Submitted:
09 October 2023
Posted:
10 October 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
| Type of Tumor (WHO Grade) | 5-Year Relative Survival Rate | ||
| Age | |||
| 20–44 | 45–54 | 55–64 | |
| Anaplastic astrocytoma (III) | 58% | 29% | 15% |
| Glioblastoma (IV) | 22% | 9% | 6% |
| Anaplastic oligodendroglioma (III) | 76% | 67% | 45% |
| FDA-Approved Therapy | YearApproved | Mechanism | Application | Dosage | Common Toxicities | Overall Survival | Other Notes |
| Lomustine (CCNU) | 1976 | Nonspecific alkylating agent that causes crosslinking of DNA and RNA | Oral | 80–110 mg/m2 every 6 weeks | Hematologic toxicity (49.7%) | 11.5 months | No benefit compared to RT alone |
| Carmustine (BCNU) | 1977 | Nonspecific alkylating agent that causes crosslinking of DNA and RNA in dividing cells; also binds to and modifies glutathione reductase | IV | 150–200 mg/m2 every 6 weeks | Pulmonary toxicity (<30%), ocular toxicity (>10%) and bone marrow suppression (>10%) | 11.75 months | No benefit compared to RT alone |
| Carmustine wafer implants (BCNU wafers) | 1996 & 2003 | Nonspecific alkylating agent that causes crosslinking of DNA and RNA in dividing cells; also binds to and modifies glutathione reductase | Directly applied during surgery | 8 wafers: 61.6 mg | Wound healing complications (12%), intracranial infection (1–10%), and cerebral edema (1–10%) | 13.9 months | High complication rate (42.7%) and expensive |
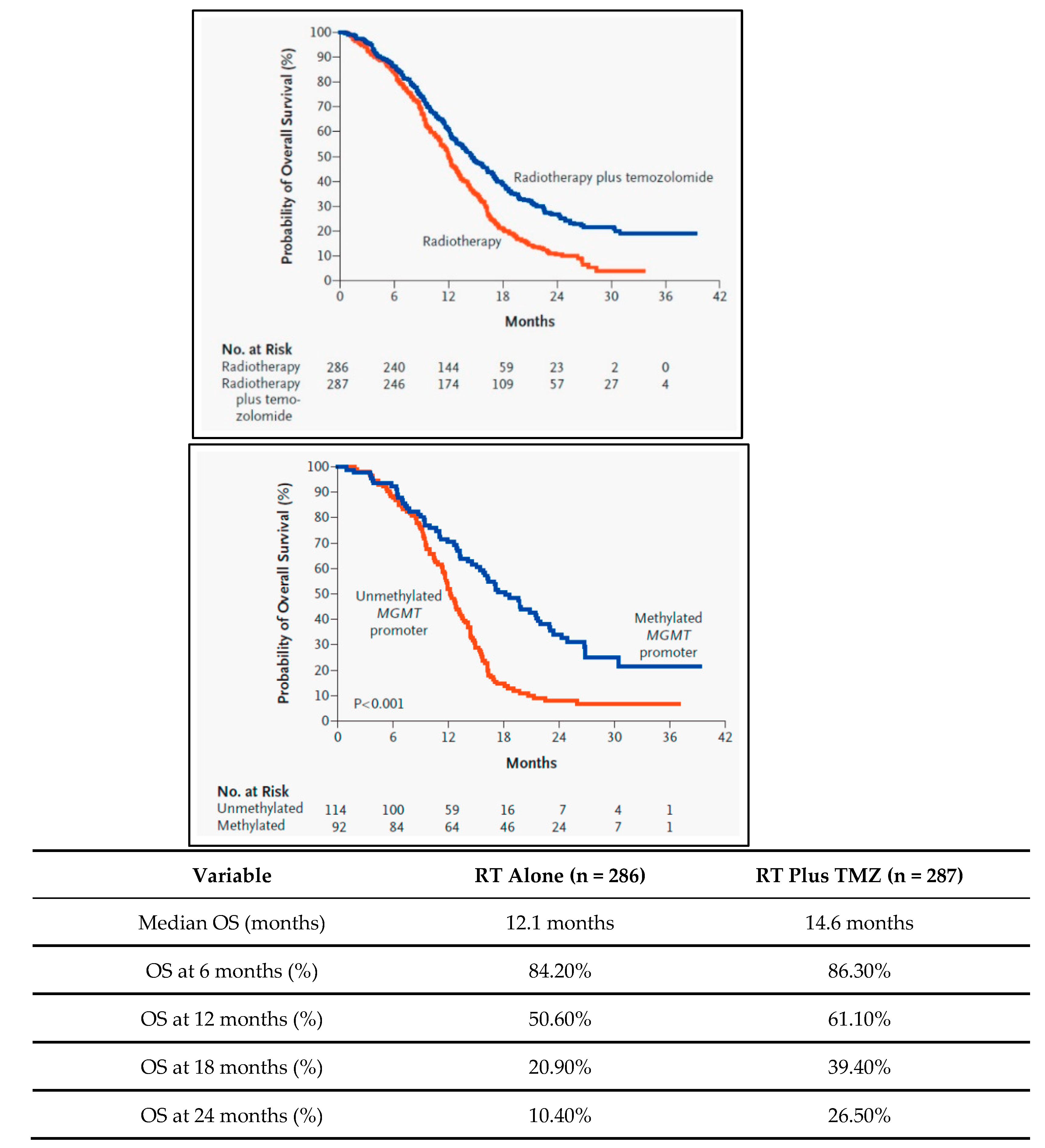
| Temozolomide (TMZ) | 2005 | Nonspecific alkylating agent that causes mismatch repair in DNA by methylation at the O6 position of guanine | Oral | 75 mg/m2 per day with RT, 150–200 mg/m2 per day | Hematologic toxicity (16%): thrombocytopenia (12%), leukopenia (7%), and neutropenia (7%) | 14.6–16.1 months | Standard of Care |
| Bevacizumab (BVZ) | 2009 | Targeted therapeutic antibody that binds and inhibits VEGF protein in tumor cells | IV | 10 mg/kg every 2 weeks | Hypertension (5.5–11.4%), thromboembolic events (3.2–11.9%), gastrointestinal perforation (1.5–5.4%), cerebral bleeding (2–5.3%), wound healing complications (0.8–3.3%), and proteinuria (2.7–11.4%) | 9.3 months (recurrent) | Used to treat symptomatic edema and radiation necrosis |
| Optune device (TTFields) | 2011 & 2015 | Low-intensity (1–3 V/cm), intermediate-frequency (200 kHz) alternating electric fields that disrupt mitosis in tumor cells | Portal device, electrodes on scalp | Greater than 18 h a day for >4 weeks | Skin toxicity (43%) and seizures (7%) | 20.5–20.9 months | Not SOC because of marginal survival benefits, expensive costs, and inconvenience for patients |
2. Transcription Factors and Epigenetic Regulation in GBM:
2.1. Examples of Transcription Factors Implicated in GBM
2.1.1. SNAI2
Role of SNAI2 in GBM:
SNAI2 and HDACs as Therapeutic Targets:
2.1.2. FOXA1: Role in Cell Differentiation and Links to GBM
2.1.3. YAP1: Implications in GBM Progression and Tumor-Suppressive Signaling
2.1.4. TWIST1: Involvement in Epithelial-to-Mesenchymal Transition (EMT)
2.1.5. ZEB1: Role in GBM Invasiveness, Link with DNA Methylation
2.1.6. NF-kB: Impact on Immune Evasion, Inflammation in GBM
2.2. Challenges in Targeting Transcription Factors
2.2.1. Structural Challenges
2.2.2. Functional Challenges
2.2.3. Contextual Challenges in GBM
2.2.4. Research Gaps
3. Emerging Therapeutic Strategies
3.1. Epigenetic Modifiers: Role in Modulating Transcription Factors, HDAC Inhibitors, etc.
3.2. Epigenetic and Transcriptional Modulation in GBM Therapy
3.2.1. Epigenetic Modifiers and Transcription Factors
3.2.2. HDAC Inhibitors
3.2.3. Challenges and Future Directions
3.3. Targeted Therapies: The Frontier of Transcription Factor Inhibition in GBM
3.3.1. Strategies for Targeting Transcription Factors
3.3.2. Challenges in Targeting Transcription Factors
3.3.3. Specific Examples in GBM
3.3.4. Future Directions
3.4. CRISPR/Cas9 Technology: Pioneering Gene Editing for Transcription Factor Targeting in GBM
3.4.1. The CRISPR/Cas9 System
3.4.2. Targeting Transcription Factors in GBM
3.4.3. Challenges and Ethical Considerations
3.4.4. Future Prospects
3.5. Challenges and Future Outlook: Bridging the Gap from Bench to Bedside
3.5.1. Clinical and Ethical Barriers
3.5.2. Future Directions
4. Personalized Immunotherapy and Neoantigen Presentation:
4.1. Neoantigens in GBM: Understanding the Basis, the Importance of Personalized Targets
4.1.1. The Basis of Neoantigens in GBM
4.1.2. Importance of Neoantigens in GBM
4.1.3. Challenges in Utilizing Neoantigens in GBM
4.1.4. Future Perspectives
4.2. Transcription Factors and Neoantigen Presentation: A Complex Interplay with Therapeutic Potential
4.2.1. Introduction: The Symbiosis of Transcription Factors and Neoantigens in GBM
4.2.2. STAT3 Pathway: A Double-Edged Sword in Immune Regulation
4.2.3. NF-κB Pathway: A Conductor in the Immune Orchestra
4.2.4. FOXP3: The Gatekeeper of Immunosuppression
4.2.5. Epigenetic Complexity: The Underlying Layers of Neoantigen Expression
4.2.6. Challenges and Future Avenues: Tackling the Complexity
4.3. Personalized Vaccine Strategies: Unraveling the Complexity Through Genetic Insights and Technological Advancements
4.3.1. Introduction: Personalized Medicine Meets Oncology
4.3.2. Decoding the Genetic Variations in GBM: A Wealth of Therapeutic Targets
4.3.3. The Genesis of Personalized Neoantigen Vaccines: From Identification to Clinical Application
4.3.4. The Roadblocks to Personalized Vaccines: Navigating the Challenges
4.3.5. The Horizon: Integrating Technologies and Therapies
4.4. Current Research and Future Directions: Latest Advancements, Ongoing Trials, and Future Expectations
4.4.1. Introduction
4.4.2. Latest Advancements
4.4.3. Ongoing Trials
4.4.4. Future Expectations
4.4.5. Conclusion
5. Conclusion
Final Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Louis DN, Perry A, Reifenberger G, et al. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: a summary. Acta Neuropathol. 2016;131(6):803-820. [CrossRef]
- Ostrom QT, Gittleman H, Truitt G, Boscia A, Kruchko C, Barnholtz-Sloan JS. CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2011-2015. Neuro Oncol. 2018;20(suppl_4):iv1-iv86. [CrossRef]
- Verhaak RG, Hoadley KA, Purdom E, et al. Integrated genomic analysis identifies clinically relevant subtypes of glioblastoma characterized by abnormalities in PDGFRA, IDH1, EGFR, and NF1. Cancer Cell. 2010;17(1):98-110. [CrossRef]
- Brennan CW, Verhaak RG, McKenna A, et al. The somatic genomic landscape of glioblastoma. Cell. 2013;155(2):462-477. [CrossRef]
- Ostrom QT, Cioffi G, Gittleman H, et al. CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2012-2016. Neuro Oncol. 2019;21(Supplement_5):v1-v100. [CrossRef]
- Stupp R, Hegi ME, Mason WP, et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10(5):459-466. [CrossRef]
- Ostrom QT, Bauchet L, Davis FG, et al. The epidemiology of glioma in adults: a "state of the science" review. Neuro Oncol. 2014;16(7):896-913. [CrossRef]
- Schwartzbaum JA, Fisher JL, Aldape KD, Wrensch M. Epidemiology and molecular pathology of glioma. Nat Clin Pract Neurol. 2006;2(9):494-503; quiz 1 p following 516. [CrossRef]
- Louis DN, Ohgaki H, Wiestler OD, et al. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007;114(2):97-109. [CrossRef]
- Weller M, van den Bent M, Tonn JC, et al. European Association for Neuro-Oncology (EANO) guideline on the diagnosis and treatment of adult astrocytic and oligodendroglial gliomas. Lancet Oncol. 2017;18(6):e315-e329. [CrossRef]
- Parsons DW, Jones S, Zhang X, et al. An integrated genomic analysis of human glioblastoma multiforme. Science. 2008;321(5897):1807-1812. [CrossRef]
- Bhat KP, Balasubramaniyan V, Vaillant B, et al. Mesenchymal differentiation mediated by NF-κB promotes radiation resistance in glioblastoma. Cancer Cell. 2013;24(3):331-346.
- Yan H, Parsons DW, Jin G, et al. IDH1 and IDH2 mutations in gliomas. N Engl J Med. 2009;360(8):765-773.
- Cancer Genome Atlas Research Network. Comprehensive genomic characterization defines human glioblastoma genes and core pathways. Nature. 2008;455(7216):1061-1068.
- Verhaak, R. G., Hoadley, K. A., Purdom, E., Wang, V., Qi, Y., Wilkerson, M. D., ... & Hayes, D. N. (2010). Integrated genomic analysis identifies clinically relevant subtypes of glioblastoma characterized by abnormalities in PDGFRA, IDH1, EGFR, and NF1. Cancer Cell, 17(1), 98-110. [CrossRef]
- Noushmehr, H., Weisenberger, D. J., Diefes, K., Phillips, H. S., Pujara, K., Berman, B. P., ... & TCGA Research Network. (2010). Identification of a CpG island methylator phenotype that defines a distinct subgroup of glioma. Cancer Cell, 17(5), 510-522. [CrossRef]
- Stupp, R., Mason, W. P., van den Bent, M. J., Weller, M., Fisher, B., Taphoorn, M. J., ... & Marosi, C. (2005). Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. New England Journal of Medicine, 352(10), 987-996. [CrossRef]
- Weller, M., van den Bent, M., Tonn, J. C., Stupp, R., Preusser, M., Cohen-Jonathan-Moyal, E., ... & Reifenberger, G. (2017). European Association for Neuro-Oncology (EANO) guideline on the diagnosis and treatment of adult astrocytic and oligodendroglial gliomas. The Lancet Oncology, 18(6), e315-e329. [CrossRef]
- Chinot, O. L., Wick, W., Mason, W., Henriksson, R., Saran, F., Nishikawa, R., ... & Cloughesy, T. (2014). Bevacizumab plus radiotherapy–temozolomide for newly diagnosed glioblastoma. The Lancet Oncology, 15(2), 200-212. [CrossRef]
- Omuro, A., & DeAngelis, L. M. (2013). Glioblastoma and other malignant gliomas: A clinical review. Neurology, 80(6), 540-554.
- Sanai, N., Polley, M. Y., McDermott, M. W., Parsa, A. T., & Berger, M. S. (2011). An extent of resection threshold for newly diagnosed glioblastomas. Neurosurgery, 68(2), 546-553. [CrossRef]
- Meyers, C. A., Smith, J. A., Bezjak, A., Mehta, M. P., Liebmann, J., Illidge, T., ... & Curran, W. (2004). Neurocognitive function and progression in patients with brain metastases treated with whole-brain radiation and motexafin gadolinium: Results of a randomized phase III trial. Journal of Clinical Oncology, 22(9), 1573-1582. [CrossRef]
- Hegi, M. E., Diserens, A. C., Gorlia, T., Hamou, M. F., de Tribolet, N., Weller, M., ... & Stupp, R. (2005). MGMT gene silencing and benefit from temozolomide in glioblastoma. New England Journal of Medicine, 352(10), 997-1003.
- Johnson, D. R., & O’Neill, B. P. (2012). Glioblastoma survival in the United States before and during the temozolomide era. Mayo Clinic Proceedings, 87(7), 673-680. [CrossRef]
- Reardon, D. A., Omuro, A., Brandes, A. A., Rieger, J., Wick, A., Sepulveda, J., ... & Sampson, J. (2017). OS10.3 Randomized Phase 3 Study Evaluating the Efficacy and Safety of Nivolumab vs Bevacizumab in Patients With Recurrent Glioblastoma: CheckMate 143. Neuro-Oncology, 19(3), iii21. [CrossRef]
- Filley, A. C., Henriquez, M., & Dey, M. (2017). Recurrent glioma clinical trial, CheckMate-143: the game is not over yet. Frontiers in Immunology, 8, 185. [CrossRef]
- Alexander, B. M., & Cloughesy, T. F. (2017). Adult Glioblastoma. Journal of Clinical Oncology, 35(21), 2402–2409.
- Hanahan, D., & Weinberg, R. A. (2011). Hallmarks of cancer: the next generation. Cell, 144(5), 646-674. [CrossRef]
- Wang, Q., Hu, B., Hu, X., Kim, H., Squatrito, M., Scarpace, L., ... & Brennan, C. (2017). Tumor Evolution of Glioma-Intrinsic Gene Expression Subtypes Associates with Immunological Changes in the Microenvironment. Cancer Cell, 32(1), 42-56.e6.
- Kaczmarek JC, Kowalski PS, Anderson DG. Advances in the delivery of RNA therapeutics: from concept to clinical reality. Genome Med. 2017;9(1):60. [CrossRef]
- Darnell JE Jr. Transcription factors as targets for cancer therapy. Nat Rev Cancer. 2002;2(10):740-749. [CrossRef]
- Meyer N, Penn LZ. Reflecting on 25 years with MYC. Nat Rev Cancer. 2008;8(12):976-990. [CrossRef]
- Yu H, Pardoll D, Jove R. STATs in cancer inflammation and immunity: a leading role for STAT3. Nat Rev Cancer. 2009;9(11):798-809. [CrossRef]
- Dang, C. V., O'Donnell, K. A., Zeller, K. I., Nguyen, T., Osthus, R. C., & Li, F. (2006). The c-Myc target gene network. Seminars in Cancer Biology, 16(4), 253-264. [CrossRef]
- Yu, H., Pardoll, D., & Jove, R. (2009). STATs in cancer inflammation and immunity: a leading role for STAT3. Nature Reviews Cancer, 9(11), 798-809. [CrossRef]
- Yang, J., & Weinberg, R. A. (2008). Epithelial-mesenchymal transition: at the crossroads of development and tumor metastasis. Developmental Cell, 14(6), 818-829. [CrossRef]
- Batlle, E., Sancho, E., Francí, C., Domínguez, D., Monfar, M., Baulida, J., & García De Herreros, A. (2000). The transcription factor Snail is a repressor of E-cadherin gene expression in epithelial tumour cells. Nature Cell Biology, 2(2), 84-89. [CrossRef]
- Friedl, P., & Alexander, S. (2011). Cancer invasion and the microenvironment: plasticity and reciprocity. Cell, 147(5), 992-1009. [CrossRef]
- Baldwin, A. S. (2001). Series introduction: the transcription factor NF-κB and human disease. Journal of Clinical Investigation, 107(1), 3-6.
- Altman, B. J., Stine, Z. E., & Dang, C. V. (2016). From Krebs to clinic: glutamine metabolism to cancer therapy. Nature Reviews Cancer, 16(10), 619-634. [CrossRef]
- Vogelstein, B., Papadopoulos, N., Velculescu, V. E., Zhou, S., Diaz, L. A., & Kinzler, K. W. (2013). Cancer genome landscapes. Science, 339(6127), 1546-1558. [CrossRef]
- Li, J., Yang, B., Zhou, Q., Wu, Y., Shang, D., Guo, Y., ... & Chen, Y. (2013). Autophagy promotes hepatocellular carcinoma cell invasion through activation of epithelial–mesenchymal transition. Carcinogenesis, 34(6), 1343-1351. [CrossRef]
- Jones PA, Baylin SB. The fundamental role of epigenetic events in cancer. Nat Rev Genet. 2002;3(6):415-428. [CrossRef]
- Esteller M. Epigenetics in cancer. N Engl J Med. 2008;358(11):1148-1159.
- Herman JG, Baylin SB. Gene silencing in cancer in association with promoter hypermethylation. N Engl J Med. 2003;349(21):2042-2054. [CrossRef]
- Feinberg AP, Vogelstein B. Hypomethylation distinguishes genes of some human cancers from their normal counterparts. Nature. 1983;301(5895):89-92. [CrossRef]
- Zhang, L., Wang, H., Zhu, J., Ding, K., & Xu, J. (2014). FGF19 (fibroblast growth factor 19) as a novel target in hepatocellular carcinoma. Biochimica et Biophysica Acta (BBA) - Reviews on Cancer, 1846(1), 152-161.
- Corcoran, R. B., Cheng, K. A., Hata, A. N., Faber, A. C., Ebi, H., Coffee, E. M., ... & Settleman, J. (2012). Synthetic lethal interaction of combined BCL-XL and MEK inhibition promotes tumor regressions in KRAS mutant cancer models. Cancer Cell, 21(1), 69-79.
- Bartel, D. P. (2009). MicroRNAs: target recognition and regulatory functions. Cell, 136(2), 215-233. [CrossRef]
- Silber, J., Lim, D. A., Petritsch, C., Persson, A. I., Maunakea, A. K., Yu, M., ... & Costello, J. F. (2012). miR-124 and miR-137 inhibit proliferation of glioblastoma multiforme cells and induce differentiation of brain tumor stem cells. Nature Communications, 3, 1126. [CrossRef]
- Ott PA, Hu Z, Keskin DB, et al. An immunogenic personal neoantigen vaccine for patients with melanoma. Nature. 2017;547(7662):217-221. [CrossRef]
- Schumacher TN, Schreiber RD. Neoantigens in cancer immunotherapy. Science. 2015;348(6230):69-74. [CrossRef]
- McGranahan N, Furness AJ, Rosenthal R, et al. Clonal neoantigens elicit T cell immunoreactivity and sensitivity to immune checkpoint blockade. Science. 2016;351(6280):1463-1469. [CrossRef]
- Schumacher, T. N., & Schreiber, R. D. (2015). Neoantigens in cancer immunotherapy. Science, 348(6230), 69-74. [CrossRef]
- Chen, D. S., & Mellman, I. (2017). Elements of cancer immunity and the cancer–immune set point. Immunity, 44(3), 221-233. [CrossRef]
- Ribas, A., & Wolchok, J. D. (2018). Cancer immunotherapy using checkpoint blockade. Science, 359(6371), 1350-1355. [CrossRef]
- Lambert SA, Jolma A, Campitelli LF, et al. The Human Transcription Factors. Cell. 2018;172(4):650-665.
- June CH, O'Connor RS, Kawalekar OU, Ghassemi S, Milone MC. CAR T cell immunotherapy for human cancer. Science. 2018;359(6382):1361-1365. [CrossRef]
- Sharma, P., & Allison, J. P. (2015). The future of immune checkpoint therapy. Cell, 161(2), 205-214. [CrossRef]
- Marusyk, A., Almendro, V., Polyak, K. (2012). Intra-tumour heterogeneity: a looking glass for cancer? Nature, 494(7437), 338–344.
- Furnari, F. B., Fenton, T., Bachoo, R. M., Mukasa, A., Stommel, J. M., Stegh, A., ... & Cavenee, W. K. (2007). Malignant astrocytic glioma: genetics, biology, and paths to treatment. Genes & Development, 21(21), 2683-2710. [CrossRef]
- Karsy M, Arslan E, Moy F. Current Progress on Understanding MicroRNAs in Glioblastoma Multiforme. Genes Cancer. 2012;3(1):3-15. [CrossRef]
- Cancer Genome Atlas Research Network. Comprehensive genomic characterization defines human glioblastoma genes and core pathways. Nature. 2008;455(7216):1061-1068.
- Bao S, Wu Q, McLendon RE, et al. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature. 2006;444(7120):756-760. [CrossRef]
- Verhaak RG, Hoadley KA, Purdom E, et al. Integrated genomic analysis identifies clinically relevant subtypes of glioblastoma characterized by abnormalities in PDGFRA, IDH1, EGFR, and NF1. Cancer Cell. 2010;17(1):98-110. [CrossRef]
- Esteller M, Garcia-Foncillas J, Andion E, et al. Inactivation of the DNA-repair gene MGMT and the clinical response of gliomas to alkylating agents. N Engl J Med. 2000;343(19):1350-1354.
- Stupp R, Hegi ME, Mason WP, et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10(5):459-466. [CrossRef]
- Lathia JD, Mack SC, Mulkearns-Hubert EE, Valentim CL, Rich JN. Cancer stem cells in glioblastoma. Genes Dev. 2015;29(12):1203-1217.
- Bouffet E, Larouche V, Campbell BB, et al. Immune Checkpoint Inhibition for Hypermutant Glioblastoma Multiforme Resulting From Germline Biallelic Mismatch Repair Deficiency. J Clin Oncol. 2016;34(19):2206-2211. [CrossRef]
- Lee, D. K., Horikoshi, M., & Roeder, R. G. (1993). Interaction of TFIID in the minor groove of the TATA element. Cell, 75(3), 487-498. [CrossRef]
- Struhl, K. (1998). Histone acetylation and transcriptional regulatory mechanisms. Genes & Development, 12(5), 599-606. [CrossRef]
- Spitz, F., & Furlong, E. E. (2012). Transcription factors: from enhancer binding to developmental control. Nature Reviews Genetics, 13(9), 613-626. [CrossRef]
- Brantley, E. C., Nabors, L. B., Gillespie, G. Y., Choi, Y. H., Palmer, C. A., Harrison, K., ... & Benveniste, E. N. (2008). Loss of protein inhibitors of activated STAT-3 expression in glioblastoma multiforme tumors: implications for STAT-3 activation and gene expression. Clinical Cancer Research, 14(15), 4694-4704. [CrossRef]
- Wang, J., Wang, H., Li, Z., Wu, Q., Lathia, J. D., McLendon, R. E., ... & Rich, J. N. (2011). c-Myc is required for maintenance of glioma cancer stem cells. PLOS ONE, 3(11), e3769. [CrossRef]
- Siegfried, Z., Simon, I., & Cedar, H. (2014). DNA methylation reprogramming and cancer: epigenetics meets metabolism. Nature Reviews Cancer, 14(7), 502-517.
- Hajra, K. M., Chen, D. Y., & Fearon, E. R. (2002). The SLUG zinc-finger protein represses E-cadherin in breast cancer. Cancer Research, 62(6), 1613-1618.
- Vega, S., Morales, A. V., Ocaña, O. H., Valdés, F., Fabregat, I., & Nieto, M. A. (2004). Snail blocks the cell cycle and confers resistance to cell death. Genes & Development, 18(10), 1131-1143. [CrossRef]
- Peinado, H., Ballestar, E., Esteller, M., & Cano, A. (2004). Snail mediates E-cadherin repression by the recruitment of the Sin3A/histone deacetylase 1 (HDAC1)/HDAC2 complex. Molecular and Cellular Biology, 24(1), 306-319.
- Marks, P., Rifkind, R. A., Richon, V. M., Breslow, R., Miller, T., & Kelly, W. K. (2001). Histone deacetylases and cancer: Causes and therapies. Nature Reviews Cancer, 1(3), 194-202. [CrossRef]
- Zhang, S., Cui, W., Xiong, F., et al. (2016). FOXA1 Promotes Tumor Progression in Prostate Cancer via the Insulin-Like Growth Factor Binding Protein 3 Pathway. PLOS ONE, 11(8), e0160379.
- Orr, B. A., et al. (2011). Yes-associated protein 1 is widely expressed in human brain tumors and promotes glioblastoma growth. Journal of Neuropathology & Experimental Neurology, 70(7), 568-577. [CrossRef]
- Elias, M. C., et al. (2005). TWIST is expressed in human gliomas and promotes invasion. Neoplasia, 7(9), 824-837. [CrossRef]
- Siebzehnrubl, F. A., et al. (2013). ZEB1 is associated with poor prognosis in glioblastoma. Tumour Biology, 34(6), 4009-4016.
- Nagai, S., et al. (2002). Concurrent inhibition of the epidermal growth factor receptor pathway by gefitinib (Iressa) and protein kinase A pathway by perifosine in malignant gliomas. Cancer Research, 62(20), 5763-5766.
- Bullock, A. N. et al. (2011). "Drugable" Transcription Factors? Chemistry & Biology, 18(11), 1313-1314.
- Lambert, M. et al. (2018). Targeting the DNA-Binding Activity of STAT3 as a New Therapeutic Avenue in Human Cancers. Oncogene, 37, 3947-3960.
- Wells, J. A., & McClendon, C. L. (2007). Reaching for High-Hanging Fruit in Drug Discovery at Protein–Protein Interfaces. Nature, 450(7172), 1001-1009. [CrossRef]
- Wang, J. et al. (2013). Pleiotropic Biological Activities of Alternatively Spliced TMPRSS2/ERG Fusion Gene Transcripts. Cancer Research, 68(20), 8516-8524.
- Spitz, F., & Furlong, E. E. M. (2012). Transcription Factors: From Enhancer Binding to Developmental Control. Nature Reviews Genetics, 13(9), 613-626. [CrossRef]
- Chen, Y. et al. (2016). Dual Phosphorylation of Suppressors of Cytokine Signaling Regulates Their Stability and Function. Journal of Biological Chemistry, 291(47), 24744-24752.
- Patel, A. P. et al. (2014). Single-Cell RNA-Seq Highlights Intratumoral Heterogeneity in Primary Glioblastoma. Science, 344(6190), 1396-1401. [CrossRef]
- Pardridge, W. M. (2019). Blood-Brain Barrier Drug Delivery of IgG Fusion Proteins with a Transferrin Receptor Monoclonal Antibody. Expert Opinion on Drug Delivery, 12(2), 207-222. [CrossRef]
- Stupp, R. et al. (2005). Radiotherapy plus Concomitant and Adjuvant Temozolomide for Glioblastoma. The New England Journal of Medicine, 352(10), 987-996. [CrossRef]
- Lu, J. et al. (2015). Novel Targeted Agents for Platelet-Derived Growth Factor Receptorα and c-KIT in Glioblastoma. Neurosurgical Focus, 38(3), E12.
- Esteller, M. (2007). Cancer Epigenomics: DNA Methylomes and Histone-Modification Maps. Nature Reviews Genetics, 8(4), 286-298. [CrossRef]
- Berger, S. L., et al. (2009). An Operational Definition of Epigenetics. Genes & Development, 23(7), 781-783. [CrossRef]
- Anastasiadou, E., et al. (2018). The Role of Non-Coding RNAs in Glioma. Cancers, 10(11), 11.
- Marks, P., et al. (2000). Histone Deacetylases and Cancer: Causes and Therapies. Nature Reviews Cancer, 1(3), 194-202. [CrossRef]
- Galanc, O., et al. (2019). HDAC Inhibitors in Glioblastoma: A Therapeutic Perspective. Frontiers in Oncology, 9, 302.
- Lee, E. Q., et al. (2015). A Multicenter, Phase II, Randomized, Non-Comparative Clinical Trial of Radiation and Temozolomide with or without Vandetanib in Newly Diagnosed Glioblastoma Patients. Clinical Cancer Research, 21(16), 3610-3618.
- Bannister, A. J., & Kouzarides, T. (2011). Regulation of Chromatin by Histone Modifications. Cell Research, 21(3), 381-395. [CrossRef]
- Eckschlager, T., et al. (2017). Histone Deacetylase Inhibitors as Anticancer Drugs. International Journal of Molecular Sciences, 18(7), 1414. [CrossRef]
- Noushmehr, H., et al. (2010). Identification of a CpG Island Methylator Phenotype that Defines a Distinct Subgroup of Glioma. Cancer Cell, 17(5), 510-522. [CrossRef]
- Vassilev, L. T., et al. (2004). In Vivo Activation of the p53 Pathway by Small-Molecule Antagonists of MDM2. Science, 303(5659), 844-848. [CrossRef]
- Sen, M., et al. (2012). First-in-Class Small Molecule Inhibitors of Signal Transducer and Activator of Transcription 3 (STAT3) That Act Through Indirect Mechanisms. Journal of Medicinal Chemistry, 55(11), 5730-5744.
- Swayze, E. E., et al. (2007). Antisense Oligonucleotides Containing Locked Nucleic Acid Improve Potency but Cause Significant Hepatotoxicity in Animals. Nucleic Acids Research, 35(2), 687-700. [CrossRef]
- Wu, S. Y., et al. (2015). Small Molecule Targeting of Transcriptional Repression in Cancer. Molecular Cancer Therapeutics, 14(6), 1422-1431.
- Lambert, M., et al. (2014). Targeting Transcription Factors for Cancer Treatment. Molecules, 19(6), 7950-7976. [CrossRef]
- Saraiva, C., et al. (2016). Nanoparticle-Mediated Brain Drug Delivery: Overcoming Blood–Brain Barrier to Treat Neurodegenerative Diseases. Journal of Controlled Release, 235, 34-47. [CrossRef]
- Schust, J., et al. (2006). Stattic: A Small-Molecule Inhibitor of STAT3 Activation and Dimerization. Chemistry & Biology, 13(11), 1235-1242. [CrossRef]
- Zhang, X., et al. (2013). A Novel Inhibitor of STAT3 Homodimerization Selectively Suppresses STAT3 Activity and Malignant Transformation. Cancer Research, 73(6), 1922-1933. [CrossRef]
- Korkolopoulou, P., et al. (2008). Activation of Nuclear Factor-κB Pathway Predicts Poor Outcome in Glioblastoma Multiforme. Clinical Neuropathology, 27(4), 228-237.
- Mullard, A. (2017). Targeting Undruggable Transcription Factors. Nature Reviews Drug Discovery, 16(7), 443-444.
- Massard, C., et al. (2016). High-Throughput Genomics and Clinical Outcome in Hard-to-Treat Advanced Cancers: Results of the MOSCATO 01 Trial. Cancer Discovery, 7(6), 586-595. [CrossRef]
- Weller, M., et al. (2017). European Association for Neuro-Oncology (EANO) guideline on the diagnosis and treatment of adult astrocytic and oligodendroglial gliomas. The Lancet Oncology, 18(6), e315-e329. [CrossRef]
- Sottoriva, A., et al. (2013). Intratumor heterogeneity in human glioblastoma reflects cancer evolutionary dynamics. Proceedings of the National Academy of Sciences, 110(10), 4009-4014. [CrossRef]
- Bhat, K. P., et al. (2013). Mesenchymal differentiation mediated by NF-κB promotes radiation resistance in glioblastoma. Cancer Cell, 24(3), 331-346.
- Wen, P. Y., et al. (2016). Updated response assessment criteria for high-grade gliomas: response assessment in neuro-oncology working group. Journal of Clinical Oncology, 28(11), 1963-1972. [CrossRef]
- Joffe, S., et al. (2001). Quality of Informed Consent: A New Measure of Understanding Among Research Subjects. JNCI Journal of the National Cancer Institute, 93(2), 139-147. [CrossRef]
- Marron, J. M., et al. (2018). Equity in Precision Medicine: Leaving No One Behind. Trends in Cancer, 4(1), 25-27.
- Ishii, T. (2017). Germ line genome editing in clinics: the approaches, objectives, and global society. Briefings in Functional Genomics, 16(1), 46-56. [CrossRef]
- Verhaak, R. G., et al. (2010). Integrated genomic analysis identifies clinically relevant subtypes of glioblastoma characterized by abnormalities in PDGFRA, IDH1, EGFR, and NF1. Cancer Cell, 17(1), 98-110. [CrossRef]
- Lathia, J. D., et al. (2015). Cancer stem cells in glioblastoma. Genes & Development, 29(12), 1203-1217.
- National Academy of Sciences. (2017). Human Genome Editing: Science, Ethics, and Governance. National Academies Press.
- Schumacher, T., & Schreiber, R. (2015). Neoantigens in cancer immunotherapy. Science, 348(6230), 69-74. [CrossRef]
- Castle, J. C., et al. (2012). Exploiting the mutanome for tumor vaccination. Cancer Research, 72(5), 1081-1091. [CrossRef]
- Hilf, N., et al. (2019). Actively personalized vaccination trial for newly diagnosed glioblastoma. Nature, 565(7738), 240-245. [CrossRef]
- Keskin, D. B., et al. (2019). Neoantigen vaccine generates intratumoral T cell responses in phase Ib glioblastoma trial. Nature, 565(7738), 234-239. [CrossRef]
- Ott, P. A., et al. (2017). An immunogenic personal neoantigen vaccine for patients with melanoma. Nature, 547(7662), 217-221. [CrossRef]
- Patel, A. P., et al. (2014). Single-cell RNA-seq highlights intratumoral heterogeneity in primary glioblastoma. Science, 344(6190), 1396-1401. [CrossRef]
- Anagnostou, V., et al. (2017). Evolution of neoantigen landscape during immune checkpoint blockade in non-small cell lung cancer. Cancer Discovery, 7(3), 264-276. [CrossRef]
- Wainwright, D. A., et al. (2015). The glioma microenvironment: Transforming stumbling blocks into stepping stones. Immunological Investigations, 44(8), 678-700.
- Jurtz, V., et al. (2017). NetMHCpan-4.0: Improved Peptide–MHC Class I Interaction Predictions Integrating Eluted Ligand and Peptide Binding Affinity Data. Journal of Immunology, 199(9), 3360-3368. [CrossRef]
- Sahin, U., et al. (2017). Personalized RNA mutanome vaccines mobilize poly-specific therapeutic immunity against cancer. Nature, 547(7662), 222-226. [CrossRef]
- Yu, H., Kortylewski, M., Pardoll, D. (2007). Crosstalk between cancer and immune cells: role of STAT3 in the tumour microenvironment. Nature Reviews Immunology, 7(1), 41-51. [CrossRef]
- Kortylewski, M. et al. (2009). Inhibiting Stat3 signaling in the hematopoietic system elicits multicomponent antitumor immunity. Nature Medicine, 15(12), 1313-1320. [CrossRef]
- Hayden, M. S., & Ghosh, S. (2012). NF-κB in immunobiology. Cell Research, 22(3), 447-462.
- Zuo, T. et al. (2007). FOXP3 is an X-linked breast cancer suppressor gene and an important repressor of the HER-2/ErbB2 oncogene. Cell, 129(7), 1275-1286.
- Gilmore, T. D., & Herscovitch, M. (2006). Inhibitors of NF-κB signaling: 785 and counting. Oncogene, 25(51), 6887-6899.
- Kortylewski, M. et al. (2009). Inhibiting Stat3 signaling in the hematopoietic system elicits multicomponent antitumor immunity. Nature Medicine, 15(12), 1313-1320. [CrossRef]
- Lawrence, T. (2009). The Nuclear Factor NF-kB Pathway in Inflammation. Cold Spring Harbor Perspectives in Biology, 1(6), a001651.
- Yu, H., Kortylewski, M., Pardoll, D. (2007). Crosstalk between cancer and immune cells: role of STAT3 in the tumour microenvironment. Nature Reviews Immunology, 7(1), 41-51. [CrossRef]
- Hayden, M. S., & Ghosh, S. (2012). NF-κB in immunobiology. Cell Research, 22(3), 447-462.
- Zuo, T. et al. (2007). FOXP3 is an X-linked breast cancer suppressor gene and an important repressor of the HER-2/ErbB2 oncogene. Cell, 129(7), 1275-1286.
- Chen, H. Z. et al. (2009). The dualistic function of cyclin D1: A concise review. Cell Cycle, 8(13), 2101-2106.
- West, A. C., & Johnstone, R. W. (2014). New and emerging HDAC inhibitors for cancer treatment. Journal of Clinical Investigation, 124(1), 30-39. [CrossRef]
- Gilmore, T. D., & Herscovitch, M. (2006). Inhibitors of NF-κB signaling: 785 and counting. Oncogene, 25(51), 6887-6899.
- Lamb, J. et al. (2013). The Connectivity Map: Using Gene-Expression Signatures to Connect Small Molecules, Genes, and Disease. Science, 313(5795), 1929-1935. [CrossRef]
- Holohan, C. et al. (2013). Cancer drug resistance: An evolving paradigm. Nature Reviews Cancer, 13(10), 714-726. [CrossRef]
- Freedman, A. et al. (2015). The Clinical Trials Process: an overview. Journal of Oncology Practice, 11(3), 253-255.
- Yu, H., Kortylewski, M., Pardoll, D. (2007). Crosstalk between cancer and immune cells: role of STAT3 in the tumour microenvironment. Nature Reviews Immunology, 7(1), 41-51. [CrossRef]
- Kortylewski, M. et al. (2009). Inhibiting Stat3 signaling in the hematopoietic system elicits multicomponent antitumor immunity. Nature Medicine, 15(12), 1313-1320. [CrossRef]
- Hayden, M. S., & Ghosh, S. (2012). NF-κB in immunobiology. Cell Research, 22(3), 447-462.
- Zuo, T. et al. (2007). FOXP3 is an X-linked breast cancer suppressor gene and an important repressor of the HER-2/ErbB2 oncogene. Cell, 129(7), 1275-1286.
- Gilmore, T. D., & Herscovitch, M. (2006). Inhibitors of NF-κB signaling: 785 and counting. Oncogene, 25(51), 6887-6899.
- Hodges, T. R., et al. (2017). Mutational burden, immune checkpoint expression, and mismatch repair in glioma: implications for immune checkpoint immunotherapy. Neuro-Oncology, 19(8), 1047-1057. [CrossRef]
- Wang, Q., et al. (2018). Landscape of multi-nucleotide variants in 125,748 human exomes and 15,708 genomes. Nature Communications, 11, 2539. [CrossRef]
- Zack, T. I., et al. (2013). Pan-cancer patterns of somatic copy number alteration. Nature Genetics, 45(10), 1134-1140. [CrossRef]
- Kreiter, S., et al. (2015). Mutant MHC class II epitopes drive therapeutic immune responses to cancer. Nature, 520, 692-696. [CrossRef]
- Ott, P. A., et al. (2017). An immunogenic personal neoantigen vaccine for patients with melanoma. Nature, 547, 217-221. [CrossRef]
- Sahin, U., et al. (2017). Personalized RNA mutanome vaccines mobilize poly-specific therapeutic immunity against cancer. Nature, 547, 222-226. [CrossRef]
- Verhaak, R. G., et al. (2010). Integrated genomic analysis identifies clinically relevant subtypes of glioblastoma characterized by abnormalities in PDGFRA, IDH1, EGFR, and NF1. Cancer Cell, 17, 98-110. [CrossRef]
- Pardoll, D. M. (2012). The blockade of immune checkpoints in cancer immunotherapy. Nature Reviews Cancer, 12, 252-264. [CrossRef]
- Mellman, I., et al. (2011). Cancer immunotherapy comes of age. Nature, 480, 480-489. [CrossRef]
- Schalper, K. A., et al. (2019). Neoadjuvant nivolumab modifies the tumor immune microenvironment in resectable glioblastoma. Nature Medicine, 25, 470-476. [CrossRef]
- Kim, S., et al. (2020). Deeper profiles and cascaded recurrent and convolutional neural networks for state-of-the-art protein secondary structure prediction. Scientific Reports, 10, 12374.
- Limon, D., et al. (2020). Glioblastoma: Molecular pathways, stem cells and therapeutic targets. Cancers, 12(4), 821.
- Weller, M., et al. (2017). Molecular neuro-oncology in clinical practice: a new horizon. The Lancet Oncology, 18, e430-e443.
- Lambin, P., et al. (2017). Radiomics: the bridge between medical imaging and personalized medicine. Nature Reviews Clinical Oncology, 14, 749-762. [CrossRef]
- Reardon, D. A., et al. (2020). CheckMate-143: a phase 3 randomized trial of nivolumab (nivo) vs bevacizumab (bev) in patients (pts) with recurrent glioblastoma (GBM). Journal of Clinical Oncology, 35(15_suppl), 2006.
- Brown, C. E., et al. (2016). Regression of Glioblastoma after Chimeric Antigen Receptor T-Cell Therapy. The New England Journal of Medicine, 375, 2561-2569. [CrossRef]
- Liau, L. M., et al. (2018). First results on survival from a large Phase 3 clinical trial of an autologous dendritic cell vaccine in newly diagnosed glioblastoma. Journal of Translational Medicine, 16, 142.
- Topol, E. J. (2019). High-performance medicine: the convergence of human and artificial intelligence. Nature Medicine, 25, 44-56. [CrossRef]
- Chin, L., et al. (2011). Comprehensive genomic characterization defines human glioblastoma genes and core pathways. Nature, 455, 1061-1068.
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).




