Submitted:
09 October 2023
Posted:
10 October 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Ethical approval
2.2. Detection of aflatoxin B1 using ELISA
2.3. Sample preparation for ELISA
2.4. New sample preparation method for sample extraction for ELISA
3. Results and Discussion
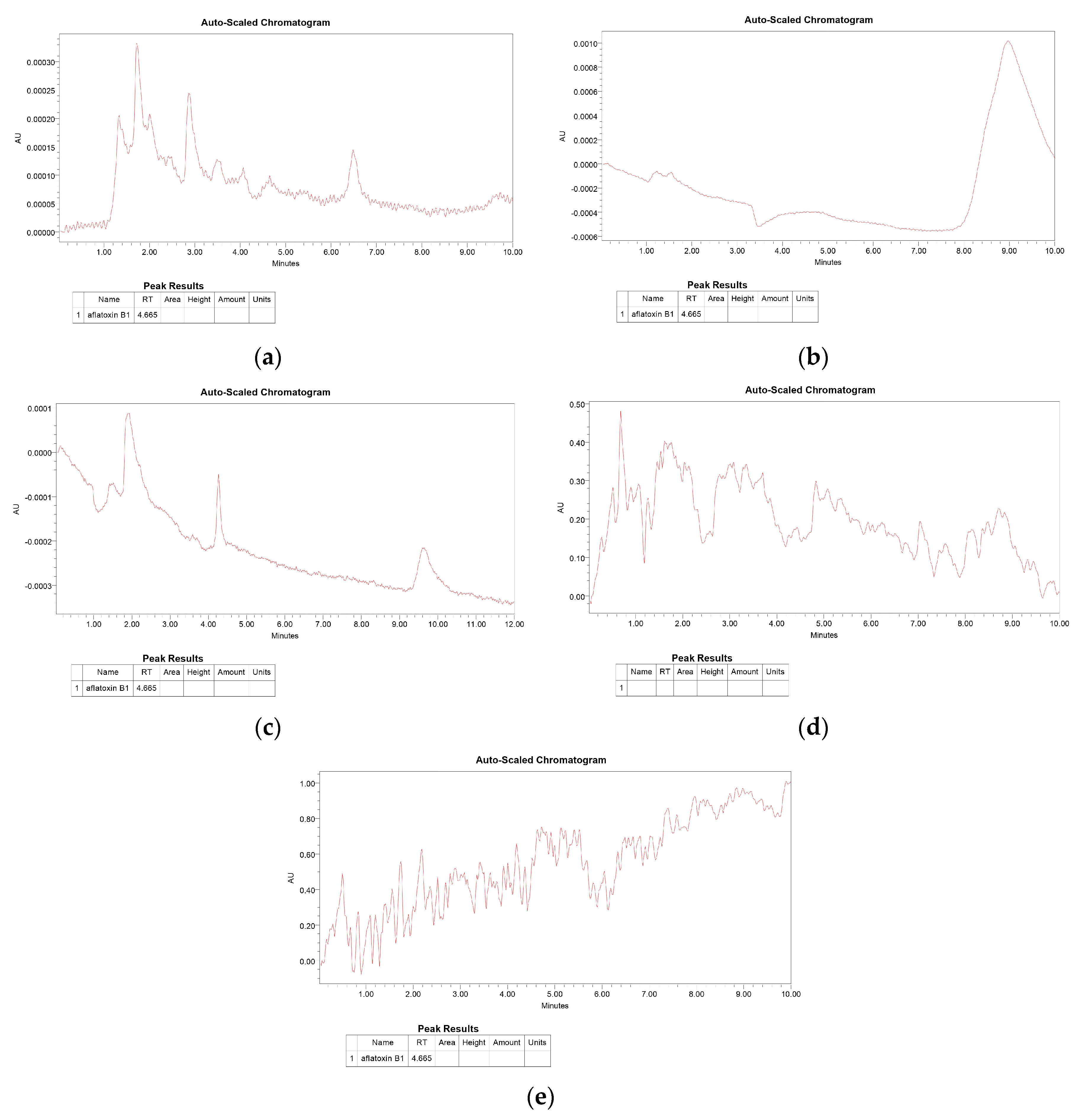
4. Conclusion
Author Contributions
Funding
Acknowledgments
References
- Yarmak, A.; Svyatkivska, E.; Prikhodko, D. Ukraine: Meat Sector Review. FAO Invest. Centre. Ctry. Highlights 2014, 14. [Google Scholar]
- Eissa, F.; Sebaei, A.S. A Comparative Study between the Top 10 Origin Countries Involved in the EU RASFF Notifications on Aflatoxins from 1997 to 2022. Microb. Risk Anal. 2023, 100277. [Google Scholar] [CrossRef]
- Wu, Q.; Xie, L.; Xu, H. Determination of Toxigenic Fungi and Aflatoxins in Nuts and Dried Fruits Using Imaging and Spectroscopic Techniques. Food Chem. 2018, 252, 228–242. [Google Scholar] [CrossRef] [PubMed]
- Kępińska-Pacelik, J.; Biel, W. Alimentary Risk of Mycotoxins for Humans and Animals. Toxins (Basel). 2021, 13, 822. [Google Scholar] [CrossRef] [PubMed]
- Wu, F. Measuring the Economic Impacts of Fusarium Toxins in Animal Feeds. Anim. Feed Sci. Technol. 2007, 137, 363–374. [Google Scholar] [CrossRef]
- Pankaj, S.K.; Shi, H.; Keener, K.M. A Review of Novel Physical and Chemical Decontamination Technologies for Aflatoxin in Food. Trends Food Sci. Technol. 2018, 71, 73–83. [Google Scholar] [CrossRef]
- Workshop for World Food Safety Day in Kazakhstan Available online: https://www.fao.org/fao-who-codexalimentarius/news-and-events/news-details/en/c/1538955.
- Cardona, T.D.; LLangantileke, S.G.; Noomhorm, A. Aflatoxin Research on Grain in Asia: Its Problems and Possible Solutions. In Mycotoxin Prevention and Control in Foodgrains; 1991.
- Eneroth, H.; Wallin, S.; Leander, K.; Nilsson Sommar, J.; Åkesson, A. Risks and Benefits of Increased Nut Consumption: Cardiovascular Health Benefits Outweigh the Burden of Carcinogenic Effects Attributed to Aflatoxin B1 Exposure. Nutrients 2017, 9, 1355. [Google Scholar] [CrossRef] [PubMed]
- Pickova, D.; Ostry, V.; Malir, F. A Recent Overview of Producers and Important Dietary Sources of Aflatoxins. Toxins (Basel). 2021, 13, 186. [Google Scholar] [CrossRef]
- Martins, C.; Vidal, A.; De Boevre, M.; De Saeger, S.; Nunes, C.; Torres, D.; Goios, A.; Lopes, C.; Assunção, R.; Alvito, P. Exposure Assessment of Portuguese Population to Multiple Mycotoxins: The Human Biomonitoring Approach. Int. J. Hyg. Environ. Health 2019, 222, 913–925. [Google Scholar] [CrossRef]
- Magan, N.; Aldred, D. Post-Harvest Control Strategies: Minimizing Mycotoxins in the Food Chain. Int. J. Food Microbiol. 2007, 119, 131–139. [Google Scholar] [CrossRef]
- Aflatoxinas Por Encima Del LMR En Cacahuetes de China // Aflatoxins above MRL in Peanuts from China Available online: https://webgate.ec.europa.eu/rasff-window/screen/notification/594751.
- Competence, E.U.; Vote, E.U. Maximum Levels for Total Aflatoxins in Certain Cereals and Cereal-Based Products Including Foods for Infants and Young Children and Associated Sampling Plans (CX/CF 22/15/9 and CL 2022/18-CF); 2022.
- Reddy, K.; Salleh, B.; Saad, B.; Abbas, H.; Abel, C.; Shier, W. An Overview of Mycotoxin Contamination in Foods and Its Implications for Human Health. Toxin Rev. 2010, 29, 3–26. [Google Scholar] [CrossRef]
- Mutegi, C.K.; Ngugi, H.K.; Hendriks, S.L.; Jones, R.B. Prevalence and Factors Associated with Aflatoxin Contamination of Peanuts from Western Kenya. Int. J. Food Microbiol. 2009, 130, 27–34. [Google Scholar] [CrossRef]
- Picinin, L.C.A.; Cerqueira, M.M.O.P.; Vargas, E.A.; Lana, Â.M.Q.; Toaldo, I.M.; Bordignon-Luiz, M.T. Influence of Climate Conditions on Aflatoxin M1 Contamination in Raw Milk from Minas Gerais State, Brazil. Food Control 2013, 31, 419–424. [Google Scholar] [CrossRef]
- Iqbal, S.Z.; Jinap, S.; Pirouz, A.A.; Ahmad Faizal, A.R. Aflatoxin M1 in Milk and Dairy Products, Occurrence and Recent Challenges: A Review. Trends Food Sci. Technol. 2015, 46, 110–119. [Google Scholar] [CrossRef]
- Reiter, E.; Zentek, J.; Razzazi, E. Review on Sample Preparation Strategies and Methods Used for the Analysis of Aflatoxins in Food and Feed. Mol. Nutr. Food Res. 2009, 53, 508–524. [Google Scholar] [CrossRef] [PubMed]
- Battilani, P.; Toscano, P.; Van der Fels-Klerx, H.J.; Moretti, A.; Camardo Leggieri, M.; Brera, C.; Rortais, A.; Goumperis, T.; Robinson, T. Aflatoxin B1 Contamination in Maize in Europe Increases Due to Climate Change. Sci. Rep. 2016, 6, 24328. [Google Scholar] [CrossRef] [PubMed]
- Report o the Scientific Committee on Food (Thirty-Fifth Series); Luxembourg, 1996.
- IARC Chemical Agents and Related Occupations; Lyon, France, 2012; Vol. 100F; ISBN 9780123864543.
- Azziz-Baumgartner, E.; Lindblade, K.; Gieseker, K.; Rogers, H.S.; Kieszak, S.; Njapau, H.; Schleicher, R.; McCoy, L.F.; Misore, A.; DeCock, K.; et al. Case-Control Study of an Acute Aflatoxicosis Outbreak, Kenya, 2004. Environ. Health Perspect. 2005, 113, 1779–1783. [Google Scholar] [CrossRef]
- Guengerich, F.P.; Johnson, W.W.; Shimada, T.; Ueng, Y.F.; Yamazaki, H.; Langouët, S. Activation and Detoxication of Aflatoxin B1. Mutat. Res. - Fundam. Mol. Mech. Mutagen. 1998, 402, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Marasas, W.F.O.; Gelderblom, W.C.A.; Shephard, G.S.; Vismer, H.F. Mycotoxins: A Global Problem. In Mycotoxins: a global problem. Mycotoxins: detection methods, management, public health and agricultural trade; CABI: UK, 2008; pp. 29–39. [Google Scholar]
- Ebrahimi, A.; Emadi, A.; Arabameri, M.; Jayedi, A.; Abdolshahi, A.; Yancheshmeh, B.S.; Shariatifar, N. The Prevalence of Aflatoxins in Different Nut Samples: A Global Systematic Review and Probabilistic Risk Assessment. AIMS Agric. Food 2022, 7, 130–148. [Google Scholar] [CrossRef]
- Juan, C.; Zinedine, A.; Moltó, J.C.; Idrissi, L.; Mañes, J. Aflatoxins Levels in Dried Fruits and Nuts from Rabat-Salé Area, Morocco. Food Control 2008, 19, 849–853. [Google Scholar] [CrossRef]
- Asghar, M.A.; Ahmed, A.; Zahir, E.; Asghar, M.A.; Iqbal, J.; Walker, G. Incidence of Aflatoxins Contamination in Dry Fruits and Edible Nuts Collected from Pakistan. Food Control 2017, 78, 169–175. [Google Scholar] [CrossRef]
- Naeem, I.; Ismail, A.; Rehman, A.U.; Ismail, Z.; Saima, S.; Naz, A.; Faraz, A.; de Oliveira, C.A.F.; Benkerroum, N.; Aslam, M.Z.; et al. Prevalence of Aflatoxins in Selected Dry Fruits, Impact of Storage Conditions on Contamination Levels and Associated Health Risks on Pakistani Consumers. Int. J. Environ. Res. Public Health 2022, 19. [Google Scholar] [CrossRef] [PubMed]
- USDA - United States Department of Agriculture. Foreign Agricultural Service. Production, Supply and Distribution. Online; 2015.
- IEA Amendoim: Produção, Exportação e a Safra 2011/2012. Análises e Indicadores do Agronegócio 2011, 11.
- Nono Levantamento. Acompanhamento Da Safra Brasileira Grãos; 2015.
- Martins, L.M.; Sant’Ana, A.S.; Fungaro, M.H.P.; Silva, J.J.; Nascimento, M. da S. do; Frisvad, J.C.; Taniwaki, M.H. The Biodiversity of Aspergillus Section Flavi and Aflatoxins in the Brazilian Peanut Production Chain. Food Res. Int. 2017, 94, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Gallo, P.; Imbimbo, S.; Alvino, S.; Castellano, V.; Arace, O.; Soprano, V.; Esposito, M.; Serpe, F.P.; Sansone, D. Contamination by Aflatoxins B/G in Food and Commodities Imported in Southern Italy from 2017 to 2020: A Risk-Based Evaluation. Toxins (Basel). 2021, 13, 368. [Google Scholar] [CrossRef] [PubMed]
- Bureau of National Statistics of the Agency for Strategic Planning and Reforms of the Republic of Kazakhstan Available online: https://www.gov.kz/memleket/entities/stat?lang=en.
- Amelin, V.G.; Karaseva, N.M.; Tretyakov, A. V. Simultaneous Determination of Trichothecene Micotoxins, Ochratoxin A, and Zearalenone in Grain and Products of Its Processing, Feed Premixes, and Meat by Gas Chromatography. J. Anal. Chem. 2013, 68, 61–67. [Google Scholar] [CrossRef]
- INTERSTATE COUNCIL FOR STANDARDIZATION, M.A.C.; (ISC) GOST 31748-2012 (ISO 16050:2003) Foodstuffs. Determination of Aflatoxin B1, and the Total Content of Aflatoxins B1, B2, G1 and G2 in Cereals, Nuts and Derived Products. High-Performance Liquid Chromatographic Method; 2013.
- Hojjati, M.; Shahbazi, S.; Askari, H.; Makari, M. Use of X-Irradiations in Reducing the Waste of Aflatoxin-Contaminated Pistachios and Evaluation of the Physicochemical Properties of the Irradiated Product. Foods 2023, 12, 3040. [Google Scholar] [CrossRef]
- GOST 30711-2001 Food-Stuffs. Methods for Detection and Determination of Aflatoxins B1 and M1 Content; 2002.
- Nuti, F.; Fernandez, F.R.; Sabatino, G.; Peroni, E.; Mulinacci, B.; Paolini, I.; Pisa, M. Di; Tiberi, C.; Lolli, F.; Petruzzo, M.; et al. A Multiple N-Glucosylated Peptide Epitope Efficiently Detecting Antibodies in Multiple Sclerosis. Brain Sci. 2020, 10, 453. [Google Scholar] [CrossRef]
- Macri, A.M.; Pop, I.; Simeanu, D.; Toma, D.; Sandu, I.; Pavel, L.L.; Mintas, O.S. The Occurrence of Aflatoxins in Nuts and Dry Nuts Packed in Four Different Plastic Packaging from the Romanian Market. Microorganisms 2021, 9, 1–12. [Google Scholar] [CrossRef]
- Eslami, M.; Mashak, Z.; Heshmati, A.; Shokrzadeh, M.; Mozaffari Nejad, A.S. Determination of Aflatoxin B 1 Levels in Iranian Rice by ELISA Method. Toxin Rev. 2015, 34, 125–128. [Google Scholar] [CrossRef]
- Ishaque, N.; Shabbir, T.; Javed, M.; Rehman, H.; Gulab, T.; Bushra, S.; Kalsoom, P.; Ali, G. Nature of Aflatoxins : Their Extraction , Analysis , and Control. 2018, 1–7. [CrossRef]
- Cole, R.J.; Dorner, J.W. Extraction of Aflatoxins from Naturally Contaminated Peanuts with Different Solvents and Solvent/Peanut Ratios. J. AOAC Int. 1994, 77, 1509–1511. [Google Scholar] [CrossRef]
- Schrenk, D.; Bignami, M.; Bodin, L.; Chipman, J.K.; del Mazo, J.; Grasl-Kraupp, B.; Hogstrand, C.; Hoogenboom, L. (Ron); Leblanc, J.; Nebbia, C.S.; et al. Risk Assessment of Aflatoxins in Food. EFSA J. 2020, 18. [Google Scholar] [CrossRef]
- Bensassi, F.; Rhouma, A.; Ghrab, M.; Bacha, H.; Rabeh Hajlaoui, M. Evaluation of Cultivar Susceptibility and Storage Periods towards Aflatoxin B1 Contamination on Pistachio Nuts. Mycotoxin Res. 2010, 26, 199–203. [Google Scholar] [CrossRef] [PubMed]
- Ostadrahimi, A.; Ashrafnejad, F.; Kazemi, A.; Sargheini, N.; Mahdavi, R.; Farshchian, M.; Mahluji, S. Aflatoxin in Raw and Salt-Roasted Nuts (Pistachios, Peanuts and Walnuts) Sold in Markets of Tabriz, Iran. Jundishapur J. Microbiol. 2014, 7, 1–6. [Google Scholar] [CrossRef]
- AL-Warshan, S.H.S.; Abed, M.M.; Mohammed, M.M. Detection of Fungi Contaminated Some Nuts and Its Ability for Aflatoxin B1 Production. IOP Conf. Ser. Earth Environ. Sci. 2022, 1060. [Google Scholar] [CrossRef]
- Gichohi-Wainaina, W.N.; Kumwenda, N.C.; Harry, M.; Matumba, L.; Njoroge, S.M.C.; Okori, P. Aflatoxin in Cereals and Groundnut from Small Holder Farming Households in Malawi. Food Addit. Contam. Part B 2022, 15, 266–274. [Google Scholar] [CrossRef] [PubMed]
- Milhome, M.A.L.; Lima, C.G.; de Lima, L.K.; Lima, F.A.F.; Sousa, D.O.B.; Nascimento, R.F. Occurrence of Aflatoxins in Cashew Nuts Produced in Northeastern Brazil. Food Control 2014, 42, 34–37. [Google Scholar] [CrossRef]
- Azer, M.; Cooper, C. Determination of Aflatoxins in Foods Using HPLC and a Commercial ELISA System. J. Food Prot. 1991, 54, 291–294. [Google Scholar] [CrossRef]
- Leong, Y.H.; Ismail, N.; Latif, A.A.; Ahmad, R. Aflatoxin Occurrence in Nuts and Commercial Nutty Products in Malaysia. Food Control 2010, 21, 334–338. [Google Scholar] [CrossRef]
- Ayejuyo, O.O.; Olowu, R.A.; Agbaje, T.O.; Atamenwan, M.; Osundiya, M.O. Enzyme - Linked Immunosorbent Assay ( Elisa ) of Aflatoxin B1 in Groundnut and Cereal Grains in Lagos , Nigeria. 2011, 1, 1–5.
- Jubeen, F.; Sher, F.; Hazafa, A.; Zafar, F.; Ameen, M.; Rasheed, T. Evaluation and Detoxification of Aflatoxins in Ground and Tree Nuts Using Food Grade Organic Acids. Biocatal. Agric. Biotechnol. 2020, 29, 101749. [Google Scholar] [CrossRef]
- Dhanshetty, M.; Elliott, C.T.; Banerjee, K. Decontamination of Aflatoxin B1 in Peanuts Using Various Cooking Methods. J. Food Sci. Technol. 2021, 58, 2547–2554. [Google Scholar] [CrossRef]
- Rastegar, H.; Shoeibi, S.; Yazdanpanah, H.; Amirahmadi, M.; Khaneghah, A.M.; Campagnollo, F.B.; Sant’Ana, A.S. Removal of Aflatoxin B1 by Roasting with Lemon Juice and/or Citric Acid in Contaminated Pistachio Nuts. Food Control 2017, 71, 279–284. [Google Scholar] [CrossRef]
- Doyle, M.P.; Marth, E.H. Bisulfite Degrades Aflatoxin: Effect of Citric Acid and Methanol and Possible Mechanism of Degradation. J. Food Prot. 1978, 41, 891–896. [Google Scholar] [CrossRef] [PubMed]
| No | Type of nut | Methanol | A mixture of sodium chloride (25 ml 30%) and citric acid (15 ml 5%) |
|---|---|---|---|
| China | |||
| 1 | Peanuts (n=7) | 0.0017±0.002 | 0.0018±0.002 |
| 2 | Walnut (n=19) | 0.06±0.04 | 0.059±0.04 |
| 3 | Almonds (n=12) | 0.0036±0.004 | 0.0029±0.004 |
| Uzbekistan | |||
| 1 | Peanuts (n=11) | 0.6±0.004 | 0.6±0.004 |
| 2 | Walnut (n=13) | 0.0016±0.004 | 0.0017±0.004 |
| 3 | Almonds (n=8) | 0.045±0.002 | 0.036±0.002 |
| 4 | Hazelnuts(n=9) | 0.021±0.001 | 0.026±0.001 |
| 5 | Pistachios(n=7) | 0.004±0.004 | 0.007±0.004 |
| р <0,05 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





