Submitted:
05 October 2023
Posted:
09 October 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Stroke Patients Infected with COVID-19
2.2. Statistics
2.3. Ethics
3. Results
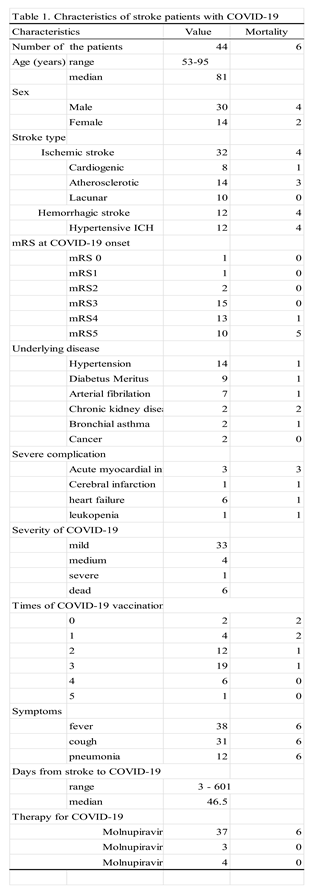
3.1. Characteristics of the Hospitalized Stroke Patients with COVID-19
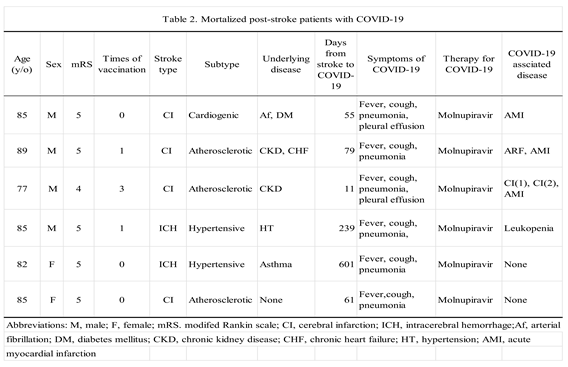
3.2. Mortalized Patients with COVID-19
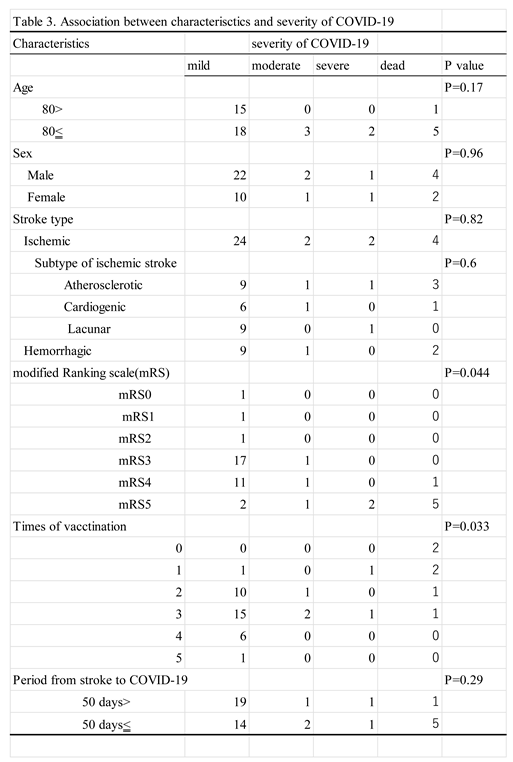
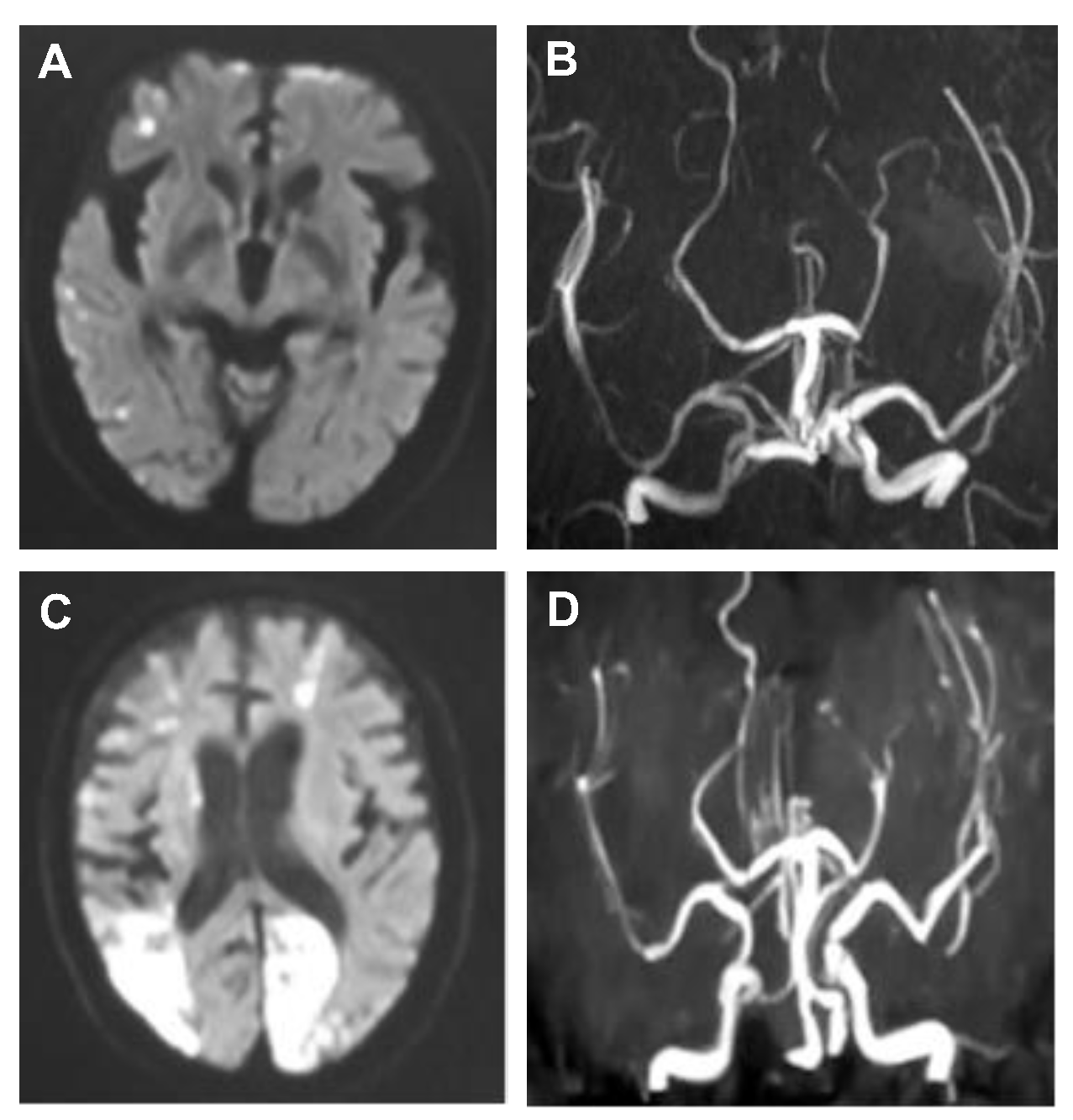
3.3. Association between Variables of the Patients
4. Discussion
5. Limitations
6. Conclusion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chow, E.J.; Uyeki, T.M.; Chu, H.Y. The effects of the COVID-19 pandemic on community respiratory virus activity. Nat. Rev. Microbiol. 2022, 21, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Lu, R.; Zhao, X.; Li, J.; Niu, P.; Yang, B.; Wu, H.; Wang, W.; Song, H.; Huang, B.; Zhu, N.; et al. Genomic characterization and epidemiology of 2019 novel coronavirus:implications for virus origins and receptor binding. Lancet. 2020, 395, 565–574. [Google Scholar] [CrossRef]
- Ye, Z.W.; Yuan, S.; Yuen, K.S.; Fung, S.Y.; Chan, C.P.; Jin, D.Y. Zoonotic origins of human coronaviruses. Int. J. Biol. Sci. 2020, 16, 1686–1697. [Google Scholar] [CrossRef] [PubMed]
- Petrosillo, N.; Viceconte, G.; Ergonul, O.; Ippolito, G.; Petersen, E. COVID-19, SARS and MERS:are they closely related? Clin. Microbiol. Infect. 2020, 26, 729–734. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Wu, Q.; Zhang, Z. Probable pangolin origin of SARS-CoV-2 associated with the COVID-19 outbreak. Curr. Biol. 2020, 30, 1346–1351. [Google Scholar] [CrossRef]
- Walls, A.C.; Park, Y.J.; Tortorici, M.A.; Wall, A.; McGuire, A.T.; Veesler, D. Structure, function, and antigenicity of the SARS-CoV-2 spike glycoprotein. Cell 2020, 181, 281–292. [Google Scholar] [CrossRef]
- Daly, J.L.; Simonetti, B.; Klein, K. ; Chen KE, Williamson MK, Antón-Plágaro C, Shoemark DK, Simón-Gracia L, Bauer M, Hollandi R, et al. Neuropilin-1 is a host factor for SARS-CoV-2 infection. Science 2020, 370, 861–865. [Google Scholar] [CrossRef]
- Ackermann, M.; Verleden, S.E.; Kuehnel, M.; Haverich, A.; Welte, T.; Laenger, F.; Vanstapel, A.; Werlein, C.; Stark, H.; Tzankov, A.; et al. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid-19. N. Engl. J. Med. 2020, 383, 120–128. [Google Scholar] [CrossRef]
- Schoggins, J.W. Interferon stimulated genes:What do they all do? Annu. Rev. Virol. 2019, 6, 567–584. [Google Scholar] [CrossRef]
- Blanco-Melo, D.; Nilsson-Payant, B.E.; Liu, W.C.; Uhl, S.; Hoagland, D.; Møller, R.; Jordan, T.X.; Oishi, K. ; Panis. M., Sachs. D. et al. Wang TT, Schwartz RE, Lim JK, et al. Imbalanced host response to SARS-CoV-2 drives development of COVID-19. Cell 2020, 181, 1036–1045. [Google Scholar] [CrossRef]
- Bastard, P.; Rosen, L.B.; Zhang, Q.; Michailidis, E.; Hoffmann, H.-H.; Zhang, Y.; Dorgham, K.; Philippot, Q.; Rosain, J.; Béziat, V.; et al. Auto-antibodies against type I IFNs in patients with life-threatening COVID-19. Science 2020, 370, eabd4585. [Google Scholar] [CrossRef]
- Broggi, A.; Ghosh, S.; Sposito, B.; Spreafico, R.; Balzarini, F.; Cascio, A.L.; Clementi, N.; De Santis, M.; Mancini, N.; Granucci, F.; et al. Type III interferons disrupt the lung epithelial barrier upon viral recognition. Science 2020, 369, 706–712. [Google Scholar] [CrossRef]
- Boudewijns, R.; Thibaut, H.J.; Kaptein, S.J.F.; Li, R.; Vergote, V.; Seldeslachts, L.; Van Weyenbergh, J.; De Keyzer, C.; Bervoets, L. ; Sharma S, et al. STAT2 signaling restricts viral dissemination but drives severe pneumonia in SARS-CoV-2 infected hamsters. Nat. Commun. 2020, 11, 5838. [Google Scholar]
- Kuba, K.; Imai, Y.; Penninger, J.M. Multiple Functions of Angiotensin-Converting Enzyme 2 and Its Relevance in Cardiovascular Diseases. Circ. J. 2013, 77, 301–308. [Google Scholar] [CrossRef]
- Semenzato, L.; Botton, J.; Drouin, J.; Baricault, B.; Vabre, C.; Cuenot, F.; Penso, L.; Herlemont, P.; Sbidian, E.; Weill, A. , et al. Antihypertensive drugs and COVID-19 risk:A cohort study of 2 million hypertensive patients. Hypertension 2021, 77, 833–842. [Google Scholar] [CrossRef] [PubMed]
- Kutsuna, S. Coronavirus disease 2019 (COVID-19): research progress and clinical practice. Glob. Heal. Med. 2020, 2, 78–88. [Google Scholar] [CrossRef] [PubMed]
- Matsunaga, N.; Hayakawa, K.; Terada, M.; Ohtsu, H.; Asai, Y.; Tsuzuki, S.; Suzuki, S.; Toyoda, A.; Suzuki, K.; Endo, M.; et al. Clinical Epidemiology of Hospitalized Patients With Coronavirus Disease 2019 (COVID-19) in Japan: Report of the COVID-19 Registry Japan. Clin. Infect. Dis. 2020, 73, e3677–e3689. [Google Scholar] [CrossRef] [PubMed]
- Altschul, D.J.; Esenwa, C.; Haranhalli, N.; Unda, S.R.; Ramos, R.d.L.G.; Dardick, J.; Fernandez-Torres, J.; Toma, A.; Labovitz, D.; Cheng, N.; et al. Predictors of mortality for patients with COVID-19 and large vessel occlusion. Interv. Neuroradiol. 2020, 26, 623–628. [Google Scholar] [CrossRef] [PubMed]
- Grasselli, G.; Greco, M.; Zanella, A.; Albano, G.; Antonelli, M.; Bellani, G.; Bonanomi, E.; Cabrini, L.; Carlesso, E.; Castelli, G.; et al. Risk Factors Associated With Mortality Among Patients With COVID-19 in Intensive Care Units in Lombardy, Italy. JAMA Intern. Med. 2020, 180, 1345–1355. [Google Scholar] [CrossRef] [PubMed]
- Mayer, C.; Woo, M.S.; Brehm, T.T.; Heyer, A.; Fischer, M.; Fischbach, F.; Bal, L.C.; Addo, M.M.; Kluge, S.; Thomalla, G.; et al. History of cerebrovascular disease but not dementia increases the risk for secondary vascular events during SARS-CoV-2 infection with presumed Omicron variant: a retrospective observational study. Eur. J. Neurol. 2023, 30, 2297–2304. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; He, Z.; Yang, J.; Guo, Q.; Weng, H.; Luo, J.; Gong, B.; Cui, W.; Ding, B.; Guo, J. Clinical Characteristics, Outcomes, and Risk Factors of Disease Severity in Patients With COVID-19 and With a History of Cerebrovascular Disease in Wuhan, China: A Retrospective Study. Front. Neurol. 2022, 12, 706478. [Google Scholar] [CrossRef]
- Aggarwal, G.; Lippi, G.; Henry, B.M. Cerebrovascular disease is associated with an increased disease severity in patients with Coronavirus Disease 2019 (COVID-19): A pooled analysis of published literature. Int. J. Stroke 2020, 15, 385–389. [Google Scholar] [CrossRef]
- Li, Y.; Li, M.; Wang, M.; Zhou, Y.; Chang, J.; Xian, Y.; Wang, D.; Mao, L.; Jin, H.; Hu, B. Acute cerebrovascular disease following COVID-19: a single center, retrospective, observational study. Stroke Vasc. Neurol. 2020, 5, 279–284. [Google Scholar] [CrossRef]
- Zhou, Y.; Li, W.; Wang, D.; Mao, L.; Jin, H.; Li, Y.; Hong, C.; Chen, S.; Chang, J.; He, Q.; et al. Clinical time course of COVID-19, its neurological manifestation and some thoughts on its management. Stroke Vasc. Neurol. 2020, 5, 177–179. [Google Scholar] [CrossRef]
- Lodigiani, C.; Iapichino, G.; Carenzo, L.; Cecconi, M.; Ferrazzi, P.; Sebastian, T.; Kucher, N.; Studt, J.-D.; Sacco, C.; Bertuzzi, A.; et al. Venous and arterial thromboembolic complications in COVID-19 patients admitted to an academic hospital in Milan, Italy. Thromb. Res. 2020, 191, 9–14. [Google Scholar] [CrossRef]
- Annie, F.; Bates, M.C.; Nanjundappa, A.; Bhatt, D.L.; Alkhouli, M. Prevalence and Outcomes of Acute Ischemic Stroke Among Patients ≤50 Years of Age With Laboratory Confirmed COVID-19 Infection. Am. J. Cardiol. 2020, 130, 169–170. [Google Scholar] [CrossRef] [PubMed]
- Kihira, S.; Schefflein, J.; Mahmoudi, K.; Rigney, B.; Delman, B.N.; Mocco, J.; Doshi, A.; Belani, P. Association of Coronavirus Disease (COVID-19) With Large Vessel Occlusion Strokes: A Case-Control Study. Am. J. Roentgenol. 2021, 216, 150–156. [Google Scholar] [CrossRef] [PubMed]
- Paganini-Hill, A.; Lozano, E.; Fischberg, G.; Perez Barreto, M.; Rajamani, K.; Ameriso, S.F.; Heseltine, P.N.; Fisher, M. Infection and risk of ischemic stroke: Differences among stroke subtypes. Stroke 2003, 34, 452–457. [Google Scholar] [CrossRef] [PubMed]
- Yaghi S, Ishida K, Torres J, et al. SARS-CoV-2 and stroke in a New York healthcare system. Stroke 2020, 51, 2002–2011.
- Rothstein, A.; Oldridge, O.; Schwennesen, H.; Do, D.; Cucchiara, B.L. Acute Cerebrovascular Events in Hospitalized COVID-19 Patients. Stroke 2020, 51, E219–E222. [Google Scholar] [CrossRef]
- Ntaios, G.; Michel, P.; Georgiopoulos, G.; Guo, Y.; Li, W.; Xiong, J.; Calleja, P.; Ostos F.; González-Ortega, G.; Fuentes, B., et al. Characteristics and outcomes in patients with COVID-19 and acute ischemic stroke: The Global COVID-19 Stroke Registry. Stroke 2020 Sep;51: e254-e258.
- Merkler, A.E.; Parikh, N.S.; Mir, S.; Gupta, A.; Kamel, H.; Lin, E.; Lantos, J.; Schenck, E.J.; Goyal, P.; Bruce, S.S.; et al. Risk of Ischemic Stroke in Patients With Coronavirus Disease 2019 (COVID-19) vs Patients With Influenza. JAMA Neurol. 2020, 77, 1366–1372. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, M.; Kleine-Weber, H. ; Schroeder, S;, Krüger, N. ; Herrler, T.; Erichsen, S.; Schiergens, T.S.; Herrler, G.; Wu,N.H.; Nitsche, A.; et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell 2020, 181, 271–280. [Google Scholar]
- Varga, Z.; Flammer, A.J.; Steiger, P.; Haberecker, M.; Andermatt, R.; Zinkernagel, A.S.; Mehra, M.R.; Schuepbach, R.A.; Ruschitzka, F.; Moch, H. Endothelial cell infection and endotheliitis in COVID-19. Lancet 2020, 395, 1417–1418. [Google Scholar] [CrossRef] [PubMed]
- Bhaskar, S.; Sinha, A.; Banach, M.; Mittoo, S.; Weissert, R.; Kass, J.S.; Rajagopal, S.; Pai, A.R.; Kutty, S. Cytokine Storm in COVID-19-Immunopathological Mechanisms, Clinical Considerations, and Therapeutic Approaches: The REPROGRAM Consortium Position Paper. Front. Immunol. 2020, 11, 1648. [Google Scholar] [CrossRef]
- Belani P, Schefflein J, Kihira S, Rigney B, Delman BN, Mahmoudi K, Mocco J, Majidi S, Yeckley J, Aggarwal A, et al. AJNR Am. J. Neuroradiol. 2020, 41, 1361–1364.
- Harrison, S.L.; Buckley, B.J.R.; Rivera-Caravaca, J.M.; Zhang, J.; Lip, G.Y.H. Cardiovascular risk factors, cardiovascular disease, and COVID-19: an umbrella review of systematic reviews. Eur. Hear. J. - Qual. Care Clin. Outcomes 2021, 7, 330–339. [Google Scholar] [CrossRef] [PubMed]
- Martí-Fàbregas, J.; Guisado-Alonso, D.; Delgado-Mederos, R. , Martínez-Domeño, A. ; Prats-Sánchez, L.; Guasch-Jiménez, M.; Cardona, P.; Núñez-Guillén, A.; Requena, M.; Rubiera, M.; et al. Impact of COVID-19 infection on the outcome of patients with ischemic stroke. Stroke 2021, 52, 3908–3917. [Google Scholar]
- Tregoning, J.S.; Flight, K.E.; Higham, S.L.; Wang, Z.; Pierce, B.F. Progress of the COVID-19 vaccine effort: viruses, vaccines and variants versus efficacy, effectiveness and escape. Nat. Rev. Immunol. 2021, 21, 626–636. [Google Scholar] [CrossRef] [PubMed]
- Akinosoglou, K.; Schinas, G.; Gogos, C. Oral Antiviral Treatment for COVID-19: A Comprehensive Review on Nirmatrelvir/Ritonavir. Viruses 2022, 14, 2540. [Google Scholar] [CrossRef]
- Moura, E.C.; Cortez-Escalante, J.; Cavalcante, F.V.; Barreto, I.C.H.C.; Sanchez, M.N.; Santos, L.M.P. Covid-19: Temporal Evolution and Immunization in the Three Epidemiological Waves, Brazil, 2020-2022. Rev. Saude. Publica. 2022, 56, 105. [Google Scholar] [CrossRef] [PubMed]
- Lauring, A.S.; Tenforde, M.W.; Chappell, J.D.; Gaglani, M.; Ginde, A.A.; McNeal, T.; Ghamande, S.; Douin, D.J.; Talbot, H.K.; Casey, J.D.; et al. Clinical severity of, and effectiveness of mRNA vaccines against, Covid-19 from Omicron, Delta, and Alpha SARS-CoV-2 variants in the United States: Prospective observational study. B.M.J. 2022, 376, e069761. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.K.; Goh, C.; Leow, A.S.; A Tambyah, P.; Ang, A.; Yap, E.S.; Tu, T.M.; Sharma, V.; Yeo, L.L.; Chan, B.P.; et al. Abstract P89: Covid-19 and Ischemic Stroke: A Systematic Review and Meta-Summary of the Literature. Stroke 2021, 52. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




