1. Introduction
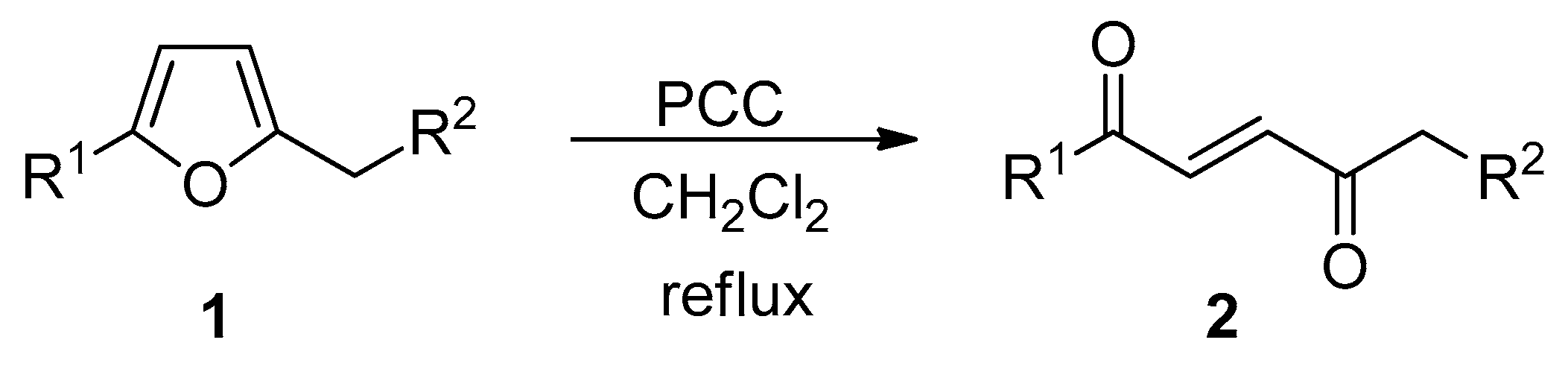
Several years ago our research group reported that the reaction of 2-alkyl and 2,5-dialkyl substituted furans
1 with pyridinium chlorochromate (PCC) in refluxing dichlorometane allowed to obtain in high yields the corresponding
E-enediones
2 (
Scheme 1) [
1,
2].
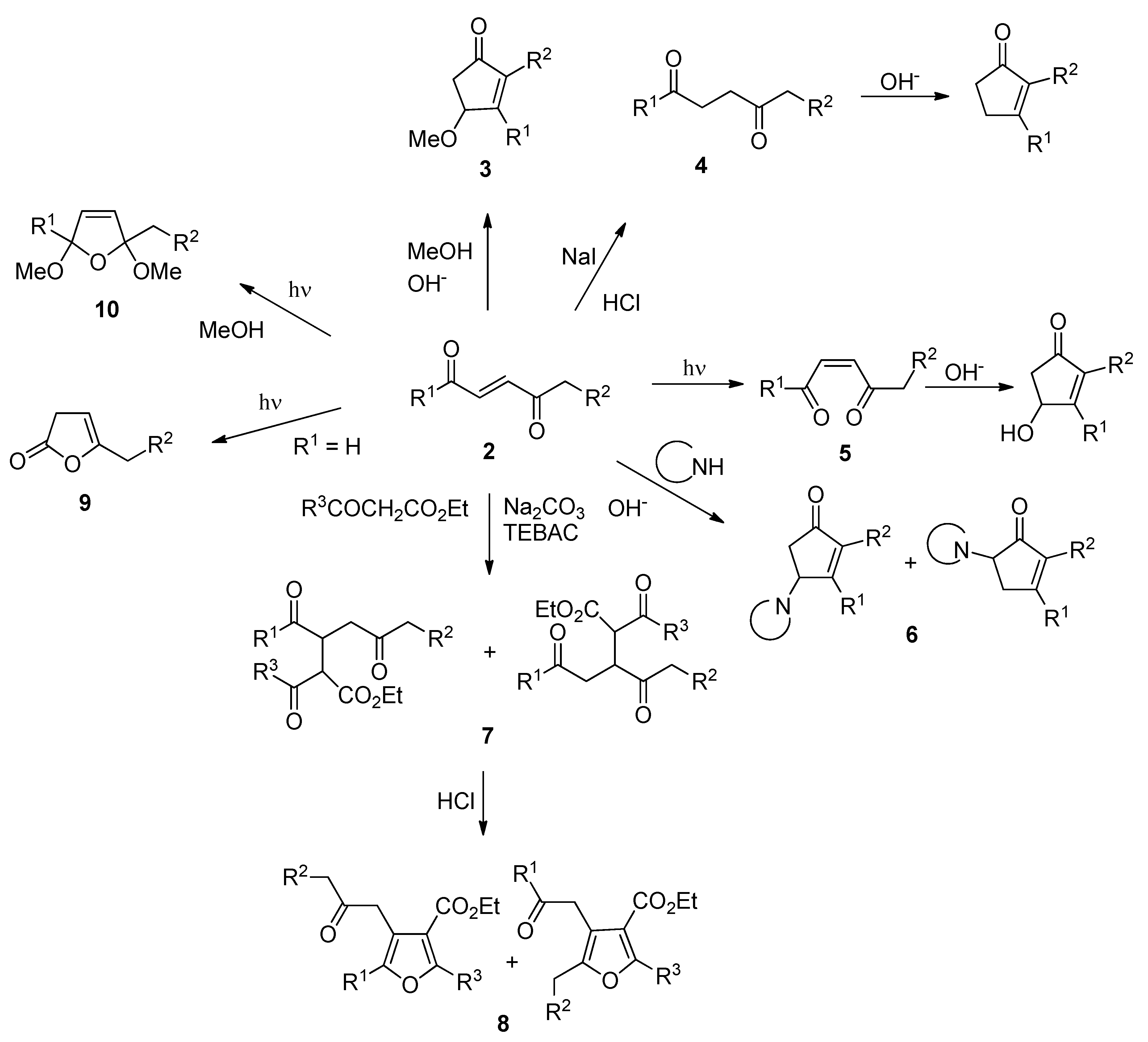
The obtained enediones allowed us to obtain several interesting applications in organic synthesis. Thus, compounds
2 can be directly converted to 4-methoxycyclopentenones
3 (
Scheme 2) [
1,
3]; the double bond can be reduced to give the compounds
4, useful intermediates in the synthesis of cyclopentenones (
Scheme 2) [
3,
4]; the photochemical isomerization of the double bond to give
5 allowed a new way for the synthesis of a pyrethroid structure (
Scheme 2) [
5]; the double bond can subjected to Michael additions allowing the formation of aminocyclopentenones
6 and tetrasubstituted furans
8 (
Scheme 2) [
6,
7]. Furthermore, irradiation of 2-alkylfuran derivatives gave 2(3H)-furanones
9, while the irradiation of
2 in methanol gave the 2,5-dimethoxydihydrofuran derivatives
10 (
Scheme 2) [
8,
9].
The PCC used to perform the reaction
1 →
2 had been prepared following the original procedure proposed by Corey [
10]. Homemade PCC was used in some other research in this field [11-18].
Recently, we attempted to perform the same reaction using 2,5-dimethylfuran as starting material. In this case, we used commercially available pyridinium chlorochromate (Sigma-Aldrich). The reaction did not give the expected result, and no reaction occurred. It is not clear why the commercially available reagent did not give the ring cleavage reaction while the reagent we prepared was able to do it. The main evident difference between the reagent is the color.
Figure 1 reports the color of both commercially available PCC and homemade PCC; commercial product shows a brilliant orange color, while homemade reagent shows a darker color, near to a brown color.
In this paper we want to discuss the origin of the observed different color, and how the possible difference in the composition can modify the reactivity of the oxidant.
2. Materials and Methods
2.1. Pyridinium Chlorochromate
Chromium trioxide (100 g, 1 mol) is rapidly added with stirring to 6 N HCl (184 ml, 1.1 mol). After 5 min the homogeneous solution is cooled at 0 °C and pyridine (79.1 g, 1 mol) is carefully adde over 10 min. Recooling to 0 °C gives a yellow-orange solid which is collected on a sintered glass filter and dried in vacuum for 1 h. Yield: 180.8 g (84%).
2.2. XRD Spectra
X-ray powder pattern of the crystal is recorded on a RICH SEIFERT powder X-ray diffractometer using Cu Kα (λ = 1.5406 Å) radiation. The sample is scanned for 2θ range of 10-60 °C at scan rate of 1 °/min.
2.3. XPS Spectra
XPS spectra were acquired with a Phoibos 100 MCD-5 (SPECS) instrument using Al Kα (146.6 eV) source operating at a constant power of 260W. Wide and detailed spectra were collected using the fixed analyzer transmission (FAT) mode of operation with channel width of 0.1 eV, and channel time 0.5 sec.
The samples were mounted in the sample holder using double sided adhesive copper tape, and they were kept under vacuum in the pre-chamber to allow for the vaporization of residual water and/or volatile compounds. The lengths of the stationary time prior to the analyses were established for all samples by monitoring the pre-chamber pressure. When the final pressure decreased below 10-8 mbar, the samples were assumed to be ‘degassed’ and ready for their transfer into the analysis chamber.
2.4. Curve-fitting procedure
The acquired XPS spectra were analyzed using a curve-fitting program(Googly) that has been fully described previously.[
19,
20] Peak areas were converted to composition in at % using established procedures and the appropriate sensitivity factors (SF) [
20].
2.5. DFT Calculation
Gaussian09 has been used for the discussions about the computed geometries [
21]. All the computations were based on the Density Functional Theory (DFT) [
22] by using the B3LYP hybrid xc functional [
23]. Geometry optimizations from the Gaussian09 program have been obtained at the B3LYP/6-311G+(d,p) level of approximation. Geometry optimizations were performed with default settings on geometry convergence (gradients and displacements), integration grid and electronic density (SCF) convergence. Redundant coordinates were used for the geometry optimization as produced by the Gaussian09 program. Analytical evaluation of the energy second derivative matrix w.r.t. Cartesian coordinates (Hessian matrix) at the B3LYP/6-31G+(d,p) level of approximation confirmed the nature of minima on the energy surface points associated to the optimized structures.
3. Results and Discussion
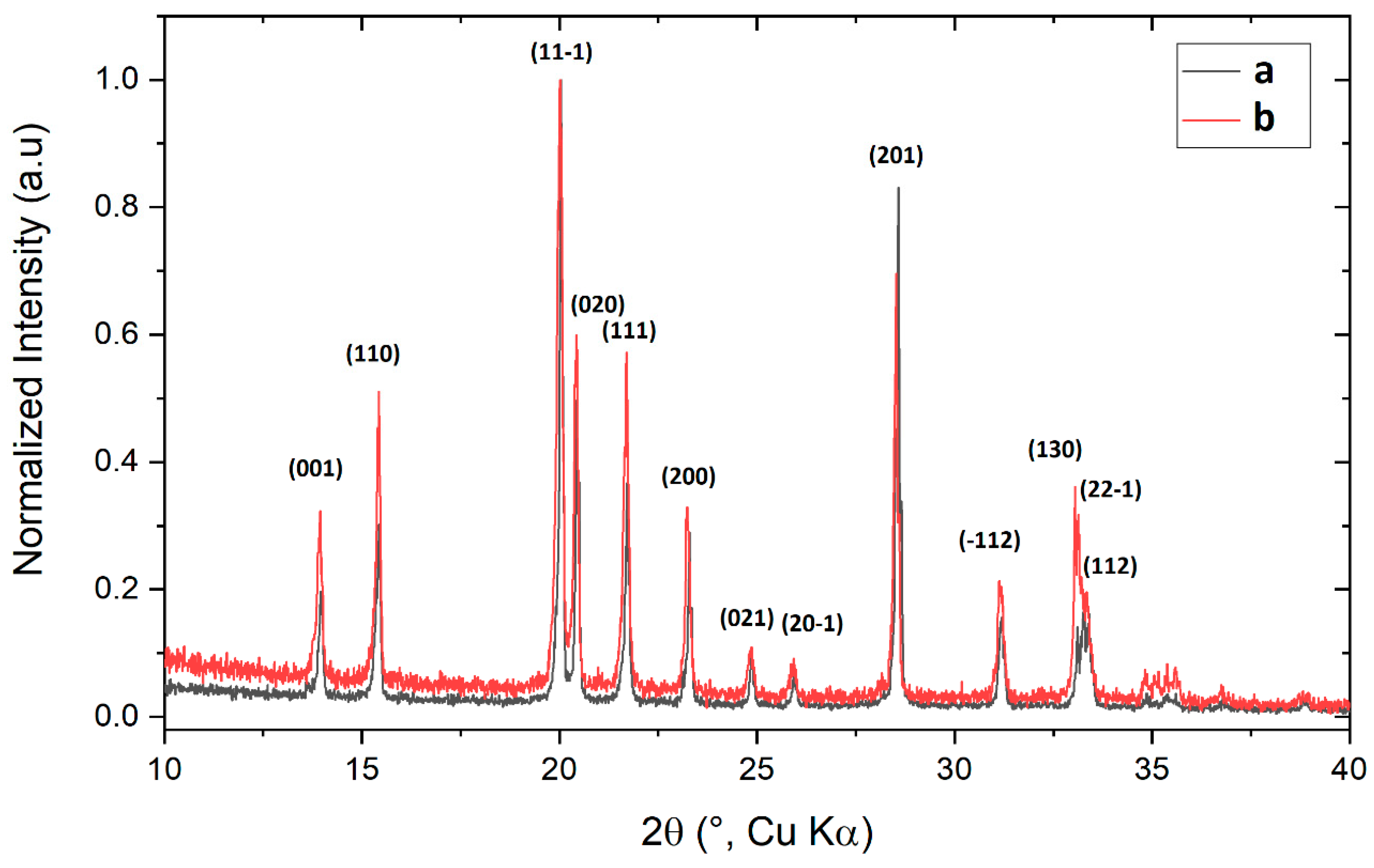
The XRD spectra of commercial PCC (
a) and homemade PCC sample (
b) are reported in
Figure 2. Sample
a was the commercially available PCC while sample
b was the homemade PCC. The results are quite similar and in agreement with previous reported results on chlorochromate derivatives [
24,
25]. The only observed differences are due to the relative height of the observed peaks. Thus, peaks relative to (001), (110), (020), (111), and (22-1) are more intense in the case of homemade PCC, while (201) peak in the commercial PCC is higher than in homemade reagent. The most relevant difference in the XRD spectra is in the relative intensities of the peaks (130), (22-1), and (112). However, those differences do not allow us to find relevant differences in the structure of the reagents.
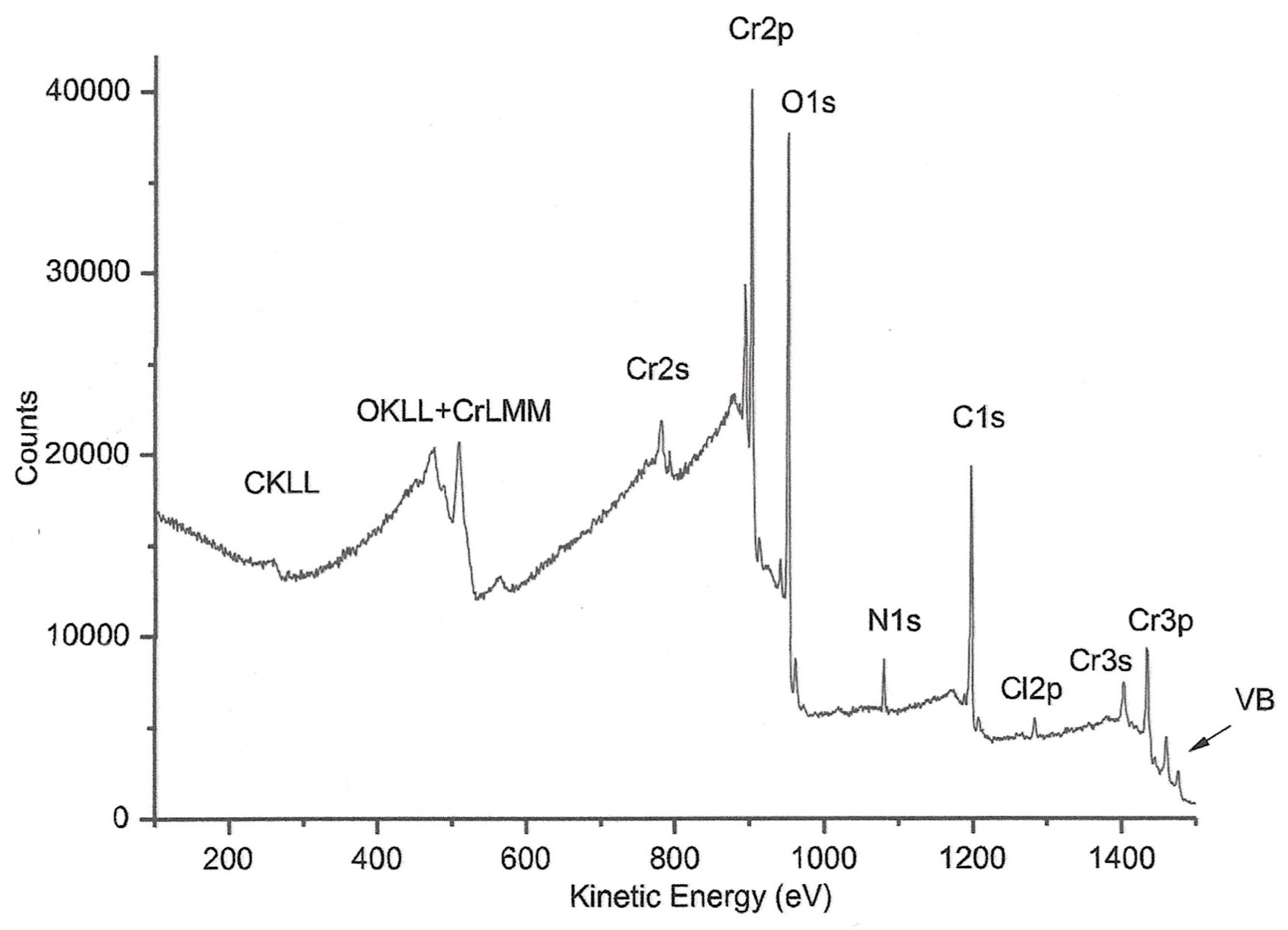
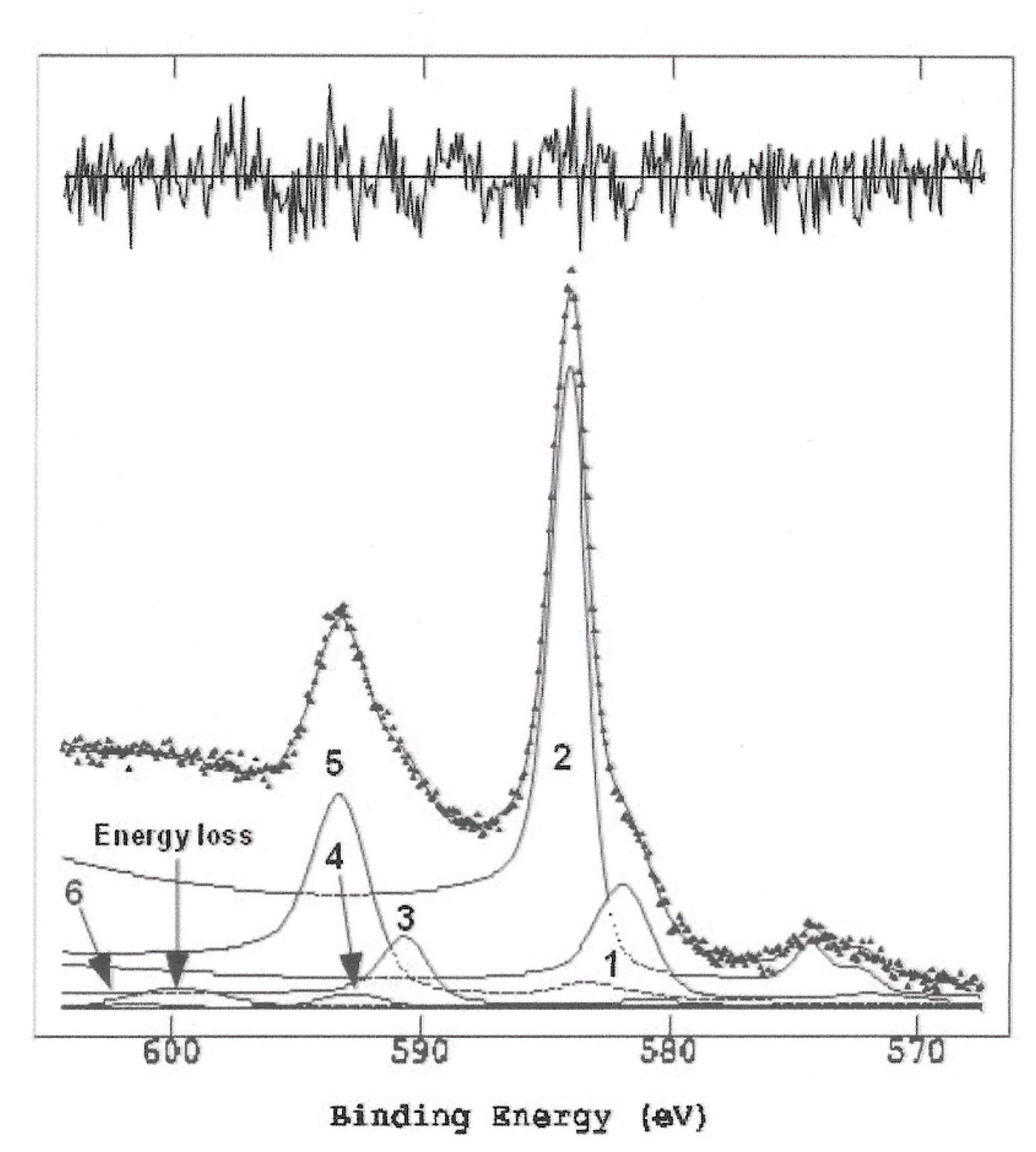
In order to have data on the possible origin of the observed color difference in homemade PCC, we performed XPS analysis of this reagent. The wide spectrum is reported in
Figure 3. The wide spectrum shows the presence of photoelectronic signals of Cr2p, C1s, O1s, N1s, Cl2p, Cr3s, Cr3p and of Auger signals C KLL, O KLL and Cr LMM. VB denotes the valence band composed of the following secondary signals: O2s, Cl3s, N2p, C2p O2p, Cl3p, Cr3d.
All the detailed regions of interest have been resolved to the component peaks. The positions of the peaks in Binding Energy, BE (eV) were corrected using the C1s peak as an internal reference of the aromatic carbon (present in the structure of the compound under examination, placed at 284.8 eV [
20]. The peak areas were normalized using sensitivity factors [
20] appropriate for the spectrometer in use.
The detailed 2p region of Chromium, resolved into the component peaks, is shown in
Figure 4 and the curve-fitting results are shown in the
Table 1. The assignments, based on the comparison of the corrected BEs with literature data [26-28] are compatible with two oxidation states present, Cr(VI) and Cr(III). In fact, the BE values resulting from the curve-fitting, relative to the main peak 2p
3/2 of Cr 2p doublet, are within the range of tabulated values for the following oxidation states: a) peak 1 at BE = 577.3 eV falls within the range of BE attributable to Cr (III); b) peak 2 at BE = 579.6 eV falls within the range of BE attributable to Cr(VI); c) metallic Cr would instead drop to a BE ~ 574 eV and is not present; d) the Cr2p region has been fitted with 6 peaks, specified in
Table 1.
The fitting was obtained taking into account that the Cr(III) satellites are ~11 eV apart for both Cr2p3/2 and Cr2p1/2 and the area ratio Sat/Cr2p is ~0.14 for both the 3/2 and the ½ [28-30]. In conclusion the Cr sample contained ~70% Cr(VI) and ~30% Cr(III).
To explain this result we have to consider how we modified the experimental procedure to isolate pyridinium chlorochromate. Because we had some difficulties to eliminate the aqueous solution from the reaction under reduced pressure, we attempted to dry the reagent adsorbing water wrapping the product in a sheet of absorbent paper. Unfortunately, we did not consider the possible oxidation of cellulose due to the reagent. The presence of green Cr(III) induced the observed change in the color of the reagent.
Anyway, it is not clear why the presence of Cr(III) induces a reactivity of the reagent towards the furan ring. It is known that Cr(III) compounds and complexes can act as catalysts in oxidation reactions [29-33].
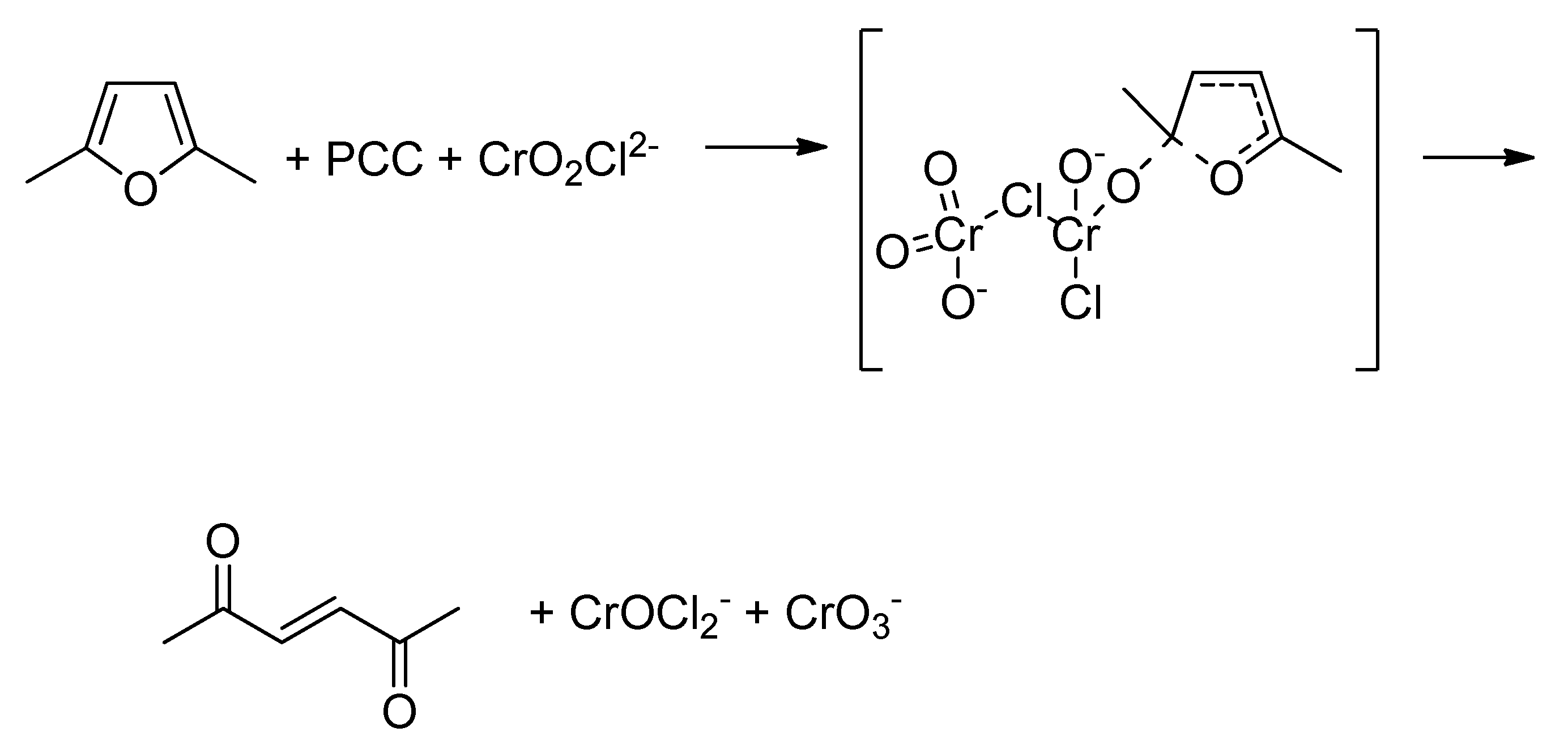
The possible catalytic effect of Cr(VI) in the presence of Cr(III) was tested performing DFT calculations. We studied this scenario: the furan ring (2,5-dimethylfuran) was oxidized by CrOCl, a Cr(III) species, while it is oxidized by PCC.
Scheme 3 depictes the reaction scheme we studied. The calulations were performed at DFT/B3LYP/6-311G+(d,p) level of theory.
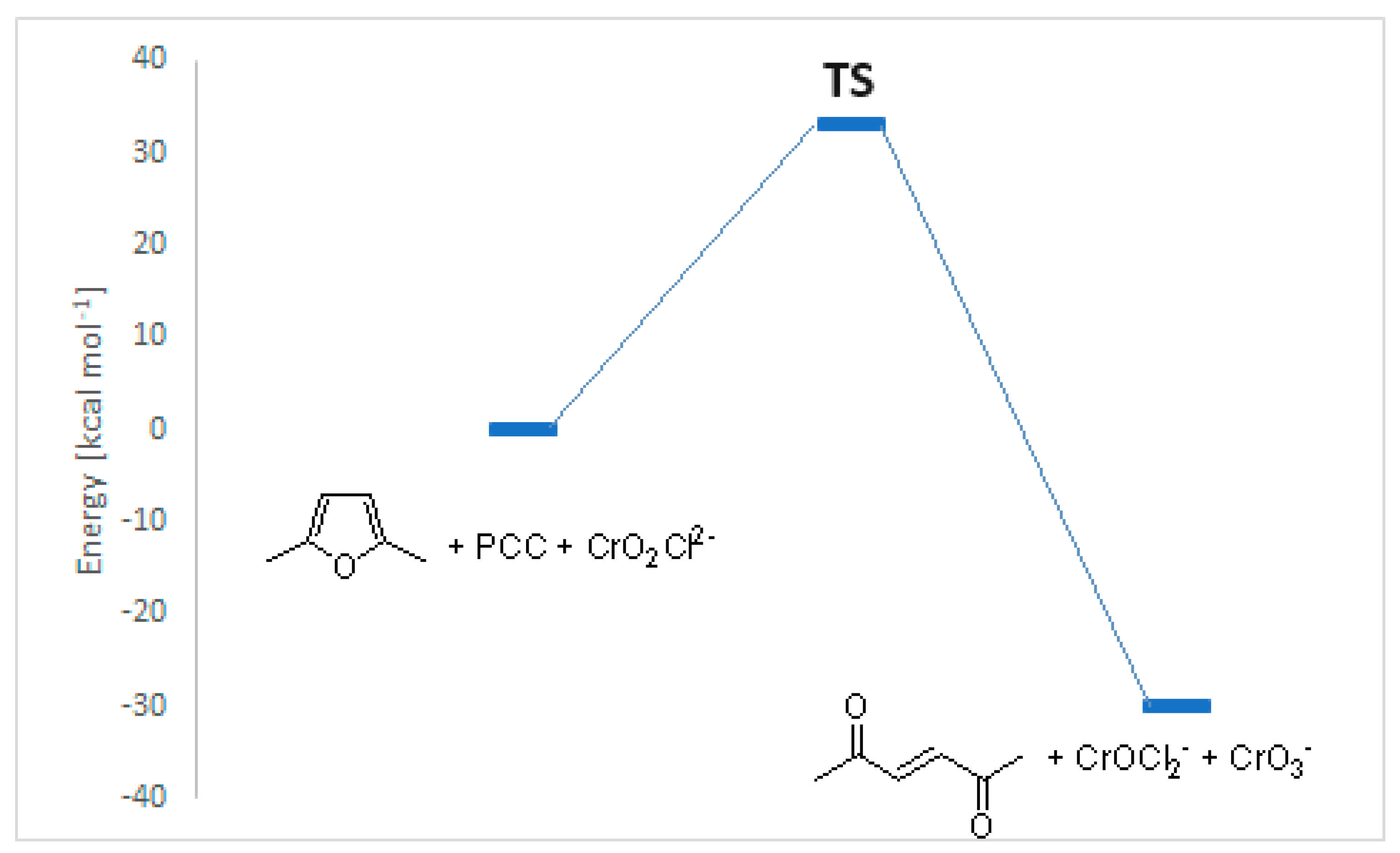
The results of the calculations are reported in
Figure 5.
Figure 5 showed that the described transformation is an exothermic reaction, with a transition state of 33 kcal mol
-1 in agreement with the esperimental condition (reflux in CH
2Cl
2). The transition state is depicted in
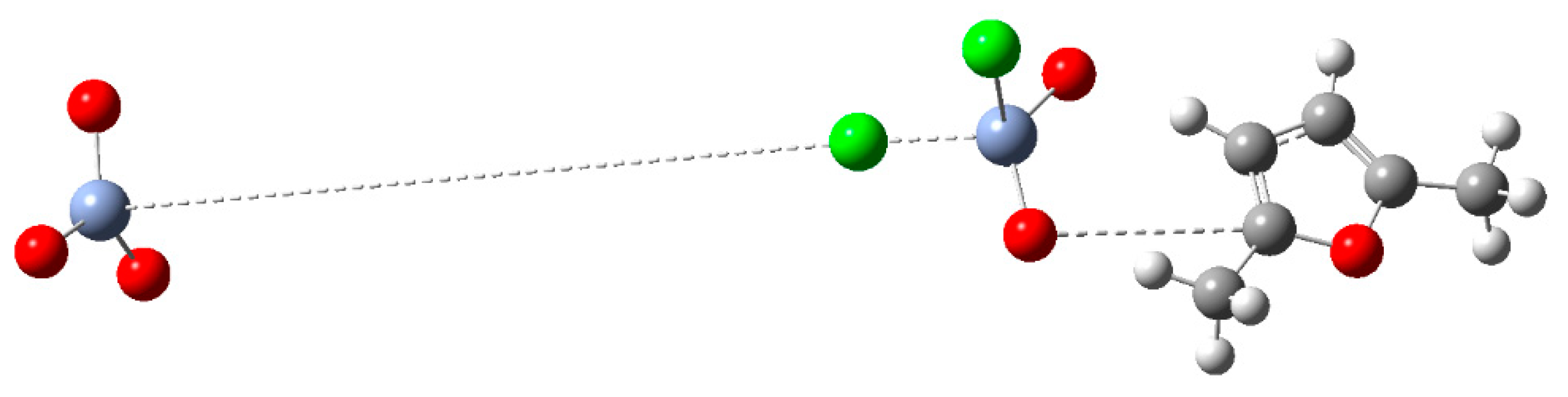
Figure 6.
In the transition state depicted in
Figure 6, while the oxygen atom of CrO
2Cl
- is forming a bond with the carbon atom of the furan ring, a bond with chlorine atom deriving from PCC is forming. The bond between Cr of PCC and chlorine atom is broken.
4. Conclusions
In this article we tried to give an explanation to an anomalous behavior found in pyridinium chlorochromate. When we prepared it many years ago, we were able to describe a furan ring opening reaction that has been the basis of considerable research activity. However, when we tried to replicate the reaction using the commercial reagent no reaction occurred. Based on the data collected in this work, we think that the reason comes from the fact that in the reagent prepared by us, due to an error in the drying process, there is a certain quantity of Cr(III), which can act as a catalyst of the reaction when it is reoxidized by pyridinium chlorochromate through a migration of a chlorine atom.
Author Contributions
“Conceptualization, M.D. investigation, F.L.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Piancatelli, G. : Scettri, A.; D'Auria M. Oxidative Ring Opening of Furan Derivatives to α,β-Unsaturated γ-Dicarbonyl Compounds, Useful Intermediates for 3-Oxocyclopentenes Synthesis. Tetrahedron 1980, 36, 661–663. [Google Scholar] [CrossRef]
- Piancatelli, G.; Scettri, A.; D'Auria, M. Pyridinium Chlorochromate: a Versatile Oxidant in Organic Synthesis. Synthesis 1982, 245.-258. [CrossRef]
- Piancatelli, G.; D'Auria, M.; D'Onofrio, F. Synthesis of 1,4-Dicarbonyl Compounds and Cyclopentenones from Furans. Synthesis 1994, 867–889. [Google Scholar] [CrossRef]
- D'Auria, M.; Piancatelli, G.; Scettri, A. A Mild and Selective Reduction of Enedicarbonyl Compounds. Synthesis 1980, 245–247. [Google Scholar] [CrossRef]
- Piancatelli, G.; D’Auria, M.; Scettri, A. Process for the synthesis of 3-keto-cyclopentene-5-oxy derivatives having insecticide activity, US Pat. 441 3145, 1981. [Google Scholar]
- D'Ascoli, R.; D'Auria, M.; De Mico, A.; Piancatelli, G.; Scettri, A. A Rapid and Efficient Route to 4- and 5-Amino-3-oxocyclopentene Derivatives. J. Org. Chem. 1980, 45, 4500–4502. [Google Scholar] [CrossRef]
- Antonioletti, R.; D'Auria, M.; Piancatelli, G.; Scettri, A. Michael Addition to trans-Enedicarbonyl Compounds: a Facile Route to 2,3,4,5-Tetra-substituted Furans. J. Chem. Soc. Perkin Trans. I 1981, 2398–2400. [Google Scholar] [CrossRef]
- D'Auria, M.; De Mico, A.; Piancatelli, G.; Scettri, A. A Facile Route to 5-Alkyl-2(3H)-furanones by Photoisomerisation of Enedicarbonyl Compounds. Tetrahedron 1982, 38, 1661–1666. [Google Scholar] [CrossRef]
- Antonioletti, R.; D'Auria, M.; Piancatelli, G.; Scettri, A. Photochemical Synthesis of 2,5-Dialkoxy- and 2,5-Diacetoxy-dihydrofurans from trans-Enedicarbonyl Compounds. Tetrahedron Lett. 1982, 2981–2984. [Google Scholar] [CrossRef]
- Corey, E. J.; Suggs, J. W. Pyridinium chlorochromate. An efficient reagent for oxidation of primary and secondary alcohols to carbonyl compounds. Tetrahedron Lett. 1975, 2647–2650. [Google Scholar] [CrossRef]
- Piancatelli, G.; Scettri, A.; D'Auria, M. The Oxidation of Furan Derivatives with Pyridinium Chlorochromate: a Novel Synthesis of 6-Hydroxy-2H-pyran-3(6H)-ones. Tetrahedron Lett. 1977, 2199–2200. [Google Scholar] [CrossRef]
- Piancatelli, G.; Scettri, A.; D'Auria, M. Pyridinium Chlorochromate in the Organic Synthesis: a Convenient Oxidation of Enol-ethers to Esters and Lactones. Tetrahedron Lett. 1977, 3483–3484. [Google Scholar] [CrossRef]
- Piancatelli, G.; Scettri, A.; D'Auria, M. Pyridinium Chlorochromate in the Organic Synthesis: a Convenient Oxidation of 5-Bromo-2-furan-derivatives into γ-Hydroxy-butenolides. Tetrahedron Lett. 1979, 1507–1508. [Google Scholar] [CrossRef]
- D'Auria, M.; D'Onofrio, F.; Piancatelli, G.; Scettri, A. Studies on Reactivity of Pyridinium Chlorochromate-Iodine System: an Efficient Method for Converting Enolsilyl Ethers into α-Iodo Ketones. Synth. Commun. 1982, 1127–1138. [Google Scholar] [CrossRef]
- Antonioletti, R.; D'Auria, M.; De Mico, A.; Piancatelli, G.; Scettri, A. The Oxidative C-C Cleavage of Phenyloxiranes by Pyridinium Chlorochromate. Synthesis 1983, 890–891. [Google Scholar] [CrossRef]
- Antonioletti, R.; D'Auria, M.; De Mico, A.; Piancatelli, G.; Scettri, A. Pyridinium Chlorochromate in Organic Synthesis. A Convenient Preparation of 4-Oxo-2-alkenethioic S-Esters. Synthesis 1984, 280-281. [CrossRef]
- Bonadies, F.; Di Fabio, R.; Bonini, C. Use of pyridinium chlorochromate as methylene oxidant in 5,6-dihydropyrans: a practical one-step preparation of the anhydromevalonolactone. J. Org. Chem. 1984, 49, 1647–1649. [Google Scholar] [CrossRef]
- Bonadies, F.; Bonini, C. Oxidation of Active Methylene Compounds by Pyridinium Chlorochromate. Synth. Commun. 1988, 18, 1573–1580. [Google Scholar] [CrossRef]
- Castle, J. E.; Chapman-Kpodo, H.; Proctor, A.; Salvi A., M. Curve-fitting in XPS using extrinsic and intrinsic background structure. J. Electron Spectrosc. Relat. Phenom. 1999, 106, 65–80. [Google Scholar] [CrossRef]
- Castle, J. E.; Salvi, A. M. Chemical state information from the near-peak region of the X-ray photoelectron background. J. Electron Spectrosc. Relat. Phenom. 2001, 114–116, 1103–1113. [CrossRef]
- Gaussian 09, Revision A.1, M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, B. Mennucci, G. A. Petersson, H. Nakatsuji, M. Caricato, X. Li, H. P. Hratchian, A. F. Izmaylov, J. Bloino, G. Zheng, J. L. Sonnenberg, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, J. A. Montgomery, Jr., J. E. Peralta, F. Ogliaro, M. Bearpark, J. J. Heyd, E. Brothers, K. N. Kudin, V. N. Staroverov, R. Kobayashi, J. Normand, K. Raghavachari, A. Rendell, J. C. Burant, S. S. Iyengar, J. Tomasi, M. Cossi, N. Rega, J. M. Millam, M. Klene, J. E. Knox, J. B. Cross, V. Bakken, C. Adamo, J. Jaramillo, R. Gomperts, R. E. Stratmann, O. Yazyev, A. J. Austin, R. Cammi, C. Pomelli, J. W. Ochterski, R. L. Martin, K. Morokuma, V. G. Zakrzewski, G. A. Voth, P. Salvador, J. J. Dannenberg, S. Dapprich, A. D. Daniels, O. Farkas, J. B. Foresman, J. V. Ortiz, J. Cioslowski, and D. J. Fox, Gaussian, Inc., Wallingford CT, 2009.
- Parr, R. G.; Yang, W. Density Functional Theory of Atoms and Molecules, Oxford University Press: Oxford, UK, 1989.
- Becke, A. D. Molecular excitation energies to high-lying bound states from time-dependent density-functional response theory: Characterization and correction of the time-dependent local density approximation ionization threshold. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef]
- Małuszyńka, H.; Czarnecki, P.; Czarnecka, A.; Pajak, Z. Structures and phase transitions in a new ferro-electric – pyridinium chlorochromate – studied by X-ray diffraction, DSC and dielectric methods. Acta Cryst. B 2012, B68, 128–136. [Google Scholar] [CrossRef]
- Suvitha, A.; Sathyanarayanamoorthi, V.; Murugakoothan, P. Growth, spectroscopy properties and DFT based PCM calculations of guanidinium chlorochromate. Spectrochim. Acta A 2013, 110, 255–261. [Google Scholar] [CrossRef]
- Desimoni, E.; Malitesta, C.; Zambonin, G.; Rivière, J. C. An x-ray photoelectron spectroscopic study of some chromium–oxygen systems. Surf. Interface Anal. 1988, 13, 173–179. [Google Scholar] [CrossRef]
- Salvi, A. M.; Castle, J. E.; Watts, J. F.; Desimoni, E. Peak fitting of the chromium 2p XPS spectrum. Appl. Surface Sci. 1995, 90, 333–341. [Google Scholar] [CrossRef]
- Aronniemi, M.; Sainio, J.; Lahtinen, J. Chemical state quantification of iron and chromium oxides using XPS: the effect of the background subtraction method. Surface Sci. 2005, 578, 108–123. [Google Scholar] [CrossRef]
- Wessjohann, L. A.; Scheid, G. Recent advances in Chromium(II)- and Chromium(III)-madiated organic synthesis. Synthesis 1999, 1–36. [Google Scholar] [CrossRef]
- Bousquet, C.; Gilheany, D. G. Chromium catalysed asymmetric alkene epoxidation. greater selectivity for an E-alkene versus its Z-isomer. Tetrahedron Lett. 1995, 36, 7739–7742. [Google Scholar] [CrossRef]
- Imanishi, H.; Katsuki, T. Unusual solvent-effect in stereochemistry of asymmetric epoxidation using a (salen)chromium(III) complex as a catalyst. Tetrahedron Lett. 1997, 38, 251–254. [Google Scholar] [CrossRef]
- Chatterjee, D.; Basak, S.; Muzart, J. Asymmetric epoxidation of alkenes with aqueous t-BuOOH catalyzed by novel chiral complexes of chromium(III) containing tridentate Schiff-base ligands. J. Mol. Catal. A: Chem. 2007, 271, 270–276. [Google Scholar] [CrossRef]
- Ikeda, H.; Nishi, K.; Tsurugi, H.; Mashima, K. Chromium-catalyzed cyclopropanation of alkenes with bromoform in the presence of 2,3,5,6-tetramethyl-1,4-bis(trimethylsilyl)-1,4-dihydropyrazine. Chem. Sci. 2020, 11, 3604–3609. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).