Submitted:
08 October 2023
Posted:
09 October 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results
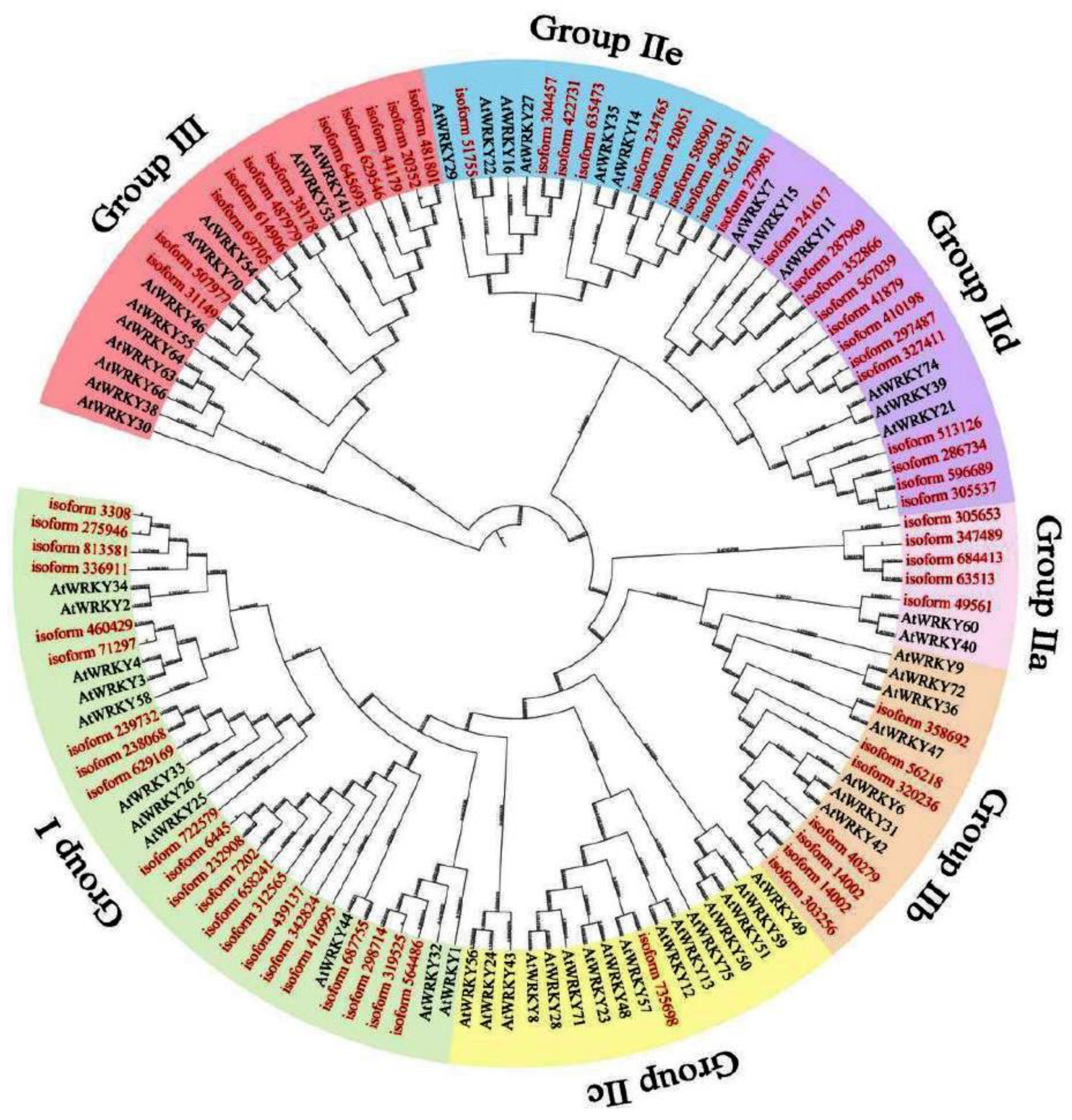
2.1. Genome-Wide Identification of WRKY Genes in I. laevigata
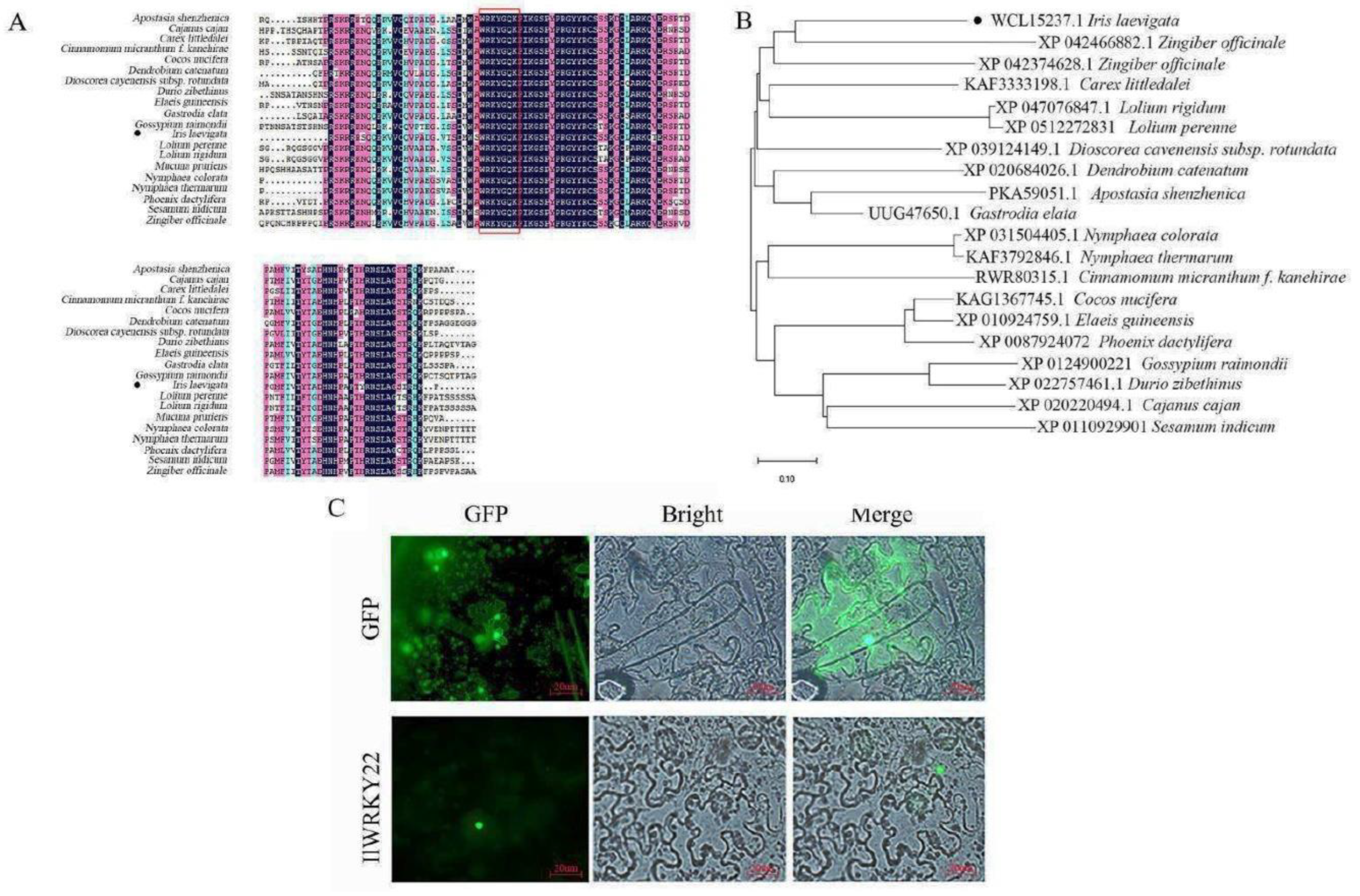
2.2. Cloning of IlWRKY22 and Subcellular localization
2.3. Overexpressing IlWRKY22 in both Arabidopsis and tobacco
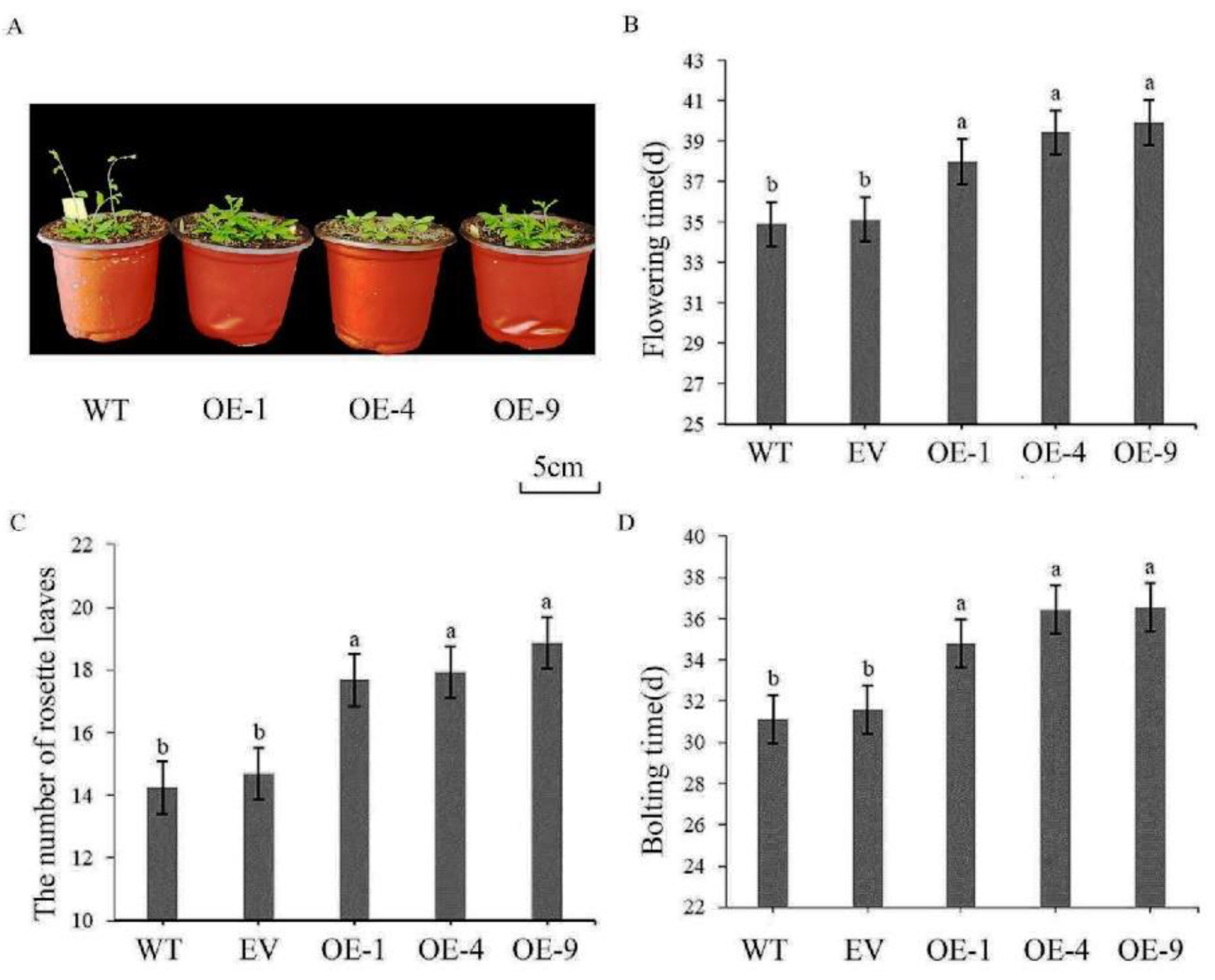
2.4. Overexpressing IlWRKY22 delays flowering in Arabidopsis thaliana
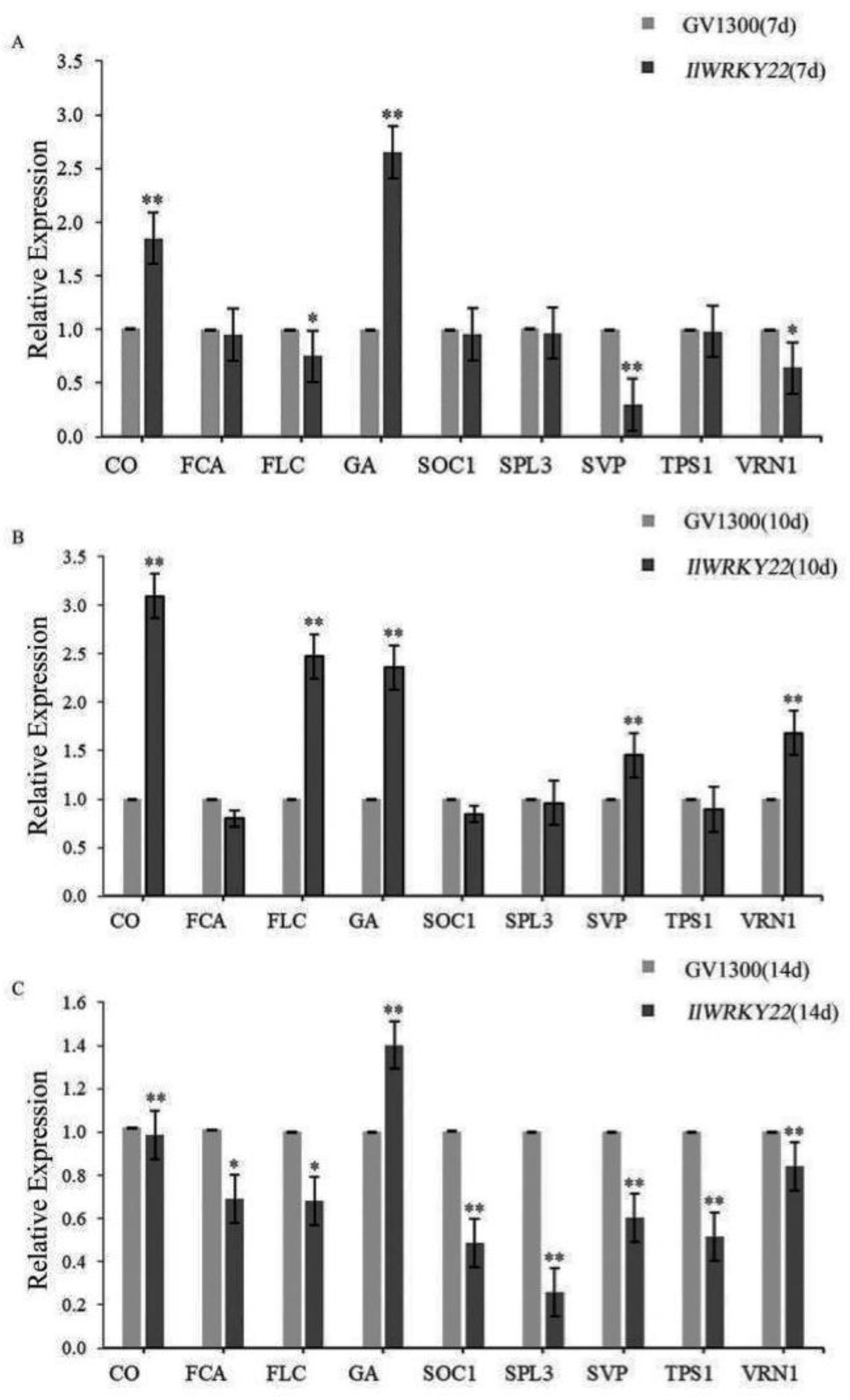
2.5. Overexpression of IlWRKY22 Modulates the Expression of Flowering Time Genes
2.6. Overexpressing IlWRKY22 in Nicotiana tabacum enhances resistance to abiotic stress
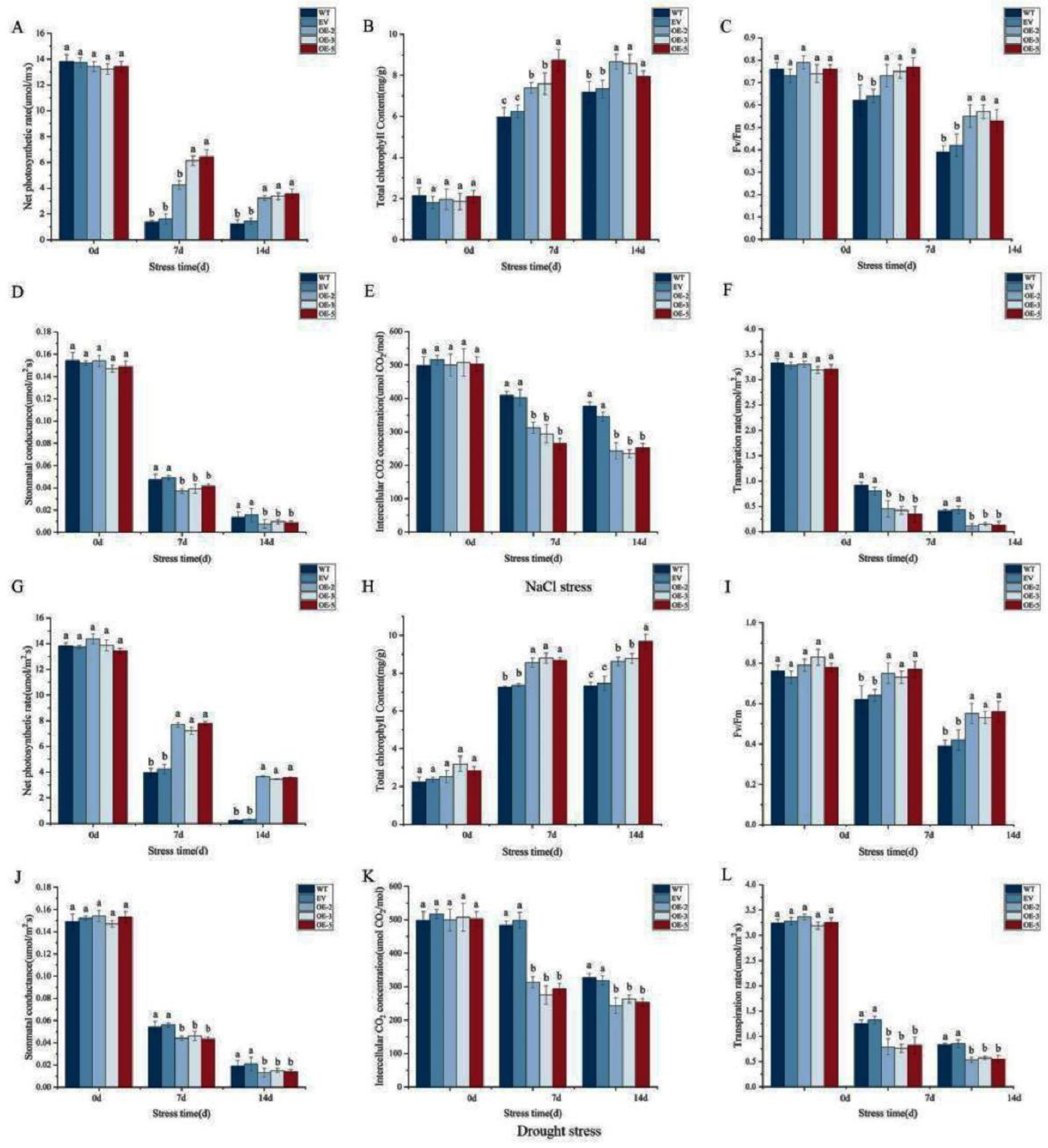
2.7. Impact of overexpressing IlWRKY22 on photosynthesis under abiotic stress
2.8. The involvement of ROS regulation in IlWRKY22-enhanced abiotic stress resistance
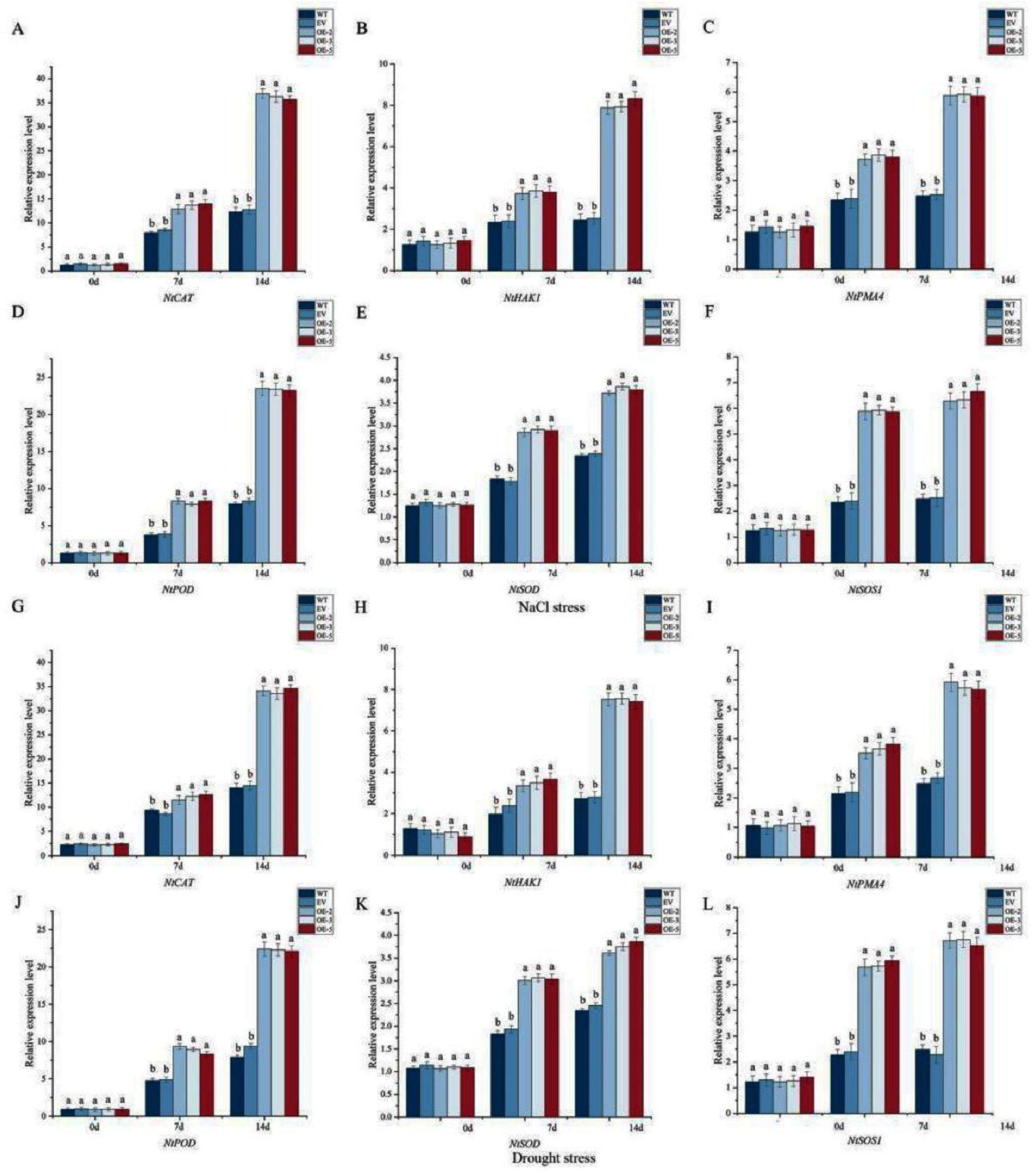
2.9. The IlWRKY22 on expression levels of stress-related genes NtCAT, NtHAK1, NtPMA4, NtPOD, NtSOD, and NtSOS1 under abiotic stress
3. Discussion
4. Materials and methods
4.1. Plant materials
4.2. Gene Sequence Identification and Phylogenetic Analysis
4.3. Gene cloning and sequence analysis
4.4. Subcellular localization
4.5. Plant transformation
4.6. Determination of flowering phenotypes and gene expression
4.7. Abiotic stress treatment
4.8. Photosynthetic parameters in tobacco s under Abiotic stress
4.9. Other physiological measurements of abiotic-treated tobacco plants
4.10. Real-time RT-PCR analyses
4.11. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wellmer, F.; Riechmann, J.L. Gene networks controlling the initiation of flower development. Trends Genet. 2010, 26, 519-527. [CrossRef]
- Rehman, S.; Bahadur, S.; Xia, W. An overview of floral regulatory genes in annual and perennial plants. Gene 2023, 885, 147699. [CrossRef]
- He, Y.; Chen, T.; Zeng, X. Genetic and Epigenetic Understanding of the Seasonal Timing of Flowering. Plant Commun 2020, 1, 100008. [CrossRef]
- Wang, F.; Li, S.; Kong, F.; Lin, X.; Lu, S. Altered regulation of flowering expands growth ranges and maximizes yields in major crops. Front. Plant Sci. 2023, 14, 1094411. [CrossRef]
- Kozlov, K.; Singh, A.; Berger, J.; Bishop-von, W.E.; Kahraman, A.; Aydogan, A.; Cook, D.; Nuzhdin, S.; Samsonova, M. Non-linear regression models for time to flowering in wild chickpea combine genetic and climatic factors. BMC Plant Biol. 2019, 19, 94. [CrossRef]
- Sun, Y.; Zhou, J.; Guo, J. Advances in the knowledge of adaptive mechanisms mediating abiotic stress responses in Camellia sinensis. Front Biosci (Landmark Ed) 2021, 26, 1714-1722. [CrossRef]
- Retraction: Exogenous application of moringa leaf extract improves growth, biochemical attributes, and productivity of late-sown quinoa. PLoS One 2022, 17, e272392.
- Zhu, J.K. Abiotic Stress Signaling and Responses in Plants. Cell 2016, 167, 313-324. [CrossRef]
- Su Wei; Xiao Liuying; Sun Guangwen; Liu Houcheng; Song Shiwei; Chen Riyuan.Functional analysis of transcription factor BcWRKY22 in low temperature-induced bolting and flowering of Brassica campestris.Molecular Plant Breeding. 2020, 18, 3862-3870.
- Shah, S.; Weinholdt, C.; Jedrusik, N.; Molina, C.; Zou, J.; Grosse, I.; Schiessl, S.; Jung, C.; Emrani, N. Whole-transcriptome analysis reveals genetic factors underlying flowering time regulation in rapeseed (Brassica napus L.). Plant Cell Environ. 2018, 41. [CrossRef]
- Golldack, D.; Luking, I.; Yang, O. Plant tolerance to drought and salinity: stress regulating transcription factors and their functional significance in the cellular transcriptional network. Plant Cell Rep. 2011, 30, 1383-1391. [CrossRef]
- Fedoroff, N.V.; Battisti, D.S.; Beachy, R.N.; Cooper, P.J.; Fischhoff, D.A.; Hodges, C.N.; Knauf, V.C.; Lobell, D.; Mazur, B.J.; Molden, D. et al. Radically rethinking agriculture for the 21st century. Science 2010, 327, 833-834. [CrossRef]
- Xia, Y.; Huang, G.; Zhu, Y. Sustainable plant disease control: biotic information flow and behavior manipulation. Sci. China Life Sci. 2019, 62, 1710-1713. [CrossRef]
- Phukan, U.J.; Jeena, G.S.; Shukla, R.K. WRKY Transcription Factors: Molecular Regulation and Stress Responses in Plants. Front. Plant Sci. 2016, 7, 760. [CrossRef]
- Khoso, M.A.; Hussain, A.; Ritonga, F.N.; Ali, Q.; Channa, M.M.; Alshegaihi, R.M.; Meng, Q.; Ali, M.; Zaman, W.; Brohi, R.D. et al. WRKY transcription factors (TFs): Molecular switches to regulate drought, temperature, and salinity stresses in plants. Front. Plant Sci. 2022, 13, 1039329. [CrossRef]
- Agarwal, P.; Reddy, M.P.; Chikara, J. WRKY: its structure, evolutionary relationship, DNA-binding selectivity, role in stress tolerance and development of plants. Mol. Biol. Rep. 2011, 38, 3883-3896. [CrossRef]
- Khoso, M.A.; Hussain, A.; Ritonga, F.N.; Ali, Q.; Channa, M.M.; Alshegaihi, R.M.; Meng, Q.; Ali, M.; Zaman, W.; Brohi, R.D. et al. WRKY transcription factors (TFs): Molecular switches to regulate drought, temperature, and salinity stresses in plants. Front. Plant Sci. 2022, 13, 1039329. [CrossRef]
- Jiang, J.; Ma, S.; Ye, N.; Jiang, M.; Cao, J.; Zhang, J. WRKY transcription factors in plant responses to stresses. J. Integr. Plant Biol. 2017, 59, 86-101. [CrossRef]
- Chen, X.; Li, C.; Wang, H.; Guo, Z. WRKY transcription factors: evolution, binding, and action. Phytopathology Research 2019, 1. [CrossRef]
- Bakshi, M.; Oelmuller, R. WRKY transcription factors: Jack of many trades in plants. Plant Signal Behav 2014, 9, e27700. [CrossRef]
- Liu, G.; Li, F.; Shi, G.; Wang, L.; Wang, L.; Fan, L. Identification of MADS-Box Transcription Factors in Iris laevigata and Functional Assessment of IlSEP3 and IlSVP during Flowering. Int. J. Mol. Sci. 2022, 23. [CrossRef]
- Yang, J.; Yu, S.; Shi, G.F.; Yan, L.; Lv, R.T.; Ma, Z.; Wang, L. Comparative analysis of R2R3-MYB transcription factors in the flower of Iris laevigata identifies a novel gene regulating tobacco cold tolerance. Plant Biol (Stuttg) 2022, 24, 1066-1075. [CrossRef]
- Chen, K.; Wang, Y.; Zhang, R.; Zhang, H.; Gao, C. CRISPR/Cas Genome Editing and Precision Plant Breeding in Agriculture. Annu. Rev. Plant Biol. 2019, 70, 667-697. [CrossRef]
- Kourelis, J.; van der Hoorn, R.; Sueldo, D.J. Decoy Engineering: The Next Step in Resistance Breeding. Trends Plant Sci. 2016, 21, 371-373. [CrossRef]
- Marks, R.A.; Hotaling, S.; Frandsen, P.B.; VanBuren, R. Representation and participation across 20 years of plant genome sequencing. Nat Plants 2021, 7, 1571-1578. [CrossRef]
- Michael, T.P.; Alba, R. The tomato genome fleshed out. Nat. Biotechnol. 2012, 30, 765-767. [CrossRef]
- The tomato genome sequence provides insights into fleshy fruit evolution. Nature 2012, 485, 635-641. [CrossRef]
- Coustham, V.; Li, P.; Strange, A.; Lister, C.; Song, J.; Dean, C. Quantitative modulation of polycomb silencing underlies natural variation in vernalization. Science 2012, 337, 584-587. [CrossRef]
- Zhang, X.; Bernatavichute, Y.V.; Cokus, S.; Pellegrini, M.; Jacobsen, S.E. Genome-wide analysis of mono-, di- and trimethylation of histone H3 lysine 4 in Arabidopsis thaliana. Genome Biol 2009, 10, R62. [CrossRef]
- He, Y. Control of the transition to flowering by chromatin modifications. Mol. Plant 2009, 2, 554-564. [CrossRef]
- Srikanth, A.; Schmid, M. Regulation of flowering time: all roads lead to Rome. Cell. Mol. Life Sci. 2011, 68, 2013-2037. [CrossRef]
- Eriksson, S.; Bohlenius, H.; Moritz, T.; Nilsson, O. GA4 is the active gibberellin in the regulation of LEAFY transcription and Arabidopsis floral initiation. Plant Cell 2006, 18, 2172-2181. [CrossRef]
- Khoso, M.A.; Hussain, A.; Ritonga, F.N.; Ali, Q.; Channa, M.M.; Alshegaihi, R.M.; Meng, Q.; Ali, M.; Zaman, W.; Brohi, R.D. et al. WRKY transcription factors (TFs): Molecular switches to regulate drought, temperature, and salinity stresses in plants. Front. Plant Sci. 2022, 13, 1039329. [CrossRef]
- Ahmad, I.; Zhu, G.; Zhou, G.; Liu, J.; Younas, M.U.; Zhu, Y. Melatonin Role in Plant Growth and Physiology under Abiotic Stress. Int. J. Mol. Sci. 2023, 24. [CrossRef]
- Chen, H.; Li, X.; Li, F.; Li, D.; Dong, Y.; Fan, Y. Bioinformatics Analysis of WRKY Family Genes in Erianthus fulvus Ness. Genes (Basel) 2022, 13. [CrossRef]
- Liu Zhi.Identification of WRKY transcription factor family in Xanthoceras sorbifolia and analysis of response patterns to abiotic stresses. Northeast Forestry University. 2020, 16.
- Liu Yuxuan. Identification and expression analysis of WRKY gene family in tomato. Shenyang Agricultural University. 2020, 13.
- Zhang, Y.; Wang, L. The WRKY transcription factor superfamily: its origin in eukaryotes and expansion in plants. BMC Evol. Biol. 2005, 5, 1. [CrossRef]
- Luo, X.; Li, C.; He, X.; Zhang, X.; Zhu, L. ABA signaling is negatively regulated by GbWRKY1 through JAZ1 and ABI1 to affect salt and drought tolerance. Plant Cell Rep. 2020, 39, 181-194. [CrossRef]
- Wang, Y.; Huang, X.; Huang, X.; Su, W.; Hao, Y.; Liu, H.; Chen, R.; Song, S. BcSOC1 Promotes Bolting and Stem Elongation in Flowering Chinese Cabbage. Int. J. Mol. Sci. 2022, 23. [CrossRef]
- Cai, Y.; Chen, X.; Xie, K.; Xing, Q.; Wu, Y.; Li, J.; Du C; Sun, Z.; Guo, Z. Dlf1, a WRKY transcription factor, is involved in the control of flowering time and plant height in rice. PLoS One 2014, 9, e102529. [CrossRef]
- Amasino, R.M.; Michaels, S.D. The timing of flowering. Plant Physiol. 2010, 154, 516-520. [CrossRef]
- Wang, H.; Chen, W.; Xu, Z.; Chen, M.; Yu, D. Functions of WRKYs in plant growth and development. Trends Plant Sci. 2023, 28, 630-645. [CrossRef]
- Liu, Y.; Yang, J.; Yang, M. [Pathways of flowering regulation in plants]. Sheng Wu Gong Cheng Xue Bao 2015, 31, 1553-1566.
- Zhou, X.; Jiang, Y.; Yu, D. WRKY22 transcription factor mediates dark-induced leaf senescence in Arabidopsis. Mol. Cells 2011, 31, 303-313. [CrossRef]
- Balti, I.; Benny, J.; Perrone, A.; Caruso, T.; Abdallah, D.; Salhi-Hannachi, A.; Martinelli, F. Identification of conserved genes linked to responses to abiotic stresses in leaves among different plant species. Funct. Plant Biol. 2020, 48, 54-71.
- Anuradha, M.; Sivaraju, K.; Krishnamurthy, V. Effect of waterlogging on physiological characteristics, yield and quality of flue-cured tobacco. Indian Journal of Plant Physiology 2013, 18, 67-70. [CrossRef]
- Elshoky, H.A.; Yotsova, E.; Farghali, M.A.; Farroh, K.Y.; El-Sayed, K.; Elzorkany, H.E.; Rashkov, G.; Dobrikova, A.; Borisova, P.; Stefanov, M. et al. Impact of foliar spray of zinc oxide nanoparticles on the photosynthesis of Pisum sativum L. under salt stress. Plant Physiol Biochem 2021, 167, 607-618. [CrossRef]
- Razi, K.; Muneer, S. Drought stress-induced physiological mechanisms, signaling pathways and molecular response of chloroplasts in common vegetable crops. Crit. Rev. Biotechnol. 2021, 41, 669-691. [CrossRef]
- Zahra, N.; Al, H.M.; Hafeez, M.B.; Rehman, A.; Wahid, A.; Siddique, K.; Farooq, M. Regulation of photosynthesis under salt stress and associated tolerance mechanisms. Plant Physiol Biochem 2022, 178, 55-69. [CrossRef]
- Skopelitis, D.S.; Paranychianakis, N.V.; Paschalidis, K.A.; Pliakonis, E.D.; Delis, I.D.; Yakoumakis, D.I.; Kouvarakis, A.; Papadakis, A.K.; Stephanou, E.G.; Roubelakis-Angelakis, K.A. Abiotic stress generates ROS that signal expression of anionic glutamate dehydrogenases to form glutamate for proline synthesis in tobacco and grapevine. Plant Cell 2006, 18, 2767-2781. [CrossRef]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol Biochem 2010, 48, 909-930. [CrossRef]
- Jaleel, C.A.; Riadh, K.; Gopi, R.; Manivannan, P.; Inès, J.; Al-Juburi, H.J.; Chang-Xing, Z.; Hong-Bo, S.; Panneerselvam, R. Antioxidant defense responses: physiological plasticity in higher plants under abiotic constraints. Acta Physiol. Plant. 2009, 31, 427-436. [CrossRef]
- Yang, J.; Yu, S.; Shi, G.F.; Yan, L.; Lv, R.T.; Ma, Z.; Wang, L. Comparative analysis of R2R3-MYB transcription factors in the flower of Iris laevigata identifies a novel gene regulating tobacco cold tolerance. Plant Biol (Stuttg) 2022, 24, 1066-1075. [CrossRef]
- Collings, D.A. Subcellular localization of transiently expressed fluorescent fusion proteins. Methods Mol Biol 2013, 1069, 227-258. [CrossRef]
- Wang, H.; Liu, Z.; Xie, J.; Li, J.; Zhang, J.; Yu, J.; Hu, L.; Zhang, G. The CaALAD Gene From Pepper (Capsicum annuum L.) Confers Chilling Stress Tolerance in Transgenic Arabidopsis Plants. Front. Plant Sci. 2022, 13, 884990. [CrossRef]
- Wang, Y.; Cao, S.; Guan, C.; Kong, X.; Wang, Y.; Cui, Y.; Liu, B.; Zhou, Y.; Zhang, Y. Overexpressing the NAC transcription factor LpNAC13 from Lilium pumilum in tobacco negatively regulates the drought response and positively regulates the salt response. Plant Physiol Biochem 2020, 149, 96-110. [CrossRef]
- Zhao, X.Y.; Liu, M.S.; Li, J.R.; Guan, C.M.; Zhang, X.S. The wheat TaGI1, involved in photoperiodic flowering, encodes an Arabidopsis GI ortholog. Plant Mol. Biol. 2005, 58, 53-64. [CrossRef]
- Sanagi, M.; Aoyama, S.; Kubo, A.; Lu, Y.; Sato, Y.; Ito, S.; Abe, M.; Mitsuda, N.; Ohme-Takagi, M.; Kiba, T. et al. Low nitrogen conditions accelerate flowering by modulating the phosphorylation state of FLOWERING BHLH4 in Arabidopsis. Proc Natl Acad Sci U S A 2021, 118. [CrossRef]
- Fukazawa, J.; Ohashi, Y.; Takahashi, R.; Nakai, K.; Takahashi, Y. DELLA degradation by gibberellin promotes flowering via GAF1-TPR-dependent repression of floral repressors in Arabidopsis. Plant Cell 2021, 33, 2258-2272. [CrossRef]
- Tayengwa, R.; Sharma, K.P.; Pierce, C.F.; Werner, B.E.; Neff, M.M. Overexpression of AtAHL20 causes delayed flowering in Arabidopsis via repression of FT expression. BMC Plant Biol. 2020, 20, 559. [CrossRef]
- Chen, Y.; Zhang, L.; Zhang, H.; Chen, L.; Yu, D. ERF1 delays flowering through direct inhibition of FLOWERING LOCUS T expression in Arabidopsis. J. Integr. Plant Biol. 2021, 63, 1712-1723. [CrossRef]
- Wang, Y.; Cui, Y.; Liu, B.; Wang, Y.; Sun, S.; Wang, J.; Tan, M.; Yan, H.; Zhang, Y. Lilium pumilum stress-responsive NAC transcription factor LpNAC17 enhances salt stress tolerance in tobacco. Front. Plant Sci. 2022, 13. [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).



