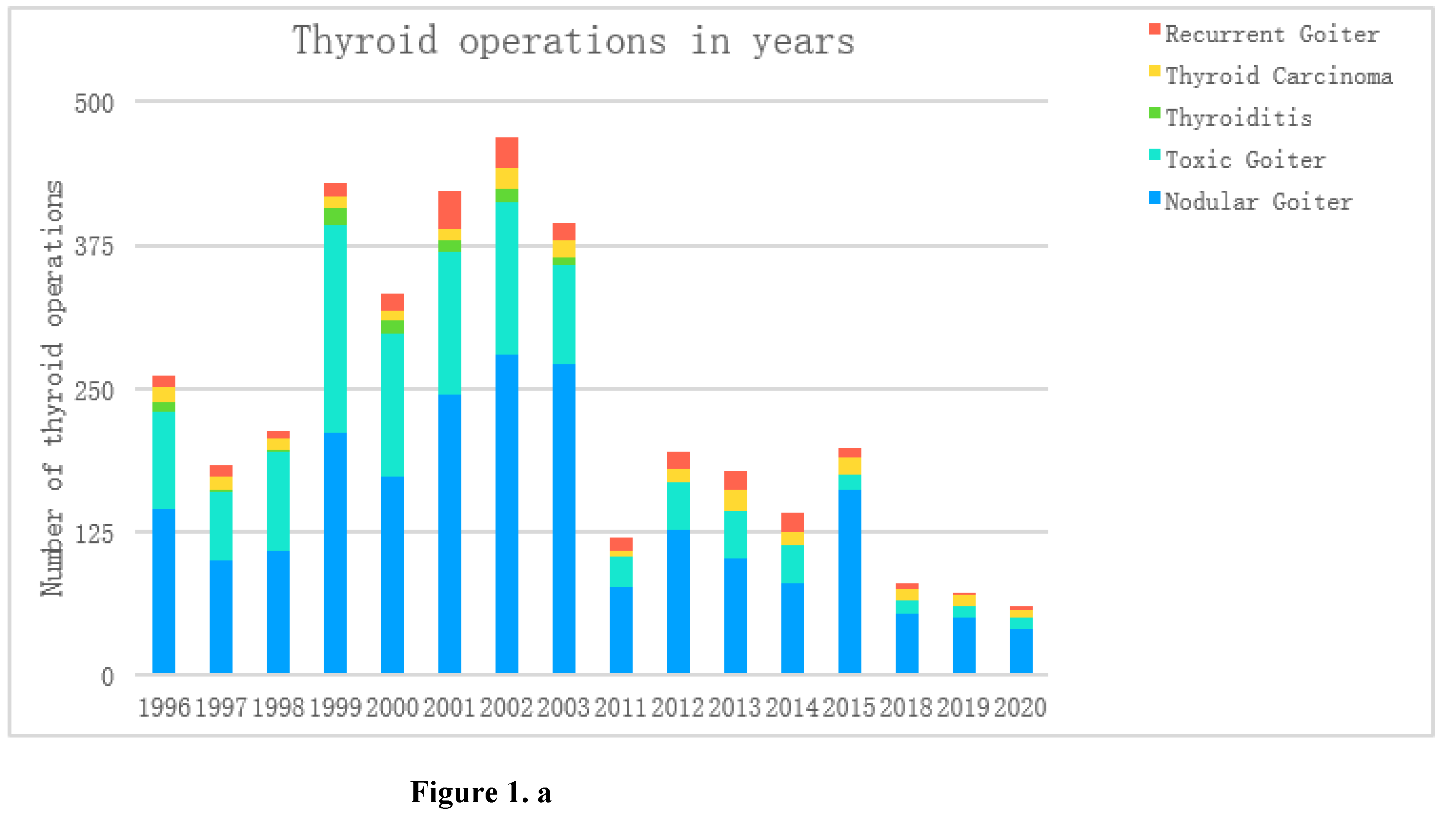
Table 3. shows the clinical diagnoses by year. The largest number of thyroid surgeries was performed in 1999-2003 (
Figure 1a);
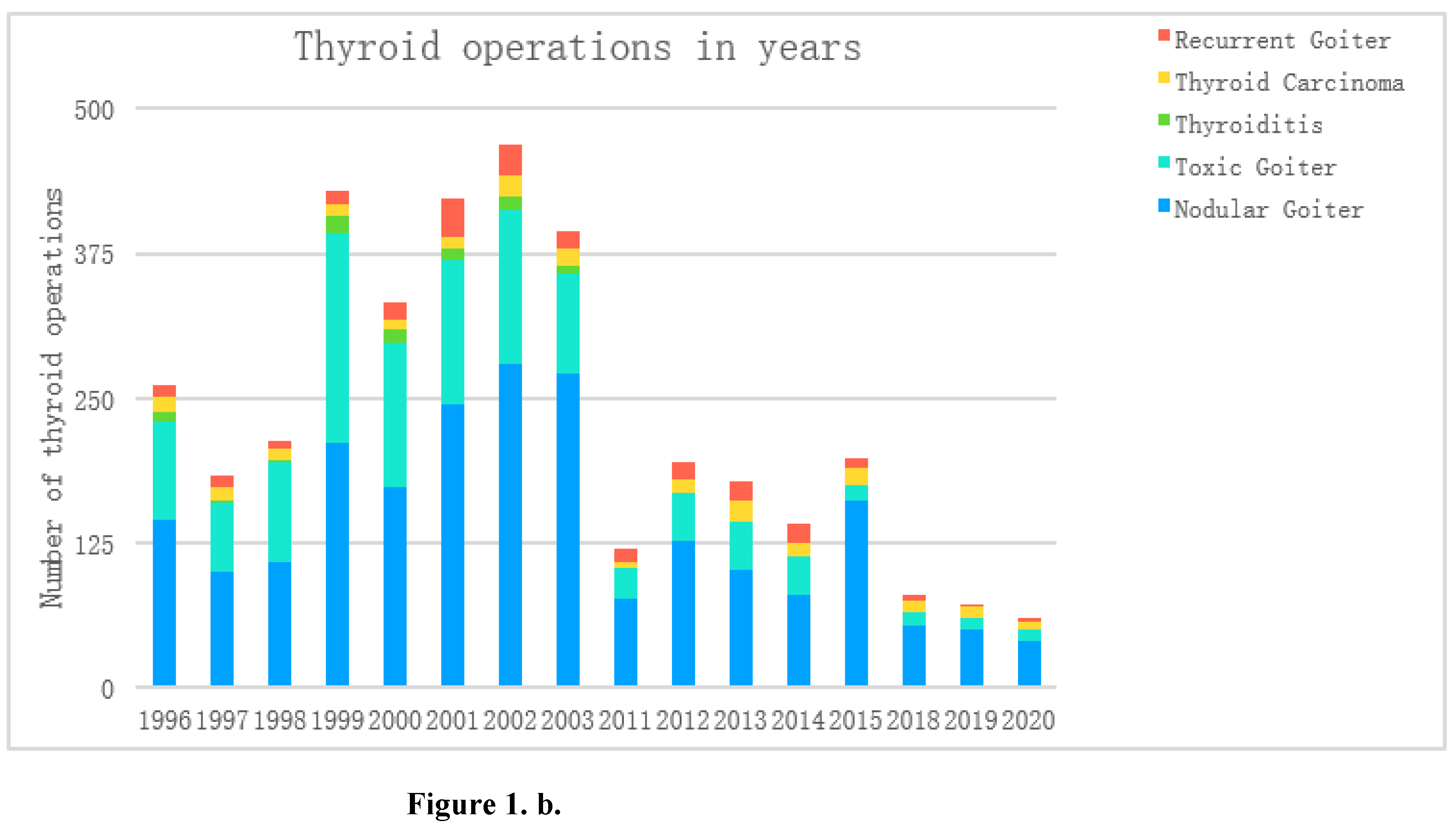
Figure 1b shows the percentage distribution of diagnoses in subsequent years, notes the increasing trend among thyroid cancers and recurrent goiter, decreasing among hyperactive goiter and relatively constant at the level of nodular goiter.
II. Type of thyroid surgery
Table 4. shows the types of thyroid surgeries performed between 1996-2003 vs. 2011-2015 and 2018-2020. At the level of statistical significance (p<0.00001), the extent of thyroid resection changed, from a clear predominance of partial thyroid resection surgeries (87.73%) in 1996-2003, to a predominance of total thyroid resection (70.8%) or total thyroid lobe resection with isthmus (20.27%) in 2011-2015 and 2018-2020. In the later years, wedge thyroid resections and cytoreductive surgeries were not performed at all. Lobectomies were not performed in the first observation period, where they accounted for 20.27% of the operations performed in 2nd period. Between 1996 and 2003, total thyroid resection was performed in only 0.62% of patients with nodular goiter and only in patients with recurrent goiter, 1.42% with toxic goiter, 1.47% with inflammatory goiter and 21.78% with thyroid cancer. In contrast, from 2011-2015, 2018-2020, the percentage of total thyroid surgery is: 91.23 in nodular goiter; 90.77% toxic goiter and 97.14% thyroid cancer (including lymph node dissection). Of the 101 patients operated on for thyroid cancer in earlier years, 55 (54.46%) underwent radicalization of surgery: in 22 (21.78%) the so-called early surgery- up to 3 days after the primary surgery, in 33 (34.02%) the late surgery- about 6 weeks after the primary treatment. Radicalization of thyroid surgery was not performed in patients with thyroid cancer in the second group of patients within 6 weeks after primary treatment.
III. Postoperative complications.
Table 5. shows complications after thyroid surgery in 1996-2003 vs. 2011-2015 and 2018-2020.
The percentage of bleeding requiring reoperation after thyroidectomy in both periods was less than 1% (0.92% vs. 0.86%), the differences were not at the level of statistical significance (p>0.05).
Clinical hypoparathyroidism, confirmed by reduced PTH levels and hypocalcemia, was statistically significantly more common among patients operated on in the later years (2011-2015 and 2018-2020), at 4.84% vs. 8.93% (p<0.00001). In both follow-up periods, it occurred twice as often in secondary than primary surgeries, respectively in the 1st period: 4.66% vs. 8.33% and 2nd: 8.42% vs. 15.19%. Biochemical hypoparathyroidism without clinical signs of hypocalcemia was monitored only in the later years and was more frequent than symptomatic hypocalcemia ( 8.93% vs. 12.78%).
Unilateral vocal fold paralysis in the immediate postoperative period was observed slightly more often in patients in the first period than in the second (8.27% vs. 6.53%); these differences were not at the level of statistical significance in both primary and secondary operations (p>0.05). The percentage of unilateral transient paresis was statistically significantly more frequent in the later years (0.78% vs. 2.11%; p=0.0006), while the percentage of permanent paralysis was significantly more frequent in the later years (7.5% vs. 4.42%; p=0.0007). No statistically significant differences were observed in the incidence of bilateral paralysis in the immediate post-operative period, transient paralysis and permanent paralysis in both observation periods (p>0.05%). These results are consistent with paralysis converted to the number of recurrent laryngeal nerves at risk of damage, where the total number of paralysis in the first period was 5.17% vs. 4.38% in the second observation period, these values were not statistically significant (p=0.1785). The statistically significantly more frequent occurrence of transient paralysis in group I (p<0.00001) and the statistically significantly less frequent occurrence of permanent paralysis in group II (p=0.0016) were again confirmed.
Among other complications in group I, the following were observed: wound infection - 4 (0.15%), urinary tract infection - 8 (0.3%), circulatory failure - 13 (90.48%), respiratory failure - 24 (0.89%) and death - 11 (0.41%). Subsequently, in the second group, 3 patients developed wound infection (0.3%), and there were no deaths in the immediate period after thyroid surgery.
IV. Secondary surgeries on the thyroid gland
Table 6. shows the clinical diagnoses and type of thyroid surgery among patients with recurrent goiter in subsequent years. Recurrent goiter was statistically significantly more frequent in the later years (4.88% vs. 7.59%). Over the years, surgery for thyroid cancer was statistically significantly more frequent in recurrent goiter (3.03% vs. 22.78%, p<0.00001), while surgery for toxic goiter was statistically significantly less frequent in later years (24.24% vs. 8.86%, p=0.0053). At the level of statistical significance, the extent of secondary surgery on the thyroid gland changed over the 25-year period, with a statistically significant prevalence of total vs. partial surgery in later years (p< 0.00001).
4. Discussion
Surgical treatment of thyroid disorders, although it is one of the most commonly performed operations in general surgery, is mainly the domain of centers specializing in endocrine surgery due to the risk of quite specific surgical complications, especially the possibility of loss of voice due to injury to the recurrent laryngeal nerve, severe post-operative hypoparathyroidism, or directly life-threatening hemorrhage [
22]. Only the large number of thyroidectomies performed annually provides the opportunity to become highly skilled in thyroid surgery, making such a center specialized in the treatment of thyroid disorders [
23,
24,
25,
26,
27].
The Department of General, Minimally Invasive and Endocrine Surgery at the Wroclaw Medical University has been involved in endocrine surgery for more than 70 years, being the reference center for the treatment of benign disorders as well as thyroid cancer in the Lower Silesia region. The last 25 years have seen many changes in thyroid surgery regarding the indications for surgical treatment, the type of thyroid surgery performed, and the rate of postoperative complications.
The authors of the paper - the so-called "young generation" - have been associated with the activities of the Department for more than 20 years, undertook a retrospective evaluation of the activities of the Department of General, Minimally Invasive and Endocrine Surgery in the last 25 years and attempted to answer the question of where thyroid surgery is heading at the beginning of the 21st? In order to isolate differences, the 25-year observation interval was divided into two time periods: early (I), which covered 1996 - 2003, and late (II), which covered 2011-2015 and 2018-2020. The eight-year periods were compared in terms of changes in demographics, indications for surgery, extent of surgery, complications, and recurrent goiter.
During the 25-year period, the percentage of patients with nodular goiter, toxic goiter and thyroid cancer referred for surgery changed at the level of statistical significance (p<0.00001). The percentage of patients operated on for thyroid cancer more than tripled after 2011 compared to patients treated between 1996 and 2003, with an increase observed especially in the diagnosis of papillary thyroid cancer (2.25 vs. 8.84%, p<0.00001), which has invariably been the most common histological type of malignant thyroid tumors for years. The percentage of other malignant neoplasms and metastatic thyroid lesions did not change (p>0.05). This is consistent with both the described trend in Poland [
16,
28] and worldwide [
17,
25,
29]. This is undoubtedly influenced by the easy availability of ultrasound, as well as the widespread performance of fine-needle aspiration biopsy [
30,
31,
32]. On the other hand, the percentage of patients operated on for toxic goiter, especially Graves disease, has statistically significantly decreased, which should be associated with the choice of radioiodine therapy as the method of choice in Graves disease and toxic adenoma [
33,
34]. In 1996 -2003, one of the indications for surgery was inflammatory goiter - Hashimoto's disease; after 2011, this diagnosis was not an indication for surgery. Perhaps this is related to the proper substitutive conservative treatment of Hashimoto's disease and there is no fibrosis of the thyroid gland causing pressure symptoms. Yes, there is still a significant percentage of operated patients with concomitant autoimmune disease, while this disease itself is no longer an indication for surgical treatment [
35]. Important to mention is the smaller volume of goiter with which patients are referred for thyroid surgery in later years, averaging 43.35 ml than in the 1990s, where the average volume was 51.26 ml, although these differences were not statistically significant (p>0.05). On the other hand, the percentage of patients with retrosternal goiter has decreased significantly, indicating that patients with objective compression symptoms are being referred for surgery much more quickly. The average age of patients undergoing surgery remains unchanged at around 54 years, while the percentage of patients over 65 years of age increased statistically significantly after 2011, which supports the fact that metric age is currently not a contraindication to thyroid surgery and safety is comparable to younger patient groups. In addition, life expectancy is lengthening and the proportion of elderly patients can be expected to increase [
36]. Over the course of 25 years, the proportion of men operated on increased from 9:1 to 5:1, and this increase was statistically significant. We also observed an increase in BMI in the group of operated patients after 2011, which is in line with the worldwide problem of obesity in recent years [
37].
Another topic analyzed was the extent of thyroid gland surgery performed. And here, over the past quarter century, we have seen a shift from partial thyroid surgery to total resection. Between 1996 and 2003, subtotal surgeries were performed in more than 90% of patients vs. 1.7% of total thyroid gland surgeries; between 2011-2015 and 2018-2020, more than 94% of total thyroid resection surgeries vs. 4% of subtotal surgeries were performed; the results are highly statistically significant (p<0.00001). What has led to such a significant change in thyroid surgery? The art of surgery is handed down from generation to generation, and this trend of partial thyroid gland surgery was present in many thyroid surgery centers in Poland. This was not the optimal extent of treatment, as postoperatively diagnosed thyroid cancer required radicalization with an almost 50% risk of surgical complications. In addition, leaving a significant amount of thyroid tissue, often with focal lesions, exposed patients to problems associated with the treatment of recurrent goiter. The choice of this extent of surgery was based on concern for potential surgical complications: damage to the parathyroid glands and the recurrent laryngeal nerve, which, located adjacent to the posterior thyroid capsule, are at much greater risk of damage during more radical surgery. Potentially leaving a portion of the thyroid fragment ostensibly protects against the risk of damage to these structures. The ability to identify the recurrent laryngeal nerve remains a separate issue. Admittedly, as early as 1994, Jatzko et al. [
38] demonstrated that identification of the RLN decreases rather than increases the risk of RLN damage and is the gold standard in thyroid surgery, but nevertheless, in many centers, including the one from which the authors of the publication come, the RLN was not routinely identified during every thyroid surgery between 1996 and 2003. An extensive discussion of the appropriate scope of thyroid surgery occurred at the beginning of the 21st century; it is between 2000 and 2005 that the largest number of articles in the medical literature on the subject appear [
39,
40,
41], which definitively shows the superiority of total thyroid or thyroid lobe surgery over partial resection surgery. In the Wroclaw center, the change in surgical strategy has been occurring slowly since 2004 due to reports in the literature about the benefits of more radical surgeries, and new trends are being introduced by the so-called young surgical generation. The turning point of change is 2011 - the introduction of neuromonitoring to the clinic where the authors work. Since then, total thyroid resection has become a commonly performed surgical procedure in both benign and cancerous goiter [
42,
43,
44].
Complications after thyroid surgery were another topic of study of the 25-year follow-up period. Theoretically, increasing the extent of surgery should affect the rate of postoperative complications. Before comparing complications from the two periods over the past 25 years, some limitations and weaknesses of the study should be mentioned. Over such a long period, the standard of perioperative care has changed. Between 1996 and 2003, preoperative ENT examination was performed in only 30% of patients, and postoperative ENT examination was performed mainly in those patients who reported phonation disorders after surgery, hence objective assessment of vocal fold paralysis is undoubtedly underestimated. The percentage of ENT examinations both before and after thyroid surgery after 2011 at our center is close to 90%. In addition, it should be noted that after 2011, some thyroid surgeries were performed with neuromonitoring. Objective assessment of postoperative hypoparathyroidism is hindered by the lack of routinely performed determination of PTH, Ca, and phosphorus in all patients in the immediate postoperative period, hence in 1996-2003 - where PTH and Ca levels were determined only in patients with clinical signs of hypocalcemia - the percentage of this complication may also be underestimated. Nevertheless, the accumulated material includes a large group of patients - 3,748, which undoubtedly allows us to draw conclusions pointing to some directions in thyroid surgery. The change in surgical strategy - transitioning from partial removal of the thyroid gland to total surgery - did not significantly affect the total number of vocal fold paralysis in the immediate post-thyroid surgery period (per RLN at risk of injury). In 1996-2003, the total number of RLN injuries was 5.17% vs. 4.38% in 2011-2015, 2018-2020, and these differences were not at the level of statistical significance (p=0.1785). Admittedly, an increase in the extent of thyroid surgery increased the percentage of transient paralysis in the second period (0.41% vs. 1.34%, p<0.00001), but this may indicate a greater exposure of the RLN during total resections. But it is interesting and quite optimistic that permanent paralysis was statistically significantly less in the later years (4.77% vs. 3.05%, p=0.0016), which convinces us to continue performing total surgeries, given the fact that there was an undoubted underestimation of RLN injuries in the earlier years. The increased extent of surgery undoubtedly increased the incidence of postoperative hypoparathyroidism after 2011 ( 4.84% vs. 8.93%, p<0.00001), but here we have no objective data as to the nature of this complication, whether it was temporary or permanent. Such results prompt us to look for new techniques to identify parathyroid glands during thyroid resection.
The rate of bleeding requiring reoperation over 25 years remains unchanged, and is low, below 1% ( 0.92% vs. 0.86%, p=0.8646). These results do not differ from those reported in the literature [
45].
Among other complications, the percentage of 11 (0.41%) deaths between 1996 and 2003 that did not occur after 2011 is noteworthy. This undoubtedly indicates an increase in overall perioperative safety in the later years.
The last analyzed topic of the 25-year period of thyroid surgery at our center was a recurrent goiter. The almost twofold increase in the number of patients with recurrent goiter in later years (4.88% vs. 7.59%, p=0.00013) is a consequence of non-radical surgeries performed in earlier years. The more than 20% rate of malignant lesions in recurrent goiter in recent years confirms that the optimal scope of surgery is primary total thyroid resection [
46,
47,
48], which should be the treatment of choice in the era of neuromonitoring.
In summary, thyroid surgery has been evolving since the beginning of the 20th century, and the last 25 years have seen advances in the diagnosis of thyroid disorders and the implementation of new technologies. In the future, it seems inevitable that the number of patients diagnosed with thyroid cancer will increase in the era when ultrasound and fine-needle aspiration biopsy are so widely available, although the possibility of active surveillance of thyroid cancer for small lesions [
49] and the possibility of using ablative techniques to treat them may also reduce the number of patients treated surgically for thyroid cancer [
50]. Total excision of the thyroid gland appears to be the optimal extent of surgery that does not significantly increase the rate of postoperative complications, and the implementation of neuromonitoring has a beneficial effect on the quality of surgical treatment, minimizing the risk of phonatory disorders after thyroid surgery. It is necessary to search for new methods to protect the parathyroid glands during thyroid surgery.
Conclusions:
In the period of 25 years (1996 -2020) in the Department of General , Minimally Invasive and Endocrine Surgery, the number of patients operated on for thyroid cancer more than tripled, twice for recurrent goiter; there is a noticeable decreasing trend in operations due to Graves disease. The scope of thyroid resection has changed, from the prevalence of partial bilateral thyroid resection in the 1990s, to practically exclusively performing total resection surgeries for both benign and malignant thyroid conditions until now. The increase in the scope of surgery has not increased the rate of total RLN paralysis, but it has increased the rate of postoperative hypoparathyroidism. The myth of the 1% complication rate in thyroid surgery has still not been achieved and requires the implementation of new technologies to minimize the risk of complications.
Author Contributions
All authors (BW, MG, MS, KS, DM, KK) made substantial contributions to the conception, design, data acquisition, analysis, and interpretation of data. We took part in drafting the article and revising it for important intellectual content. We agreed to submit it to the current journal. We also gave final approval of the version to be published and decided to be accountable for all aspects of the work. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Bioethics Committee of Wroclaw Medical University, Wroclaw, Poland (Signature number: KB-280/2020; May 13, 2020).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study. Data Availability Statement: The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Acknowledgments
the authors are grateful to all the staff at the study center who contributed to this work.
Conflicts of Interest
The authors report no conflicts of interest in this work.
Abbreviations
IONM- Intraopertive nerve monitoring; RLN- recurrent laryngeal nerve; RLN at risk - recurrent laryngeal nerve at risk of damage ; BMI - body mass index; GD- Graves disease; ENT – Ear Nose Throat Specialist; ECG – electrocardiogram; Ca - total calcium; PTH - parathormone.
References
- Choroby tarczycy u Polaków. Statystyki, objawy, leczenie. Available online: http://zdrowie.wprost.pl/medycyna/choroby/10398657/choroby-tarczycy-u-polakow-statystyki-objawy-leczenie.html. (accessed on 15 October 2022).
- Fortuny, J.V.; Guigard, S.; Karenovics, W.; Triponez, F. Surgery of the thyroid: recent developments and perspective. Swiss Med Wkly. 2015, 145, 14144. [Google Scholar] [CrossRef] [PubMed]
- Terris, D.J.; et al. Incisions in thyroid and parathyroid surgery. In Surgery and Parathyroid Surgery, 2nd ed.; SAUNDERS: Philadelphia, USA, 2013; pp. 403–406. [Google Scholar]
- Halsted, W.S. The operative story of goitre. The author’s operation. J Am Med Assoc. 1920, 74, 693–694. [Google Scholar]
- Mitrecic, M.Z.; Kaplan, E.L.; Gaz, R.D. Mitrecic, M.Z.; Kaplan, E.L.; Gaz, R.D., et al. History of thyroid and parathyroid surgery. In:. Surgery of the thyroid and parathyroid glands.; SAUNDERS: Philadelphia, USA, 2003; pp. 3–14.
- Hannan, S.A. The magnificent seven: a history of modern thyroid surgery. Int J Surg. 2006, 4, 187–191. [Google Scholar] [CrossRef] [PubMed]
- Patel, N.; Scott-Coombes, D. Impact of surgical volume and surgical outcome assessing registers on the quality of thyroid surgery. Best Pract Res Clin Endocrinol Metab. 2019, 33. [Google Scholar] [CrossRef] [PubMed]
- Shedd, D.P.; Burget, G.C. Identification of the recurrent laryngeal nerve. Arch Surg. 1966, 92, 861–864. [Google Scholar] [CrossRef] [PubMed]
- Randolph, G.W.; Dralle, H.; Abdullah, H.; Barczynski, M.; Bellantone, R.; Brauckhoff, M.; Carnaille, B.; Cherenko, S.; Chiang, F.Y.; Dionigi, G.; et al. Electrophysiologic recurrent laryngeal nerve monitoring during thyroid and parathyroid surgery: international standards guideline statement. Laryngoscope 2011, 121. [Google Scholar] [CrossRef] [PubMed]
- Barczyński, M.; Randolph, G.W.; Cernea, C.R.; Dralle, H.; Dionigi, G.; Alesina, P.F.; Mihai, R.; Finck, C.; Lombardi, D.; Hartl, D.M.; et al. External branch of the superior laryngeal nerve monitoring during thyroid and parathyroid surgery: International Neural Monitoring Study Group Standards Guideline Statement. Laryngoscope 2013, 123. [Google Scholar] [CrossRef]
- Orloff, L.A.; Wiseman, S.M.; Bernet, V.J.; Fahey, T.J., 3rd; Shaha, A.R.; Shindo, M.L.; Snyder, S.K.; Stack, B.C., Jr.; Sunwoo, J.B.; Wang, M.B. American Thyroid Association Statement on Postoperative Hypoparathyroidism: Diagnosis, Prevention, and Management in Adults. Thyroid 2018, 28, 830–841. [Google Scholar] [CrossRef] [PubMed]
- Barbieri, D.; Indelicato, P.; Vinciguerra, A.; Salerno, E.; Battista, R.A.; Di Marco, F.; Giordano, L.; Luce, F.L.; Bondi, S.; Trimarchi, M.; Bussi, M. The impact of near-infrared autofluorescence on postoperative hypoparathyroidism during total thyroidectomy: a case-control study. Endocrine 2023, 79, 392–399. [Google Scholar] [CrossRef] [PubMed]
- de Vries, L.H.; Aykan, D.; Lodewijk, L.; Damen, J.A.A.; Borel Rinkes, I.H.M.; Vriens, M.R. Outcomes of Minimally Invasive Thyroid Surgery - A Systematic Review and Meta-Analysis. Front Endocrinol (Lausanne). 2021, 12, 719397. [Google Scholar] [CrossRef]
- Lee, C.R.; Chung, W.Y. ; Robotic surgery for thyroid disease. Minerva Chir. 2015, 70, 331–339. [Google Scholar] [CrossRef] [PubMed]
- Anuwong, A.; Ketwong, K.; Jitpratoom, P.; Sasanakietkul, T.; Duh, Q.Y. Safety and Outcomes of the Transoral Endoscopic Thyroidectomy Vestibular Approach. JAMA Surg. 2018, 153, 21–27. [Google Scholar] [CrossRef]
- Wojciechowska, U.; Didkowska, J. Zachorowania i zgony na nowotwory złośliwe w Polsce. Krajowy Rejestr Nowotworów, Narodowy Instytut Onkologii im. Marii Skłodowskiej-Curie – Państwowy Instytut Badawczy. Avaliable online: http://onkologia.org.pl/raporty (accessed on 23.12.2021).
- Li, M.; Dal Maso, L.; Vaccarella, S. Global trends in thyroid cancer incidence and the impact of overdiagnosis. Lancet Diabetes Endocrinol. 2020, 8, 468–470. [Google Scholar] [CrossRef] [PubMed]
- Vaccarella, S.; Franceschi, S.; Bray, F.; Wild, C.P.; Plummer, M.; Dal Maso, L. Worldwide Thyroid-Cancer Epidemic? The Increasing Impact of Overdiagnosis. N Engl J Med. 2016, 375, 614–617. [Google Scholar] [CrossRef] [PubMed]
- Kahaly, G.J. Management of Graves Thyroidal and Extrathyroidal Disease: An Update. J Clin Endocrinol Metab. 2020, 105, 3704–3720. [Google Scholar] [CrossRef]
- Patel, K.N.; Yip, L.; Lubitz, C.C.; Grubbs, E.G.; Miller, B.S.; Shen, W.; Angelos, P.; Chen, H.; Doherty, G.M.; Fahey, T.J., 3rd; et al. The American Association of Endocrine Surgeons Guidelines for the Definitive Surgical Management of Thyroid Disease in Adults. Ann Surg. 2020, 271, e21–e93. [Google Scholar] [CrossRef] [PubMed]
- Papini, E.; Monpeyssen, H.; Frasoldati, A.; Hegedüs, L. 2020 European Thyroid Association Clinical Practice Guideline for the Use of Image-Guided Ablation in Benign Thyroid Nodules. Eur Thyroid J. 2020, 9, 72–185. [Google Scholar] [CrossRef] [PubMed]
- Cherenfant, J.; Gage, M.; Mangold, K.; Du, H.; Moo-Young, T.; Winchester, D.J.; Prinz, R.A. Trends in thyroid surgery in Illinois. Surgery 2013, 154, 1016–1023. [Google Scholar] [CrossRef] [PubMed]
- Al-Qurayshi, Z.; Robins, R.; Hauch, A.; Randolph, G.W.; Kandil, E. Association of Surgeon Volume With Outcomes and Cost Savings Following Thyroidectomy: A National Forecast. JAMA Otolaryngol Head Neck Surg. 2016, 142, 32–39. [Google Scholar] [CrossRef]
- Gourin, C.G.; Tufano, R.P.; Forastiere, A.A.; Koch, W.M.; Pawlik, T.M.; Bristow, R.E. Volume-based trends in thyroid surgery. Arch Otolaryngol Head Neck Surg. 2010, 136, 1191–1198. [Google Scholar] [CrossRef] [PubMed]
- van Gerwen, M.; Alsen, M.; Alpert, N.; Sinclair, C.; Taioli, E. Trends for In- and Outpatient Thyroid Cancer Surgery in Older Adults in New York State, 2007-2017. J Surg Res. 2022, 273, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekhar, S.S.; Randolph, G.W.; Seidman, M.D.; Rosenfeld, R.M.; Angelos, P.; Barkmeier-Kraemer, J.; Benninger, M.S.; Blumin, J.H.; Dennis, G.; Hanks, J.; et al. Clinical Practice Guideline: improving voice outcomes after thyroid surgery. Otolaryngology–Head and Neck Surgery 2013, 148, 1–37. [Google Scholar] [CrossRef] [PubMed]
- Jeannon, J.P.; Orabi, A.A.; Bruch, G.A.; Abdalsalam, H.A.; Simo, R. Diagnosis of recurrent laryngeal nerve palsy after thyroidectomy: A systematic review. International Journal of Clinical Practice 2009, 63, 624–629. [Google Scholar] [CrossRef]
- Główny Urząd Statystyczny. Stan zdrowia ludności Polski w 2019 r. Available online: http://stat.gov.pl/obszary-tematyczne/zdrowie/zdrowie/stan-zdrowia-ludnosci-polski-w-2019-r-,26,1.html. (accessed on 12 December 2021).
- Miranda-Filho, A.; Lortet-Tieulent, J.; Bray, F.; Cao, B.; Franceschi, S.; Vaccarella, S.; Dal Maso, L. Thyroid cancer incidence trends by histology in 25 countries: a population-based study. Lancet Diabetes Endocrinol. 2021, 9, 225–234. [Google Scholar] [CrossRef] [PubMed]
- Roman, B.R.; Morris, L.G.; Davies, L. The thyroid cancer epidemic, 2017 perspective. Curr Opin Endocrinol Diabetes Obes. 2017, 24, 332–336. [Google Scholar] [CrossRef] [PubMed]
- Seib, C.D.; Sosa, J.A. Evolving Understanding of the Epidemiology of Thyroid Cancer. Endocrinol Metab Clin North Am. 2019, 48, 23–35. [Google Scholar] [CrossRef] [PubMed]
- Zaridze, D.; Maximovitch, D.; Smans, M.; Stilidi, I. Thyroid cancer overdiagnosis revisited. Cancer Epidemiol. 2021, 74, 102014. [Google Scholar] [CrossRef] [PubMed]
- Subekti, I.; Pramono, L.A. Current Diagnosis and Management of Graves' Disease. Acta Med Indones. 2018, 50, 177–182. [Google Scholar] [PubMed]
- Wiersinga, W.M.; Poppe, K.G.; Effraimidis, G. Hyperthyroidism: aetiology, pathogenesis, diagnosis, management, complications, and prognosis. Lancet Diabetes Endocrinol. 2023, 11, 282–298. [Google Scholar] [CrossRef] [PubMed]
- Gan, T.; Randle, R.W. The Role of Surgery in Autoimmune Conditions of the Thyroid. Surg Clin North Am. 2019, 99, 633–648. [Google Scholar] [CrossRef] [PubMed]
- Inversini, D.; Morlacchi, A.; Melita, G.; Del Ferraro, S. , Boeri, C.; Portinari, M.; Cancellieri, A.; Frattini, F.; Rizzo, A.G.; Dionigi, G. Thyroidectomy in elderly patients aged ≥70 years. Gland Surg. 2017, 6, 587–590. [Google Scholar] [CrossRef] [PubMed]
- Canu, G.L.; Medas, F.; Cappellacci, F.; Podda, M.G.; Romano, G.; Erdas, E.; Calò, P.G. Can thyroidectomy be considered safe in obese patients? A retrospective cohort study. BMC Surg. 2020, 20, 275. [Google Scholar] [CrossRef] [PubMed]
- Jatzko, G.R.; Lisborg, P.H.; Muller, M.G.; Vette, V.M. Recurrent nerve palsy after thyroid operations-principal nerve identification and a literature review. Surgery 1994, 115, 139–144. [Google Scholar] [PubMed]
- Colak, T.; Akca, T.; Kanik, A.; Yapici, D.; Aydin, S. Total versus subtotal thyroidectomy for the management of benign multinodular goiter in an endemic region. ANZ J Surg. 2004, 74, 974–978. [Google Scholar] [CrossRef] [PubMed]
- Robert, J.; Mariéthoz, S.; Pache, J.C.; Bertin, D.; Caulfield, A.; Murith, N.; Peytremann, A.; Goumaz, M.; Garcia, B.; Martin-Du Pan, R.; et al. Short- and long-term results of total vs subtotal thyroidectomies in the surgical treatment of Graves' disease. Swiss Surg. 2001, 7, 20–24. [Google Scholar] [CrossRef] [PubMed]
- Tezelman, S.; Borucu, I.; Senyurek Giles, Y.; Tunca, F.; Terzioglu, T. The change in surgical practice from subtotal to near-total or total thyroidectomy in the treatment of patients with benign multinodular goiter. World J Surg. 2009, 33, 400–405. [Google Scholar] [CrossRef] [PubMed]
- Wojtczak, B.; Kaliszewski, K.; Sutkowski, K.; Głód, M.; Barczyński, M. The learning curve for intraoperative neuromonitoring of the recurrent laryngeal nerve in thyroid surgery. Langenbecks Arch Surg. 2017, 402, 701–708. [Google Scholar] [CrossRef] [PubMed]
- Wojtczak, B.; Kaliszewski, K.; Sutkowski, K.; Głód, M.; Barczyński, M. Evaluating the introduction of intraoperative neuromonitoring of the recurrent laryngeal nerve in thyroid and parathyroid surgery. Arch Med Sci. 2018, 14, 321–328. [Google Scholar] [CrossRef]
- Wojtczak, B.; Kaliszewski, K.; Sutkowski, K.; Bolanowski, M.; Barczyński, M. A functional assessment of anatomical variants of the recurrent laryngeal nerve during thyroidectomies using neuromonitoring. Endocrine 2018, 59, 82–89. [Google Scholar] [CrossRef]
- Edafe, O.; Cochrane, E.; Balasubramanian, S.P. Reoperation for Bleeding After Thyroid and Parathyroid Surgery: Incidence, Risk Factors, Prevention, and Management. World J Surg. 2020, 44, 1156–1162. [Google Scholar] [CrossRef] [PubMed]
- Miccoli, P.; Frustaci, G.; Fosso, A.; Miccoli, M.; Materazzi, G. Surgery for recurrent goiter: complication rate and role of the thyroid-stimulating hormone-suppressive therapy after the first operation. Langenbecks Arch Surg. 2015, 400, 253–258. [Google Scholar] [CrossRef] [PubMed]
- Wojtczak, B.; Barczyński, M. Intermittent neural monitoring of the recurrent laryngeal nerve in surgery for recurrent goiter. Gland Surg. 2016, 5, 481–489. [Google Scholar] [CrossRef] [PubMed]
- Głód, M.; Marciniak, D.; Kaliszewski, K.; Sutkowski, K.; Rudnicki, J.; Bolanowski, M.; Wojtczak, B. Analysis of Risk Factors for Phonation Disorders after Thyroid Surgery. Biomedicines 2022, 10, 2280. [Google Scholar] [CrossRef] [PubMed]
- Chou, R.; Dana, T.; Haymart, M.; Leung, A.M.; Tufano, R.P.; Sosa, J.A.; Ringel, M.D. Active Surveillance Versus Thyroid Surgery for Differentiated Thyroid Cancer: A Systematic Review. Thyroid 2022, 32, 351–367. [Google Scholar] [CrossRef] [PubMed]
- Tufano, R.P.; Pace-Asciak, P.; Russell, J.O.; Suárez, C.; Randolph, G.W.; López, F.; Shaha, A.R.; Mäkitie, A.; Rodrigo, J.P.; Kowalski, L.P.; et al. Update of Radiofrequency Ablation for Treating Benign and Malignant Thyroid Nodules. The Future Is Now. Front Endocrinol (Lausanne). 2021, 12, 698689. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).