1. Introduction
Amyotrophic lateral sclerosis (ALS) is an adult-onset motor neuron and multisystem disease [
1] that is mainly characterized by progressive motor symptoms, such as muscle weakness, muscle atrophy, and spasticity. Over the past twenty years, several clinical studies have highlighted that clinical presentation in ALS can be quite heterogeneous [2, 3]. Up to 50% of ALS cases are first identified with cognitive dysfunctions [
4], which may worsen and show different profiles across stages of the disease [5-9]. Executive and behavioral dysfunctions may have prognostic implications [10, 11]. Moreover, in ALS, motor and cognitive components appear to worsen in parallel, especially when the bulbar function is involved [8, 12]. Notably, advanced neuroimaging studies revealed widespread damage to extra-motor networks underlying cognitive and behavioral functions during disease progression [13-15]. Consequently, ALS-specific cognitive and behavioral impairments are more frequent in more advanced disease stages [7, 9]. However, cognitive and motor involvement may present with distinct trajectories across the disease course, suggesting a differential vulnerability of motor and non-motor cortical networks in different disease phenotypes [5, 13]. Increasing evidence suggests that cognitive and behavioral impairment in ALS overlaps with pathological and genetic features, as TDP-43 pathologic burden has been associated with cognitive impairment [16, 17] and
C9orf72 repeat expansion has been revealed in ALS patients with rapid cognitive decline and poor survival [18, 19].
The cognitive profile generally described in ALS includes deficits in verbal fluency, language, social cognition, and executive functions [
4]. Conversely, visuospatial abilities in their visuo-perceptual and representational components are not assessed systematically; for instance, the most used assessment tool, the Edinburgh Cognitive and Behavioural Amyotrophic Lateral Sclerosis Screen (ECAS) [
20], does not tap these cognitive aspects.
Evidence in ALS suggested that the visuo-representational and the visuo-perceptual abilities play a crucial role in managing activities of daily living and in preserving patients’ well-being [
21], such as in spatial orientation mediated by environmental cues [
22]. Moreover, visuo-representational and visuo-perceptual abilities participate in generating, retaining, and transforming visual images [
23], processing overall configuration of perceptual stimuli, appreciating their position, and performing mental operations on their spatial representation [
24]. In terms of neural correlates, visuo-representational and visuo-perceptual functions are mediated by wide, distributed neural network including the parietal lobes, the lateral prefrontal cortex, the medial temporal lobes, the inferior temporal cortex, the occipital cortex, and the basal ganglia, particularly in the right hemisphere [
24].
A useful battery to explore both the perceptual component and the representational component of the visuospatial abilities, independent from motor impairment, is the Battery for Visuospatial Abilities (BVA, known in Italy as TeRaDiC; [25-27]), available in English and Italian. Yet, to date, no study applied the BVA in the ALS.
The present pilot study aims at filling the literature gap on the impairment of visuospatial abilities in ALS by means of the 8 subtests included in the BVA. We expected to observe an impairment in visuospatial abilities in ALS, with some subtests possibly showing impairment of selected visuospatial skills. To identify an early impairment of these functions in the ALS course will help improve clinical management.
2. Materials and Methods
2.1. Participants
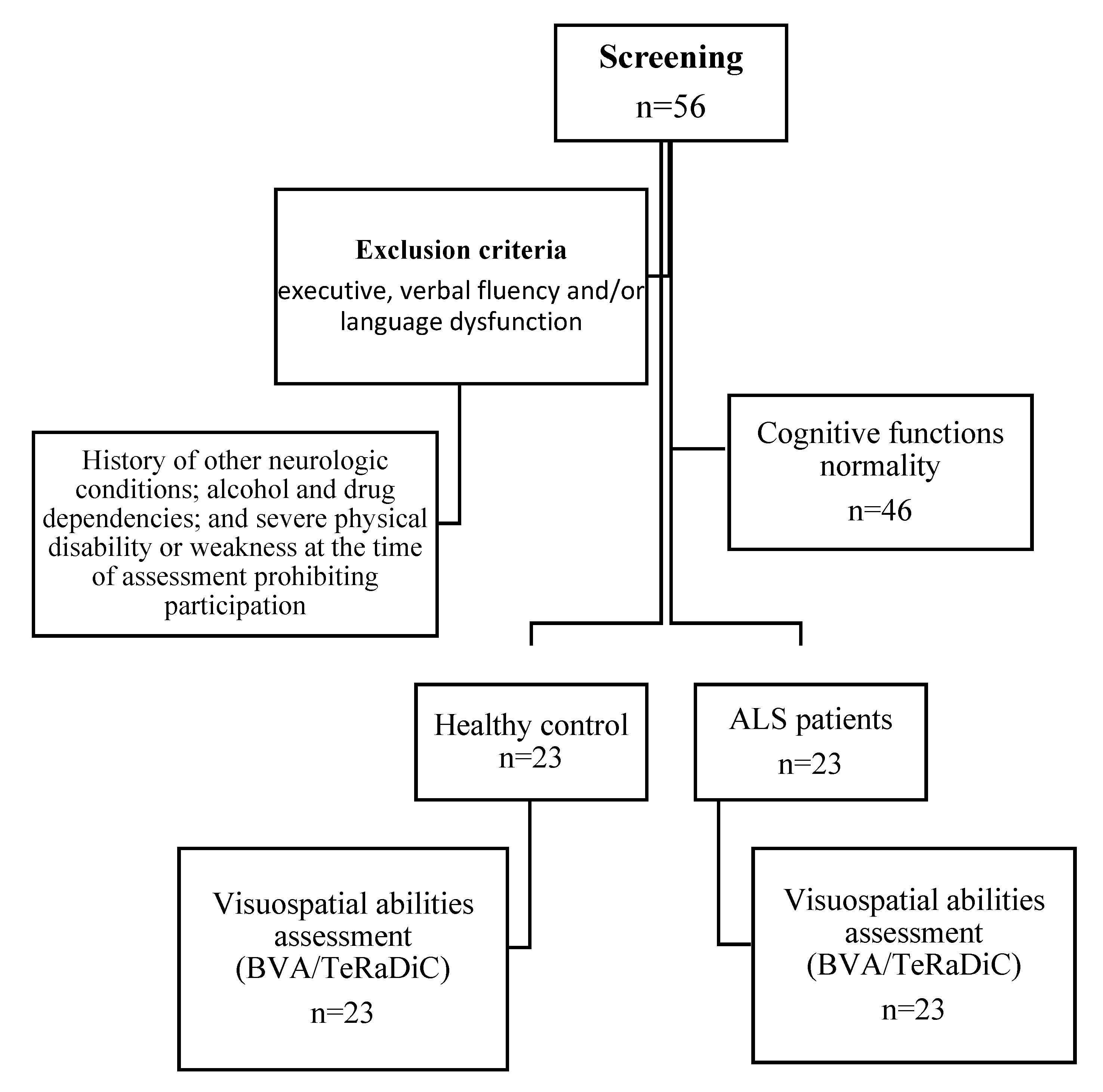
Twenty-three right-handed patients with definite or clinical/laboratory-supported probable ALS, according to the El-Escorial revised criteria [
28], showing classic (n=7), bulbar (n=2), flail limbs (n=11) and 3 pyramidal phenotypes [
29], were included. These patients were prospectively recruited across the First Division of Neurology of the University of Campania “Luigi Vanvitelli” (Naples, Italy) between December 2021 and April 2022. Exclusion criteria were history of other neurologic or psychological conditions; alcohol or drug addiction. Genetic analysis was performed in all patients, exploring
C9orf72 repeat expansion and mutations of
SOD1,
TARDBP, and
FUS/TLS. No mutations of these genes were reported.
Twenty-three age- and education-matched healthy adults were additionally recruited as healthy controls (HC) group through research volunteer panels held by the First Division of Neurology of “Luigi Vanvitelli” University (Naples, Italy), non–blood caregivers of patients with ALS, and local community noticeboards.
Ethics approval was obtained from the Ethics Committee of the University of Campania “Luigi Vanvitelli” in Naples, Italy (Protocol nr. 591/2018). All participants provided informed consent to participate in the study according to the Declaration of Helsinki.
Figure 1.
Flowchart showing the enrollment of the participants in the study.
Figure 1.
Flowchart showing the enrollment of the participants in the study.
2.2. Materials
ALSFRS-R: the ALSFRS-R is a disease-specific 12-item tool assessing patients’ functional abilities to perform independent tasks. The questionnaire is structured on a 5-point scale ranging from 4 to 0, where 4 indicates no loss of function and 0 indicates total loss of function. The ALSFRS-R includes four scales, each measuring one domain affected by the disease [
30].
ECAS: The ECAS is a short screening test (15–20 min) assessing cognitive impairment in ALS [
20], providing sub-scores for language, fluency, executive, memory, and visuospatial abilities. Language is evaluated by naming, comprehension, and spelling. Fluency is measured by free production of words beginning with the letter "s" and a restrained production of words beginning with the letter "t" but with only four letters. Executive functions are measured by a reverse digit span, alternation of letters and numbers, inhibitory sentence completion, and social cognition. The memory subscale includes measurements of immediate recall, delayed percentage retention, and delayed recognition. Finally, visuospatial abilities are measured with dot and cube counting, and number location.
BVA - perceptual subtests: this battery consisted of four tasks: each item is composed of a stimulus presented on the left and the four-choice display presented on the right [
27]. Items are presented one at a time, and participants are required to point to the only item identical to the stimulus among the distracters without time constraints. Scoring procedures assign one point for each correct response; no penalty is computed for wrong responses. The first subtest is line length judgment (LLJ), in which participants are required to identify the line with the same length as the stimulus in the four-choice display. Item complexity increases during the task as linear differences between stimuli and distracters gradually decrease (score range: 0–20). The second subtest is line orientation judgment (LOJ), in which participants must identify, in the four-choice display, the line with the same orientation as the stimulus presented on the left side. The difference in orientation between stimulus and distracters is 30° in half of the items and 15° in the remaining trials. In the first seven items, distracters (of the same length as the stimulus) are arranged as a sunburst, while in the last three items, distracters are randomly spread on the four-choice display (score range: 0–10). The third subtest is angle width judgment (AWJ), in which participants should identify the angle with the same width as the stimulus in the four-choice display. The distracters differ from 15° to 90° from the stimulus (score range: 0–10). The fourth subtest is pointing position identification (PPI): participants are required to identify the square with the same configuration of 1–3 embedded points as the stimulus. Distracters in the four-choice display have the same number of points as the stimulus but in different spatial arrangements (score range: 0–12).
BVA—representational subtests: The four tasks included in this section assess participants’ ability to mentally represent spatial relationships; three of them include a four-choice display, as above, and the last task has a different arrangement [
27]. Each correct response is assigned one point. The first subtest is mental rotation: participants are required to mentally rotate bidimensional stimuli (the italic capital letter L or S, with small white or black circles at the extremities) on the horizontal plane and to identify the only item in the display matching it. The four-choice displays enclose the stimulus item, rotated by 45°, 90°, 135°, or 180°, together with three mirror forms of the stimulus (distracters), printed at different degrees of rotation. Prior to the task, participants receive two practice trials that can be solved with the aid of solid items (score range: 0–10). The second subtest is complex figure identification (CFI, shape recognition): participants have to identify the only figure matching the nonsense geometrical shape (not easily described verbally and of increasing complexity) presented on the left side in the four-choice display. Two practice trials are given before the task (score range: 0–10). The third subtest is hidden figure identification (HFI): stimuli consist of nonsense, complex geometrical patterns. Participants must disassemble each stimulus in their minds to identify, among the four complex geometrical patterns shown in the four-choice display, the only shape exactly embedded in the stimulus. Two practice trials are given (score range: 0–10). The fourth subtest is mental construction: in this task, participants must mentally assemble bidimensional stimuli. Stimuli consist of squares randomly subdivided into four parts, that are randomly shown on the right side of the display. In every trial, the examiner names two of these components, and participants must identify with which side they are contiguous in the stimulus (printed on the left side). Solid stimuli are used to explain the task in two practice trials. Two questions are foreseen for each trial; each correct response is scored 1 point (score range: 0–20).
2.3. Statistical Analyses
We used the Mann–Whitney test (U-test) or Pearson’s chi-squared test (χ2) to compare the patients with ALS and HC on demographics (i.e., age, education, and sex), BVA-perceptual subtests, and BVA-representational subtests.
We employed Spearman's correlation analyses to explore the associations between the clinical features (i.e., disease duration, ALSFRS-R, and UMN) and the accuracy in BVA subtests. Finally, we reported the percentage of ALS and HC with age- and education-adjusted scores in BVA subtests below normative data [
27]. All multiple comparisons were corrected by the Bonferroni procedure, where the corrected p-value lower than 0.05 was considered statistically significant. All analyses were performed using the IBM Statistical Package for Social Science (SPSS) version 25 (Chicago, IL, USA).
3. Results
Patients with ALS and HC did not differ in demographics (
Table 1).
Spearman's correlation analyses did not show significant associations between ALS clinical features and the accuracy in visuo-perceptual and visuo-representational BVA subtests (
Table 2).
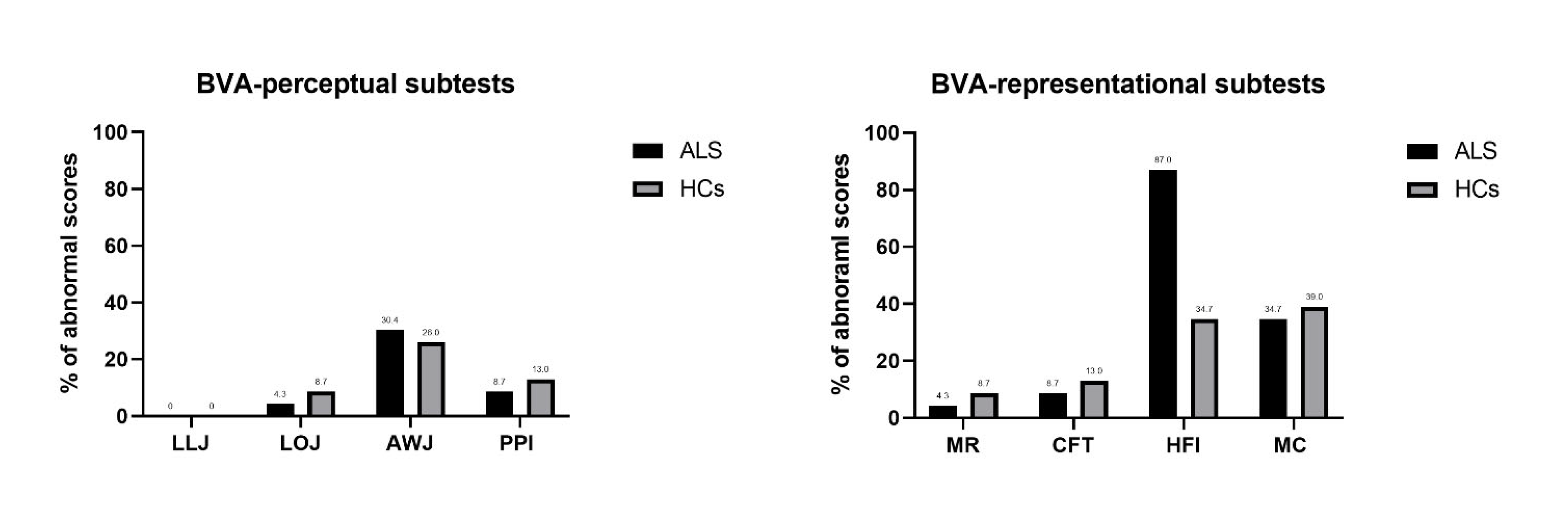
Compared with HC, ALS group performed worse on the BVA perceptual LLJ and LOJ subtests and on the BVA-representational HFI subtest (
Table 3). Since deficits in language or executive functions could impact these results, we also ran Mann–Whitney analyses considering only the subgroup of ALS free from impairments in executive functions and/or language disturbances (as assessed on ECAS, n=19), which basically confirmed the pattern above (see Supplementary Material 1).
Figure 2 reported the percentage of abnormal scores in BVA tasks for ALS and HC.
4. Discussion
In the present study, we examined the profile of visuospatial abilities in patients with ALS by BVA, a battery assessing the visuospatial abilities. The battery minimized the role of motor disorders as it requires only simple pointing or verbal responses, as per individual patients’ preferences. Overall, our results showed that ALS patients scored worse than the HC in several visuo-perceptual and visual-representational tasks, independently from co-occurrent clinically evident executive and language deficits. Some visuo-perceptual tasks (LLJ and LOJ) and one visual-representational task (HFI) were sensitive to identify visuospatial impairments in patients with ALS, with below cut-off scores in visual-representational abilities (HFI) occurring in more than 80% of our cohort of early ALS. On these bases, the common practice of assessing the visuo-perceptual skills in ALS exclusively by means of screening tests (mainly focusing on visuo-perceptual abilities) might underestimate visuospatial deficits, usually related to posterior cortical atrophy and often associated with the risk of cognitive deterioration in neurological disorders (e.g., Parkinson’s disease [
31]).
Visuo-spatial impairments have been reported in several neurological disorders. In dementias, such as dementia with Lewy bodies, vascular dementia, and Alzheimer’s disease (AD), visuospatial deficits have been widely reported, although often neglected [
32]. In fMRI studies on AD patients compared to HC, hypoactivation in visual task-related regions, such as the V5 area, the superior parietal lobe, the parieto-occipital cortex, and the premotor cortices have been observed in association with some compensatory hyperactivation in the inferior parietal lobule; these abnormalities were interpreted as the pathophysiological basis for visuospatial disorientation in patients with AD [33, 34]. In Parkinson’s disease, visual perception deficits are frequent and likely related to the potential pathophysiological role of basal ganglia and limbic structures in visuospatial functions [
35].
In ALS, cognitive deficits are often reported in verbal fluency, language, social cognition, executive functions, and verbal memory, while visuospatial abilities appear to be less impaired [
4]. Nonetheless, Boeve & Graff-Radford [
36] found different degrees of impairment of cognitive abilities, including the visuospatial ones, in patients with
C9orf72 repeat expansions showing ALS and/or the frontotemporal dementia phenotype (c9FTD/ALS). In this subset of patients, in addition to bifrontal and cingulate cortex atrophy, structural MRI revealed parietal and occipital atrophy that could be part of the MRI signature pattern of c9FTD/ALS [37, 38]; this evidence might help explain the evidence of visuospatial dysfunction in this as well as in other phenotypes of ALS. Particularly, frontotemporal lobar degeneration with ubiquitin and TDP-43 positive neuronal inclusions may be associated with ALS, "progressive supranuclear palsy-like" syndrome, in which early behavioral disturbances, and marked visuospatial impairment are observed [
39]. In contrast, Crockford and colleagues found no significant differences in visuospatial abilities, explored by ECAS, across disease stages [
9], while lower cognitive abilities in ALS-specific functions and more behavioral alterations have been observed during disease course. Probably, assessment tools more specific for detecting impairments in both components of visuospatial abilities, independent from motor disability, such as BVA, might reveal these cognitive dysfunctions in the early stages of the disease, suggesting the potential benefits of specific cognitive training protocols in ALS patients as well as in other neurological disorders [40, 41]. Indeed, from a clinical point of view, the integrity of visual and visuospatial abilities could play a pivotal role in using brain-computer interface (BCI) technology for improving communication abilities, assessing cognitive functions, and controlling external devices in patients with motor disabilities (for a review see [
42]).
Although we obtained interesting insights on the visuospatial impairment in ALS, the generalization of the present findings is limited by the small sample size and the cross-sectional design. Moreover, an additional limit is the lacking inclusion of a positive-control group of subjects or of a subset of patients carrying C9orf72 repeat expansions. Further research might also address the anatomical and functional correlates of visuospatial defects in ALS.
5. Conclusions
The present study suggested an early impairment of visuospatial abilities in ALS, involving both perceptual and representational abilities, as assessed by BVA. In clinical practice, our findings provide new insight into multi-domain cognitive assessment in ALS to monitor disease progression effectively and organize care management properly. Further research on functional connectivity correlates of visuospatial functions might be important to better comprehend the impairment of extra-motor brain networks and address the dynamics of the spreading pathology in ALS.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org. Table S1: Comparison between the subgroup of ALS free from impairments in executive functions and/or language disturbances (ALS) and healthy controls (HC) in age- and education-adjusted perceptive and representational tasks of BVA.
Author Contributions
Conceptualization: Mattia Siciliano, Luigi Trojano and Francesca Trojsi; Data curation: Minoo Sharbafshaaer, Mattia Siciliano and Carla Passaniti; Formal analysis: Mattia Siciliano, Valeria Sant’Elia and Luigi Trojano; Investigation, Minoo Sharbafshaaer, Carla Passaniti, Valeria Sant’Elia, Marcello Silvestro, Antonio Russo and Sabrina Esposito; Methodology: Sabrina Esposito and Luigi Trojano; Project administration, Francesca Trojsi; Resources, Carla Passaniti, Valeria Sant’Elia, Marcello Silvestro, Antonio Russo and Sabrina Esposito; Software: Mattia Siciliano; Supervision, Antonio Russo, Gioacchino Tedeschi, Luigi Trojano and Francesca Trojsi; Validation: Mattia Siciliano; Visualization: Marcello Silvestro and Gioacchino Tedeschi; Writing – original draft: Minoo Sharbafshaaer and Francesca Trojsi; Writing – review & editing: Mattia Siciliano, Gioacchino Tedeschi, Luigi Trojano and Francesca Trojsi. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Institutional Review Board (or Ethics Committee) of University of Campania “Luigi Vanvitelli”, Naples (protocol code 591/2018, 01.08.2018).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Data will be available upon request to the corresponding author.
Acknowledgments
We thank all the study participants.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Hardiman, O.; Al-Chalabi, A.; Chiò, A.; Corr, E. M.; Logroscino, G.; Robberecht, W.; Shaw, P. J.; Simmons, Z.; van den Berg, L. H. Amyotrophic lateral sclerosis. Nat rev dis primers. 2017, 3, 17071. [Google Scholar] [CrossRef] [PubMed]
- Chiò, A.; Calvo, A.; Moglia, C.; Mazzini, L.; Mora, G.; PARALS study group. Phenotypic heterogeneity of amyotrophic lateral sclerosis: a population based study. J. Neurol. Neurosurg. Psychiatry 2011, 82, 740–746. [Google Scholar] [CrossRef]
- A Goutman, S.; Hardiman, O.; Al-Chalabi, A.; Chió, A.; Savelieff, M.G.; Kiernan, M.C.; Feldman, E.L. Recent advances in the diagnosis and prognosis of amyotrophic lateral sclerosis. Lancet Neurol. 2022, 21, 480–493. [Google Scholar] [CrossRef]
- Beeldman, E.; Raaphorst, J.; Twennaar, M.K.; de Visser, M.; A Schmand, B.; de Haan, R.J. The cognitive profile of ALS: a systematic review and meta-analysis update. J. Neurol. Neurosurg. Psychiatry 2015, 87, 611–619. [Google Scholar] [CrossRef] [PubMed]
- Consonni, M.; Bella, E.D.; Bersano, E.; Lauria, G. Cognitive and behavioural impairment in amyotrophic lateral sclerosis: A landmark of the disease? A mini review of longitudinal studies. Neurosci. Lett. 2021, 754, 135898. [Google Scholar] [CrossRef] [PubMed]
- Consonni, M.; Bella, E.D.; Bersano, E.; Telesca, A.; Lauria, G. Cognitive reserve is associated with altered clinical expression in amyotrophic lateral sclerosis. Amyotroph. Lateral Scler. Front. Degener. 2020, 22, 237–247. [Google Scholar] [CrossRef]
- Trojsi, F.; Santangelo, G.; Caiazzo, G.; Siciliano, M.; Ferrantino, T.; Piccirillo, G.; Femiano, C.; Cristillo, V.; Monsurrò, M.R.; Esposito, F.; Tedeschi, G. Neuropsychological assessment in different King's clinical stages of amyotrophic lateral sclerosis. Amyotroph Lateral Scler Frontotemporal Degener. 2016, 17, 228–235. [Google Scholar] [CrossRef] [PubMed]
- Chiò, A.; Moglia, C.; Canosa, A.; Manera, U.; Vasta, R.; Brunetti, M.; Barberis, M.; Corrado, L.; D'Alfonso, S.; Bersano, E.; et al. Cognitive impairment across ALS clinical stages in a population-based cohort. Neurology 2019, 93, e984–e994. [Google Scholar] [CrossRef]
- Crockford, C.; Newton, J.; Lonergan, K.; Chiwera, T.; Booth, T.; Chandran, S.; Colville, S.; Heverin, M.; Mays, I.; Pal, S.; et al. ALS-specific cognitive and behavior changes associated with advancing disease stage in ALS. Neurology 2018, 91, e1370–e1380. [Google Scholar] [CrossRef]
- Elamin, M.; Phukan, J.; Bede, P.; Jordan, N.; Byrne, S.; Pender, N.; Hardiman, O. Executive dysfunction is a negative prognostic indicator in patients with ALS without dementia. Neurology 2011, 76, 1263–1269. [Google Scholar] [CrossRef]
- Chio, A.; Ilardi, A.; Cammarosano, S.; Moglia, C.; Montuschi, A.; Calvo, A. Neurobehavioral dysfunction in ALS has a negative effect on outcome and use of PEG and NIV. Neurology 2012, 78, 1085–1089. [Google Scholar] [CrossRef] [PubMed]
- Trojsi, F.; Caiazzo, G.; Di Nardo, F.; Fratello, M.; Santangelo, G., Siciliano, M.; Femiano, C.; Russo, A.; Monsurrò, M. R.; Cirillo, M.; Tedeschi, G.; Esposito, F. High angular resolution diffusion imaging abnormalities in the early stages of amyotrophic lateral sclerosis. J Neurol Sci. 2017, 380, 215–222.
- Basaia, S.; Agosta, F.; Cividini, C.; Trojsi, F.; Riva, N.; Spinelli, E.G.; Moglia, C.; Femiano, C.; Castelnovo, V.; Canu, E.; Falzone, Y.; Monsurrò, M.R.; Falini, A.; Chiò, A.; Tedeschi, G.; Filippi, M. Structural and functional brain connectome in motor neuron diseases: A multicenter MRI study. Neurology 2020, 95, e2552–e2564. [Google Scholar] [CrossRef]
- Trojsi, F.; Caiazzo, G.; Corbo, D.; Piccirillo, G.; Cristillo, V.; Femiano, C.; Ferrantino, T.; Cirillo, M.; Monsurrò, M.R.; Esposito, F.; Tedeschi, G. Microstructural changes across different clinical milestones of disease in amyotrophic lateral sclerosis. PLoS One 2015, 10, e0119045. [Google Scholar] [CrossRef] [PubMed]
- Sorrentino, P.; Rucco, R.; Jacini, F.; Trojsi, F.; Lardone, A.; Baselice, F.; Femiano, C.; Santangelo, G.; Granata, C.; Vettoliere, A.; Monsurrò, M.R.; Tedeschi, G.; Sorrentino, G. Brain functional networks become more connected as amyotrophic lateral sclerosis progresses: a source level magnetoencephalographic study. Neuroimage Clin. 2018, 20, 564–571. [Google Scholar] [CrossRef] [PubMed]
- Brettschneider, J.; Libon, D.J.; Toledo, J.B.; Xie, S.X.; McCluskey, L.; Elman, L.; Geser, F.; Lee, V.M.-Y.; Grossman, M.; Trojanowski, J.Q. Microglial activation and TDP-43 pathology correlate with executive dysfunction in amyotrophic lateral sclerosis. Acta Neuropathol. 2012, 123, 395–407. [Google Scholar] [CrossRef] [PubMed]
- Cykowski, M.D.; Powell, S.Z.; Peterson, L.E.; Appel, J.W.; Rivera, A.L.; Takei, H.; Chang, E.; Appel, S.H. Clinical Significance of TDP-43 Neuropathology in Amyotrophic Lateral Sclerosis. J. Neuropathol. Exp. Neurol. 2017, 76, 402–413. [Google Scholar] [CrossRef]
- Cooper-Knock, J.; Hewitt, C.; Highley, J.R.; Brockington, A.; Milano, A.; Man, S.; Martindale, J.; Hartley, J.; Walsh, T.; Gelsthorpe, C.; et al. Clinico-pathological features in amyotrophic lateral sclerosis with expansions in C9ORF72. Brain 2012, 135, 751–764. [Google Scholar] [CrossRef]
- Irwin, D.J.; McMillan, C.T.; Brettschneider, J.; Libon, D.J.; Powers, J.; Rascovsky, K.; Toledo, J.B.; Boller, A.; Bekisz, J.; Chandrasekaran, K.; et al. Cognitive decline and reduced survival inC9orf72expansion frontotemporal degeneration and amyotrophic lateral sclerosis. J. Neurol. Neurosurg. Psychiatry 2013, 84, 163–169. [Google Scholar] [CrossRef]
- Niven, E.; Newton, J.; Foley, J.; Colville, S.; Swingler, R.; Chandran, S.; Bak, T.H.; Abrahams, S. Validation of the Edinburgh Cognitive and Behavioural Amyotrophic Lateral Sclerosis Screen (ECAS): A cognitive tool for motor disorders. Amyotroph. Lateral Scler. Front. Degener. 2015, 16, 172–179. [Google Scholar] [CrossRef]
- Prell, T.; Witte, O.W.; Gunkel, A.; Grosskreutz, J. Cognitive deficits have only limited influence on health-related quality of life in amyotrophic lateral sclerosis. Aging Ment. Heal. 2020, 24, 1963–1967. [Google Scholar] [CrossRef]
- Gardini, S.; Concari, L.; Pagliara, S.; Ghetti, C.; Venneri, A.; Caffarra, P. Visuo-spatial imagery impairment in posterior cortical atrophy: a cognitive and SPECT study. Behav neurol. 2011, 24, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Meneghetti, C.; De Beni, R.; Pazzaglia, F.; Gyselinck, V. The role of visuo-spatial abilities in recall of spatial descriptions: A mediation model. Learn. Individ. Differ. 2011, 21, 719–723. [Google Scholar] [CrossRef]
- Possin, K.L. Visual spatial cognition in neurodegenerative disease. Neurocase 2010, 16, 466–487. [Google Scholar] [CrossRef] [PubMed]
- Angelini, R.; Grossi, D. Rational therapy of the constructive disorders. In La terapia razionale dei disordini costruttivi; Ed. Centro di Riabilitazione S. Lucia: Roma, Italy, 1993. [Google Scholar]
- Trojano, L.; Fragassi, N.A.; Chiacchio, L.; Izzo, O.; Izzo, G.; Di Cesare, G.; Cristinzio, C.; Grossi, D. Relationships between Constructional and Visuospatial Abilities in Normal Subjects and in Focal Brain-damaged Patients. J. Clin. Exp. Neuropsychol. 2004, 26, 1103–1112. [Google Scholar] [CrossRef]
- Trojano, L.; Siciliano, M.; Pedone, R.; Cristinzio, C.; Grossi, D. Italian normative data for the Battery for Visuospatial Abilities (TERADIC). Neurol. Sci. 2015, 36, 1353–1361. [Google Scholar] [CrossRef]
- Brooks, B.R.; Miller, R.G.; Swash, M.; Munsat, T.L. El Escorial revisited: Revised criteria for the diagnosis of amyotrophic lateral sclerosis. Amyotroph. Lateral Scler. Other Motor Neuron Disord. 2000, 1, 293–299. [Google Scholar] [CrossRef]
- Chiò, A.; Calvo, A.; Moglia, C.; Mazzini, L.; Mora, G.; PARALS study group. Phenotypic heterogeneity of amyotrophic lateral sclerosis: a population based study. J. Neurol. Neurosurg. Psychiatry 2011, 82, 740–746. [Google Scholar] [CrossRef]
- Cedarbaum, J.M.; Stambler, N.; Malta, E.; Fuller, C.; Hilt, D.; Thurmond, B.; Nakanishi, A. The ALSFRS-R: a revised ALS functional rating scale that incorporates assessments of respiratory function. J. Neurol. Sci. 1999, 169, 13–21. [Google Scholar] [CrossRef]
- Kehagia, A.A.; Barker, R.A.; Robbins, T.W. Cognitive Impairment in Parkinson’s Disease: The Dual Syndrome Hypothesis. Neurodegener. Dis. 2012, 11, 79–92. [Google Scholar] [CrossRef]
- Biswas, A.; Pal, A.; Pandit, A.; Roy, A.; Guin, D.; Gangopadhyay, G.; Senapati, A.K. Study of visuospatial skill in patients with dementia. Ann. Indian Acad. Neurol. 2016, 19, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Thiyagesh, S.N.; Farrow, T.F.; Parks, R.W.; Accosta-Mesa, H.; Young, C.; Wilkinson, I.D.; Hunter, M.D.; Woodruff, P.W. The neural basis of visuospatial perception in Alzheimer's disease and healthy elderly comparison subjects: an fMRI study. Psychiatry res. 2009, 172, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.-G.; Lee, H.-W. Stereoscopic Depth Perception and Visuospatial Dysfunction in Alzheimer’s Disease. Healthcare 2021, 9, 157. [Google Scholar] [CrossRef]
- Caproni, S.; Muti, M.; Di Renzo, A.; Principi, M.; Caputo, N.; Calabresi, P.; Tambasco, N. Subclinical visuospatial impairment in Parkinson's disease: the role of Basal Ganglia and limbic system. Front. Neurol. 2014, 5, 152. [Google Scholar] [CrossRef]
- Boeve, B.F.; Graff-Radford, N.R. Cognitive and behavioral features of c9FTD/ALS. Alzheimer's Res. Ther. 2012, 4, 29–29. [Google Scholar] [CrossRef] [PubMed]
- Whitwell, J.L.; Weigand, S.D.; Boeve, B.F.; Senjem, M.L.; Gunter, J.L.; DeJesus-Hernandez, M.; Rutherford, N.J.; Baker, M.; Knopman, D.S.; Wszolek, Z.K.; et al. Neuroimaging signatures of frontotemporal dementia genetics: C9ORF72, tau, progranulin and sporadics. Brain 2012, 135, 794–806. [Google Scholar] [CrossRef] [PubMed]
- Agosta, F.; Ferraro, P.M.; Riva, N.; Spinelli, E.G.; Domi, T.; Carrera, P.; Copetti, M.; Falzone, Y.; Ferrari, M.; Lunetta, C.; et al. Structural and functional brain signatures of C9orf72 in motor neuron disease. Neurobiol. Aging 2017, 57, 206–219. [Google Scholar] [CrossRef] [PubMed]
- Rusina, R.; Kovacs, G.G.; Fiala, J.; Hort, J.; Ridzoň, P.; Holmerová, I.; Ströbel, T.; Matěj, R. FTLD-TDP with motor neuron disease, visuospatial impairment and a progressive supranuclear palsy-like syndrome: broadening the clinical phenotype of TDP-43 proteinopathies. A report of three cases. BMC Neurol. 2011, 11, 50–50. [Google Scholar] [CrossRef]
- Nousia, A.; Siokas, V.; Aretouli, E.; Messinis, L.; Aloizou, A.-M.; Martzoukou, M.; Karala, M.; Koumpoulis, C.; Nasios, G.; Dardiotis, E. Beneficial Effect of Multidomain Cognitive Training on the Neuropsychological Performance of Patients with Early-Stage Alzheimer’s Disease. Neural Plast. 2018, 2018, 1–9. [Google Scholar] [CrossRef]
- De Luca, R.; Latella, D.; Maggio, M.G.; Di Lorenzo, G.; Maresca, G.; Sciarrone, F.; Militi, D.; Bramanti, P.; Calabrò, R.S. Computer assisted cognitive rehabilitation improves visuospatial and executive functions in Parkinson’s disease: Preliminary results. NeuroRehabilitation 2019, 45, 285–290. [Google Scholar] [CrossRef]
- Carelli, L.; Solca, F.; Faini, A.; Meriggi, P.; Sangalli, D.; Cipresso, P.; Riva, G.; Ticozzi, N.; Ciammola, A.; Silani, V.; et al. Brain-Computer Interface for Clinical Purposes: Cognitive Assessment and Rehabilitation. BioMed Res. Int. 2017, 2017, 1–11. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).