Submitted:
07 October 2023
Posted:
08 October 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
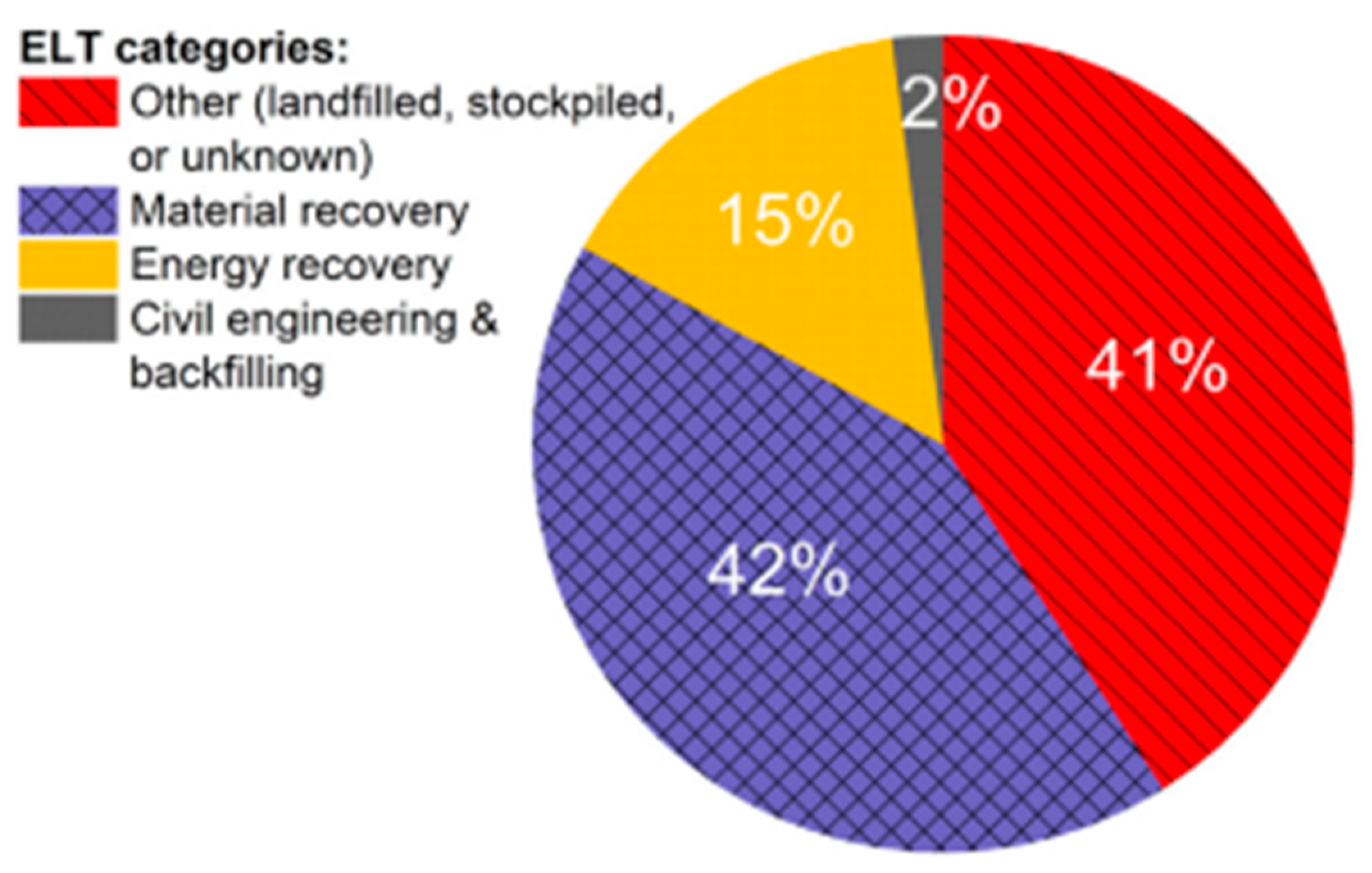
- Tyre industry responsibility: Recovery, recycling and disposal of waste tyres is a responsibility of tyre producers. They finance these activities through an eco-fee, which is charged on the original sale price. This model is used in some European countries, Brazil, South Africa, Russia, Ukraine, and South Korea.
- Tax system: Each producer pays to the government a disposal duty that is added to the cost of the new tyre. Recovery organizations are financed by the government and they are responsible for the management of the used tyres. The following countries apply this model: Canada, Croatia, Latvia, Denmark, and Slovak Republic.
- Free market system: Dedicated enterprises are operating independently on recovering and recycling waste tyres. This model is applied in Austria, Germany, Ireland, New Zealand, Switzerland, Argentina, China, India, Indonesia, Japan, Malaysia, Mexico, Saudi Arabia, Thailand, UK, and USA.
1.1. Products of pyrolysis of car tyres waste; composition and characteristics
1.2. Influence of the process parameters on tyre pyrolysis
2. Materials and Methods
3. Results
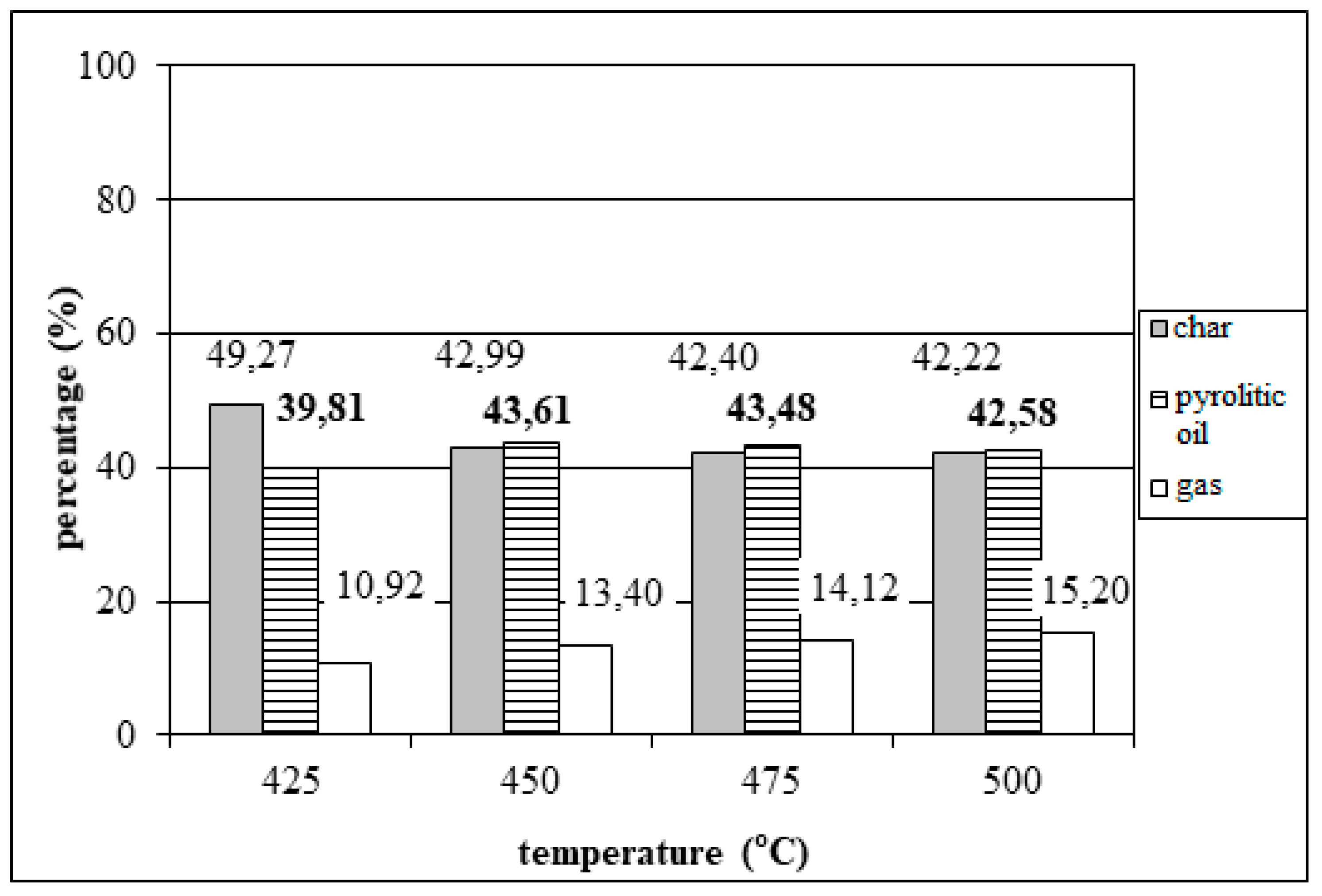
3.1. Effect of temperature on tyre pyrolysis
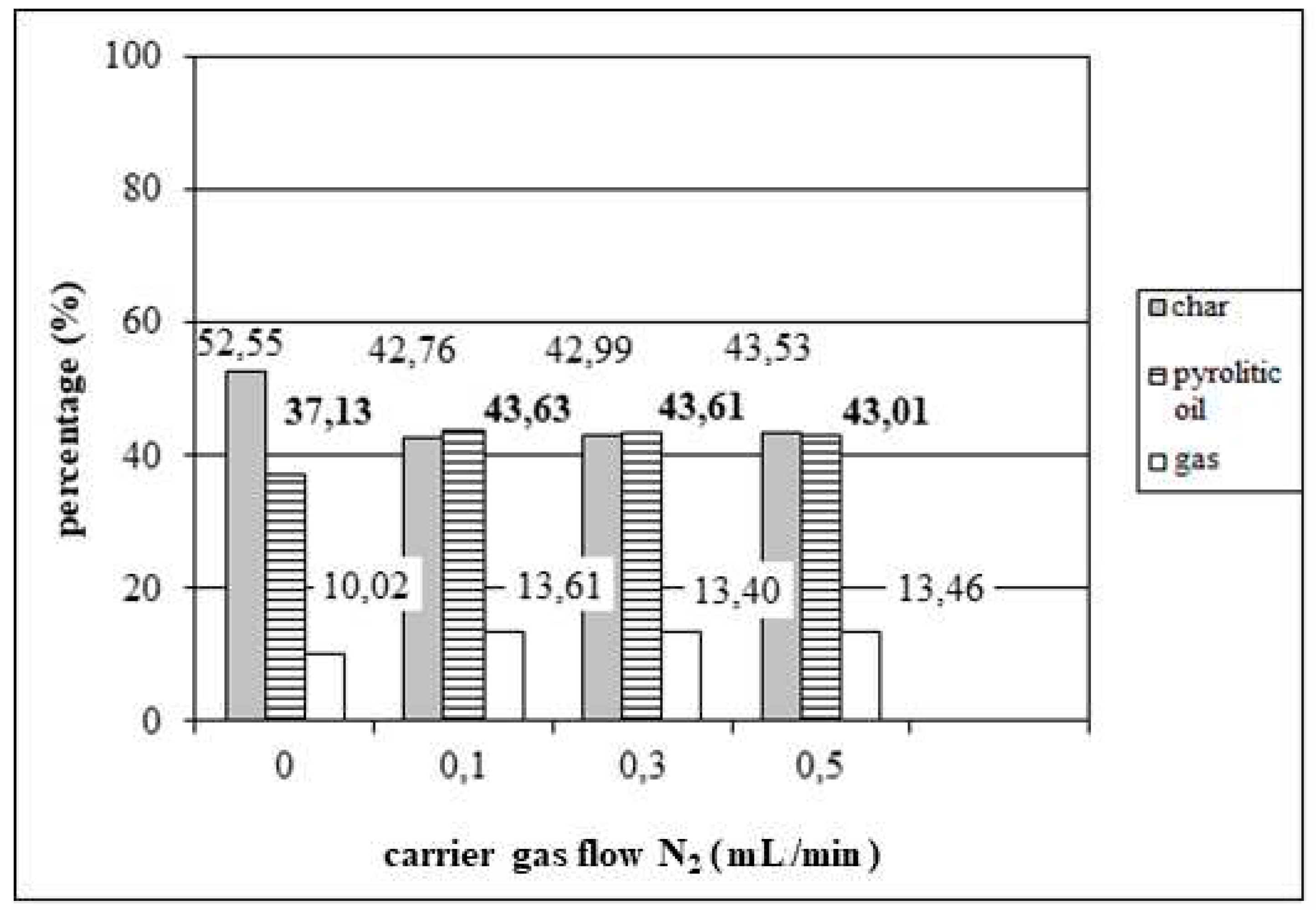
3.2. Effect of inert gas flow on tyre pyrolysis
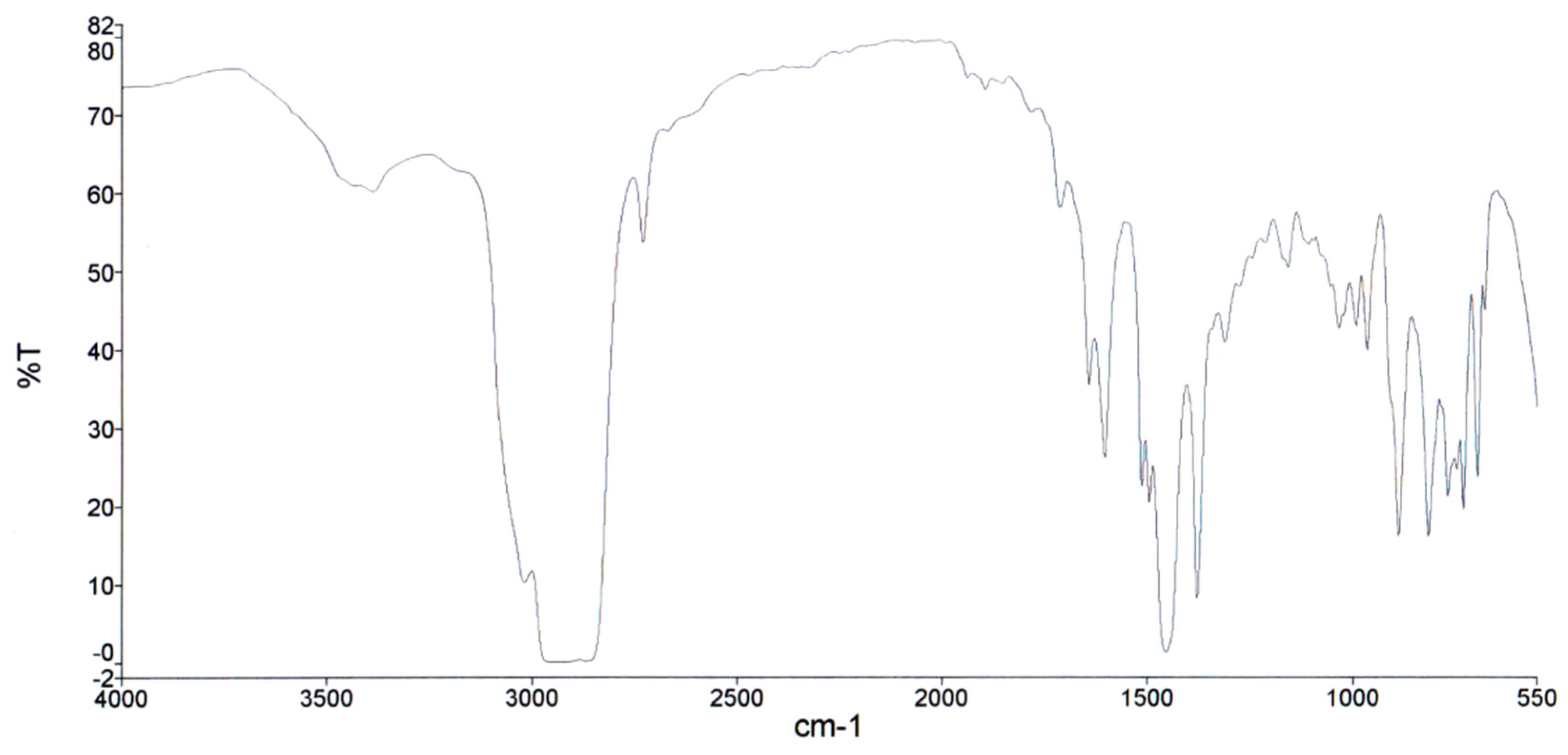
3.3. Calorimetry, chemical and proximate analysis
3.4. Proximate analysis of pyrolysis char
4. Discussion
5. Conclusions
- Research on the influence of the process parameters on the maximum yield of pyrolysis oil during the pyrolysis of tyres in a fixed bed reactor shows that the optimal conditions are: reaction time of 120 min, reactor temperature of 450 °C, inert gas flow of 100 mL/min, at an installed heating power of 1000 W and a heating rate of 14°C/min.
- Under optimal conditions, the pyrolysis of tyres waste yields a product consisting of: 43.63% m/m pyrolysis oil, 13.61% m/m gas and 42.76% m/m solid residue, all in mass percentages, that is, the total conversion of raw materials into pyrolysis oil and gas of 57.24% is achieved.
- Pyrolysis oil obtained from tyres is the most suitable for use for energy purposes due to its high heating value (42 MJ/kg), which is close to the heating value of higher quality coals (43 MJ/kg) and the heating value of oil (44 MJ/kg). The results of FTIR analysis of the pyrolysis oil show the following content in mass percentages: aromatic compounds 32.59%, paraffins 51.06% and naphthenes 16.35%.
- The low content of sulfur (0.407%) in the obtained pyrolysis oil also indicates its potential for use as an energy source. The prescribed value of sulfur content in heating oil is 1% m/m, for 4 types of liquid petroleum fuels (light special LS, light L, medium LUS and heavy oil LUT).
- Pyrolysis char or carbon black obtained by pyrolysis of tyres waste also has a high calorific value 31 MJ/kg, and can be used as a solid fuel as well as an adsorbent, catalyst or catalyst carrier after the activation process.
Author Contributions
Funding
Conflicts of Interest
References
- How Many Cars Are There in the World? [Internet]. PD Insurance Blog. 2022. Available on: https://www.pd.com.au/blogs/how-many-cars-in-the-world/.
- Valentini, F.; Pegoretti A. End-of-life options of tyres. A review. Adv Ind Eng Polym Res. 2022, 5, 4, 203–213. [CrossRef]
- Radley-Gardner, O; Beale, H.; Zimmermann, R. Editors. Fundamental Texts On European Private Law [Internet]. Hart Publishing; 2016 [cited 2023 Sep 4]. Available from: http://www.bloomsburycollections.com/book/fundamental-texts-on-european-private-law-1.
- Dong, Y.; Zhao, Y.; Hossain, M.dU.; He, Y. , Liu, P. Life cycle assessment of vehicle tires: A systematic review. Clean Environ Syst. 2021, 2, 100033. [Google Scholar] [CrossRef]
- Gao, N.; Wang, F.; Quan, C.; Santamaria, L.; Lopez, G.; Williams, P.T. Tire pyrolysis char: Processes, properties, upgrading and applications. Prog Energy Combust Sci 2022, 93, 101022. [Google Scholar] [CrossRef]
- Han, W.; Han, D.; Chen, H. Pyrolysis of Waste Tires: A Review. Polymers. 2023, 15, 7, 1604. [CrossRef] [PubMed]
- Papuga, S.; Djurdjevic, M.; Ciccioli, A.; Vecchio Ciprioti, S. Catalytic Pyrolysis of Plastic Waste and Molecular Symmetry Effects: A Review. Symmetry 2023, 15, 38. [Google Scholar] [CrossRef]
- Cafiero, L.; Castoldi, E.; Tuffi, R.; Vecchio Ciprioti, S. Identification and characterization of plastics from small appliances and kinetic analysis of their thermally activated pyrolysis. Polym. Degrad. Stab. 2014, 109, 307–318. [Google Scholar] [CrossRef]
- Vouvoudi, E.C.; Achilias, D.S. Pyrolytic degradation of common polymers present in packaging materials. J. Therm. Anal. Calorim. 2019, 138, 2683–2689. [Google Scholar] [CrossRef]
- Vouvoudi, E.C.; Achilias, D.S. Polymer packaging waste recycling: study of the pyrolysis of two blends via TGA. J. Therm. Anal. Calorim. 2020, 142, 1891–1895. [Google Scholar] [CrossRef]
- Tuffi, R.; D’Abramo, S.; Cafiero, L.M.; Trinca, E.; Vecchio Ciprioti, S. Thermal behavior and pyrolytic degradation kinetics of polymeric mixtures from waste packaging plastics. Express Polym. Lett. 2018, 12, 82–99. [Google Scholar] [CrossRef]
- Esposito, L.; Cafiero, L.; De Angelis, D.; Tuffi, R.; Vecchio Ciprioti, S. Valorization of the plastic residue from a WEEE treatment plant by pyrolysis. Waste Manag. 2020, 112, 1–10. [Google Scholar] [CrossRef]
- Hoang, A.T.; Nguyen, T.H.; Nguyen, H.P. Scrap tire pyrolysis as a potential strategy for waste management pathway: a review. Energy Sources Part Recovery Util Environ Eff. 2020, 1–18. [Google Scholar] [CrossRef]
- Juma, M.; Kore, Z. Pyrolysis and combustion of scrap tire. 2006; Pet Coal. 2006, 48, 1, 15-26.
- Cunliffe, A.M., Williams, P.T. Composition of oils derived from the batch pyrolysis of tyres. J Anal Appl Pyrolysis. 1998, 44, 2, 131–52. [CrossRef]
- Kaminsky, W. Recycling of polymers by pyrolysis. J Phys IV. 1993, 3, C7-1543-C7-1552. [CrossRef]
- Cheung, K.Y.; Lee, K.L.; Lam, K.L.; Lee C.W.; Hui CW. Integrated kinetics and heat flow modelling to optimise waste tyre pyrolysis at different heating rates. Fuel Process Technol. 2011, 92, 5, 856–63. [CrossRef]
- Quek, A. , Balasubramanian, R. Liquefaction of waste tires by pyrolysis for oil and chemicals—A review. J Anal Appl Pyrolysis. 2013, 101, 1–16. [Google Scholar] [CrossRef]
- Luo, W.; Wan, J.; Fan, Z.; Hu, Q.; Zhou, N.; Xia, M.; Song, M.; Qi, Z.; Zhou, Z. In-situ catalytic pyrolysis of waste tires over clays for high quality pyrolysis products. Int J Hydrog Energy. 2021, 46, 9, 6937–44. [Google Scholar] [CrossRef]
- Lee, J.M.; Lee, J.S.; Kim, J.R.; Kim, S.D. Pyrolysis of waste tires with partial oxidation in a fluidized-bed reactor. Energy. 1995, 20, 10, 969–76. [Google Scholar] [CrossRef]
- Dai, X.; Yin, X.; Wu, C.; Zhang, W.; Chen, Y. Pyrolysis of waste tires in a circulating fluidized-bed reactor. Energy. 2001, 26, 4, 385–99. [Google Scholar] [CrossRef]
- Chen, G.; Sun, B.; Li, J.; Lin, F.; Xiang, L.; Yan, B. Products distribution and pollutants releasing characteristics during pyrolysis of waste tires under different thermal process. J Hazard Mater. 2022, 424, 127351. [Google Scholar] [CrossRef]
- Nkosi, N.; Muzenda, E. A Review and Discussion of Waste Tyre Pyrolysis and Derived Products. In Proceedings of the World Congress on Engineering, London, United Kingdom, 2-4 July 2014. [Google Scholar]
- Yaqoob, H.; Teoh, Y.H.; Sher, F.; Jamil, M.A.; Murtaza, D.; Al Qubeissi, M.; Ui Hassan, M.; Mutjaba, M.A. Current Status and Potential of Tire Pyrolysis Oil Production as an Alternative Fuel in Developing Countries. Sustainability. 2021, 13, 6, 3214. [Google Scholar] [CrossRef]
- Williams, P.T. Pyrolysis of waste tyres: A review. Waste Manag. 2013, 33, 8, 1714–28. [Google Scholar] [CrossRef]
- Hita, I.; Arabiourrutia, M.; Olazar, M.; Bilbao, J.; Arandes, J.M.; Castaño, P. Opportunities and barriers for producing high quality fuels from the pyrolysis of scrap tires. Renew Sustain Energy Rev. 2016, 56, 745–59. [Google Scholar] [CrossRef]
- Charitopoulou, M.A.; Stefanidis, S.D.; Lappas, A.A.; Achilias, D.S. Catalytic pyrolysis of polymers with brominated flame-retardants originating in waste electric and electronic equipment (WEEE) using various catalysts. Sustain. Chem. Pharm. 2022, 26, 100612. [Google Scholar] [CrossRef]
- Tomic, T.; Kremer, I.; Vecchio Ciprioti, S.; Schneider, D.R. Efficiency of municipal packaging waste recovery chain and suitability of separated residual waste fractions for use in alternative fuels production. J. Environ. Manag. 2022, 322, 15, 116056. [Google Scholar] [CrossRef] [PubMed]
- Kremer, I.; Tomić, T.; Katančić, Z.; Hrnjak-Murgić, Z.; Erceg, M.; Vecchio Ciprioti, S.; Schneider, D.R. Effect of Zeolite Catalyst on the Pyrolysis Kinetics of Multi-Layered Plastic Food Packaging. Symmetry 2022, 14, 1362. [Google Scholar] [CrossRef]
- United States Environmental Protection Agency: Scarp Tire Cleanup Guidebook: A Resource for Solid Waste Managers Across the United States [Internet]. 2006. Available online: https://nepis.epa.gov/Exe/ZyNET.exe/P100DCR8.TXT?ZyActionD=ZyDocument&Client=EPA&Index=2006+Thru+2010&Docs=&Query=&Time=&EndTime=&SearchMethod=1&TocRestrict=n&Toc=&TocEntry=&QField=&QFieldYear=&QFieldMonth=&QFieldDay=&IntQFieldOp=0&ExtQFieldOp=0&XmlQuery=&File=D%3A%5Czyfiles%5CIndex%20Data%5C06thru10%5CTxt%5C00000031%5CP100DCR8.txt&User=ANONYMOUS&Password=anonymous&SortMethod=h%7C&MaximumDocuments=1&FuzzyDegree=0&ImageQuality=r75g8/r75g8/x150y150g16/i425&Display=hpfr&DefSeekPage=x&SearchBack=ZyActionL&Back=ZyActionS&BackDesc=Results%20page&MaximumPages=1&ZyEntry=1&SeekPage=x&ZyPURL (accessed on 3rd September 2023).
- Cheung, K.Y.; Lee. K.L.; Lam, K.L.; Chan, T.Y.; Lee, C.W.; Hui, C.W. Operation strategy for multi-stage pyrolysis. J Anal Appl Pyrolysis. 2011, 91, 1, 165–82. [CrossRef]
- Abdallah, R.; Juaidi, A.; Assad, M.; Salameh, T.; Manzano-Agugliaro, F. Energy Recovery from Waste Tires Using Pyrolysis: Palestine as Case of Study. Energies. 2020, 13, 7, 1817. [Google Scholar] [CrossRef]
- Jasminská, N.; Brestovič, T.; Čarnogurská, M. The effect of temperature pyrolysis process of used tires on the quality of output products. Acta Mech Autom. 2013, 7,1, 20–5. [Google Scholar] [CrossRef]
- Lee, S.M.; Lee, S.H.; Roh, J.S. Analysis of Activation Process of Carbon Black Based on Structural Parameters Obtained by XRD Analysis. Crystals. 2021, 11,2, 153. [Google Scholar] [CrossRef]
- Kuśmierek, K.; Świątkowski, A.; Kotkowski, T.; Cherbański, R.; Molga, E. Adsorption on activated carbons from end-of-life tyre pyrolysis for environmental applications. Part I. preparation of adsorbent and adsorption from gas phase. J Anal Appl Pyrolysis. 2021, 157, 105205. [Google Scholar] [CrossRef]
- Jones, I.; Preciado-Hernandez, J.; Zhu, M.; Zhang, J.; Zhang, Z.; Zhang, D. Utilisation of spent tyre pyrolysis char as activated carbon feedstock: The role, transformation and fate of Zn. Waste Manag. 2021, 126, 549–58. [Google Scholar] [CrossRef]
- Mikulova, Z.; Sedenkova, I.; Matejova, L.; Večeř, M.; Dombek, V. Study of carbon black obtained by pyrolysis of waste scrap tyres. J Therm Anal Calorim. 2013, 111,2, 1475–81. [Google Scholar] [CrossRef]
- Shah, J.; Jan, M.R.; Mabood, F.; Shahid, M. Conversion of Waste Tyres into Carbon Black and their Utilization as Adsorbent. J Chin Chem Soc. 2006, 53, 5, 1085–9. [Google Scholar] [CrossRef]
- Matandabuzo, M.; Dovorogwa, D. Activated Carbons from Waste Tyre Pyrolysis: Application. In: Bartoli M, Giorcelli M, editors. Recent Perspectives in Pyrolysis Research [Internet]. IntechOpen; 2022 [cited 2023 Sep 21]. Available from: https://www.intechopen.com/chapters/80798.
- Muttil, N.; Jagadeesan, S.; Chanda, A.; Duke, M.; Singh, S.K. Production, Types, and Applications of Activated Carbon Derived from Waste Tyres: An Overview. Appl Sci. 2022, 13,1, 257. [Google Scholar] [CrossRef]
- De Marco Rodriguez, I.; Laresgoiti, M.F.; Cabrero, M.A.; Torres, A.; Chomón, M.J.; Caballero, B. Pyrolysis of scrap tyres. Fuel Process Technol. 2001, 72,1, 9–22. [Google Scholar] [CrossRef]
- Roy, C.; Labrecque, B.; De Caumia, B. Recycling of scrap tires to oil and carbon black by vacuum pyrolysis. Resour Conserv Recycl. 1990, 4,3, 203–13. [Google Scholar] [CrossRef]
- Choi, G.G.; Oh, S.J.; Kim, J.S. Non-catalytic pyrolysis of scrap tires using a newly developed two-stage pyrolyzer for the production of a pyrolysis oil with a low sulfur content. Appl Energy. 2016, 170, 140–7. [Google Scholar] [CrossRef]
- Laresgoiti, M.F.; De Marco, I.; Torres, A.; Caballero, B.; Cabrero, M.A.; Chomón, M.J. Chromatographic analysis of the gases obtained in tyre pyrolysis. J Anal Appl Pyrolysis. 2000, 55,1, 43–54. [Google Scholar] [CrossRef]
- Environmental Factors of Waste Tire Pyrolysis, Gasification, and Liquefaction. California Integrated Waste Management Board; 1995. Report No.: 1364.
- Chatterjee, R.; Sajjadi, B.; Chen, W.Y.; Mattern, D.L.; Hammer, N.; Raman, V.; Dorris, A. Effect of Pyrolysis Temperature on PhysicoChemical Properties and Acoustic-Based Amination of Biochar for Efficient CO2 Adsorption. Front Energy Res. 2020, 8, 85. [Google Scholar] [CrossRef]
- Banar, M.; Akyıldız, V.; Özkan, A.; Çokaygil, Z.; Onay, Ö. Characterization of pyrolytic oil obtained from pyrolysis of TDF (Tire Derived Fuel). Energy Convers Manag. 2012, 62, 22–30. [Google Scholar] [CrossRef]
- Kar, Y. Catalytic pyrolysis of car tire waste using expanded perlite. Waste Manag. 2011, 8, 1772–82. [Google Scholar] [CrossRef]
- Miranda, M.; Pinto, F.; Gulyurtlu, I.; Cabrita, I. Pyrolysis of rubber tyre wastes: A kinetic study. Fuel. 2013, 103, 542–52. [Google Scholar] [CrossRef]
- Li, S.Q.; Yao, Q.; Chi, Y.; Yan, J.H.; Cen, K.F. Pilot-Scale Pyrolysis of Scrap Tires in a Continuous Rotary Kiln Reactor. Ind Eng Chem Res. 2004, 43,17, 5133–45. [Google Scholar] [CrossRef]
- Islam, M.R.; Joardder, M.U.H.; Hasan, S.M.; Takai, K.; Haniu, H. Feasibility study for thermal treatment of solid tire wastes in Bangladesh by using pyrolysis technology. Waste Manag. 2011, 31, 9–10, 2142–9. [CrossRef]
- Akkouche, N.; Balistrou, M.; Loubar, K.; Awad, S.; Tazerout, M. Heating rate effects on pyrolytic vapors from scrap truck tires. J Anal Appl Pyrolysis. 2017, 123, 419–29. [Google Scholar] [CrossRef]
- Williams, P.T.; Beslerm, S.; Taylor, D.T. The pyrolysis of scrap automotive tyres. Fuel. 1990, 69,12, 1474–82. [Google Scholar] [CrossRef]
- Clark, C. , USA, editors. Scrap tire technology and markets. Park Ridge, NJ: Noyes Data Corp; 1993. 316 p. (Pollution technology review).
- Laresgoiti, M.F.; Caballero, B.M.; De Marco, I.; Torres, A.; Cabrero, M.A.; Chomón, M.J. Characterization of the liquid products obtained in tyre pyrolysis. J Anal Appl Pyrolysis. 2004, 71,2, 917–34. [Google Scholar] [CrossRef]
- Berrueco, C.; Esperanza, E.; Mastral, F.J.; Ceamanos, J.; García-Bacaicoa, P. Pyrolysis of waste tyres in an atmospheric static-bed batch reactor: Analysis of the gases obtained. J Anal Appl Pyrolysis. 2005, 74, 1–2, 245–53. [CrossRef]
- Xu, F.; Wang, B.; Yang, D.; Ming, X.; Jiang, Y.; Hao, J.; Qiao, Y.; Tian, Y. TG-FTIR and Py-GC/MS study on pyrolysis mechanism and products distribution of waste bicycle tire. Energy Convers Manag. 2018, 175, 288–97. [Google Scholar] [CrossRef]
- Rofiqulislam, M.; Haniu, H.; Rafiqulalambeg, M. Liquid fuels and chemicals from pyrolysis of motorcycle tire waste: Product yields, compositions and related properties. Fuel. 2008, 87, 13–14, 3112–22. [CrossRef]
- Zabaniotou, A.A.; Stavropoulos, G. Pyrolysis of used automobile tires and residual char utilization. J Anal Appl Pyrolysis. 2003, 70, 2, 711–22. [Google Scholar] [CrossRef]
- Chang, Y.M. On pyrolysis of waste tire: Degradation rate and product yields. Resour Conserv Recycl. 1996, 2, 125–39. [Google Scholar] [CrossRef]
- Barbooti, M.M.; Mohamed, T.J.; Hussain, A.A.; Abas, F.O. Optimization of pyrolysis conditions of scrap tires under inert gas atmosphere. J Anal Appl Pyrolysis. 2004, 72,1, 165–70. [Google Scholar] [CrossRef]
- Yongrong, Y.; Jizhong, C.; Guibin, Z. Technical advance on the pyrolysis of used tires in China. China-Japan International Academic Symposium, Environmental Problem in Chinese Iron-Steelmaking industries and Effective Technology Transfer, Sendai, Japan, 6th March 2000. 20 March.
- Murena, F. Kinetics of sulphur compounds in waste tyres pyrolysis. J Anal Appl Pyrolysis. 2000, 56,2, 195–205. [Google Scholar] [CrossRef]
- Acevedo, B.; Fernández, A.M.; Barriocanal, C. Identification of polymers in waste tyre reinforcing fibre by thermal analysis and pyrolysis. J Anal Appl Pyrolysis. 2015, 111, 224–32. [Google Scholar] [CrossRef]
- Suhanya, M.; Thirumarimurugan, M.; Kannadasan, T. Recovery of oil from waste tyres using pyrolysis method: a review. IJRET, 2013, 1, 81–90. [Google Scholar]
- Kremer, I.; Tomić, T.; Katančić, Z.; Erceg, M.; Papuga, S.; Vuković, J.P.; Schneider, D.R. Catalytic pyrolysis of mechanically non-recyclable waste plastics mixture: Kinetics and pyrolysis in laboratory-scale reactor. J Environ Manage. 2021, 296, 113145. [Google Scholar] [CrossRef]
- Kremer, I.; Tomić, T.; Katančić, Z.; Hrnjak-Murgić, Z.; Erceg, M.; Schneider, D.R. Catalytic decomposition and kinetic study of mixed plastic waste. Clean Technol Environ Policy. 2021, 23,3, 811–27. [Google Scholar] [CrossRef]
- Papuga, S.; Gvero, P.; Vukic, L. Temperature and time influence on the waste plastics pyrolysis in the fixed bed reactor. Therm Sci. 2016, 20,2, 731–41. [Google Scholar] [CrossRef]
- Castaldi, M.J.; Kwon, E. An Investigation of the Thermal Degradation Mechanisms of a Waste Tire Through Chemical Analysis Including Hydrocarbons, Benzene Derivatives, and Polycyclic Aromatic Hydrocarbons (PAHs) at High Temperature. In: 16th Annual North American Waste-to-Energy Conference [Internet]. Philadelphia, Pennsylvania, USA: ASMEDC; 2008 [cited 2023 Sep 3]. 97–105. Available from: https://asmedigitalcollection.asme.org/NAWTEC/proceedings/NAWTEC16/42932/97/328538.
- Ramírez Arias, A.M.; Moreno-Piraján, J.C.; Giraldo, L. Kinetic Study of Waste Tire Pyrolysis Using Thermogravimetric Analysis. ACS Omega. 2022, 7,19, 16298–305. [Google Scholar] [CrossRef]
- Menares, T.; Herrera, J.; Romero, R.; Osorio, P.; Arteaga-Pérez, L.E. Waste tires pyrolysis kinetics and reaction mechanisms explained by TGA and Py-GC/MS under kinetically-controlled regime. Waste Manag. 2020, 102, 21–9. [Google Scholar] [CrossRef]
- Al-Salem, S.M. Valorisation of End of Life Tyres (ELTs) in a Newly Developed Pyrolysis Fixed-Bed Batch Process. Process Saf Environ Prot. 2020, 138, 167–75. [Google Scholar] [CrossRef]
- Al-Salem, S.M. Pyrolysis of end of life tyres reclaimed from lorry trucks: part i – oil recovery and characterisation. 2021, 133, 107–12. [CrossRef]
- Al-Salem, S.M.; Lettieri, P.; Baeyens, J. Kinetics and product distribution of end of life tyres (ELTs) pyrolysis: A novel approach in polyisoprene and SBR thermal cracking. J Hazard Mater. 2009, 172, 2–3, 1690–4. [CrossRef]
- Wang, H.; Hu, H.; Yang, Y.; Liu, H.; Tang, H.; Xu, S.; Li, A.; Yao, H. Effect of high heating rates on products distribution and sulfur transformation during the pyrolysis of waste tires. Waste Manag. 2020, 118, 9–17. [Google Scholar] [CrossRef]
- Rekalic, V.; Vitorovic, O. Analytical tests in technological production: principles and procedures [Original Serbian: Analitička ispitivanja u tehnološkoj proizvodnji: principi i postupci]. Faculty of Technology and Metallurgy, Belgrade, Serbia, 1988.
- Aydın, H.; İlkılıç, C. Optimization of fuel production from waste vehicle tires by pyrolysis and resembling to diesel fuel by various desulfurization methods. Fuel, 2012; 102, 605–12. [CrossRef]
- Rada, E.C.; Ragazzi, M.; Panaitescu, V.N. Energy recovery from tyres waste through thermal option. UPB Scientific Bulletin, Series D: Mechanical Engineering, 2012.
- Hopa, D.Y.; Yilmaz, A.; Bahtli, T.A. Recovery of waste tyres by pyrolysis in a fixed bed reactor for liquid fuel production: effects of pyrolysis conditions on oil yield. Res Eng Struct Mater. 2017, 3, 186–91. [Google Scholar] [CrossRef]
- Islam, M.R.; Beg, M.R.A.; Haniu, H. Fuel Based Liquids From Scrap Tire By Pyrolysis Technology. In Proceedings of the 3rd BSME-ASME International Conference on Thermal Engineering, Dhaka, Bangladesh. Dhaka, Bangladesh; 2006. [Google Scholar]
- González, J.F.; Encinar, J.M.; Canito, J.L.; Rodrı́guez, J.J. Pyrolysis of automobile tyre waste. Influence of operating variables and kinetics study. J Anal Appl Pyrolysis. 2001, 58–59, 667–83. [Google Scholar] [CrossRef]
| Parameter | %m/m |
|---|---|
| Moisture content | 0.77 ± 0.01 |
| Ash content | 5.25 ± 0.01 |
| Char residue | 31.90 ± 0.01 |
| Fixed carbon | 26.65 ± 0.01 |
| Combustible substances | 93.98 ± 0.02 |
| Volatile matter | 67.29 ± 0.01 |
| Temperature | Oil yield (%m/m) | Char yield (%m/m) | Gas yield (%m/m) |
|---|---|---|---|
| 425 | 39.81 | 49.27 | 10.92 |
| 450 | 43.61 | 42.99 | 13.40 |
| 475 | 43.48 | 42.40 | 14.12 |
| 500 | 42.58 | 42.22 | 15.22 |
| Flow rate (mL/min) | Oil yield (%m/m) | Char yield (%m/m) | Gas yield (%m/m) |
|---|---|---|---|
| 0 | 37.13 | 52.55 | 10.02 |
| 100 | 43.63 | 42.76 | 13.61 |
| 300 | 43.61 | 42.99 | 13.40 |
| 500 | 43.01 | 43.53 | 13.46 |
| Property | Method | Result | Unit |
| S content | ISO 20487 | 0.407 | %m/m |
| FTIR Spectrometry | CEI IEC 590 | - | - |
| C aromatic | CEI IEC 590 | 32.59 | %m/m |
| C paraffin | CEI IEC 590 | 51.06 | %m/m |
| C naphthene | CEI IEC 590 | 16.35 | %m/m |
| Parameter | % |
| Moisture content | 0.73 ± 0.01 |
| Ash content | 12.11 ± 0.01 |
| Char residue | 93.83 ± 0.01 |
| Fixed carbon | 81.72 ± 0.01 |
| Combustible substances | 87.16 ± 0.01 |
| Volatile substances | 5.44 ± 0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





