1. Introduction
Cisplatin (cis-diaminedichloroplatinum [II]) is an antineoplastic drug widely used in chemotherapy practice for the treatment of various human cancers [
1,
2,
3,
4]. However, its usage has been limited due to cisplatin-caused various side effects, especially different toxicities [
3,
4,
5,
6]. It has been shown, that these toxicoses are the result of the accumulation of reactive oxygen species (ROS) [
1,
2,
3,
6,
7]. Cisplatin induces the formation of ROS, which in turn can interact with DNA, lipids, proteins, leading to lipids peroxidation and DNA damage [
8,
9,
10]. The induction of oxidative stress and ROS formation is considered another mechanism of cisplatin action [
1,
2,
7,
9]. Cisplatin increases the production of free oxygen radicals and decreases the antioxidants, thus resulting in the disturbance of the oxidant/antioxidant balance [
10]. It is well known, that in normal physiological conditions the presence of ROS is vital for normal function of cells. In order to promote normal cellular physiological function and survival, redox homeostasis regulation is maintained in the cell [
11]. High amount of ROS mostly accumulated due to imbalance in the ROS production and its elimination process. This imbalance is formed due to either increased production of oxidants or decreased levels of antioxidants or both [
9,
11]. Cisplatin disrupts the oxidant/antioxidant balance by inducing the formation of ROS and by decreasing of antioxidants. Disturbed redox homeostasis leads to oxidative damage to biomolecules, such as proteins, lipids, and nucleic acids [
9,
11]. These damages resulting harmful effects on cells. In contrast, ROS level modulation contributes to the regulation of cell survival, death, differentiation and proliferation [
12].
The main targets of ROS are lipids, which are oxidized when interacting with oxidants. These process is known as lipid peroxidation, which leads to the formation of lipoperoxyl radicals and lipid hydro peroxides [
12,
13,
14]. As highly reactive compounds, lipid peroxides are also able to propagate further generation of ROS, or degrade into reactive compounds capable of cross linking DNA and proteins. Lipid hydro peroxides have been recognized as key mediators of cellular disease and death [
12,
13]. Lipid peroxidation products are involved in the intracellular signaling mechanisms that determine the cell’s final fate [
8]. ROS generated by cisplatin could also increase lipid peroxidation, which alters enzymes and structural proteins, and direct the cell to an apoptotic pathway [
12,
13,
14].
As already noted, the cause of side effects of cisplatin is oxidative stress. To mitigate the side effects of antitumor drug cisplatin, dexamethasone is used as concomitant agent in chemotherapy practice [
15,
16,
17]. Dexamethasone is synthetic glucocorticoid hormone. It is known that glucocorticoids, including dexamethasone acts as an anti-inflammatory and immunosuppressant agent [
15,
16,
17]. Dexamethasone induced alterations in lipid peroxidation products and antioxidants content in wistar albino rats [
16,
17,
18].
Thus, it turns out that both the antitumor drug cisplatin and dexamethasone, which is used as a concomitant agent, stimulate the formation of reactive oxygen species and increase the degree of lipid peroxidation [
4,
7,
14,
15,
16,
17,
18]. From this point of view, the study of lipid peroxidation processes with separate and combined use of cisplatin and dexamethasone is of particular interest. This work is devoted to the study of quantitative changes of lipid peroxidation products in the nuclei of cells of some tissues of rats after the separate and combined use of cisplatin and dexamethasone.
2. Materials and methods
Experiments were conducted according to the “International Recommendations on Carrying out of Biomedical Researches with use of Animals” (CIOMS, 1985; 2016), to the “Human Rights and Biomedicine the Oviedo Convention” (CE, 1997), to the European Convention for the Protection of Vertebral Animals Used for Experimental and Other Scientific Purposes (CE, 2005) and approved by the National Center of Bioethics (Armenia).
The investigation was performed on adult albino rats (120-150 g weight, 16 rats). The animals were divided into 4 groups. The group 1 was a control group of animals without treatment. Animals of group 2 and group 4 received a single dose of cisplatin (8 mg/kg). Cisplatin was injected peritoneal. Exposition time for cisplatin was 24 hour. The group 3 was treated with dexamethasone (4 mg/kg, injected peritoneal). Exposition time for dexamethasone was 4 hours. Animals from the group 4 were received the same single dose of dexamethasone within 20 hours after the cisplatin injection (4 hours before decapitation). All animals were killed by decapitation through appropriate time after the inhalation anesthesia with chloroform. Then, animals were sacrificed, and the brain and kidney tissues were extracted from each group of animals and used for isolation of nuclei by the method of Blobel and Potter [
19].
The nuclear fraction of brain and kidney tissues of rats has been used for estimation the quantity of lipid peroxidation primary products. The primary products of lipid peroxidation are lipid hydro peroxides, which form conjugate double bonds in the fatty acid molecule - diene conjugates and triene conjugates. The primary products of lipids peroxidation were estimated by the method of Volchegorsky [
20].
The principle of the method is based on the determination of the content of lipid peroxidation products in biological material by absorption of monochromatic light flux in the ultraviolet spectrum after its extraction by heptane-isopropyl alcohol mixture. This mixture of heptane-isopropyl alcohol is extracted lipids of nuclei from brain and kidney tissues. Heptane extracts mainly neutral lipids and isopropanol extracts phospholipids. In this way, it is possible to identify lipoperoxidation products (diene and triene conjugates) in extracts of different lipid classes. In the lipid extracts of each phase, measurements are made at 220 nm (absorption of double bonds in unsaturated fatty acids), 232 nm (absorption reflects the content of diene conjugates) and 278 nm (absorption depends on the content of ketodienes and conjugated trienes) [
20].
After the estimation of optical density at appropriate wavelengths, it is possible to calculate the oxidation index. The oxidation index is equal to the ratio of optical densities determined at different wavelengths (232nm, 278nm) and absorption of unsaturated fatty acids (220 nm), i.e., 232/220; 278/220 [
20].
2.1. Statistical analysis
All results were expressed as Median±SE from 4 independent experiments. Statistical analysis was performed using paired Student’s t-test for grouped data. Statistically significance is reported at P < 0.05 (*).
3. Results and discussion
The process of lipid peroxidation begins with the attack of free radicals on polyunsaturated fatty acids. As a result, lipid radicals are formed, which in turn can initiate new radicals, starting a chain reaction [
12,
13,
14]. Since unsaturated fatty acids are the raw material for lipid peroxidation, our research has been started by their quantitative evaluation in heptane and isopropanol phases, extracted from nuclei of brain and kidney tissues of rats
included in four experimental groups. As it has already been mentioned
, հeptane dissolves neutral lipids, and isopropyl alcohol dissolves mainly phospholipids [
20]
.
The obtained data are presented in table 1 (
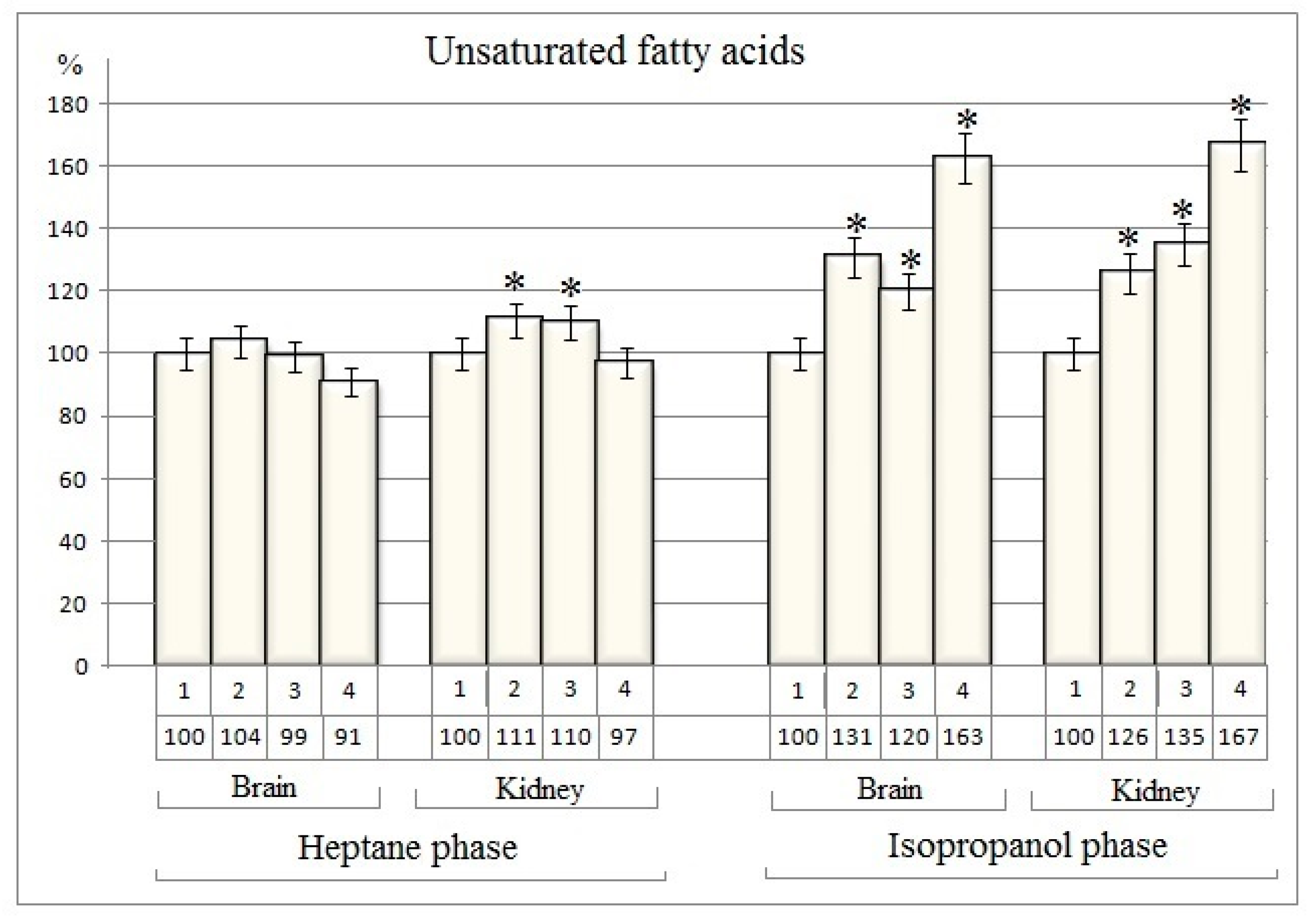
Table 1). The data received indicate that there are no changes in the amount of unsaturated fatty acids in the heptane phase from rat brain nuclei after separate use of cisplatin and dexamethasone, as well as with their combined action (
Table 1).
Only an 11% and 10% increase of unsaturated fatty acids quantity is recorded in heptane phase from rat kidney nuclei after the cisplatin and dexamethasone separate action respectively (
Table 1 and
Figure 1). In the same time the quantity of unsaturated fatty acids were increased in isopropanol phase from nuclear fraction of investigated tissues both with the separate action of cisplatin and dexamethasone and with their combined action (
Table 1).
In comparison to the baseline group, the
changes in the amount of unsaturated fatty acids in the isopropanol phase from rat
brain nuclei are 31%, 20% and 63%, respectively after separate action of of cisplatin and dexamethasone and in case of its combined injection (
Figure 1).
In the isopropanol phase from rat
kidney nuclei, the changes in the amount of unsaturated fatty acids are 26%, 35% and 67%, respectively after separate action of of cisplatin and dexamethasone and in case of its combined injection (
Figure 1).
It is known that cisplatin, like other anticancer drugs, is capable of suppressing and blocking all processes that support cell growth. Cisplatin suppresses all cell metabolic processes, including lipid metabolism [
21]
. The increase in the amount of unsaturated fatty acids in the isopropanol phase is probably a result of suppression of lipid metabolism, cleavage of various lipids [
21]
.
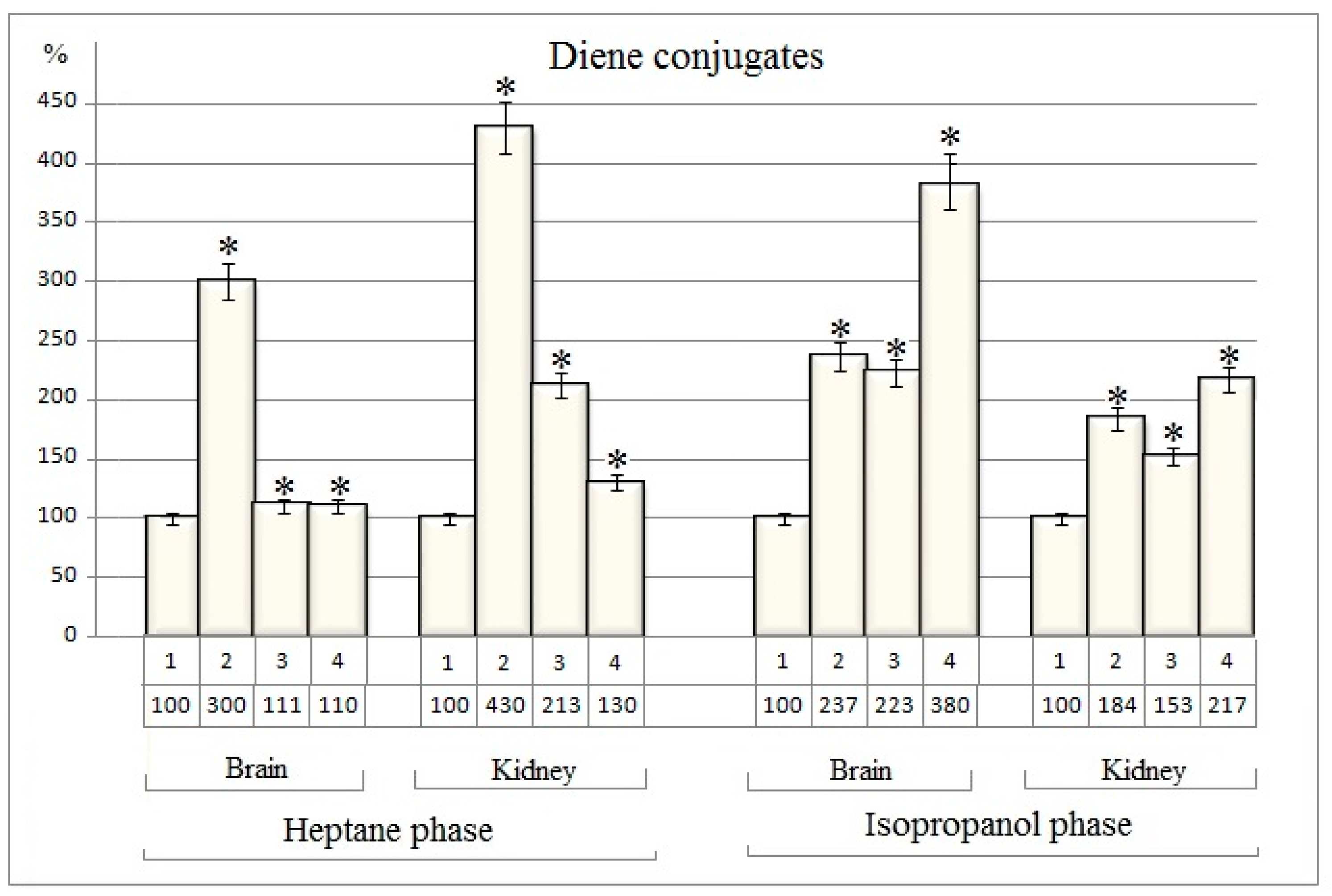
The results of quantitative evaluation of diene conjugates are given in table 2 (Table 2). The data obtained indicate significant changes in the amount of diene conjugates in both the heptane and isopropanol phases of investigated nuclear fractions in comparison to the baseline group
(Table 2).
These quantitative changes expressed as percentages are shown in
Figure 2.
The amount of diene conjugates in the heptane phase both extracted from the nuclear fractions of brain and kidney tissues of rats increases by 200% and 330 % after the separate injection of cisplatin, while dexamethasone increases the quantity
of dien conjugates by only 11% in the nuclei of the brain tissue. In the nuclear fraction of kidney tissue
dexamethasone increases the quantity
of diene conjugates by 113% (
Table 2 and Figure2).
In case of cisplatin and dexamethasone combined use the quantity of diene conjugates
in the heptane phase of brain nuclei increased by only 10% and in the kidney nuclei by 30% in comparison to the baseline group
(Figure 2). In fact, the stimulatory effect of cisplatin is completely suppressed in the presence of
dexamethason. (
Figure 2).
The stimulating effect of cisplatin and dexamethasone is also observed in the isopropanol phase from the nuclei of the brain and kidney tissues. When used separately, cisplatin increases quantity of diene conjugates by 137% and 84% respectively in the brain and kidney nuclear fractions (Figure 2). Unlike the heptane phase, in the phospholipid content isopropanol phase the stimulating effects of cisplatin and dexamethasone seem to be summed up in case of combined use of these drugs. In this case, the amount of diene conjugates increases by 280% and 117%, respectively, in the nuclei of the brain and kidney tissues of rats (Figure 2).
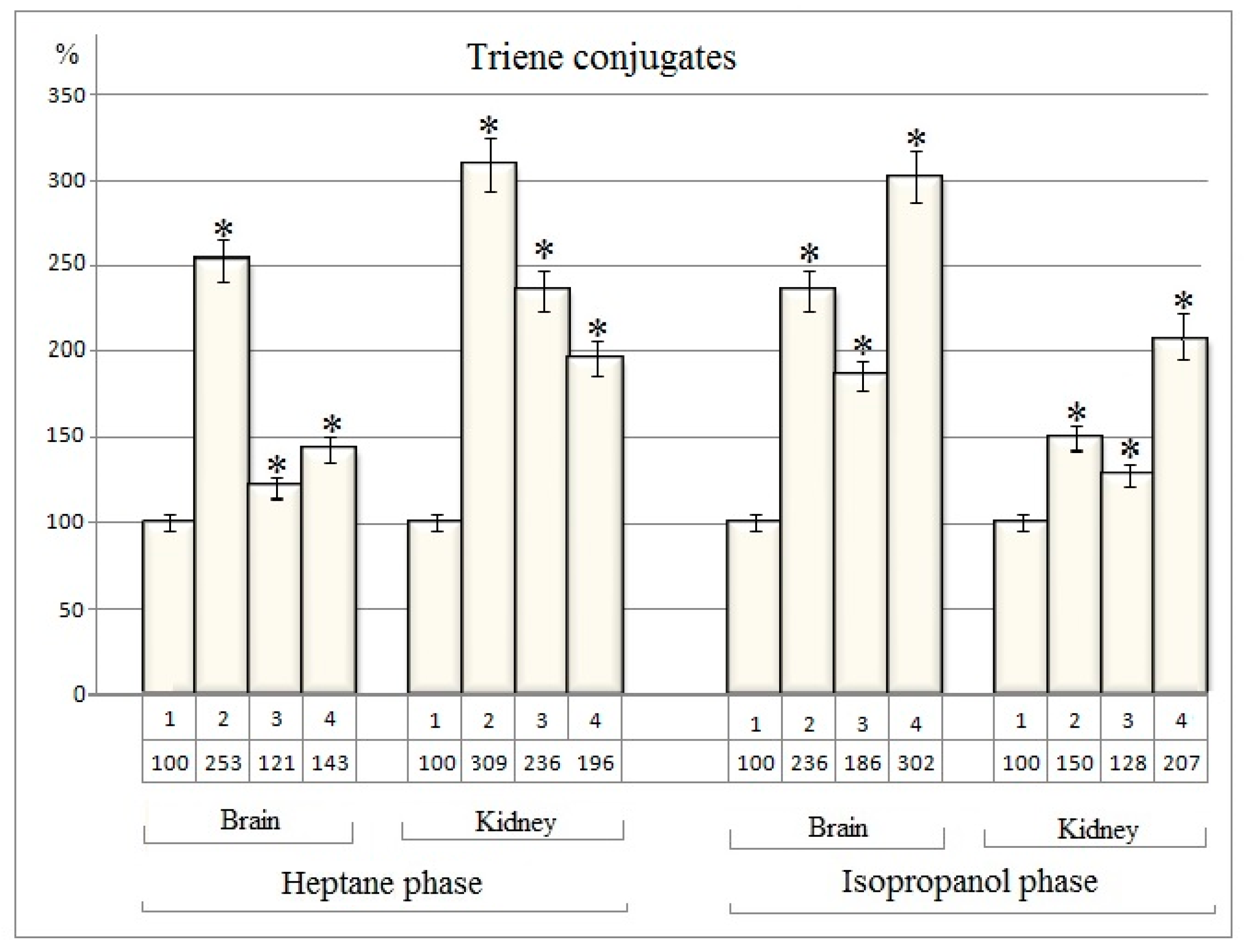
Quantitative analysis data for triene conjugates extracted from the nuclear fractions of brain and kidney tissues of rats are presented in the table 3 (
Table 3).
The obtained data indicate that, the quantity of triene conjugates in heptane phases of both nuclear fractions of brain and kidney tissues are growing significantly in all investigating experimental groups (Table 3)
.
Thus, after exposure to cisplatin in nuclear fractions of rat brain and kidney tissues the quantity of triene conjugates are increased by 153% and 209%, respectively, compared to baseline (
Figure 3). After the separate action of dexamethasone the alterations are relatively small and amount to 21% and 136%
respectively in nuclear fractions of rat brain and kidney tissues (
Figure 3).
After the separate action of dexamethasone in the nuclear fraction of the rat brain, the change in the amount of triene conjugates is only 21%, while in kidney tissue it is 136% (
Figure 3).
In case of combined use of cisplatin and dexamethasone the quantitative change of triene conjugates in heptane phase from brain nuclei (43%) is lower than in the case of cisplatin separate injection, but are higher than the dexamethasone effect (21%) (
Figure 3). In heptane phase from nuclear fraction of kidney cells the quantity of triene conjugates increases by 96% in comparission to baseline after the cisplatin and dexamethasone combined action.
Significant changes are also registered in the isopropanol phase (
Figure 3).
Thus, cisplatin increases the amount of triene conjugates in the brain nuclei by 136%, and in the kidney by 50% (
Figure 3).
Dexamethasone separate injection increases the amount of triene conjugates in the nuclear fraction of brain by 86% and in the kidney nuclei by 28%. The joint injection of these drugs increases the amount of triene conjugates of the isopropanol phase of brain nuclei by 202% and kidney nuclei by 107%. In this case, the additive effect of cisplatin and dexamethasone is recorded (
Figure 3).
Based on the results of quantitative assessment of lipid peroxidation products in investigated experimental groups the oxidation index for diene and triene conjugates both from heptane and isopropanol phases were calculated (Table 4). Oxidation index is the ratio of optical densities recorded at wavelengths of 232 nm and 278 nm and optical absorption values at 220 nm (unsaturated fatty acids) [
20]
. The results are shown in Table 4 (Table 4). Accepting the values recorded in the control versions as 100%, the changes in the oxidative index values expressed in % after exposure to cisplatin and dexamethasone separate and combined action were calculated.
Thus, oxidative index values calculated for diene and triene conjugates increased to a different extent as a result of both cisplatin and dexamethasone separate and combined exposure (Table 4). The exception is the values of oxidative indexes, calculated for triene conjugates of isopropanol phase from kidney nuclei after the dexamethasone separate action. In this case no change was registered (Table 4). For diene and triene conjugates, rather high percentage changes of the oxidation index are registered, especially in the heptane phase obtained from brain and kidney nuclei after separate exposure to cisplatin
(Table 4). The calculated values for the isopropanol phase after single exposure to cisplatin is relatively low (Table 4).
In the case of separate injection of dexamethasone, the changes in the oxidation index calculated for diene and triene conjugates in the investigated phases are smaller than the changes caused by cisplatin (Table 4). An interesting pattern is observed in the heptane phase after combined exposure to cisplatin and dexamethasone. In this case, the obtained values
take an average position from the values recorded as a result of separate use of these drugs (Table 4). In the isopropanol (containing phospholipids) phase this pattern is maintained only for renal diene conjugates
(Table 4).
4. Conclusions
Thus, summarizing the results of the research, it should be noted that both exposure to the cisplatin and dexametasone separate and combined use causes profound changes in the content of lipid peroxidation products (diene and triene conjugates) of rat brain and kidney nuclear fractions in all investigated experimental groups. The obtained quantitative changes of diene and triene conjugates and its oxidation indexes indicate the activation of the lipid peroxidation process with separate and combined use of cisplatin and dexamethasone. Other researchers also testify to similar effects of cisplatin and dexamethasone when used separately. Both cisplatin and dexamethasone induce the formation of oxidative stress. As a result, lipid peroxidation is activated to varying degrees [
1,
2,
3,
15,
16,
17]
. Dexamethasone induced alterations in lipid peroxidation products, antioxidants content in wistar albino rats [
15,
16,
17,
18].
It should be noted that the enhancing effect of cisplatin is much greater than the effect of dexamethasone. As a result of these alterations, the values of the oxidation index in the studied nuclear preparations change accordingly. With the combined use of cisplatin and dexamethasone there is some antagonism in the action of these drugs. Instead of the supposed summation of the effects of enhancing lipid peroxidation processes, “masking” of the effect of cisplatin with dexamethasone is observed. Thus, it was assumed that exactly such antagonism revealed during combined action helps mitigate the side effects of cisplatin with dexamethasone.
Author Contributions
Conceptualization, E.G.; Writing-Review&Editing, E.G.; Supervision, E.G.; Methodology, Z.Y.; Software, Z.Y.; Validation, Z.Y.; Formal analysis, Z.Y.; Investigation, Z.Y., A.H. and N.H.; Writing–Original Draft, Z.Y. and N.H.; Vizualization, N.H.; Resources, A.H.; Data Curation, Z.Y. and N.H. All authors have read and agreed to the published version of the manuscript.
Funding
This study received no external funding. The work was carried out at the Interfaculty Research Laboratory of Structural Biophysics with basic financing (Department of Biophysics, Faculty of Biology, Yerevan State University, Republic of Armenia).
Institutional Review Board Statement
Experiences were fulfilled according to the “International Recommendations on carrying out of biomedical Researches with use of Animals” (CIOMS, 2016), to the “Human Rights and Biomedicine the Oviedo Convention” (CE, 1997), to the European Convention for the Protection of Vertebral Animals Used for Experimental and Other Scientific Purposes (CE, 2005) and approved by the National Center of Bioethics (Armenia).
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Aldossary, S.A. Review on pharmacology of cisplatin: clinical use, toxicity and mechanism of resistance of cisplatin. Biomed. Pharmacol. J. 2019, 12, 7–15. [Google Scholar] [CrossRef]
- Jadon., A.S.; Bhadauriya, P.; Sharma, M. An integrative review of cisplatin: the first metal anti-tumor drug. Journal of Drug Delivery and Therapeutics. (JDDT) 2019, 9, 673–677. [Google Scholar]
- Dasari, S.; Njiki, S.; Mbemi, A.; Yedjou, C.G.; Tchounwou, P.B. Pharmacological effects of cisplatin combination with natural products in cancer chemotherapy. Int. J. Mol. Sci. 2022, 23, 1–25. [Google Scholar] [CrossRef]
- Tchounwou, P. B.; Dasari, S.; Noubissi, F. K.; Ray, P.; Kumar, S. Advances in our understanding of the molecular mechanisms of action of cisplatin in cancer therapy. J. Exp. Pharmacol. 2021, 13, 308–323. [Google Scholar] [CrossRef] [PubMed]
- Stone, J.B.; DeAngelis, L.M. Cancer Treatment-induced neurotoxicity: a focus on newer treatments. Nat. Rev. Clin. Oncol. 2016, 13, 92–105. [Google Scholar] [CrossRef] [PubMed]
- Hashem, R.M.; Safwar, G.M.; Rashed, L.A.; Bakry, S. Biochemical findings on cisplatin-induced oxidative neurotoxicity in rats. Int J Adv.Res. 2015, 3, 1222–1234. [Google Scholar]
- Yang, H.; Villani, R.M.; Wang, H.; Simpson, M.J.; Roberts, M.S.; Tang, M.; Liang, X. The role of cellular reactive oxygen species in cancer chemotherapy. Journal of Experimental and Clinical Cancer Research. 2018, 37, 1–10. [Google Scholar] [CrossRef]
- Habtemariam, S. Modulation of reactive oxygen species in health and disease. Antioxidants 2019, 8, 513–516. [Google Scholar] [CrossRef] [PubMed]
- Sidharta, B.R.A.; Purwanto, B.; Wasita, B.; Widyaningsih, V.; Soetrisno, O.E. Single or Divided Administration of Cisplatin Can Induce Inflammation and Oxidative Stress in Male Sprague-Dawley Rats. Indones Biomed J. 2022, 14, 164–171. [Google Scholar] [CrossRef]
- Singh, R.; Manna, P.P. Reactive oxygen species in cancer progression and its role in therapeutics. Explore Med. 2022, 3, 43–57. [Google Scholar] [CrossRef]
- Gaschler M., M.; Stockwell, B.R. Lipid peroxidation in cell death. Biochem. and Biophys. Res. Commun. 2017, 482, 419–425. [Google Scholar] [CrossRef]
- Mirzaei, S.; Hushmandi, K.; Zabolian, A.; Saleki, H.; Torabi, S.M.R.; Ranjbar, A.; SeyedSaleh, S.; Sharifzadeh, S.O.; Khan, H.; Ashrafizadeh, M.; et al. Elucidating role of reactive oxygen species (ROS) in cisplatin chemotherapy: A focus on molecular pathways and possible therapeutic strategies. Molecules 2021, 26, 1–37. [Google Scholar] [CrossRef]
- Hauck, A. K.; Bernlohr, D. A. Oxidative stress and lipotoxicity. Journal of Lipid Research 2016, 57, 1977–1986. [Google Scholar] [CrossRef]
- Casares, C.; Ramı´rez-Camacho, R.; Trinidad, A.; Rolda´n, A.; Jorge, E.; Garcı´a-Berrocal, J. R. Reactive oxygen species in apoptosis induced by cisplatin: review of physiopathological mechanisms in animal models. Eur Arch Otorhinolaryngol 2012, 269, 2455–2459. [Google Scholar] [CrossRef] [PubMed]
- Cook, A. M.; McDonnella, A. M.; Lakea, R.A.; Nowaka, A. K. Dexamethasone co-medication in cancer patients undergoing chemotherapy causes substantial immunomodulatory effects with implications for chemo-immunotherapy strategies. Oncoimmunology 2016, 5, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Chow, R.; Warr, D.G.; Navari, R.M.; Tsao, M.; Milakovic, M.; Popovic, M.; Chiu, L.; Lam, H.; DeAngelis, C. Efficacy and safety of 1-day versus 3-day dexamethasone for the prophylaxis of chemotherapy-induced nausea and vomiting: a systematic review and meta-analysis of randomized controlled trials. J Hosp Manag Health Policy 2018, 2, 1–13. [Google Scholar] [CrossRef]
- Sverediuk, Y.A.; Pelykh, V.Y. Changes of lipid oxidation products content in ventricles of the rat heart as a result of electrolyte-steroid cardiomyopathy and correction of this condition. Journal of Education, Health and Sport. 2020, 10, 272–280. [Google Scholar] [CrossRef]
- Alahmar, A.T.; Al Jothery, A.H.T.; Al-Daami, Q.J.; Abbas, A.; Al-Hassnawi, A.T.S. The effect of coenzyme Q10 on dexamethasone-induced oxidative stress in rats testes. Med J Babylon 2023, 20, 130–135. [Google Scholar]
- Blobel, G.; Potter, V.R. Nuclei from rat liver: isolation method that combines purity with high yield. Science 1966, 154, 1662–1665. [Google Scholar] [CrossRef] [PubMed]
- Volchegorsky, I.A.; Nalimov, A.G.; Yarovinsky, B.G.; Livshits, R.I. Comparison of different approaches to the determination of lipid peroxidation products in heptane-isopropanol blood extracts. Question. honey. chemistry 1989, 35, 127–131, (Article in Russian). [Google Scholar]
- Plathow, C.H.; Weber, W.A. Tumor Cell Metabolism Imaging. Journal of Nuclear Medicine 2008, 4, 43S–63S. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).