1. Introduction
Eggs are a high-quality protein source for humans that contains several essential amino acids, consequently helping build and maintain the body’s muscle mass. With the increase in the public demand for eggs, commercial layer producers tend to operate at an intensive scale to increase maximal hen-day egg production. However, it well known that animal-rearing conditions under intensive production systems create stressful conditions. Environmental stressors including temperature, humidity, and stocking density are the main factors affecting animal welfare, health, and productivity [1]. Geng et al. [2] reported that stocking density in rearing spaces has become one of the most important environmental and management factors for modern intensive animal husbandry. Nevertheless, in order to increase the total egg production per housing unit, many egg producers endeavor to decrease the payback period and increase their net income by increasing the number of hens per cage at maximum capacity [3]. With increasing cage density (342 to 690 cm2/hen), egg performance and ME efficiency of egg production decreased significantly in hens kept in cages at a density of 342 cm2 per hen [4]. A dense environment also has been associated with detrimental impacts including decreased egg production and egg mass [5], decreased laying rate, and increased levels of noxious gas emissions from the litter [6]. A study carried out by Wang et al. [7] revealed that laying hens reared in high stocking density had reduced laying rate and decreased eggshell indices such as shell color, strength, and thickness. Physiologically, birds kept at high stocking density may be sensitive to oxidative stress [8,9]. Incharoen et al. [10] noted that nutritional modification might be a key factor to ameliorate stress from high stocking density in laying hens. Thus, addition of specific antioxidants to the diet could be one efficient approach to alleviate the negative impact of stress [11].
Recently, gamma-oryzanol in the rice bran layer has been identified as a potent natural antioxidant due to its capacity to prevent lipid peroxidation and the resulting oxidative stress [12]. It contains a mixture of ferulic acid esters and phytosterols (sterols and triterpenic alcohols) [13,14]. Antioxidant components of gamma-oryzanol such as 24-methylenecycloartanyl ferulate, cycloartenyl ferulate, campesteryl ferulate, and β-sitosteryl ferulate, are able to inhibit lipid peroxidation and free radical production and scavenge the free radicals from the body [15]. Ferulic acid in particular not only scavenges free radicals, but also increases the activity of enzymes that are responsible for scavenging free radicals and inhibits enzymes that catalyze the production of free radicals [16]. Furthermore, the structure of gamma-oryzanol components is similar to that of cholesterol and can reduce oxidative stress and maintain the functionality of cells [17].
As a lipid-soluble nutrient, vitamin E takes on a vital function as a peroxyl radical-scavenging antioxidant and inhibitor of lipid peroxidation by breaking chain propagation [18]. Natural vitamin E consists of 8 different analogues: α-, β-, γ- and δ-tocopherol; and α-, β-, γ- and δ-tocotrienol [19]. Among these, α-tocopherol has been mainly used as a supplement in livestock feed. Previous studies revealed that compared with a basal diet without vitamin E supplementation, dietary α-tocopherol acetate (200 to 500 mg/kg) enhanced the antioxidant capacity in hens [20,21,22]. Zhao et al. [23] reported that dietary natural tocopherol at a dosage of 100 mg/kg enhanced laying performance and tocopherol deposition as well as egulated serum cholesterol concentrations and improved antioxidant status. Although tocopherol is the most acceptable to use in domestic animal production, Serbinova et al. [24] reported higher antioxidant activity with tocotrienol than with α-tocopherol against lipid peroxidation in rat liver microsomes. Thus, it appears that vitamin E tocotrienol has greater potency. Furthermore, tocotrienols possess powerful antioxidant, neuroprotective, anti-cancer, and cholesterol regulatory activities that often differ from the properties of tocopherols [25].
We hypothesized that supplementing gamma-oryzanol and vitamin E tocotrienols or their combination would be an effective nutritional strategy to lessen the detrimental impact of high cage density in laying hens. Hence, the current research was conducted to evaluate the effect of diets supplemented with or without GO and VE on the productivity, egg quality, and immune- and health-related mRNA abundance in laying hens reared at different cage densities.
2. Materials and Methods
2.1. Animal, diet and management
Hy-Line Brown layers purchased from a commercial farm in Phitsanulok province were used. The gamma-oryzanol (98.0% purity) and vitamin E tocotrienols (60.0 % total tocotrienols and 30.0 % total tocopherols) products were extracted from the rice bran of Oryza sativa Linne (Gramineae) and obtained from Oryza Oil & Fat Chemical Co., Ltd., Japan. All animals were reared in wire cages in tunnel-ventilated houses equipped with an evaporative cooling system to control the ambient temperature. Throughout the duration of the experiment, the average temperature remained consistent at 28±2 °C, accompanied by a relative humidity range of 60–65%. LED artificial lighting was at a consistent photoperiod (17L:7D). At 54-weeks of age, a total of 120 laying hens with identical body weight and egg uniformity were allocated into 8 groups with 5 replicates per group (3 layers/replicate). A completely randomized experiment designed with a 4×2 factorial arrangement of treatments was used. Dietary treatments were a basal diet without supplementation (CON) and three other diets with the same chemical composition as CON, but supplemented with 200 ppm gamma-oryzanol (GO-200), 200 ppm vitamin E tocotrienols (VE-200) or 200 ppm gamma-oryzanol + 200 ppm vitamin E tocotrienols (GE-400). The first 4 groups of hens were kept in wire cages with a low density of 840 cm
2/hen (LCD). Laying hens in the other 4 treatments were confined to a wire cage with a high density of 420 cm
2/hen (HCD). During the 54 to 62 weeks of age, all hens had free access to clean drinking water and feed. Diets were formulated in accordance with the nutrient requirement recommendation of NRC [26] (
Table 1).
2.2. Laying performance and egg quality measurements
All eggs were carefully collected twice daily (at 6:00 A.M. and 6:00 P.M.) from each cage and counted per replication. We also recorded the weight of each collected egg on a daily basis, while monitoring the remaining feed on a weekly basis. Parameters analyzed included hen-day production (HDE), average egg weight (AEW), average daily feed intake (ADFI), egg mass, and feed conversion ratio (FCR). Additionally, we collected 10 eggs from each group on a weekly basis to assess eggshell breaking strength (ESBS), eggshell thickness (EST), eggshell ratio (ESR), yolk ratio (YR), albumen ratio (AR), albumen height (AH), yolk color (YC) and Haugh unit (HU). These parameters were evaluated using a TA-XT2 Plus Analyzer (Stable Microsystems, UK) following the methods described by Likittrakulwong et al. [27].
2.3. Sample Collection
At 62 weeks of age, blood samples were collected from five hens from each group. They were taken by venipuncture from the wing vein, and blood was saved into collection tubes using a sterile syringe, kept in blood collection tubes, and stored at 4 ºC in a refrigerator. Blood samples were mixed with an anticoagulant solution [ethylene diamine tetraacetic acid; (EDTA)] and then used for mRNA abundance analysis of hydroxyl-3-methyl-glutaryl coenzyme A reductase (HMGCR) and heat shock protein 70 (HSP-70) [10]. After blood collection, hens were sacrificed under mild anesthesia. Whole visceral organs were pulled out of the abdomen and placed on a clean aluminum tray. Using sterile equipment, the spleen was removed and cut into a small pieces of 4-5 mm thickness, rapidly frozen in liquid nitrogen, and kept at -80 ˚C until mRNA abundance analysis of interleukin-12 subunit beta (IL-12β) and interferon gamma (IFN- γ). Approximately 30 mg of spleen tissue from each treatment were homogenized with the TissueRuptor homogenizer (Qiagen GmbH, Hilden, Germany) in 350 µl of RLT buffer (RNeasy Mini RNA isolation kit, Qiagen GmbH, Hilden, Germany) and stored at -80˚C for RNA extraction.
2.4. mRNA abundance analysis
Total RNA was isolated using the RNeasy Mini RNA isolation kit (Qiagen GmbH, Hilden, Germany) and eluted in 50 µl RNase-free water. The concentration of total RNA was measured using a nanodrop Quawell UV–VIS Spectrophotometer Q5000 (Quawell Technology, Inc., San Jose, CA, USA). One µg of total RNA from each sample was used for first-strand cDNA synthesis, which was performed using the RevertAidTM first strand cDNA synthesis kit (Fermentas, Burlington, Canada), following the manufacturer’s recommendations. One µl of first-strand cDNA from each sample was used as the template for semi-quantitative RT-PCR analysis. PCR amplification was performed using specific primers [28, 29, 30] (
Table 2). Quantitative Real-time RT-PCR (qPCR) was performed as previously described by Incharoen et al. [10] to measure the levels of HMGCR, HSP70, IL-12β, IFN-γ and beta-actin (internal control) mRNA. The reactions were performed in triplicate in a MyGo Pro real-time PCR instrument (IT-IS Life Science Ltd., Mahon, Cork, Ireland). The relative mRNA abundance was analyzed using MyGoPro qPCR software (IT-IS Life Science Ltd., Mahon, Cork, Ireland). Results of real-time PCR were analyzed by the 2-ΔΔCt method [31]. The mRNA abundance of these genes was normalized to beta-actin.
2.5. Statistical Analysis
A two-way analysis of variance of the data on egg performance and quality using the general linear model procedure of SPSS version 17.0 (SPSS Inc., Chicago, IL, USA) was performed according to a 4×2 factorial arrangement of treatments, including dietary supplementation and cage density as the main effects and the respective interactions. One-way ANOVA was used to determine differences in mRNA abundance. Statistically significant means were compared using Duncan’s Multiple Range Test and a probability level of P < 0.05 was considered significant.
3. Results
3.1. Egg production response to dietary supplementation and cage density
Compared with the CON group, average egg weight (AEW) tended to be greater in the GO-200 and VE-200 groups, and was greater (P < 0.01) in the GE-400 group (
Table 3). Egg mass was greater (P < 0.01) in all supplemented treatments compared with the CON group. In addition, FCR improved in the GO-200 and VE-200 groups, with the best responses (P < 0.01) detected in the GE-400 group. Data on average daily feed intake (ADFI) and hen-day egg production (HDE) did not differ among dietary treatments. Compared with the LCD condition, in HCD the hens experienced a decrease (P < 0.01) in ADFI, HDE, and egg mass, while there were no changes observed in AEW and FCR.
The ADFI, AEW, HDE, FCR, and egg mass of laying hens was influenced by the interaction between dietary supplementation and cage density. Except for the GE-400-fed birds, results pertaining to ADFI in birds raised under HCD conditions did not demonstrate a significant variance across the different dietary treatments. Birds in GE-400 exhibited the lowest (P < 0.01) ADFI compared with other groups. CON-fed hens maintained in HCD tended to have lower HDE and egg mass compared with other groups, and had the lowest values (P < 0.01) compared with the GO-200 group. Results of AEW in birds maintained under HCD revealed higher values in all groups that received dietary antioxidants, with the greatest response observed in the GE-400 group (P < 0.01). Additionally, all antioxidant-fed hens reared at HCD had better (P < 0.01) FCR and the GO-200 and GE-400 groups had the lowest FCR values.
3.2. Dietary supplementation and cage density influenced egg quality
Compared with the CON group, all of the supplemented groups had better responses in various egg quality parameters (except for the AR) including ESBS, EST, ESR, YR, AH, YC, and HU (
Table 4). Furthermore, the highest response (P < 0.01) in these collected parameters was detected in the VE-200 group. Compared with the LCD environment, the HCD-housed hens exhibited a significant reduction (P < 0.01) in ESBS, ESR, YR, AR, and AH. There was no effect on EST, YC, and HU. In addition, there was an interaction between dietary supplementation, specifically of gamma-oryzanol and vitamin E tocotrienols with cage density on ESBS, ESR, YR, AH, YC, and HU. Furthermore, compared with the CON group, HCD-housed hens fed GO-200 and GE-400 had a notable increase in ESR. Compared with the CON birds raised in the HCD environment, the GO-200 group exhibited the highest AH and the lowest YC. However, there were no significant differences observed among dietary treatments regarding EST, AR, and HU of laying hens housed in the HCD situation.
3.3. Stress-, lipid metabolism- and immune-related genes
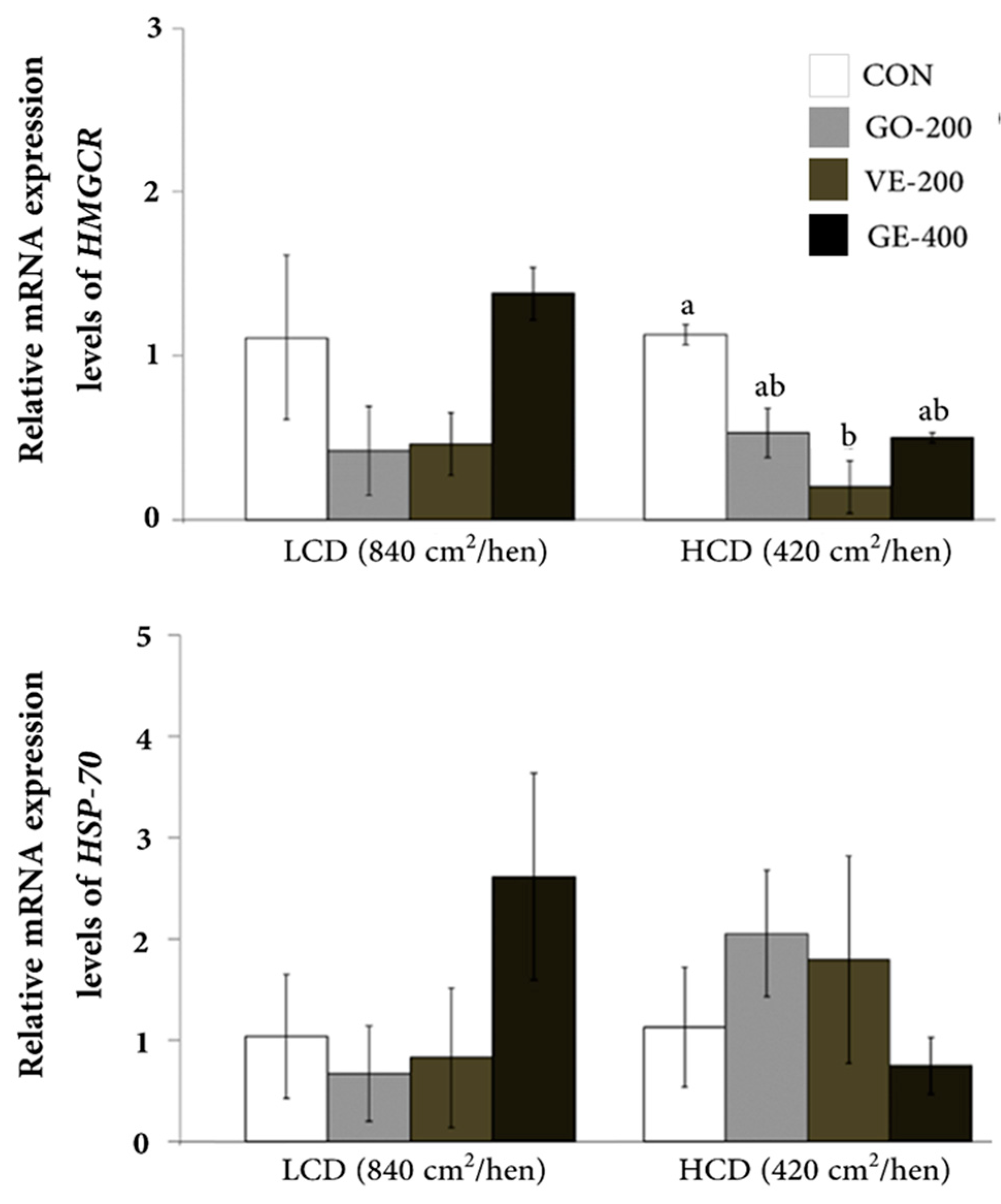
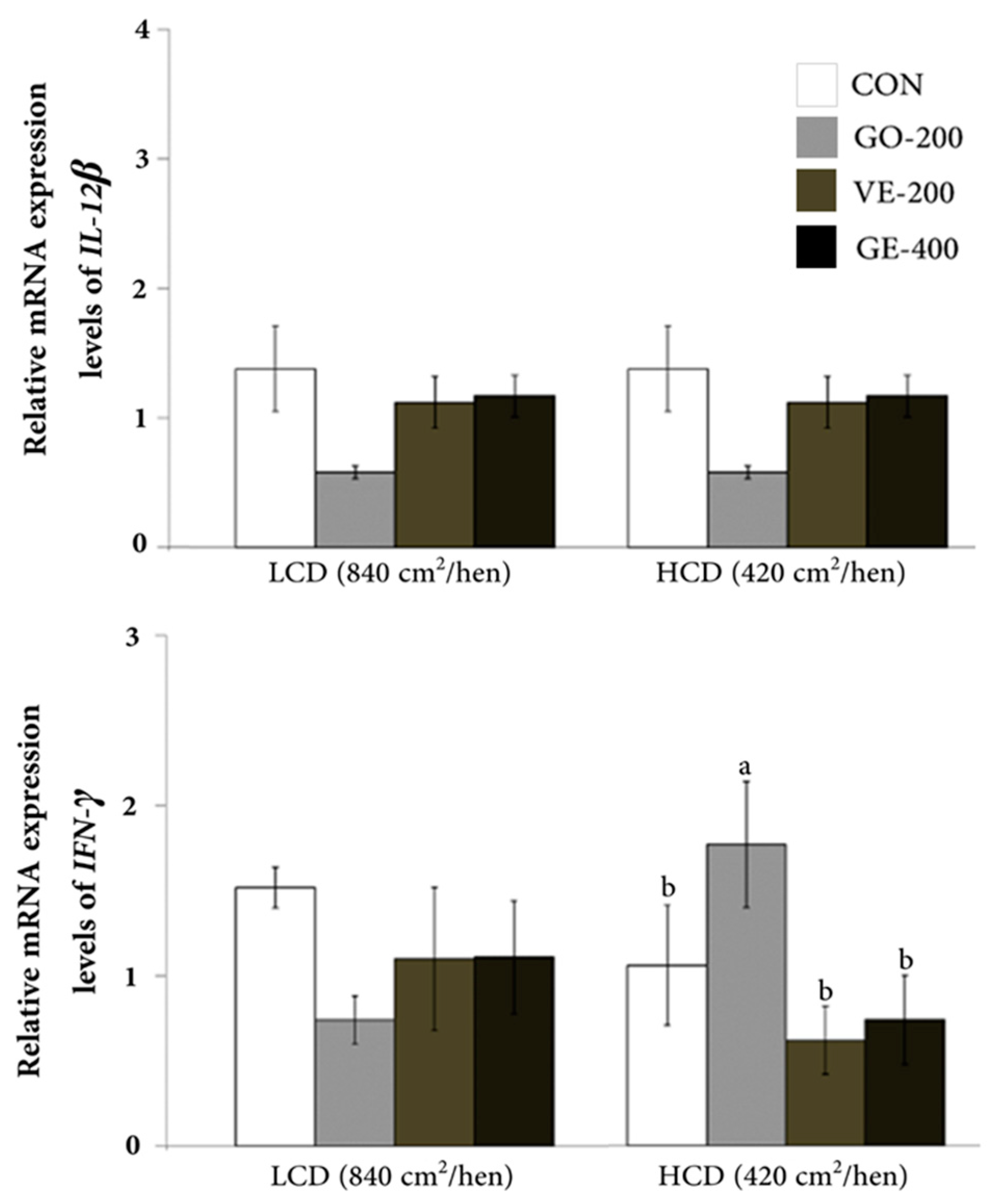
In the LCD conditions, the hens had minimal differences in mRNA abundance of HMGCR, HSP70, IL-12 β, and IFN- γ genes across all dietary treatments (
Figure 1 and
Figure 2). However, HCD-housed hens had a decrease (P < 0.05) in HMGCR abundance within the VE-200 group compared with the CON group. Among hens kept in HCD-conditions, those fed GO-200 exhibited the highest level of IFN-γ mRNA abundance relative to other diets. However, there was no difference in abundance of HSP70 and IL-12 β in HCD-reared hens regardless of diet.
4. Discussion
The reduction in ADFI, HDE, egg mass, ESBS, ESR, YR, and AH observed in laying hens raised under the HCD condition agreed with the recent study from Incharoen et al. [10] in which hens raised on HCD under heat stress displayed decreased egg performance and eggshell-breaking strength. Similarly, a lower HDE and daily egg mass [5], a reduction of ME efficiency in egg production [4], and a detrimental impact on laying rate and litter quality resulting in high levels of noxious gas emission were reported previously [6]. In broilers, there is evidence confirming that increasing stocking density decreased feed intake, body weight, weight gain, and FCR [32, 33]. Goo et al. [34] also reported that broilers reared under heat stress and high stocking density decreased performance with a negative impact on breast meat quality. During the starter period, studies have observed that high stocking density is associated with a reduction in feed intake and weight gain in White Pekin ducks [35] and geese [36]. Furthermore, research studies have provided scientific evidence that housing birds in a densely stocked environment can lead to elevated ambient temperatures surrounding the birds and lowering body heat dissipation resulting in heat stress conditions [33, 37]. Thus, this overwhelming evidence underscores that monitoring environmental factors and adjusting nutritional management practices accordingly is crucial to minimize harmful outcomes on health and welfare.
To our knowledge, there is no published research on the potential benefits of incorporating gamma-oryzanol and vitamin E tocotrienols, either individually or in combination, into poultry diets with regards to reducing the detrimental effects of oxidative stress caused by HCD conditions. However, Minatel et al. [12] demonstrated that gamma-oryzanol in the rice bran layer is a potent natural antioxidant due to its capacity to prevent lipid peroxidation and the resulting oxidative stress. In addition, López-Revuelta et al. [17] noted that the structure of gamma-oryzanol components is analogous to that of cholesterol, meaning that it can help reduce oxidative stress and support the normal functioning of cells. Previous studies have also reported that gamma-oryzanol has the potential to positively affect the immune system, lipid levels in blood, antioxidant capabilities, and better efficiency for the animal to avoid heat stress [38, 39, 40]. Functionally, due to its effectiveness as an antioxidant and inhibitor of lipid peroxidation by breaking chain propagation, tocopherol is the generic form of vitamin E used in feed [18]. Previous research provided evidence that hens fed a diet containing α-tocopherol acetate displayed stronger antioxidant capacity than control-fed birds [20, 21, 22]. Recently, Zhao et al. [23] reported that dietary tocopherol content (100 mg/kg diet) increased egg-laying performance and tocopherol deposition as well as regulated serum cholesterol concentration and improved antioxidant status. Despite these data, the study conducted in rat liver microsomes by Serbinova et al. [24], demonstrated that compared with α-tocopherol, tocotrienol had greater antioxidant properties in regards to lipid peroxidation. Thus, there is a clear benefit in generating more data on the relevance of tocotrienol in poultry diets.
Decreased productivity and poor egg quality were observed in hens raised under HCD conditions, possibly due to reduced digestibility caused by heat stress [41]. However, there is some evidence suggesting that antioxidants delivered through supplementation in the diet can minimize oxidative stress [42, 43] leading to enhanced growth and feed efficiency, and optimizing nutrient utilization. Other reported that high dietary concentrations of gamma-oryzanols, tocotrienols, and other bioactive components in rice bran oil improved growth performance of broiler chickens [44, 45]. The present data demonstrating a significant impact on various aspects of egg production and quality, and AEW and FCR under HCD conditions in response to feeding gamma-oryzanol and vitamin E tocotrienols support and add to those findings. Hence, dietary supplementation with oryzanols and vitamin E tocotrienol could have a synergistic positive effect on hen’s performance specifically by minimizing oxidative stress. As such, these compounds can improve nutrient digestibility and consequently enhance productivity during stressful periods. It is important to conduct further research in order to confirm and better understand the specific mechanisms whereby these nutrients have a positive impact on the animal.
The levels of mRNA transcription were confirmed using quantitative real-times RT-PCR. In our results, a significant effect of LCD situation on the expression levels of HMGCR, HSP70, IL-12β, and IFN-γ were not observed among 4 dietary groups. However, the expression levels of HMGCR and IFN - γ in the blood were significantly impacted by the HCD condition, whereas there was no significant difference for HSP70 and IL-12β.
The study of Sohn et al. [30] reported greater abundance of HMGCR, but not HSP70, in blood of chickens exposed to stress. A similar finding was reported by Incharoen et al. [10] where HMGCR abundance was lower in HCD-reared laying hens fed with dietary germinated paddy rice containing several bioactive compounds (vitamins, gamma-oryzanol, and γ-amino butyric acid) [46]. The lower HMGCR abundance in the HCD-housed hens that received gamma-oryzanol alone or combined with vitamin E tocotrienols (VE-200) could be taken as indication of a reduction in stressful conditions as reported by Sohn et al. [30]. This idea is further supported by data from Zavoshy et al. [47] where feeding vitamin E isomers (tocopherol and tocotrienols) and oryzanol (also contained in rice bran oil) led to lower total cholesterol and LDL-C levels by inhibiting HMG-CoA reductase, i.e., the rate-limiting enzyme in de novo cholesterol synthesis. Thus, based on our findings, providing dietary gamma-oryzanol and vitamin E tocotrienols may have a mitigating effect on the stress status caused by HCD conditions.
Interferon γ is a vital cytokine synthesized primarily by type 1 T helper cells and plays a crucial role in the activation of macrophages [48, 49]. In avian species, IFN-γ represents a natural component of the immune system [50] and its abundance has been detected in laying hen [10], duck [51] and goose [52]. Thus, the lower abundance of IFN-γ [53] in broiler chickens exposed to heat stress or in birds raised in environmental conditions with higher endotoxin levels underscore the usefulnes of this cytokine as a marker of stressful conditions in avian species [54]. Despite the lack of differences in the abundance of IFN-γ due to diet under LCD conditions, the fact that dietary GO-200 in hens raised in an HCD environment led to greater IFN-γ mRNA abundance suggests that nutrition may play a role in the function of this cytokine. Lee et al. [55] noted that high levels of IFN- γ have been associated with protective immune responses to parasitic infections. In fact, Gao et al. [51] suggested that IFN-γ has the potential to inhibit viral activity in ducks. Thus, the greater mRNA abundance of IFN-γ in birds fed gamma-oryzanol suggests that this compound might aid in mitigating the detrimental impacts of the HCD environment by enhancing the immune response.
5. Conclusions
Hens housed in 420 cm2/hen HCD conditions produced lower productivity, egg quality, and eggshell hardness than hens kept in LCD conditions (840 cm2/hen). However, dietary GO or VE at a level 200 ppm, either individually or in combination can improve performance and egg and shell quality as well as regulate mRNA abundance of immune- and stress-related genes. Thus, we conclude that dietary antioxidants should be part of a nutritional strategy to mitigate the negative impacts on laying hens reared under HCD conditions.
Author Contributions
Conceptualization, T.I.; methodology, T.I.; validation, T.I., R.S., R.C. and W.T.; formal analysis, T.I., W.L.; investigation, T.I., W.L., R.S., W.T. and J.J.L.; resources, T.I. and R.S.; data curation, T.I.; writing—original draft preparation, T.I., R.S. and W.L.; writing—review and editing, T.I. and J.J.L.; visualization, T.I. and W.L.; supervision, T.I. and J.J.L.; project administration, T.I.; funding acquisition, T.I. All authors have read and agreed to the published version of the manuscript.
Funding
This experiment has received partial funding from the Benergies Agritrade Co., Ltd., Thailand via the Division of Research and Innovation, Naresuan University (grant number: R2560A181). Additionally, our research is also partially supported by the Reinventing University Program 2023, The Ministry of Higher Education, Science, Research and Innovation (MHESI), Thailand (grant number: R2566A039).
Institutional Review Board Statement
Animal handling techniques and procedures were approved by the Naresuan University Agricultural Animal Care and Use Committee (approval number: 60 01 015), based on the Ethics of Animal Experimentation of the National Research Council of Thailand.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data presented in this research are available on request from the corresponding author.
Acknowledgments
The authors are grateful to acknowledge the support of the Faculty of Agriculture Natural Resource and Environment, Naresuan University for the successful completion of this research project.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Son, J.; Kim, H.J.; Hong, E.C.; Kang, H.K. Effects of stocking density on growth performance, antioxidant status, and meat quality of finisher broiler chickens under high temperature. Antioxidants 2022, 11(5), 871. [Google Scholar] [CrossRef]
- Geng, A.L.; Liu, H.G.; Zhang, Y.; Zhang, J.; Wang, H.H.; Chu, Q.; Yan, Z.X. Effects of indoor stocking density on performance, egg quality, and welfare status of a native chicken during 22 to 38 weeks. Poult. Sci. 2020, 99(1), 163–171. [Google Scholar] [CrossRef] [PubMed]
- Saki, A.A.; Zamani, P.; Rahmati, M.; Mahmoudi, H. The effect of cage density on laying hen performance, egg quality, and excreta minerals. J. Appl. Poult. Res. 2012, 21(3), 467–475. [Google Scholar] [CrossRef]
- Jalal, M.A.; Scheideler, S.E.; Marx, D. Effect of bird cage space and dietary metabolizable energy level on production parameters in laying hens1. Poult. Sci. 2006, 85(2), 306–311. [Google Scholar] [CrossRef] [PubMed]
- Anderson, K.E.; Davis, G.S.; Jenkins, P.K.; Carroll, A.S. Effects of bird age, density, and molt on behavioral profiles of two commercial layer strains in cages. Poult. Sci. 2004, 83(1), 15–23. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.K.; Park, S.B.; Jeon, J.J.; Kim, H.S.; Kim, S.H.; Hong, E.; Kim, C.H. Effect of stocking density on laying performance, egg quality and blood parameters of Hy-Line Brown laying hens in an aviary system. Eur. Poult. Sci. 2018, 82. [Google Scholar] [CrossRef]
- Wang, J.; Qiu, L.; Gong, H.; Celi, P.; Yan, L.; Ding, X.; Bai, S.; Zeng, Q.; Mao, X.; Xu, S.; Wu, C.; Zhang, K. Effect of dietary 25-hydroxycholecalciferol supplementation and high stocking density on performance, egg quality, and tibia quality in laying hens. Poult. Sci. 2020, 99(5), 2608–2615. [Google Scholar] [CrossRef]
- Simitzis, P.E.; Kalogeraki, E.; Goliomytis, M.; Charismiadou, M.A.; Triantaphyllopoulos, K.; Ayoutanti, A.; Niforou, K.; Hager-Theodorides, A.L.; Deligeorgis, S.G. Impact of stocking density on broiler growth performance, meat characteristics, behavioural components and indicators of physiological and oxidative stress. Br. Poult. Sci. 2012, 53(6), 721–730. [Google Scholar] [CrossRef]
- Wu, Y.; Li, J.; Qin, X.; Sun, S.; Xiao, Z.; Dong, X.; Shahid, M.S.; Yin, D.; Yuan, J. Proteome and microbiota analysis reveals alterations of liver-gut axis under different stocking density of Peking ducks. PLoS ONE 2018, 13(10), e0198985. [Google Scholar] [CrossRef]
- Incharoen, T.; Roytrakul, S.; Likittrakulwong, W. Dietary germinated paddy rice and stocking density affect egg performance, serum biochemical properties, and proteomic and transcriptomic response of laying hens exposed to chronic heat stress. Proteomes 2021, 9(4), 48. [Google Scholar] [CrossRef]
- Li, W.; Wei, F.; Xu, B.; Sun, Q.; Deng, W.; Ma, H.; Bai, J.; Li, S. Effect of stocking density and alpha-lipoic acid on the growth performance, physiological and oxidative stress and immune response of broilers. Asian-Australas. J. Anim. Sci. 2019, 32(12), 1914–1922. [Google Scholar] [CrossRef]
- Minatel, I.O.; Francisqueti, F.V.; Corrêa, C.R.; Lima, G.P. Antioxidant activity of γ-oryzanol: A complex network of interactions. Int. J. Mol. Sci. 2016, 17(8), 1107. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Godber, J.S. Purification and identification of components of gamma-oryzanol in rice bran Oil. J. Agric. Food Chem. 1999, 47(7), 2724–2728. [Google Scholar] [CrossRef] [PubMed]
- Lerma-García, M.J.; Herrero-Martínez, J.M.; Simó-Alfonso, E.F.; Mendonça, C.R.B.; Ramis-Ramos, G. Composition, industrial processing and applications of rice bran γ-oryzanol. Food Chem. 2009, 115(2), 389–404. [Google Scholar] [CrossRef]
- Islam, M.S.; Yoshida, H.; Matsuki, N.; Ono, K.; Nagasaka, R.; Ushio, H.; Guo, Y.; Hiramatsu, T.; Hosoya, T.; Murata, T.; Hori, M.; Ozaki, H. Antioxidant, free radical-scavenging, and NF-kappaB-inhibitory activities of phytosteryl ferulates: structure-activity studies. J. Pharmacol Sci. 2009, 111(4), 328–337. [Google Scholar] [CrossRef]
- Ketsawatsakul, U.; Whiteman, M.; Halliwell, B. A reevaluation of the peroxynitrite scavenging activity of some dietary phenolics. Biochem. Biophys. Res. Commun. 2000, 279(2), 692–699. [Google Scholar] [CrossRef]
- López-Revuelta, A.; Sánchez-Gallego, J.I.; Hernández-Hernández, A.; Sánchez-Yagüe, J.; Llanillo, M. Increase in vulnerability to oxidative damage in cholesterol-modified erythrocytes exposed to t-BuOOH. Biochim. Biophys. Acta. 2005, 1734(1), 74–85. [Google Scholar] [CrossRef]
- Niki, E. Role of vitamin E as a lipid-soluble peroxyl radical scavenger: in vitro and in vivo evidence. Free Radic. Biol. Med. 2014, 66(8), 3–12. [Google Scholar] [CrossRef]
- Zingg, J.M. Molecular and cellular activities of vitamin E analogues. Mini Rev. Med. Chem. 2007, 7(5), 543–558. [Google Scholar] [CrossRef]
- Jena, B.; Panda, N.; Patra, R.; Mishra, P.; Behura, N.; Panigrahi, B. Supplementation of vitamin E and C reduces oxidative stress in broiler breeder hens during summer. Food Nutr. Sci. 2013, 4(8A), 33–37. [Google Scholar] [CrossRef]
- Zaghari, M.; Sedaghat, V.; Shivazad, M. Effect of vitamin E on reproductive performance of heavy broiler breeder hens. J. Appl. Poult. Res. 2013, 22(4), 808–813. [Google Scholar] [CrossRef]
- Yang, J.; Ding, X.M.; Bai, S.P.; Wang, J.P.; Zeng, Q.F.; Peng, H.W.; Xuan, Y.; Su, Z.W.; Zhang, K.Y. Effects of dietary vitamin E supplementation on laying performance, hatchability, and antioxidant status in molted broiler breeder hens. J. Appl. Poul.t Res. 2021, 30(3), 100184. [Google Scholar] [CrossRef]
- Zhao, Z.; Huang, J.; Jin, Q.; Wang, X. Influence of oryzanol and tocopherols on thermal oxidation of rice bran oil during the heating process at Chinese cooking temperatures. LWT 2021, 142, 111022. [Google Scholar] [CrossRef]
- Serbinova, E.; Kagan, V.; Han, D.; Packer, L. Free radical recycling and intramembrane mobility in the antioxidant properties of alpha-tocopherol and alpha-tocotrienol. Free Radic. Biol. Med. 1991, 10(5), 263–275. [Google Scholar] [CrossRef] [PubMed]
- Sen, C.K.; Khanna, S.; Roy, S. Tocotrienols: Vitamin E beyond tocopherols. Life Sci. 2006, 78(18), 2088–2098. [Google Scholar] [CrossRef] [PubMed]
- NRC. Nutrient requirements of poultry, 9th ed., National Academies Press: Washington DC, USA, 1994. [CrossRef]
- Likittrakulwong, W.; Moonsatan, S.; Incharoen, T. Enhancement of tibia bone and eggshell hardness through the supplementation of bio-calcium derived from fish bone mixed with chelated trace minerals and vitamin D3 in laying duck diet. Vet. Anim. Sci. 2021, 14, 100204. [Google Scholar] [CrossRef] [PubMed]
- Beloor, J.; Kang, H.; Kim, Y.; Subramani, V.K.; Jang, I.S.; Sohn, S.H.; Moon, Y.S. The effect of stocking density on stress related genes and telomeric length in broiler chickens. Anim. Biosci. 2010, 23(4), 437–443. [Google Scholar] [CrossRef]
- Brisbin, J.T.; Gong, J.; Parvizi, P.; Sharif, S. Effects of lactobacilli on cytokine expression by chicken spleen and cecal tonsil cells. Clin. Vaccine Immunol. 2010, 17(9), 1337–1343. [Google Scholar] [CrossRef]
- Sohn, S.H.; Subramani, V.K.; Moon, Y.S.; Jang, I.S. Telomeric DNA quantity, DNA damage, and heat shock protein gene expression as physiological stress markers in chickens. Poult. Sci. 2012, 91(4), 829–836. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 2001, 25(4), 402–408. [Google Scholar] [CrossRef]
- Sun, Z.W.; Yan, L.; G, Y.Y.; Zhao, J.P.; Lin, H.; Guo, Y.M. Increasing dietary vitamin D3 improves the walking ability and welfare status of broiler chickens reared at high stocking densities. Poult. Sci. 2013, 92(12), 3071–3079. [Google Scholar] [CrossRef] [PubMed]
- Cengiz, Ö.; Köksal, B.H.; Tatlı, O.; Sevim, Ö.; Ahsan, U.; Üner, A.G.; Ulutaş, P.A.; Beyaz, D.; Büyükyörük, S.; Yakan, A.; Önol, A.G. Effect of dietary probiotic and high stocking density on the performance, carcass yield, gut microflora, and stress indicators of broilers. Poult. Sci. 2015, 94(10), 2395–2403. [Google Scholar] [CrossRef] [PubMed]
- Goo, D.; Kim, J.H.; Park, G.H.; Delos Reyes, J.B.; Kil, D.Y. Effect of heat stress and stocking density on growth performance, breast meat quality, and intestinal barrier function in broiler chickens. Animals 2019, 9(3), 107. [Google Scholar] [CrossRef] [PubMed]
- Xie, M.; Jiang, Y.; Tang, J.; Wen, Z.G.; Huang, W.; Hou, S.S. Effects of stocking density on growth performance, carcass traits, and foot pad lesions of White Pekin ducks. Poult. Sci. 2014, 93(7), 1644–1648. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.L.; Xue, J.J.; Huang, X.F.; Chen, Y.; Wang, Q.G.; Zhang, S.; Wang, C. Effect of stocking density on growth performance, feather quality, serum hormone, and intestinal development of geese from 1 to 14 days of age. Poult Sci. 2021, 100(11), 101417. [Google Scholar] [CrossRef]
- Bessei, W. Welfare of broilers: a review. World’s Poult. Sci. J. 2006, 62(3), 455–466. [Google Scholar] [CrossRef]
- Berger, A.; Rein, D.; Schäfer, A.; Monnard, I.; Gremaud, G.; Lambelet, P.; Bertoli, C. Similar cholesterol-lowering properties of rice bran oil, with varied gamma-oryzanol, in mildly hypercholesterolemic men. Eur. J. Nutr. 2005, 44(3), 163–173. [Google Scholar] [CrossRef]
- Klongpityapong, P.; Supabphol, R.; Supabphol, A. Antioxidant effects of gamma-oryzanol on human prostate cancer cells. Asian Pac. J. Cancer Prev. 2013, 14(9), 5421–5425. [Google Scholar] [CrossRef]
- Wilson, T.A.; Nicolosi, R.J.; Woolfrey, B.; Kritchevsky, D. Rice bran oil and oryzanol reduce plasma lipid and lipoprotein cholesterol concentrations and aortic cholesterol ester accumulation to a greater extent than ferulic acid in hypercholesterolemic hamsters. J. Nutr. Biochem. 2007, 18(2), 105–112. [Google Scholar] [CrossRef]
- Hai, L.; Rong, D.; Zhang, Z.Y. The effect of thermal environment on the digestion of broilers. J. Anim. Physiol. Anim. Nutr. 2000, 83(2), 57–64. [Google Scholar] [CrossRef]
- Fang, Y.Z.; Yang, S.; Wu, G. Free radicals, antioxidants, and nutrition. Nutrition 2002, 18(10), 872–879. [Google Scholar] [CrossRef]
- Velasco, V.; Williams, P. Improving meat quality through natural antioxidants. Chil. J. Agric. Res. 2011, 71(2), 313–322. [Google Scholar] [CrossRef]
- Punia, S.; Kumar, M.; Sandhu, K.S. Rice-bran oil: An emerging source of functional oil. J. Food Process. Preserv. 2021, 45(4), e15318. [Google Scholar] [CrossRef]
- Kang, H.K.; Kim, C.H. Effects of dietary supplementation with rice bran oil on the growth performance, blood parameters, and immune response of broiler chickens. J. Anim. Sci. Technol. 2016, 58, 12. [Google Scholar] [CrossRef]
- Oh, S.K.; Hwang, P.S.; Kim, K.J.; Kim, Y.K.; Lee, J.H. Changes in nutritional components throughout germination in paddy rice and brown rice. J. Food Sci. Nutr. 2010, 15(2), 113–119. [Google Scholar] [CrossRef]
- Zavoshy, R.; Noroozi, M.; Jahanihashemi, H. Effect of low calorie diet with rice bran oil on cardiovascular risk factors in hyperlipidemic patients. J. Res. Med. Sci. 2012, 17(7), 626–631. [Google Scholar]
- Wigley, P.; Kaiser, P. Avian cytokines in health and disease. Braz. J. Poult. Sci. 2003, 5(1), 1–14. [Google Scholar] [CrossRef]
- Giansanti, F.; Giardi, M.F.; Botti, D. Avian cytokines-an overview. Curr. Pharm. Des. 2006, 12(24), 3083–3099. [Google Scholar] [CrossRef]
- Cardenas-Garcia, S.; Dunwoody, R.P.; Marcano, V.; Diel, D.G.; Williams, R.J.; Gogal, R.M.; Brown, C.C.; Miller, P.J.; Afonso, C.L. Effects of chicken interferon gamma on newcastle disease virus vaccine immunogenicity. PLoS ONE 2016, 11(7), e0159153. [Google Scholar] [CrossRef]
- Gao, P.; Fan, L.; Du, H.; Xiang, B.; Li, Y.; Sun, M.; Kang, Y.; Chen, L.; Xu, C.; Li, Y.; Ren, T. Recombinant duck interferon gamma inhibits H5N1 influenza virus replication in vitro and in vivo. J. Interferon Cytokine Res. 2018, 38(7), 290–297. [Google Scholar] [CrossRef]
- Li, H.T.; Ma, B.; Mi, J.W.; Jin, H.Y.; Xu, L.N.; Wang, J.W. Molecular cloning and functional analysis of goose interferon gamma. Vet. Immunol. Immunopathol. 2007, 117(1-2), 67–74. [Google Scholar] [CrossRef] [PubMed]
- Ohtsu, H.; Yamazaki, M.; Abe, H.; Murakami, H.; Toyomizu, M. Heat stress modulates cytokine gene expression in the spleen of broiler chickens. J. Poult. Sci. 2015, 52(4), 282–287. [Google Scholar] [CrossRef]
- Roque, K.; Shin, K.M.; Jo, J.H.; Kim, H.A.; Heo, Y. Relationship between chicken cellular immunity and endotoxin levels in dust from chicken housing environments. J. Vet. Sci. 2015, 16(2), 173–177. [Google Scholar] [CrossRef]
- Lee, S.H.; Lillehoj, H.S.; Lillehoj, E.P.; Cho, S.M.; Park, D.W.; Hong, Y.H.; Chun, H.K.; Park, H. J. Immunomodulatory properties of dietary plum on coccidiosis. Comp. Immunol. Microbiol. Infect. Dis. 2008, 31(5), 389–402. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).