Submitted:
07 October 2023
Posted:
10 October 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
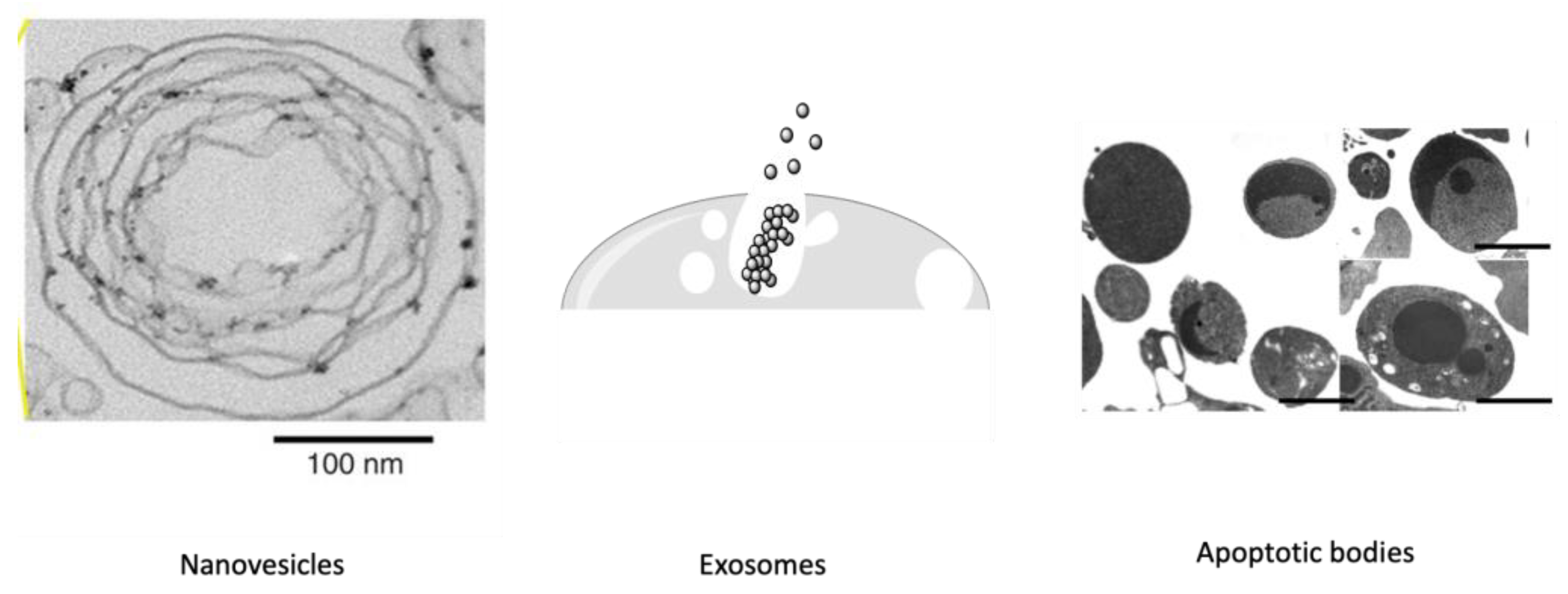
1.1. Exosome Biogenesis.
1.1. Microvesicles and Their Biogenesis.
2. Stem Cell-Based EVs
2.1. Stem Cell-Based EVs in Peripheral Nerve Regeneration
2.1. Stem Cell-Based EVs in Central Nervous System Regeneration
3. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Akers, J.C., D. Gonda, R. Kim, B.S. Carter, and C.C. Chen, Biogenesis of extracellular vesicles (EV): exosomes, microvesicles, retrovirus-like vesicles, and apoptotic bodies. Journal of neuro-oncology 2013, 113, 1–11. [CrossRef]
- Yáñez-Mó, M. P. R.-M. Siljander, Z. Andreu, A. Bedina Zavec, F.E. Borràs, E.I. Buzas, K. Buzas, E. Casal, F. Cappello, and J. Carvalho, Biological properties of extracellular vesicles and their physiological functions. Journal of extracellular vesicles 2015, 4, 27066. [Google Scholar]
- Regev-Rudzki, N., D. W. Wilson, T.G. Carvalho, X. Sisquella, B.M. Coleman, M. Rug, D. Bursac, F. Angrisano, M. Gee, and A.F. Hill, Cell-cell communication between malaria-infected red blood cells via exosome-like vesicles. Cell 2013, 153, 1120–1133. [Google Scholar] [CrossRef]
- Waters, C.M. and B.L. Bassler, Quorum sensing: cell-to-cell communication in bacteria. Annu. Rev. Cell Dev. Biol. 2005, 21, 319–346. [Google Scholar] [CrossRef]
- Wolf, P. The nature and significance of platelet products in human plasma. British journal of haematology 1967, 13, 269–288. [Google Scholar] [CrossRef] [PubMed]
- Desrochers, L.M., M. A. Antonyak, and R.A. Cerione, Extracellular vesicles: satellites of information transfer in cancer and stem cell biology. Developmental cell 2016, 37, 301–309. [Google Scholar] [CrossRef]
- Ma, P., Y. Pan, W. Li, C. Sun, J. Liu, T. Xu, and Y. Shu, Extracellular vesicles-mediated noncoding RNAs transfer in cancer. Journal of hematology & oncology 2017, 10, 1–11. [Google Scholar]
- Kim, S.-M. and H.-S. Kim, Engineering of extracellular vesicles as drug delivery vehicles. Stem cell investigation 2017, 4. [Google Scholar]
- AYGAN, B., M. KAYA, E. CANSEVER MUTLU, and İ. KÜÇÜK, THE ROLE OF EXOSOMES IN DISEASES AND THEIR USE FOR DIAGNOSIS AND THERAPEUTIC PURPOSE. Beykent Üniversitesi Fen ve Mühendislik Bilimleri Dergisi 2021, 14.
- Thomaidou, A.C. M. Goulielmaki, A. Tsintarakis, P. Zoumpourlis, M. Toya, I. Christodoulou, and V. Zoumpourlis, miRNA-Guided Regulation of Mesenchymal Stem Cells Derived from the Umbilical Cord: Paving the Way for Stem-Cell Based Regeneration and Therapy. International Journal of Molecular Sciences 2023, 24, 9189. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q., X. Zhuang, J. Mu, Z.-B. Deng, H. Jiang, L. Zhang, X. Xiang, B. Wang, J. Yan, and D. Miller, Corrigendum: Delivery of therapeutic agents by nanoparticles made of grapefruit-derived lipids. Nature Communications 2016, 7.
- Harding, C.V., J.E. Heuser, and P.D. Stahl, Exosomes: looking back three decades and into the future. 2013.
- D'Souza-Schorey, C. and J.W. Clancy, Tumor-derived microvesicles: shedding light on novel microenvironment modulators and prospective cancer biomarkers. Genes & development 2012, 26, 1287–1299. [Google Scholar]
- Battistelli, M. and E. Falcieri, Apoptotic bodies: particular extracellular vesicles involved in intercellular communication. Biology 2020, 9, 21. [Google Scholar] [CrossRef]
- Di Vizio, D., J. Kim, M.H. Hager, M. Morello, W. Yang, C.J. Lafargue, L.D. True, M.A. Rubin, R.M. Adam, and R. Beroukhim, Oncosome formation in prostate cancer: association with a region of frequent chromosomal deletion in metastatic disease. Cancer research 2009, 69, 5601–5609. [CrossRef]
- Levental, K.R. and I. Levental, Giant plasma membrane vesicles: models for understanding membrane organization. Current topics in membranes 2015, 75, 25–57. [Google Scholar]
- Ingato, D., J.U. Lee, S.J. Sim, and Y.J. Kwon, Good things come in small packages: Overcoming challenges to harness extracellular vesicles for therapeutic delivery. Journal of Controlled Release 2016, 241, 174–185. [CrossRef]
- Mutlu, E.C., Ö. Kaya, M. Wood, I. Mager, K.Ç. Topkara, Ç. Çamsarı, A. Birinci Yildirim, A. Çetinkaya, D. Acarel, and J. Odabaşı Bağcı, Efficient doxorubicin loading to isolated dexosomes of immature JAWSII cells: Formulated and characterized as the bionanomaterial. Materials 2020, 13, 3344.
- Raposo, G. and W. Stoorvogel, Extracellular vesicles: exosomes, microvesicles, and friends. Journal of Cell Biology 2013, 200, 373–383. [Google Scholar] [CrossRef]
- Tkach, M. and C. Théry, Communication by extracellular vesicles: where we are and where we need to go. Cell 2016, 164, 1226–1232. [Google Scholar] [CrossRef]
- Mathivanan, S., C.J. Fahner, G.E. Reid, and R.J. Simpson, ExoCarta 2012: database of exosomal proteins, RNA and lipids. Nucleic acids research 2012, 40, D1241–D1244. [CrossRef]
- Kim, D.-K., B. Kang, O.Y. Kim, D.-s. Choi, J. Lee, S.R. Kim, G. Go, Y.J. Yoon, J.H. Kim, and S.C. Jang, EVpedia: an integrated database of high-throughput data for systemic analyses of extracellular vesicles. Journal of extracellular vesicles 2013, 2, 20384. [CrossRef]
- Keller, S., M.P. Sanderson, A. Stoeck, and P. Altevogt, Exosomes: from biogenesis and secretion to biological function. Immunology letters 2006, 107, 102–108. [CrossRef] [PubMed]
- Stuffers, S., C. Sem Wegner, H. Stenmark, and A. Brech, Multivesicular endosome biogenesis in the absence of ESCRTs. Traffic 2009, 10, 925–937. [CrossRef] [PubMed]
- Henne, W.M., N.J. Buchkovich, and S.D. Emr, The ESCRT pathway. Developmental cell 2011, 21, 77–91.
- Maas, S.L., X.O. Breakefield, and A.M. Weaver, Extracellular vesicles: unique intercellular delivery vehicles. Trends in cell biology 2017, 27, 172–188. [CrossRef] [PubMed]
- Ailawadi, S., X. Wang, H. Gu, and G.-C. Fan, Pathologic function and therapeutic potential of exosomes in cardiovascular disease. Biochimica et Biophysica Acta (BBA) - Molecular Basis of Disease 2015, 1852, 1–11. [CrossRef] [PubMed]
- Barile, L., T. Moccetti, E. Marbán, and G. Vassalli, Roles of exosomes in cardioprotection. European heart journal 2017, 38, 1372–1379.
- Schorey, J.S., Y. Cheng, P.P. Singh, and V.L. Smith, Exosomes and other extracellular vesicles in host–pathogen interactions. EMBO reports 2015, 16, 24–43. [CrossRef]
- Villarroya-Beltri, C., F. Baixauli, C. Gutiérrez-Vázquez, F. Sánchez-Madrid, and M. Mittelbrunn. Sorting it out: regulation of exosome loading. in Seminars in cancer biology. 2014. Elsevier.
- György, B., T.G. Szabó, M. Pásztói, Z. Pál, P. Misják, B. Aradi, V. László, E. Pállinger, E. Pap, and A. Kittel, Membrane vesicles, current state-of-the-art: emerging role of extracellular vesicles. Cellular and molecular life sciences 2011, 68, 2667–2688. [CrossRef]
- Bebawy, M., A. Roseblade, F. Luk, T. Rawling, A. Ung, and G.E. Grau, Cell-derived microparticles: new targets in the therapeutic management of disease. Journal of Pharmacy & Pharmaceutical Sciences 2013, 16, 238–253.
- Piccin, A., W.G. Murphy, and O.P. Smith, Circulating microparticles: pathophysiology and clinical implications. Blood reviews 2007, 21, 157–171. [CrossRef]
- Al-Nedawi, K., B. Meehan, J. Micallef, V. Lhotak, L. May, A. Guha, and J. Rak, Intercellular transfer of the oncogenic receptor EGFRvIII by microvesicles derived from tumour cells. Nature cell biology 2008, 10, 619–624. [CrossRef] [PubMed]
- Sedgwick, A.E., J.W. Clancy, M. Olivia Balmert, and C. D’Souza-Schorey, Extracellular microvesicles and invadopodia mediate non-overlapping modes of tumor cell invasion. Scientific reports 2015, 5, 1–14.
- Blanton, H., J. Jaccard, J. Klick, B. Mellers, G. Mitchell, and P.E. Tetlock, Transparency should trump trust: Rejoinder to McConnell and Leibold (2009) and Ziegert and Hanges (2009). 2009.
- McConnell, R.E., J.N. Higginbotham, D.A. Shifrin Jr, D.L. Tabb, R.J. Coffey, and M.J. Tyska, The enterocyte microvillus is a vesicle-generating organelle. Journal of Cell Biology 2009, 185, 1285–1298.
- Cocucci, E. and J. Meldolesi, Ectosomes and exosomes: shedding the confusion between extracellular vesicles. Trends in cell biology 2015, 25, 364–372. [Google Scholar] [CrossRef]
- Till, J. and E. McCulloch, A direct measurement of the radiation sensitivity of normal mouse bone marrow cells. Radiation research 2011, 175, 145–149. [Google Scholar] [CrossRef]
- Till, J. and E. McCulloch, A direct measurement of the radiation sensitivity of normal bone marrow cells. Radiat Res 1961, 14, 1419–1430. [Google Scholar] [CrossRef]
- Allahverdiyev, A., Somatik ve Kök Hücre Kültür Sistemlerinin Temel İlkeleri. İstanbul: Nobel Tıp Kitabevi 2018, 1.
- Schofield, R. The relationship between the spleen colony-forming cell and the haemopoietic stem cell. Blood cells 1978, 4, 7–25. [Google Scholar] [PubMed]
- Can, A., Kök Hücre: biyolojisi, türleri ve tedavide kullanımları. Akademisyen Tıp Kitabevleri, Ankara 2014.
- Nawaz, M., F. Fatima, K.C. Vallabhaneni, P. Penfornis, H. Valadi, K. Ekström, S. Kholia, J.D. Whitt, J.D. Fernandes, and R. Pochampally, Extracellular vesicles: evolving factors in stem cell biology. Stem cells international 2016, 2016.
- Takahashi, K., K. Tanabe, M. Ohnuki, M. Narita, T. Ichisaka, K. Tomoda, and S. Yamanaka, Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 2007, 131, 861–872. [CrossRef]
- Alvarez, C.V., M. Garcia-Lavandeira, M. Garcia-Rendueles, E. Diaz-Rodriguez, A.R. Garcia-Rendueles, S. Perez-Romero, T.V. Vila, J.S. Rodrigues, P.V. Lear, and S.B. Bravo, Defining stem cell types: understanding the therapeutic potential of ESCs, ASCs, and iPS cells. Journal of molecular endocrinology 2012, 49, R89–R111.
- Golchin, A. and T.Z. Farahany, Biological products: cellular therapy and FDA approved products. Stem cell reviews and reports 2019, 15, 166–175. [Google Scholar] [CrossRef]
- Wang, Z.-g., Z.-y. He, S. Liang, Q. Yang, P. Cheng, and A.-m. Chen, Comprehensive proteomic analysis of exosomes derived from human bone marrow, adipose tissue, and umbilical cord mesenchymal stem cells. Stem Cell Research & Therapy 2020, 11, 1–11.
- Chen, W., L. Lv, N. Chen, and E. Cui, Immunogenicity of mesenchymal stromal/stem cells. Scandinavian Journal of Immunology 2023, 97, e13267. [CrossRef]
- Yi T, Song SU. Immunomodulatory properties of mesenchymal stem cells and their therapeutic applications. Arch Pharm Res. 2012, 35, 213–221. [CrossRef]
- Tianyi Wu, Yang Liu, Bin Wang and Gang Li, The roles of mesenchymal stem cells in tissue repair and disease modification. Current Stem Cell Research and Therapy 2014, 9, 424–431. [CrossRef]
- Karnas, E., P. Dudek, and E.K. Zuba-Surma, Stem cell-derived extracellular vesicles as new tools in regenerative medicine-Immunomodulatory role and future perspectives. Frontiers in Immunology 2023, 14, 1120175. [CrossRef]
- Squillaro, T., G. Peluso, and U. Galderisi, Clinical trials with mesenchymal stem cells: an update. Cell transplantation 2016, 25, 829–848. [CrossRef]
- Khalyfa, A. and D. Gozal, Exosomal miRNAs as potential biomarkers of cardiovascular risk in children. Journal of translational medicine 2014, 12, 1–12. [Google Scholar] [CrossRef]
- Li, T., Y. Yan, B. Wang, H. Qian, X. Zhang, L. Shen, M. Wang, Y. Zhou, W. Zhu, and W. Li, Exosomes derived from human umbilical cord mesenchymal stem cells alleviate liver fibrosis. Stem cells and development 2013, 22, 845–854. [CrossRef] [PubMed]
- Takahara, K., M. Ii, T. Inamoto, T. Nakagawa, N. Ibuki, Y. Yoshikawa, T. Tsujino, T. Uchimoto, K. Saito, and T. Takai, microRNA-145 mediates the inhibitory effect of adipose tissue-derived stromal cells on prostate cancer. Stem cells and development 2016, 25, 1290–1298. [CrossRef]
- Bai, L., H. Shao, H. Wang, Z. Zhang, C. Su, L. Dong, B. Yu, X. Chen, X. Li, and X. Zhang, Effects of mesenchymal stem cell-derived exosomes on experimental autoimmune uveitis. Scientific reports 2017, 7, 4323. [CrossRef]
- Potter, D.R., B.Y. Miyazawa, S.L. Gibb, X. Deng, P.P. Togaratti, R.H. Croze, A.K. Srivastava, A. Trivedi, M. Matthay, and J.B. Holcomb, Mesenchymal stem cell-derived extracellular vesicles attenuate pulmonary vascular permeability and lung injury induced by hemorrhagic shock and trauma. The journal of trauma and acute care surgery 2018, 84, 245.
- Zhang, Y., Y. Pan, Y. Liu, X. Li, L. Tang, M. Duan, J. Li, and G. Zhang, Exosomes derived from human umbilical cord blood mesenchymal stem cells stimulate regenerative wound healing via transforming growth factor-β receptor inhibition. Stem cell research & therapy 2021, 12, 1–14.
- Yuan, X., D. Li, X. Chen, C. Han, L. Xu, T. Huang, Z. Dong, and M. Zhang, Extracellular vesicles from human-induced pluripotent stem cell-derived mesenchymal stromal cells (hiPSC-MSCs) protect against renal ischemia/reperfusion injury via delivering specificity protein (SP1) and transcriptional activating of sphingosine kinase 1 and inhibiting necroptosis. Cell death & disease 2017, 8, 3200.
- Haga, H., I.K. Yan, K. Takahashi, A. Matsuda, and T. Patel, Extracellular vesicles from bone marrow-derived mesenchymal stem cells improve survival from lethal hepatic failure in mice. Stem cells translational medicine 2017, 6, 1262–1272. [CrossRef] [PubMed]
- Lee, M., T. Liu, W. Im, and M. Kim, Exosomes from adipose-derived stem cells ameliorate phenotype of Huntington's disease in vitro model. European Journal of Neuroscience 2016, 44, 2114–2119. [CrossRef] [PubMed]
- Bian, S., L. Zhang, L. Duan, X. Wang, Y. Min, and H. Yu, Extracellular vesicles derived from human bone marrow mesenchymal stem cells promote angiogenesis in a rat myocardial infarction model. Journal of molecular medicine 2014, 92, 387–397. [CrossRef]
- Xu, K., D. Ma, G. Zhang, J. Gao, Y. Su, S. Liu, Y. Liu, J. Han, M. Tian, and C. Wei, Human umbilical cord mesenchymal stem cell-derived small extracellular vesicles ameliorate collagen-induced arthritis via immunomodulatory T lymphocytes. Molecular Immunology 2021, 135, 36–44. [CrossRef] [PubMed]
- Keshtkar, S., M. Kaviani, F.S. Sarvestani, M.H. Ghahremani, M.H. Aghdaei, I.H. Al-Abdullah, and N. Azarpira, Exosomes derived from human mesenchymal stem cells preserve mouse islet survival and insulin secretion function. EXCLI journal 2020, 19, 1064.
- Yan, Y., W. Jiang, Y. Tan, S. Zou, H. Zhang, F. Mao, A. Gong, H. Qian, and W. Xu, hucMSC exosome-derived GPX1 is required for the recovery of hepatic oxidant injury. Molecular Therapy 2017, 25, 465–479. [CrossRef]
- Sheykhhasan, M., N. Kalhor, A. Sheikholeslami, M. Dolati, E. Amini, and H. Fazaeli, Exosomes of mesenchymal stem cells as a proper vehicle for transfecting miR-145 into the breast cancer cell line and its effect on metastasis. BioMed Research International 2021, 2021.
- Guo, Q., J. Yan, T. Song, C. Zhong, J. Kuang, Y. Mo, J. Tan, D. Li, Z. Sui, and K. Cai, microRNA-130b-3p contained in MSC-derived EVs promotes lung cancer progression by regulating the FOXO3/NFE2L2/TXNRD1 axis. Molecular Therapy-Oncolytics 2021, 20, 132–146. [CrossRef] [PubMed]
- Sharma, A., R. Kulkarni, H. Sane, N. Awad, A. Bopardikar, A. Joshi, S. Baweja, M. Joshi, C. Vishwanathan, and N. Gokulchandran, Phase 1 clinical trial for intravenous administration of mesenchymal stem cells derived from umbilical cord and placenta in patients with moderate COVID-19 virus pneumonia: results of stage 1 of the study. American Journal of Stem Cells 2022, 11, 37.
- Doeppner, T.R., B. Kaltwasser, M. Teli, E. Bretschneider, M. Bähr, and D.M. Hermann, Effects of acute versus post-acute systemic delivery of neural progenitor cells on neurological recovery and brain remodeling after focal cerebral ischemia in mice. Cell death & disease 2014, 5, e1386–e1386.
- Han, Y., D. Seyfried, Y. Meng, D. Yang, L. Schultz, M. Chopp, and D. Seyfried, Multipotent mesenchymal stromal cell–derived exosomes improve functional recovery after experimental intracerebral hemorrhage in the rat. Journal of neurosurgery 2018, 131, 290–300.
- Doeppner, T.R., J. Herz, A. Görgens, J. Schlechter, A.-K. Ludwig, S. Radtke, K. de Miroschedji, P.A. Horn, B. Giebel, and D.M. Hermann, Extracellular vesicles improve post-stroke neuroregeneration and prevent postischemic immunosuppression. Stem cells translational medicine 2015, 4, 1131–1143. [CrossRef]
- Han, D., C. Wu, Q. Xiong, L. Zhou, and Y. Tian, Anti-inflammatory mechanism of bone marrow mesenchymal stem cell transplantation in rat model of spinal cord injury. Cell biochemistry and biophysics 2015, 71, 1341–1347. [CrossRef]
- de Rivero Vaccari, J.P., F. Brand III, S. Adamczak, S.W. Lee, J. Perez-Barcena, M.Y. Wang, M.R. Bullock, W.D. Dietrich, and R.W. Keane, Exosome-mediated inflammasome signaling after central nervous system injury. Journal of neurochemistry 2016, 136, 39–48.
- Vilaça-Faria, H., A.J. Salgado, and F.G. Teixeira, Mesenchymal stem cells-derived exosomes: a new possible therapeutic strategy for Parkinson’s disease? Cells 2019, 8, 118. [CrossRef]
- Fan, C.-G., Q. -J. Zhang, F.-W. Tang, Z.-B. Han, G.-S. Wang, and Z.-C. Han, Human umbilical cord blood cells express neurotrophic factors. Neuroscience letters 2005, 380, 322–325. [Google Scholar] [CrossRef]
- Caplan, A.I. and J.E. Dennis, Mesenchymal stem cells as trophic mediators. Journal of cellular biochemistry 2006, 98, 1076–1084. [Google Scholar] [CrossRef]
- Zhou, Y., H. Xu, W. Xu, B. Wang, H. Wu, Y. Tao, B. Zhang, M. Wang, F. Mao, and Y. Yan, Exosomes released by human umbilical cord mesenchymal stem cells protect against cisplatin-induced renal oxidative stress and apoptosis in vivo and in vitro. Stem cell research & therapy 2013, 4, 1–13. [Google Scholar]
- De Miroschedji, K.N.M. , Human platelet lysate derived extracellular vesicles for use in medicine. 2019, Google Patents.
- Patel, N.A., L. D. Moss, J.-Y. Lee, N. Tajiri, S. Acosta, C. Hudson, S. Parag, D.R. Cooper, C.V. Borlongan, and P.C. Bickford, Long noncoding RNA MALAT1 in exosomes drives regenerative function and modulates inflammation-linked networks following traumatic brain injury. Journal of neuroinflammation 2018, 15, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Yu, B., X. -R. Li, and X.-M. Zhang, Mesenchymal stem cell-derived extracellular vesicles as a new therapeutic strategy for ocular diseases. World journal of stem cells 2020, 12, 178. [Google Scholar] [CrossRef] [PubMed]
- Mead, B., E. Cullather, N. Nakaya, Y. Niu, C. Kole, Z. Ahmed, and S. Tomarev, Viral delivery of multiple miRNAs promotes retinal ganglion cell survival and functional preservation after optic nerve crush injury. Experimental eye research 2020, 197, 108071. [Google Scholar] [CrossRef] [PubMed]
- Mead, B., X. Chamling, D.J. Zack, Z. Ahmed, and S. Tomarev, TNFα-mediated priming of mesenchymal stem cells enhances their neuroprotective effect on retinal ganglion cells. Investigative ophthalmology & visual science 2020, 61, 6–6. [Google Scholar]
- Mead, B., A. Logan, M. Berry, W. Leadbeater, and B.A. Scheven, Paracrine-mediated neuroprotection and neuritogenesis of axotomised retinal ganglion cells by human dental pulp stem cells: comparison with human bone marrow and adipose-derived mesenchymal stem cells. PloS one 2014, 9, e109305. [Google Scholar] [CrossRef] [PubMed]
- Katsuda, T., R. Tsuchiya, N. Kosaka, Y. Yoshioka, K. Takagaki, K. Oki, F. Takeshita, Y. Sakai, M. Kuroda, and T. Ochiya, Human adipose tissue-derived mesenchymal stem cells secrete functional neprilysin-bound exosomes. Scientific reports 2013, 3, 1197. [Google Scholar] [CrossRef]
- Mendes-Pinheiro, B., S. I. Anjo, B. Manadas, J.D. Da Silva, A. Marote, L.A. Behie, F.G. Teixeira, and A.J. Salgado, Bone marrow mesenchymal stem cells' secretome exerts neuroprotective effects in a Parkinson's disease rat model. Frontiers in bioengineering and biotechnology 2019, 7, 294. [Google Scholar] [CrossRef]
- Chen, H.-X., F. -C. Liang, P. Gu, B.-L. Xu, H.-J. Xu, W.-T. Wang, J.-Y. Hou, D.-X. Xie, X.-Q. Chai, and S.-J. An, Exosomes derived from mesenchymal stem cells repair a Parkinson’s disease model by inducing autophagy. Cell death & disease 2020, 11, 288. [Google Scholar]
- Ribeiro, C.A., J. S. Fraga, M. Grãos, N.M. Neves, R.L. Reis, J.M. Gimble, N. Sousa, and A.J. Salgado, The secretome of stem cells isolated from the adipose tissue and Wharton jelly acts differently on central nervous system derived cell populations. Stem cell research & therapy 2012, 3, 1–7. [Google Scholar]
- Li, Y., Y. Tang, and G.-Y. Yang, Therapeutic application of exosomes in ischaemic stroke. Stroke and Vascular Neurology 2021, 6. [Google Scholar]
| INVESTİGATED DİSEASE | EV SOURCES | Used EVs | THERAPEUTİC MECHANİSM | Ref. |
|---|---|---|---|---|
| Liver Fibrosis | Human Umbilical Cord MSC | Exosome | By inhibiting the epithelial-to-mesenchymal transition (EMT) by increasing E-cadherin-positive cells and decreasing N-cadherin- and vimentin-positive cells | Li et al, 2013[55] |
| Prostate Cancer | Human Adipose-derived stromal cell MSC | Exosome | BclxL activity decreased by miR-145 decreased proliferation and increased apoptosis | Takahara et al, 2016 [56] |
| Autoimmune Uveoretinitis | Human Umbilical Cord MSCs | Exosome | By inhibiting the migration of inflammatory cells | Bai et al, 2017 [57] |
| Acute Lung Injury | Human Bone Marrow-Derived MSCs | Exosomes and Microvesicles | Regulate immunity and reduce Pulmonary capillary permeability by Delivery of Angiopoietin-1 mRNA | Potter et al, 2018 [58] |
| Wound Healing | Human Umbilical Cord MSCSs | Exosome | Reduces scar formation and myofibroblast accumulation by transfer of microRNAs and suppression of TGF-β | Zhang et al, 2021 [59] |
| Kidney Injury | Induced Pluripotent Stem Cell iPSC-MSCs | Exosomes and Microvesicles | By transporting the specificity protein (SP1) to renal tubular epithelial cells, it increases the expression of sphingosine kinase 1 and inhibits necroptosis | Yuan et al, 2017 [60] |
| Osteoporosis | Induced Pluripotent Stem Cell iPSC-MSCs | Exosome | Protection against bone loss by activation of the PI3K/Akt signaling pathway | Haga et al, 2017 [61] |
| Huntington's Disease | Human Adipose-derived stromal cell MSCs | Exosome | Reducing mHtt aggregate level, improving abnormal apoptotic protein level by regulating PGC-1, phospho-CREB | Lee et al, 2016 [62] |
| Myocardial Infarction | Human Bone Marrow-Derived MSCs | Exosomes and Microvesicles | Protecting heart tissue from ischemic damage by promoting neoangiogenesis | Bian et al, 2014 [63] |
| Rheumatoid Arthritis | Human Umbilical Cord MSCs | Exosomes and Microvesicles | Reducing joint inflammation, synovial hyperplasia, and cartilage destruction by decreasing T cell proliferation | Xu et al, 2021 [64] |
| Diabetes-Type 1 | Human Wharton's Jelly-Derived MSCs | Exosome | It increases the insulin content and improves normoglycemia with the increase in VEGF expression | Keshthar et al, 2020 [65] |
| Diabetes-Type 2 | Human Umbilical Cord MSCs | Exosome | It restores phosphorylation (tyrosine domain) of insulin receptor substrate 1 and protein kinase B, promotes expression and membrane translocation of glucose transporter 4 in muscle, and increases glycogen storage in the liver to maintain glucose homeostasis, thereby inhibiting β-cell apoptosis. | Yan et al, 2018 [66] |
| Breast Cancer | Human Adipose-derived stromal cell MSCs | Exosome | Overexpression of miR-145 leads to the downregulation of ROCK1, which inhibits cell proliferation and suppresses metastasis. | Sheykhhasan et al, 2021 [67] |
| Lung Cancer | Human Umbilical Cord MSCs | Exosomes and Microvesicles | miR-130b-3p directly targeted FOXO3, and FOXO3 upregulated Keap1 expression to downregulate NFE2L2, thereby inhibiting TXNRD1. FOXO3 overexpression or silencing of NFE2L2 or TXNRD1 decreased lung cancer cell proliferation, invasion, and migration but increased apoptosis. | Guo et al, 2021 [68] |
| Gastric Cancer | Human Bone Marrow-Derived MSCs | Exosome | Inhibition of gastric cancer cell migration and invasion by miR-221 transfection | Kim et al, 2017 [8] |
| Covid-19 | Human Umbilical Cord MSCs | Exosome | A clinical trial of the treatment of chronic cough is still ongoing | Sharma et al, 2022 [69] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





