Submitted:
08 October 2023
Posted:
09 October 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Recruitment of patients
2.2. Blood Collection
2.3. RNA Extraction
2.5. Validation of lncRNA Gene Expression in Blood by Real-time Quantitative PCR (RT-qPCR)
2.6. Statistical and Bioinformatics Analyses
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Giau VV, Bagyinszky E, An SSA (2019) Potential Fluid Biomarkers for the Diagnosis of Mild Cognitive Impairment. Int J Mol Sci. [CrossRef]
- Shen XN, Niu LD, Wang YJ, Cao XP, Liu Q, Tan L, Zhang C, Yu JT (2019) Inflammatory markers in Alzheimer's disease and mild cognitive impairment: a meta-analysis and systematic review of 170 studies. J Neurol Neurosurg Psychiatry. [CrossRef]
- Mayo Clinic. (n.d.). Dementia: Symptoms and causes. https://www.mayoclinic.org/diseases-conditions/dementia/symptoms-causes/syc-20352013.
- Wang J, Zhu S, Meng N, He Y, Lu R, Yan GR (2019) ncRNA-Encoded Peptides or Proteins and Cancer. Mol Ther. [CrossRef]
- De Felice B, Montanino C, Oliva M, Bonavita S, Di Onofrio V, Coppola C (2020) MicroRNA Expression Signature in Mild Cognitive Impairment Due to Alzheimer's Disease. Mol Neurobiol. [CrossRef]
- Wei ZD, Shetty AK (2022) Can mild cognitive impairment and Alzheimer's disease be diagnosed by monitoring a miRNA triad in the blood? Ageing Cell. [CrossRef]
- Zhan F, Yang J, Lin S, Chen L (2022) miRNA-Based Signature to Predict the Development of Alzheimer's Disease. Comb Chem High Throughput Screen. [CrossRef]
- Santoro M, Nociti V, Lucchini M, Loiodice M, Centofanti F, Botta A, Losavio FA, De Fino C, Mirabella M (2020) A pilot study of lncRNAs expression profile in serum of progressive multiple sclerosis patients. Eur Rev Med Pharmacol Sci. [CrossRef]
- Zhu J, Fu H, Wu Y, Zheng X (2013) Function of lncRNAs and approaches to lncRNA-protein interactions. Sci China Life Sci. [CrossRef]
- Wu YY, Kuo HC (2020) Functional roles and networks of non-coding RNAs in the pathogenesis of neurodegenerative diseases. J Biomed Sci. [CrossRef]
- Serebrovska ZO, Serebrovska TV, Kholin VA, Tumanovska LV, Shysh AM, Pashevin DA, Goncharov SV, Stroy D, Grib ON, Shatylo VB, Bachinskaya NY, Egorov E, Xi L, Dosenko VE (2019) Intermittent Hypoxia-Hyperoxia Training Improves Cognitive Function and Decreases Circulating Biomarkers of Alzheimer's Disease in Patients with Mild Cognitive Impairment: A Pilot Study. Int J Mol Sci. [CrossRef]
- Harris, C. R. et al. Array programming with NumPy. Nature 585, 357–362 (2020). [CrossRef]
- Virtanen, P. et al. SciPy 1.0: fundamental algorithms for scientific computing in Python. Nat. Methods 17, 261–272 (2020). [CrossRef]
- McKinney, W. Data Structures for Statistical Computing in Python. Proc. 9th Python Sci. Conf. 56–61 (2010). 2010. [CrossRef]
- Hunter, J. D. Matplotlib: A 2D graphics environment. Comput. Sci. Eng. 9, 90–95 (2007). [CrossRef]
- Waskom, M. Seaborn: Statistical Data Visualization. J. Open Source Softw. 6, 3021 (2021). [CrossRef]
- Kang, J. et al. RNAInter v4.0: RNA interactome repository with redefined confidence scoring system and improved accessibility. Nucleic Acids Res. 50, D326–D332 (2022). [CrossRef]
- Lin, Y. et al. RNAInter in 2020: RNA interactome repository with increased coverage and annotation. Nucleic Acids Res. 48, D189–D197 (2020). [CrossRef]
- Python Software Foundation. ‘glob - Unix style pathname pattern expansion.’ Python Documentation. 2020. Available at: https://docs.python.org/3/library/glob.html. (Accessed: 14th June 2023). 20 June.
- Raudvere, U. et al. G:Profiler: A web server for functional enrichment analysis and conversions of gene lists (2019 update). Nucleic Acids Res. 47, W191–W198 (2019). [CrossRef]
- Paul Shannon, 1 et al. Cytoscape: A Software Environment for Integrated Models. Genome Res. 13, 426 (1971). [CrossRef]
- Merico, D., Isserlin, R., Stueker, O., Emili, A. & Bader, G. D. Enrichment map: A network-based method for gene-set enrichment visualization and interpretation. PLoS One 5, (2010). [CrossRef]
- Chin, C. H. et al. cytoHubba: Identifying hub objects and sub-networks from complex interactome. BMC Syst. Biol. 8, S11 (2014). 2014. [CrossRef]
- Shobeiri, P. et al. Circulating long non-coding RNAs as novel diagnostic biomarkers for Alzheimer’s disease (AD): A systematic review and meta-analysis. PLoS One 18, 1–17 (2023). [CrossRef]
- Dong, L. X. et al. LncRNA NEAT1 promotes Alzheimer’s disease by down regulating micro-27a-3p. Am. J. Transl. Res. 13, 8885–8896 (2021).
- The lncRNA MIR31HG. (2023).
- Wu, Y. et al. lncRNA Neat1 regulates neuronal dysfunction post-sepsis via stabilization of hemoglobin subunit beta. Mol. Ther. 30, 2618–2632 (2022). [CrossRef]
- Khodayi, M., Khalaj-Kondori, M., Feizi, M. A. H., Bonyadi, M. J. & Talebi, M. Plasma Lncrna Profiling Identified Bc200 and Neat1 Lncrnas As Potential Blood-Based Biomarkers for Late-Onset Alzheimer’S Disease. EXCLI J. 21, 772–785 (2022). [CrossRef]
- Breton A, Casey D, Arnaoutoglou NA (2019) Cognitive tests for the detection of mild cognitive impairment (MCI), the prodromal stage of dementia: Meta-analysis of diagnostic accuracy studies. Int J Geriatr Psychiatry. [CrossRef]
- Anderson ND (2019) State of the science on mild cognitive impairment (MCI). CNS Spectr. [CrossRef]
- Jalaiei A, Asadi MR, Sabaie H, Dehghani H, Gharesouran J, Hussen BM, Taheri M, Ghafouri-Fard S, Rezazadeh M (2021) Long Non-Coding RNAs, Novel Offenders or Guardians in Multiple Sclerosis: A Scoping Review. Front Immunol. [CrossRef]
- Wang, L. et al. Dexmedetomidine had neuroprotective effects on hippocampal neuronal cells via targeting lncRNA SHNG16 mediated microRNA-10b-5p/BDNF axis. Mol. Cell. Biochem. 469, 41–51 (2020). [CrossRef]
- Pang, X., Su, Z., Sun, Y., Zhang, W. & Wang, H. Long noncoding RNA SNHG16 reduced ketamine-induced neurotoxicity in human embryonic stem cell-derived neurons. J. Chem. Neuroanat. 94, 39–45 (2018). [CrossRef]
- Teng, H., Li, M., Qian, L., Yang, H. & Pang, M. Long non-coding RNA SNHG16 inhibits the oxygen-glucose deprivation and reoxygenation-induced apoptosis in human brain microvascular endothelial cells by regulating miR-15a-5p/bcl-2. Mol. Med. Rep. 22, 2685–2694 (2020).
- LncRNA H19 promotes inflammatory response induced by cerebral ischemia- reperfusion injury through regulating miR-138-5p /p65 Running title : H19/miR-138-5p/p65 axis in cerebral ischemia-reperfusion injury. 1–44.
- Guo, S., Wang, H. & Yin, Y. Microglia Polarization From M1 to M2 in Neurodegenerative Diseases. Front. Aging Neurosci. 14, 1–16 (2022). [CrossRef]
- Zhong, L., Liu, P., Fan, J. & Luo, Y. Long non-coding RNA H19: Physiological functions and involvements in central nervous system disorders. Neurochem. Int. 148, 105072 (2021). [CrossRef]
- Zhang, Y. Y. et al. Silenced lncRNA H19 and up-regulated microRNA-129 accelerates viability and restrains apoptosis of PC12 cells induced by Aβ25-35 in a cellular model of Alzheimer’s disease. Cell Cycle 20, 112–125 (2021). [CrossRef]
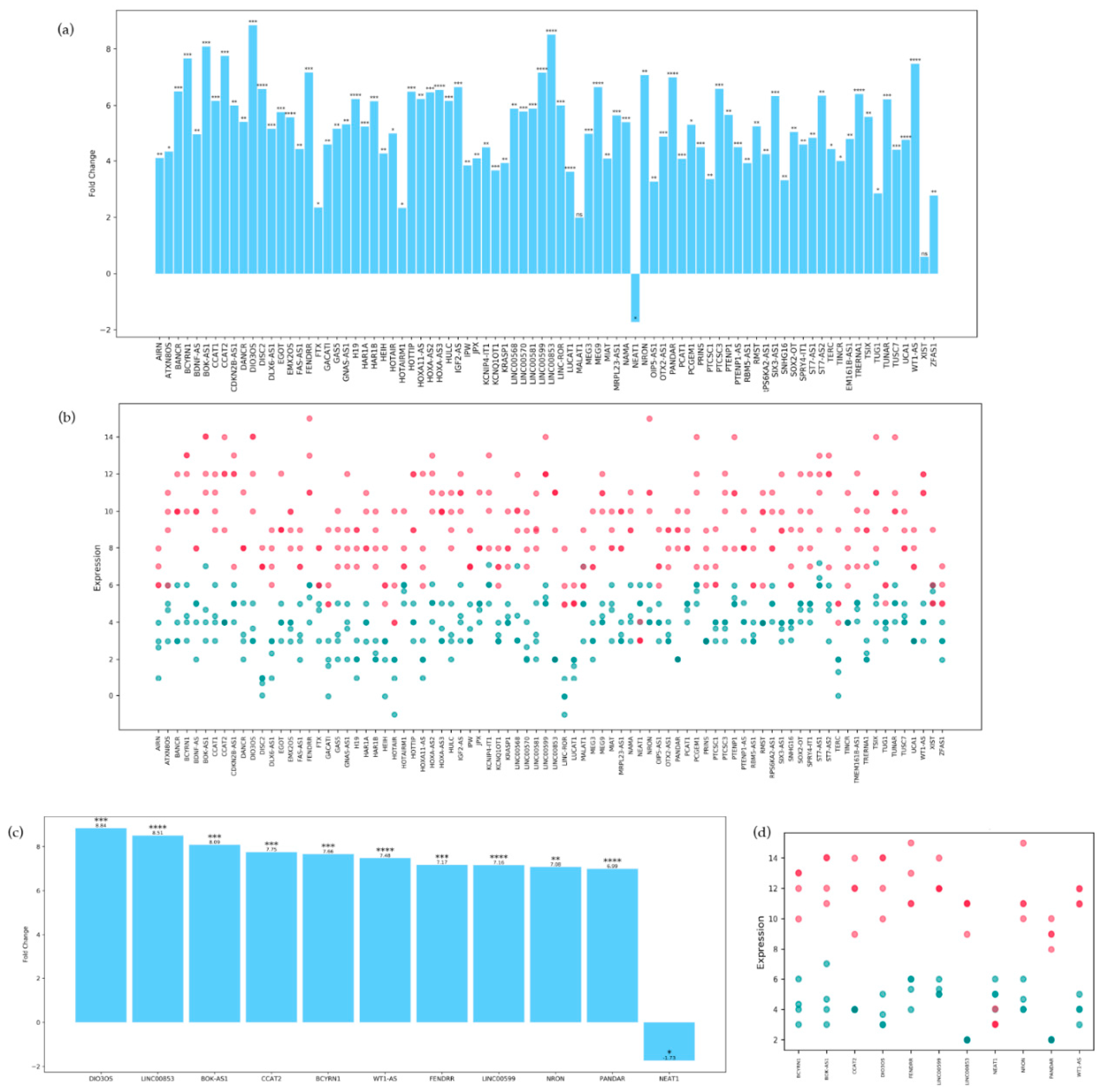
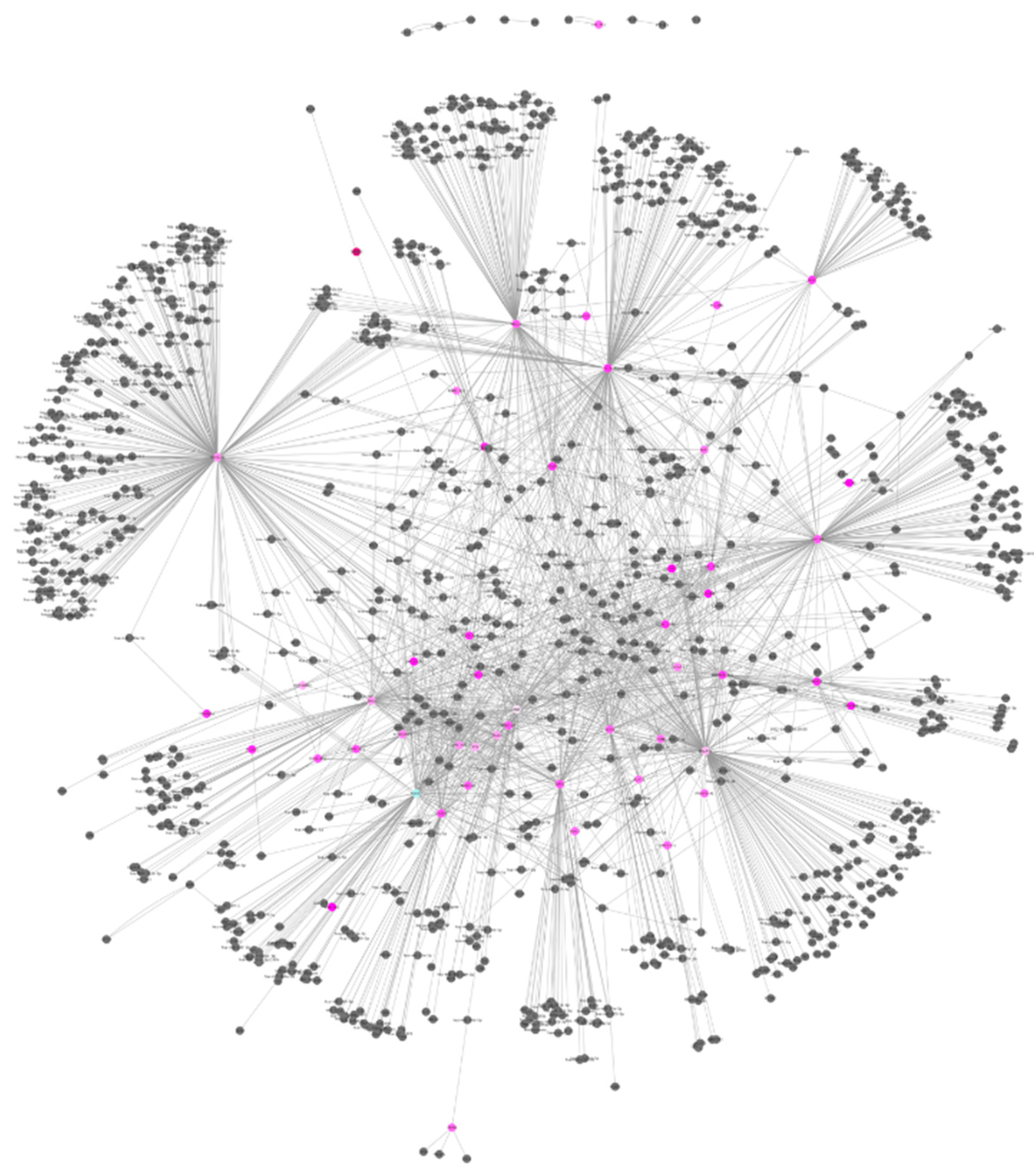
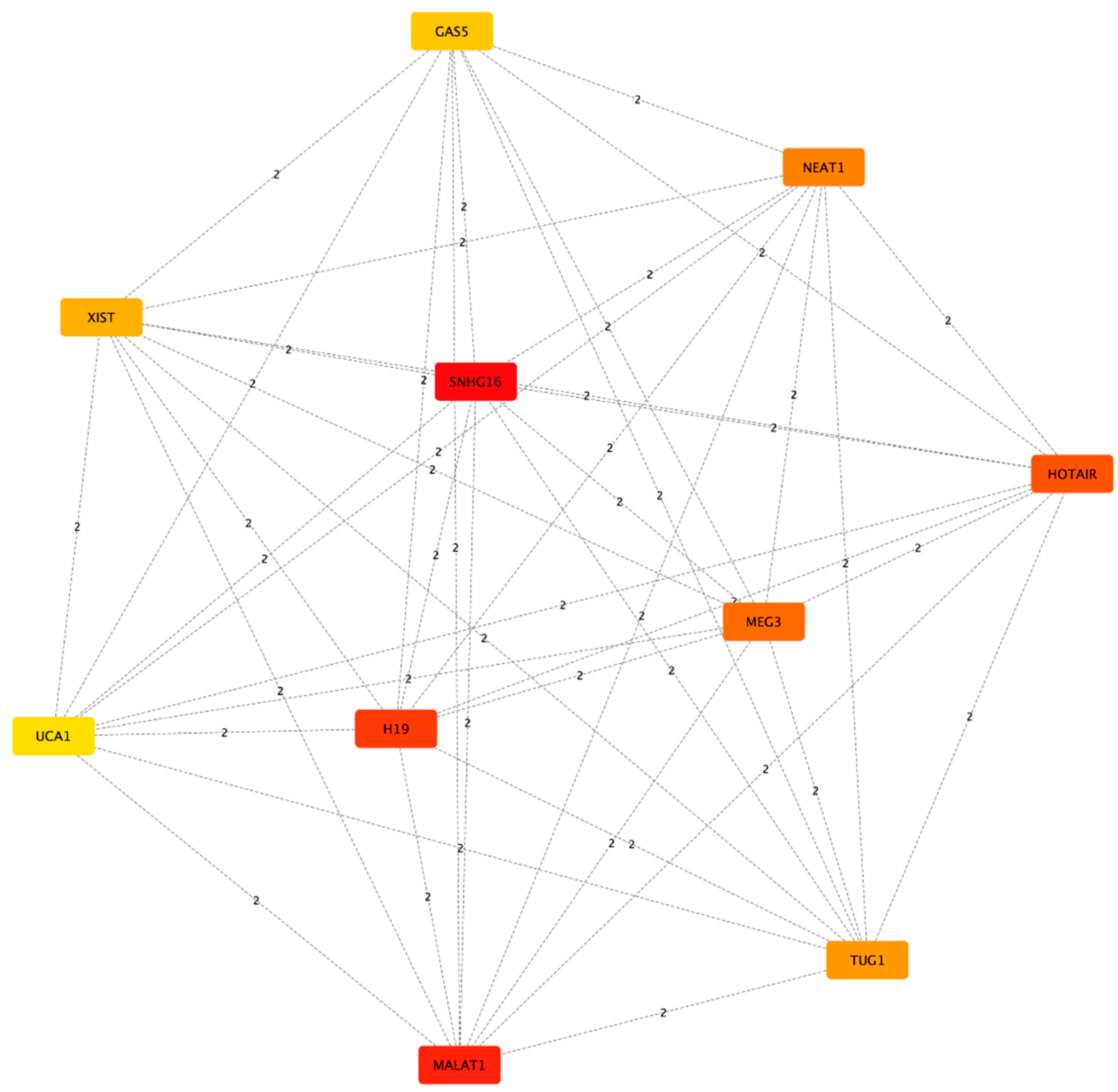
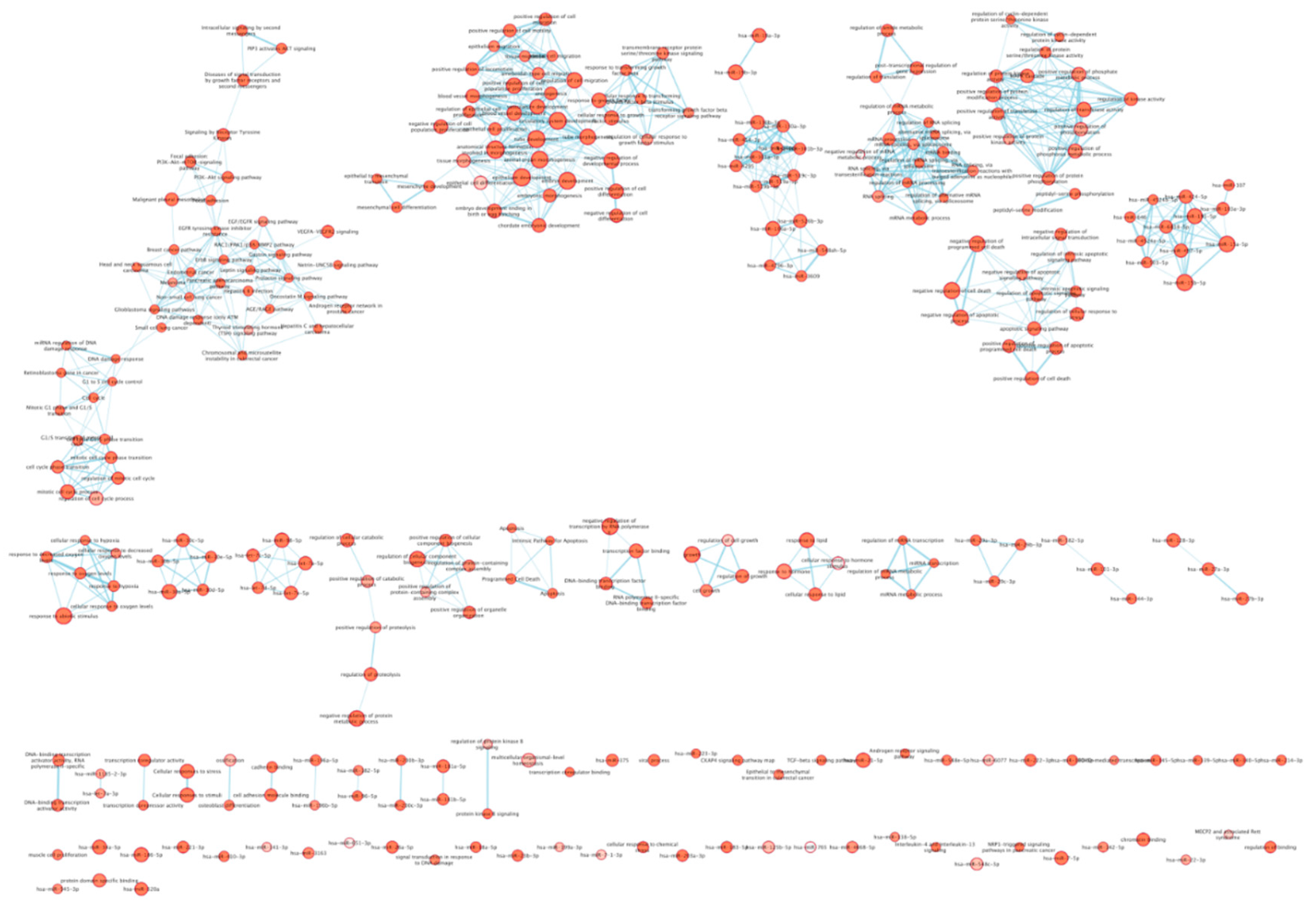
| Clinical characteristics | Value | MCI Patients | Healthy Controls |
|---|---|---|---|
| Age at our observation (years) | Mean | 59.07 ± 18.76 | 62.25 ± 11.26 |
| Range | 49-65 | 52-66 | |
| Age at symptoms onset (years) | Mean | 55.17 ±10.35 | - |
| Range | 55.17 ±10.35 | - | |
| Sex | Male | 4 | 5 |
| Female | 6 | 5 | |
| Mini-Mental State Examination (MMSE) score | - | 22.48 (±2.06) | 28.47 (±1.93) |
| Other cerebrovascular pathology | - | None | None |
| Metabolic/endocrine disease | - | None | None |
| Rank | Name | Score |
|---|---|---|
| 1 | SNHG16 | 281.0 |
| 2 | MALAT1 | 202.0 |
| 3 | H19 | 174.0 |
| 4 | HOTAIR | 153.0 |
| 5 | MEG3 | 135.0 |
| 6 | NEAT1 | 102.0 |
| 7 | TUG1 | 95.0 |
| 8 | XIST | 84.0 |
| 9 | GAS5 | 78.0 |
| 10 | UCA1 | 63.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





