1. Introduction
Foodborne diseases pose a significant public health concern worldwide, leading to substantial morbidity and mortality (WHO, 2017). Every year, millions of people suffer from foodborne diseases that are globally important because of their high incidence and the costs that they impose on society. Meat, an excellent protein source for human beings, is a perishable food that is easily contaminated, resulting in economic and health losses. The contamination of meat with Enterobacteriaceae is a significant public health concern with far-reaching consequences for both consumers and the food industry. These pathogens, including Escherichia coli (E. coli O157:H7), Salmonella spp., Campylobacter jejuni, Yersinia enterocolitica, Klebsiella spp., causing severe illnesses such as salmonellosis, hemolytic uremic syndrome, and hemorrhagic colitis (Vogt & Dippold, 2005). In animals, Enterobacteriaceae are predominantly present in the gastrointestinal tract and contaminates meat during slaughter (Brichta-Harhay et al., 2008). Therefore, their prevalence in livestock warrants comprehensive investigation (Bintsis, Litopoulou-Tzanetaki, & Robinson, 2000). In most countries, including Saudi Arabia, camels, cattle, and sheep are primary meat sources, and the microbiological safety of meat is crucial, given the scale of their consumption. Slaughterhouses are critical points in the meat production process where many studies have shown that the prevalence of E. coli and Salmonella spp. on carcasses due to improper slaughter practices and fed-animal hygiene (Brichta-Harhay et al., 2008). Along the same lines, outlets have been documented as a source of Enterobacteriaceae prevalence in the raw meats (Ahmad et al., 2013; Bohaychuk et al., 2006; Bosilevac et al., 2015; Jaja, Green, & Muchenje, 2018). The contamination of Enterobacteriaceae can occur during slaughtering and handling practices causing health risks. The health risks of E. coli and Salmonella spp. pose are not uniform and depend on the specific strains involved. Some E. coli strains, such as enterohemorrhagic E. coli (EHEC), can cause severe bloody diarrhea, kidney failure, and even death, while certain Salmonella Enteritidis and S. Typhimurium are responsible for the majority of human salmonellosis cases (CDC, 2010).
According to the Centers for Disease Control and Prevention (CDCP), Salmonella spp. causes approximately 1.35 million infections, 26,500 hospitalizations, and 420 deaths in the United States annually. While in Europe, Salmonella spp. was reported as the second most frequent causative agent of foodborne and the second cause of bacterial inflammation of the small intestine in Germany (Meyer, Thiel, Ullrich, & Stolle, 2010; Terentjeva et al., 2017). Another area of concern is the potential for cross-contamination in slaughterhouses and butcher shops. Despite the ability to eliminate pathogens during cooking, the risk posed by cross-contamination with other foods, such as ready-to-eat food (Todd, Greig, Bartleson, & Michaels, 2009). A study carried out by Brichta-Harhay et al. (2008) found that the prevalence of E. coli O157:H7 on cattle carcasses ranged from 0% to 3.6%, while Salmonella spp. prevalence ranged from 0% to 1.8% on carcasses. In retail outlets, the presence of E. coli and Salmonella spp. can be influenced by cross-contamination, temperature control, and the overall quality of the meat (Bohaychuk et al., 2006). understanding the prevalence and load of Enterobacteriaceae contamination present on the hides and carcasses of animals during processing is a significant prerequisite for risk assessment and management. However, comprehensive research into the prevalence and diversity genera of Enterobacteriaceae, particularly in camels, remains limited. This study, therefore, aims to elucidate the prevalence of Enterobacteriaceae in camels, cattle, and sheep during slaughtering and presentation in butcher shops in Al-Ahsa governate.
2. Materials and Methods
2.1. Sample Design
A random sampling technique was employed to ensure the unbiased selection of sampling units. This method facilitated the random selection of samples based on the number of animals slaughtered on sampling days. Data was gathered from two high-throughput municipality slaughterhouses (located in the north and the south) and six butcher shops located in Al-Ahsa governate (Eastern province, Saudi Arabia) between August and October 2022. To comprehensively understand microbial diversity among consumed meat, the study focused on three distinct animal types, camels, cattle, and sheep. All animal intent to slaughter were subjected to comprehensive veterinary examination.
2.2. Sample Collection
A comprehensive collection of 120 samples from camels, cattle, and sheep was undertaken from two distinct slaughterhouses and six butcher shops. From each animal variety, 40 samples (20 specimens from slaughterhouse, and the same from butcher shops) were analyzed. All animals were subjected to comprehensive veterinary inspection before and after the slaughtering phase. The samples at the slaughterhouses were obtained during slaughtering phase, after evisceration process, whereas in butcher shops, they were collected from the display refrigerators. Approximately 250g of each carcass were aseptically taken from different parts of the carcass including neck, chest, backchain, belly, and legs. The same weight was collected from butcher shops from different parts of the carcasses. All samples were placed in sterile plastic bags and stored in an ice box to avoid microbial development. The samples, then, transferred to the laboratories within 4 - 8 hours for microbiological analyses.
2.3. Microbiological analyses
2.3.1. Enumerating of Total Viable Count
The Total Viable Count (TVC) of the samples was enumerated using standard microbiological techniques. Briefly, 25g of each collected sample was placed into sterile stomacher bag containing 225 mL of Buffered Peptone Water (BPW) (Oxoid, UK), and completely homogenized using stomacher (Seward Medical Ltd., London, UK) at 200 rpm for 3 min. Following homogenization, serial decimal dilutions of the samples (up to 10-6) were prepared. The samples, then, were cultured in plate count agar (PCA) (Oxoid, UK) in three replicates. The plates were incubated at 37 ˚C for 48 hours, allowing bacterial growth. The plates with colonies of 30 to 300 were only considered for enumeration TVC. The final results were presented in log10 CFU/g.
2.3.2. Presumptive testing for Enterobacteriaceae
All meat samples were analyzed for detection of the presence of any member of Enterobacteriaceae by weighing 25 g of each meat sample aseptically and placed into sterile stomacher bag containing 225 ml of sterile 0.1 % buffered peptone water (BPW) (Oxoid, UK and homogenized (Cox et al., 2010). For Salmonella spp. pre-enrichment was carried out by incubating the mixture at 37 ˚C for 24 h. Aliquots of 100µL were transferred into 100 mL. Tetrathionate Broth (TTB) (Oxoid, UK) tubes containing potassium iodide and iodine solution, as recommended by the manufacturer, and incubated again at 37 ˚C for 24 h for enrichment. Aliquots of 1ml from each final dilution was added on Petri dishes containing different agars of MacConkey (MCA) (Oxoid, UK), Eosin Methylene Blue (EMB) (Oxoid, UK), Salmonella-Shigella (SS) (Oxoid, UK), and incubated for a minimum of 24 h until visible colonies were observed. All suspected colonies were subculture based on their phenotypic appearances as following: colonies that appeared on MacConkey agar (MCA) as lactose and non-lactose fermenters, were subculture separately on different MCA and Salmonella-Shigella (SS) agar. while colonies had dark centered and colonies had green metallic sheen were subculture on Salmonella-Shigella (SS) agar and Eosin methylene blue (EMB) agar respectively and subsequently screened on sorbitol MacConkey agar (SMAC) as described by (Cox et al., 2010).
2.3.3. Confirmation of Identification
Biochemical testing
All isolated bacteria were subjected to preliminary standard biochemical test for identification. Presumptively identified members of Enterobacteriaceae were further screened by Analytical Profile Index API® 20E (BioMérieux®, Inc. France), following the manufacturer’s instructions.
Molecular Testing
The identification of isolated strains was performed using Extract-N-Amp™ Tissue PCR Kit (XNAT2R, Sigma-Aldrich Pty Ltd., Germany) according to the manufacturer’s instruction (White, Bruns, Lee, & Taylor, 1990). For identification, 16SRNA sequences for each isolated strain were used. For amplification, universal primers NS1 (forward 5’- AGA GTT TGA TCM TGG CTC AG-3′) and NS2 (reverse 5’-ACGGYTACCTTGTTACGACTT-3′) were used (Przemieniecki et al., 2016). The polymerase chain reaction (PCR) was performed using a Rotor-Gene 6000 thermocycler (Corbett Life Science, Qiagen, Australia) as following procedure: 3 min initial denaturation at 95 °C, 35 cycles of denaturation (30 s at 95 °C), annealing (30 s at 55 °C), extension (1.5 min at 72 °C) and a final extension at 72 °C for 7 min. Each 25 µL PCR reaction mixture contained 12.5 µL of No-ROX Kit, 3.5 µL deionized water, 2 µL each of 10 µM forward and reverse primer and 5 µL of the extracted DNA. Sequencing was performed by Macrogen Inc. (South Korea). All sequences were assembled by Seqman program of DNASTAR 7.1 software (DNASTAR Inc., Madison, WI, USA).
2.4. Statistical Analysis
The data were analyzed using the Statistical Package for the Social Science (SPSS) version 26 (IBM Corporation, Armonk, New York, United States). Descriptive statistics were used to describe the frequency of Enterobacteriaceae along the camels, cattle abs sheep production chain. The statistical significance of the differences in counts and Enterobacteriaceae prevalence between different sources was determined. A p-value < 0.05 was considered statistically significant.
3. Results and Discussion
3.1. Total Viable Count Enumeration
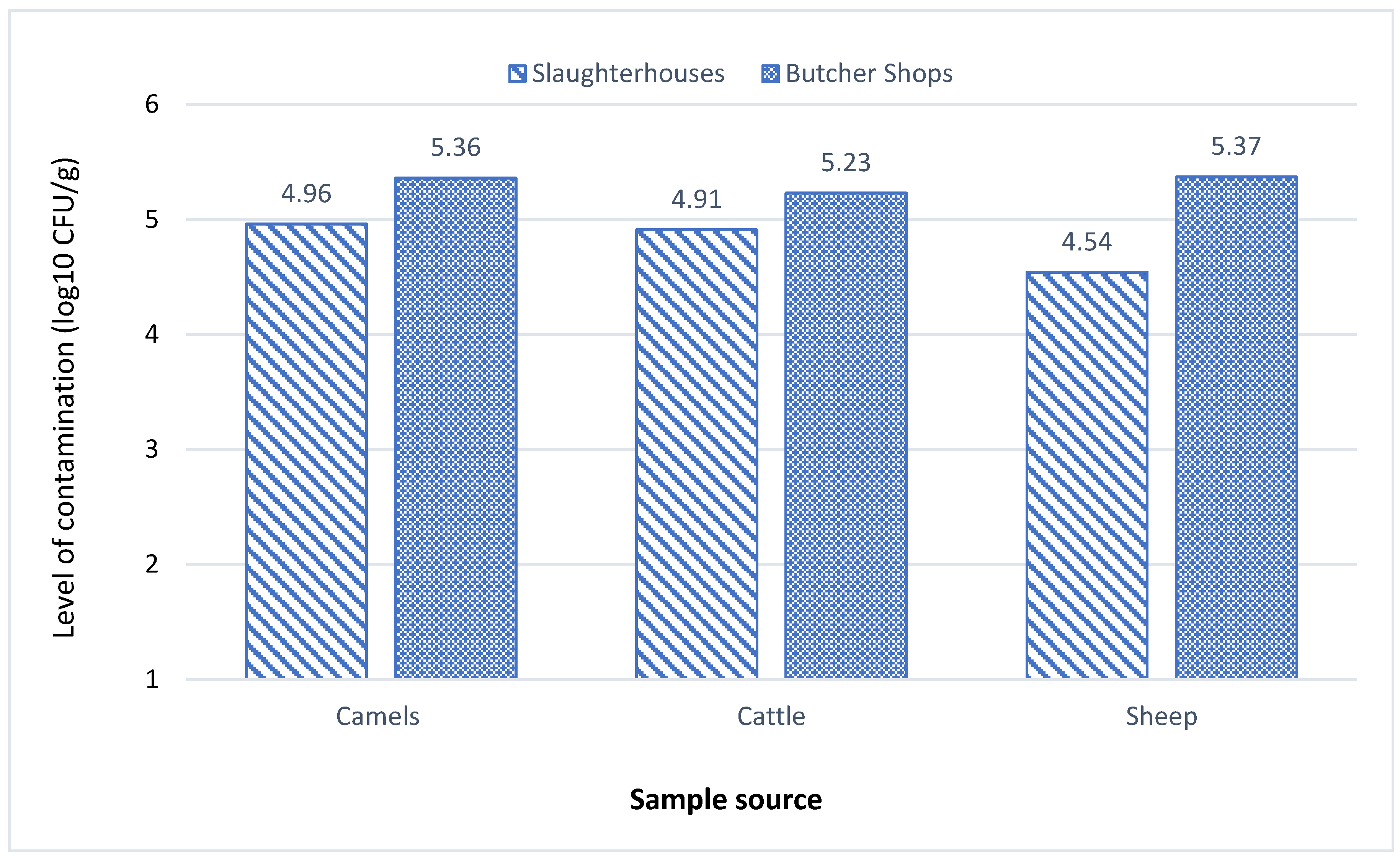
The total viable bacteria (TVC) in samples gathered from two slaughterhouses and six butcher shops was evaluated using morphological methods.
Table 1 shows the contamination levels of camel, cattle, and sheep samples in these establishments. The mean TVC of camel samples ranged from log10 3.3 to log10 6.2 CFU/g in slaughterhouses and log10 2.8 to log10 6.9 CFU/g in butcher shops. Out of 20 camel samples, half from the slaughterhouses fell within the critical limits of the microbiological criteria for foodstuffs created by G.C.C Standardization Organization (GSO 1016:2015) (G.S.O., 2015), while the remaining samples were within standard limits. Three out 20 butcher shops samples exceeded the limits with ≥ log10 6 CFU/g, 12 were within critical limits, and the remaining five were within standard limits. Similarly, the average TVC of cattle samples varied from log10 3.8 to log10 5.7 CFU/g in slaughterhouses and from log10 4.2 to log10 6.7 CFU/g in butcher shops. Half of the 20 cattle samples from slaughterhouses were within critical limits and the remaining were within standard limits. While 3 cattle samples from butcher shops surpassed the standard limits with ≥ log10 6 CFU/g, and nine samples were within the critical limit.
In contrast, sheep samples demonstrated lower bacterial contamination levels, with TVC means ranging from log10 3.4 to log10 6.6 CFU/g in slaughterhouses and from log10 3 to log10 6.9 CFU/g in butcher shops. Two sheep samples were within the critical limits with log10 5 CFU/g, and only 1 sample exceeded standard limits with log10 6.6 CFU/g. Concurrently, 5 sheep samples from butcher shops showed TVC values exceeding standard limits with ≥ log10 6 CFU/g, 9 samples were within the critical limits with log10 5 CFU/g, while the remaining samples were within standard limits. In alignment with previous research (Ali, Farooqui, Khan, Khan, & Kazmi, 2010; Bhandare, Sherikar, Paturkar, Waskar, & Zende, 2007).The TVC levels found in this study were significantly higher in butcher shops than in slaughterhouses. This heightened contamination in butcher shops can be attributed to the proximity handling of different meat types, which facilitates cross-contamination, increased handling by numerous individuals, improper sanitation of utensils, and extended display times, providing ample opportunity to multiply bacteria. This could be attributed to the fact that different types of meat are often handled nearby in butcher shops, allowing bacteria to transfer from one place to another (cross-contamination). In addition, more hands touching meat products in butcher shops is the potential for introducing bacteria, in addition to improper utensils sanitation, and the long exposure time in displaying, which allows bacteria more time to multiply (Nychas, Skandamis, Tassou, & Koutsoumanis, 2008).
3.2. Prevalence of Total Coliform and E. coli
Table 1 summarizes the presence of coliforms and
E. coli in the evaluated samples. The assessment aimed to explore the overall hygiene quality and safety practices when handling carcasses in both slaughterhouses and butcher shops. In this study, coliforms were found in all samples. Out of 60 samples of camel, cattle, and sheep carcasses collected from slaughterhouses, 55% of camel and cattle samples exhibited 1.1x103 MPN/g, while 20% of sheep samples showed the same. Interestingly, 60% of samples collected from retail shops had 1.1x103 MPN/g. The results from retail shops mirrored those from slaughterhouses, with 60% of camel samples, 55% of cattle samples, and 45% of sheep samples presenting with 1.1x103 MPN/g. As the high prevalence of coliforms may attributed to inadequate sanitary conditions and poor general hygiene during the slaughtering practices and handling carcasses. In addition, coliforms can proliferate from -2 to 37 °C (Jay, Loessner, & Golden, 2005) which allows bacteria multiplying during display in shops.
Moreover, detection of
E. coli was carried out irrespective of pathogenic or nonpathogenic strain to estimate the level of hygiene. Among isolated Enterobacteriaceae genera,
E. coli was the most predominant genera as shown in
Table 2. Out of 120 samples,
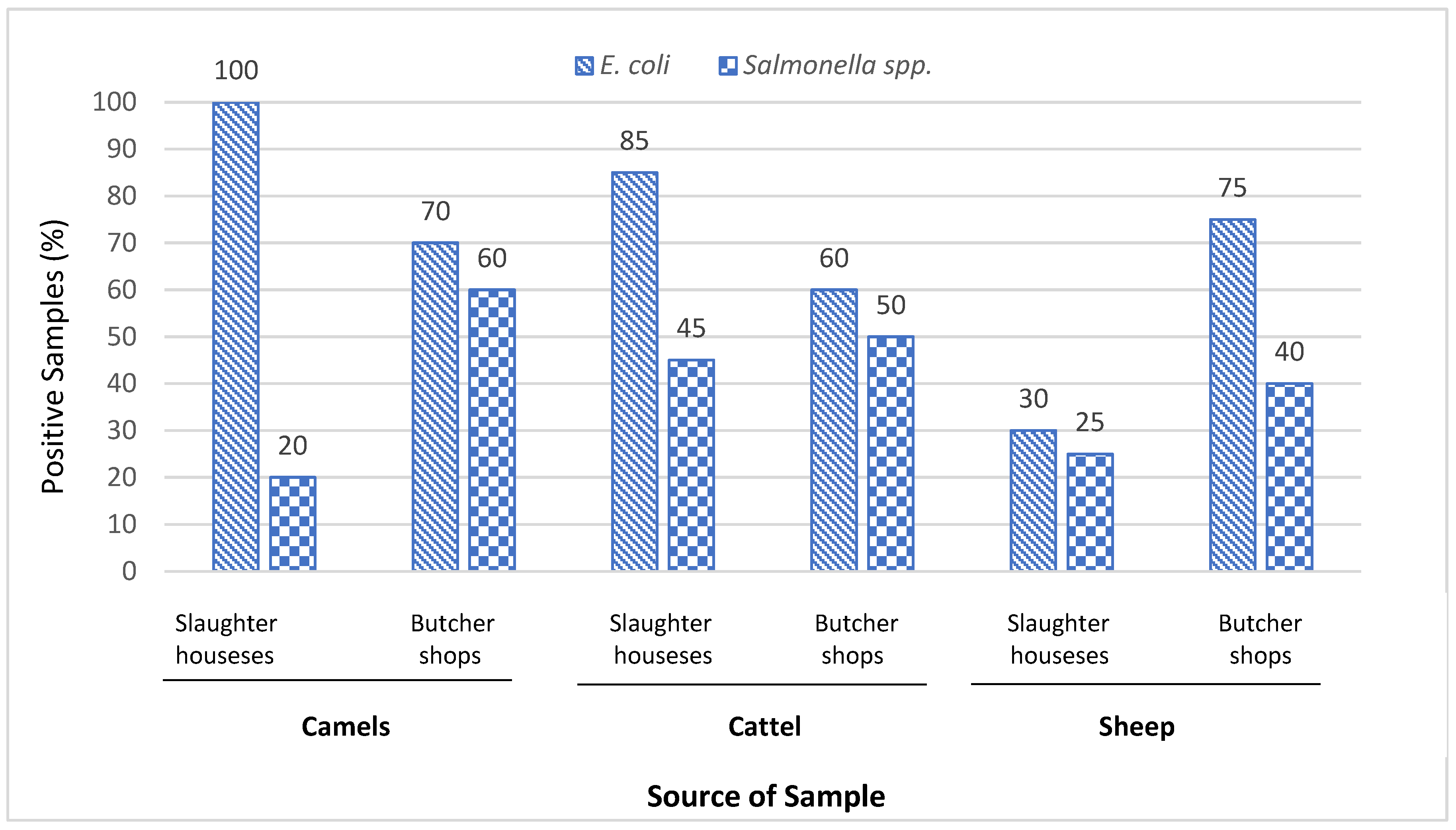
E. coli. was found in total 84 (70%) of camels, cattle and sheep samples in slaughterhouses (35.8%) and butcher shops (34.1%) which were higher than established limits in guidelines (Álvarez-Astorga, 2002). In detail,
E. coli was found to be positive in all camel carcasses (100%), 17 (85%) of cattle carcasses, and 6 (30%) of sheep carcasses obtained from slaughterhouses. While in butcher shops, the raw meat-cut samples contaminated with
E. coli were found in 14 (70%) camel samples, 12 (60%) of cattle samples, and 15 (75%) of sheep samples. There were no significant differences (P>0.05) in the occurrence of
E. coli between the two slaughterhouses, nor between butcher shops.
The presence of E. coli in food is a significant concern for public health and food safety. The bacteria of E. coli are typically found in the lower intestine of warm-blooded organisms. While many strains of E. coli are harmless, certain serotypes, including E. coli O157:H7, Shiga toxin-producing E. coli (STEC), and enterohemorrhagic E. coli (EHEC), poses serious illnesses (Bhaskar, 2017). These pathogens carry several different virulence factors, controlled by genes located on chromosomes, plasmids or phages (Abdalla et al., 2022). Animals are considered the main source of E. coli found in fresh meat. This mainly due to the abundance and natural presence of this bacteria in digestive system of many animals (Abdalla et al., 2022). The presence of E. coli in carcasses and raw meat typically indicates fecal contamination, which can occur during slaughtering, handling, packaging, or from cross-contamination with equipment, surfaces, or other foods (Ranasinghe et al., 2022). This presents a significant risk to public health and can result in foodborne illnesses (Zerabruk, Retta, Muleta, & Tefera, 2019).
The high rate of E. coli contamination is due to unhygienic practices, which is also an indication of the presence of unacceptable levels of other pathogens. It is worth mentioning that the samples of carcasses in this study were obtained after the evisceration phase. Thus, it is likely that worker hands and utensils used during slaughter process are one of the major causes of the E. coli prevalence in carcasses and meat cut samples. Similar results have been reported for abattoirs and retail outlets in Lahore found that 63 (45%) out of 140 samples were contaminated with E. coli (Ahmad et al., 2013). Previous studies have documented the prevalence of E. coli in meats. A study carried out in Nigeria reviled that the presence of E. coli in different types of meat including beef, pork, chicken, and mutton were 23.6%. Similarly, E. coli the was the most frequently isolated bacteria with 45.4% among all tested samples carried out in pig slaughterhouse in South Africa (Abdalla et al., 2022). The current study reviled in higher level of contamination in comparison with the study conducted in Ethiopia by Mohammed, Shimelis, Admasu, and Feyera (2014) found the prevalence of E. coli in meat samples collected from abattoirs was a 15.89%.
3.3. Detection of Salmonella spp.
The second most predominant isolated genera were
Salmonella spp. Out of total 120 different samples obtained from slaughterhouses and butcher shops, 48 (40%) tested positive for
Salmonella spp. distributed on 16 (13.3%) in camels, 19 (15.8%) in cattle, and 13 (10.8%) in sheep (
Table 2). A total of 48 different isolated of
Salmonella spp. were recovered from the positive sample’s comparison of two different strains in which S. enterica serotype Paratyphi A with 47 (39%) positives followed by S. arizonae with only 1 (0.8%) positive isolate as illustrated in
Table 2.
Figure 1 shows the prevalence of
Salmonella spp. and
E. coli in camel, cattle and sheep samples collected from slaughterhouses and butcher shops. In general, the samples of butcher showed more contamination with
Salmonella spp. (25%), compared with slaughterhouses (15%). S. paratyhpi A is causal agent for serious disease called paratyphoid fever, causing an estimated 5.4 million illnesses worldwide (Sanderson, Liu, Tang, & Johnston, 2015). As per the GSO 1016:2015, FAO/WHO (2005) and Health Protection Agency (2009), the
Salmonella spp. must not be detected in meat and meat products samples intended to be consumed by human (G.S.O., 2015).
Salmonella spp., a member of the Enterobacteriaceae family, are the second most common cause of global foodborne infections. Animal products, especially meat, are recognized as primary vectors transmitting Salmonella spp. to humans. In this study, the prevalence of Salmonella spp., in carcasses and meat cuts of camels, cattle, and sheep sourced from both slaughterhouses and butcher shops was investigated. According to our knowledge, a single study research was carried out by Mandour and Altabary (2014) on the microbial quality of camel and mutton carcasses at Al-Ahsa abattoirs. The findings revealed a 40% prevalence of Salmonella spp. in animal carcasses and meat cuts, aligning with several studies (Barkocy-Gallagher et al., 2003; El-Sharkaway, Samaha, & El-Galil, 2016; Sallam, Mohammed, Hassan, & Tamura, 2014). However, this contrasts entirely with the findings of with the previous research carried out by Mandour and Altabary (2014) who detected no Salmonella spp. in samples from the same region..
The elevated levels of Salmonella spp. across various animals underscore the suboptimal hygiene standards and practices during the slaughtering process. Additionally, exposing carcasses and meat cuts to high temperatures prior to refrigeration could markedly lead to acceleration of Salmonella spp. growth and other foodborne microorganisms. The prevalence of Salmonella spp. in this study was higher than some previous studies (Ahmad et al., 2013; Hyeon et al., 2011; Nurye & Demlie, 2021). However, some other research have reported more than 60% prevalence in raw meat samples (Ekli, Adzitey, & Huda, 2019; HATHAI & Yamaguchi, 2012). Based on the current results, S. enteric Paratyphi A, was the predominant serovars found in camels, cattle, and sheep samples with 39%. Whereas S. arizonae was found in one sample (0.8%). The distribution of Salmonella serovars in raw meat including beef, lambs and poultry can vary considerably among different regions of the world (Altaf Hussain et al., 2020). The difference could be influenced by local environmental factors. For instance, S. enteritidis was identified as the most prevalent contaminant in another study, with a rate of 37.5% (Sallam et al., 2014).
The rising incidence of Salmonella-induced foodborne illnesses underscores the urgency of addressing this public health concern. This study indicates that camels, cattle and sheep carcasses and meat cuts obtained from slaughterhouse and butcher shops in Al-Ahsa are heavily contaminated with Salmonella spp. this level of contamination in beef suggests poor sanitary conditions of raw meat production where it is being produced. Such extensive contamination points to inadequate sanitation in meat production facilities. Potential sources of contamination include fecal matter near butchering sites, direct contact during skinning, and contaminated water used for rinsing meat (McEvoy et al., 2000). Designing slaughtering lines to facilitate hygienic operations is critical. Nevertheless, effectively enforcing sanitary practices, including the regular disinfection of working tools, is crucial in mitigating the risk of microbiological contamination of carcasses.
3.4. Prevalence of other Enterobacteriaceae genera
Twenty-five different strains belonging to nineteen Enterobacteriaceae genera were isolated from camel, cattle and sheep samples obtained from slaughterhouses and butcher shops (
Table 2). Out of total 120 samples, the most occurrence genera isolated form carcasses and butcher shops were
E. coli 84 (70%) and
Salmonella spp. 47 (39.1%) samples. Other genera including
Proteus spp.,
Citrobacter spp., and
Serratia spp. were also identified in 16 (13.3%), 8 (6.6%), 4 (3.3%) of samples respectively. Meanwhile,
Klebsiella spp.,
Shigella spp., and
Yersinia spp. were also confirmed in meat samples at 1.6% and 0.83% and 0.83% respectively. The occurrence of pathogens belongs to Enterobacteriaceae family including
Salmonella spp.,
Shigella spp., and Yersinia spp. in meat and meat products may possess significant risks to human health. These bacteria cause foodborne illnesses, leading to severe complications and even death, particularly in vulnerable populations like young children, the elderly, pregnant women, and immunocompromised individuals (Gwida, Hotzel, Geue, & Tomaso, 2014). Despite the common practice in Saudi Arabia of cooking meat at high temperatures that sufficient to eliminate any present pathogens, it does not guarantee a reduction in the risk associated with cross-contamination. This is particularly concerning in the context of other types of food, such as fruits and vegetables, which are often consumed raw. Thus, the potential for cross-contamination presents a significant risk to other foods, underscoring the need for attention and precautionary measures (Bosilevac et al., 2015).
5. Conclusions
The present findings evidenced a considerable prevalence of pathogens, including E. coli, Salmonella spp. and other Enterobacteriaceae genera, across different types of livestock. The data indicates that animal carcasses exhibited high contamination levels of E. coli and Salmonella spp., with a rate of 70% and 40% respectively. E. coli was predominating among all other microorganisms with 70%. The carcasses of camels had 100%, cattle 58% and sheep 30%. While in butcher shops, E. coli had 70%, 60% and 75% in camel, cattle and sheep meat cuts respectively. Meanwhile, Salmonella spp. was positive in 40% of camel samples, 47.5% of cattle samples, and 32.5% in sheep samples, collected from both slaughterhouses and butcher shops. Twenty-five Enterobacteriaceae genera were also confirmed using PCR. Where sheep’s samples had the highest occurrence of Enterobacteriaceae with 15 different genera followed by camels and cattle samples with 14 different genera. Interestingly, identical diversity was observed in the samples from camels and cattle, which also contained 14 different genera. In conclusion, the pronounced prevalence of Enterobacteriaceae among camel, cattle, and sheep carcasses raises significant concerns regarding food safety. These findings underscore the need to enhance hygiene practices and implement stringent microbial monitoring procedures in slaughterhouses and butcher shops. The high prevalence of these pathogens in livestock highlights the potential risks to public health, illuminating the critical importance of further research in this area to prevent foodborne illnesses. Designing slaughtering lines to facilitate hygienic operations is evidently critical. Nevertheless, the effective enforcement of sanitary practices, including the regular disinfection of working tools, plays a crucial role in mitigating the risk of microbiological contamination of carcasses.
Author Contributions
Fahad Al- Asmari (F.A): Writing – review & editing, Supervision, Project administration, Funding acquisition, read and agreed to the published version of the manuscript. Siddiq Hamad (S.H): Data curation, Writing – original draft, preparation, read and agreed to the published version of the manuscript. Salah Al-Hashidi (S.A): Data curation, Writing – original draft, preparation, read and agreed to the published version of the manuscript.
Funding
The authors extend their appreciation to the Deputyship for Research & Innovation, Ministry of Education in Saudi Arabia for funding this research work (Project number INST114).
Data Availability Statement
Data Available when requested.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Abdalla, S.E.; Abia, A.L.; Amoako, D.G.; Perrett, K.; Bester, L.A.; Essack, S.Y. Food animals as reservoirs and potential sources of multidrug-resistant diarrheagenic E. coli pathotypes: Focus on intensive pig farming in South Africa. Onderstepoort Journal of Veterinary Research 2022, 89, 1963. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, M.; Sarwar, A.; Najeeb, M.; Nawaz, M.; Anjum, A.; Ali, M.; Mansur, N. Assessment of microbial load of raw meat at abattoirs and retail outlets. J. Anim. plant sci 2013, 23, 745–748. [Google Scholar]
- Ali, N.H.; Farooqui, A.; Khan, A.; Khan, A.Y.; Kazmi, S.U. Microbial contamination of raw meat and its environment in retail shops in Karachi, Pakistan. The Journal of Infection in Developing Countries 2010, 4, 382–388. [Google Scholar]
- Altaf Hussain, M.; Wang, W.; Sun, C.; Gu, L.; Liu, Z.; Yu, T.;... Hou, J. Molecular Characterization Of Pathogenic Salmonella Spp From Raw Beef In Karachi, Pakistan. Antibiotics, 2020; 9, 73.
- Álvarez-Astorga, e. a. Microbiological quality of retail chicken by-products in Spain. Meat science 2002, 62, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Barkocy-Gallagher, G.A.; Arthur, T.M.; Rivera-Betancourt, M.; Nou, X.; Shackelford, S.D.; Wheeler, T.L.; Koohmaraie, M. Seasonal prevalence of Shiga toxin–producing Escherichia coli, including O157: H7 and non-O157 serotypes, and Salmonella in commercial beef processing plants. Journal of food protection 2003, 66, 1978–1986. [Google Scholar] [CrossRef] [PubMed]
- Bhandare, S.G.; Sherikar, A.; Paturkar, A.; Waskar, V.; Zende, R. A comparison of microbial contamination on sheep/goat carcasses in a modern Indian abattoir and traditional meat shops. Food control 2007, 18, 854–858. [Google Scholar] [CrossRef]
- Bhaskar, S. (2017). Foodborne diseases—disease burden. In Food safety in the 21st century (pp. 1-10): Elsevier.
- Bintsis, T.; Litopoulou-Tzanetaki, E.; Robinson, R.K. Existing and potential applications of ultraviolet light in the food industry–a critical review. Journal of the Science of Food and Agriculture 2000, 80, 637–645. [Google Scholar] [CrossRef]
- Bohaychuk, V.; Gensler, G.; King, R.; Manninen, K.; Sorensen, O.; Wu, J. ;... McMullen, L. Occurrence of pathogens in raw and ready-to-eat meat and poultry products collected from the retail marketplace in Edmonton, Alberta, Canada. Journal of Food Protection 2006, 69, 2176-2182.
- Bosilevac, J.M.; Gassem, M.A.; Al Sheddy, I.A.; Almaiman, S.A.; Al-Mohizea, I.S.; Alowaimer, A.; Koohmaraie, M. Prevalence of Escherichia coli O157: H7 and Salmonella in camels, cattle, goats, and sheep harvested for meat in Riyadh. Journal of food protection 2015, 78, 89–96. [Google Scholar] [CrossRef]
- Brichta-Harhay, D.M.; Guerini, M.N.; Arthur, T.M.; Bosilevac, J.M.; Kalchayanand, N.; Shackelford, S.D. ;... Koohmaraie, M. Salmonella and Escherichia coli O157: H7 contamination on hides and carcasses of cull cattle presented for slaughter in the United States: an evaluation of prevalence and bacterial loads by immunomagnetic separation and direct plating methods. Applied and Environmental Microbiology 2008, 74, 6289-6297.
- CDC. (2010). What is Salmonellosis?. Retrieved from http: www.cdc.gov/ Salmonella/-general/index.
- Cox, N., Richardson, L., Cason, J., Buhr, R., Vizzier-Thaxton, Y., Smith, D., . . . Doyle, M. (2010). Comparison of
neck skin excision and whole carcass rinse sampling methods for microbiological evaluation of broiler
carcasses before and after immersion chilling. Journal of food protection 2010, 73, 976–980. [CrossRef]
- Ekli, R.; Adzitey, F.; Huda, N. (2019). Prevalence of resistant Salmonella spp. isolated from raw meat and liver of cattle in the Wa Municipality of Ghana. Paper presented at the IOP Conference Series: Earth and Environmental Science.
- El-Sharkaway, S.; Samaha, I.A.; El-Galil, H. Prevalence of pathogenic microorganisms in raw meat products from retail outlets in Alexandria province. Alexandria Journal of Veterinary Sciences 2016, 51, 374–380. [Google Scholar]
- G.S.O. (2015). microbiological criteria for foodstuffs In (Vol. GSO 1016).
- Gwida, M.; Hotzel, H.; Geue, L.; Tomaso, H. (2014). Occurrence of Enterobacteriaceae in raw meat and in human samples from Egyptian retail sellers. International scholarly research notices, 2014.
- HATHAI, T.; Yamaguchi, R. Molecular characterization of antibiotic-resistant Salmonella isolates from retail meat from markets in Northern Vietnam. Journal of food protection 2012, 75, 1709–1714. [Google Scholar] [CrossRef] [PubMed]
- Hyeon, J.-Y., Chon, J.-W., Hwang, I.-G., Kwak, H.-S., Kim, M.-S., Kim, S.-K., . . . Seo, K.-H. (2011). Prevalence, Antibiotic Resistance, and Molecular Characterizatio of Salmonella Serovars in Retail Meat Products. Journal of food protection 2011, 74, 161–166. [CrossRef] [PubMed]
- Jaja, I.F.; Green, E.; Muchenje, V. Aerobic mesophilic, coliform, Escherichia coli, and Staphylococcus aureus counts of raw meat from the formal and informal meat sectors in South Africa. International Journal of Environmental Research and Public Health 2018, 15, 819. [Google Scholar] [CrossRef] [PubMed]
- Jay, J.M.; Loessner, M.J.; Golden, D.A. (2005). The HACCP and FSO systems for food safety. Modern Food Microbiology, 497-515.
- Mandour, M.A.; Altabary, G.F. Slaughtered at Al-Ahsaa Abattoir, Saudi Arabia. Journal of Animal and Veterinary Advances 2014, 13, 1179–1184. [Google Scholar]
- McEvoy, J.; Doherty, A.; Finnerty, M.; Sheridan, J.; McGuire, L.; Blair, I.. . . Harrington, D. The relationship between hide cleanliness and bacterial numbers on beef carcasses at a commercial abattoir. Letters in Applied Microbiology 2000, 30, 390–395. [Google Scholar] [CrossRef]
- Meyer, C.; Thiel, S.; Ullrich, U.; Stolle, A. Salmonella in raw meat and by-products from pork and beef. Journal of food protection 2010, 73, 1780–1784. [Google Scholar] [CrossRef]
- Mohammed, O.; Shimelis, D.; Admasu, P.; Feyera, T. Prevalence and antimicrobial susceptibility pattern of E. coli isolates from raw meat samples obtained from abattoirs in Dire Dawa City, eastern Ethiopia. Int J Microbiol Res 2014, 5, 35–39. [Google Scholar]
- Nurye, M.; Demlie, M. Assessment of hygienic practices and microbial quality of meat at slaughterhouses and butcher’s shops in West Hararghe Zone, Ethiopia. Abyssinia Journal of Science and Technology 2021, 6, 32–41. [Google Scholar]
- Nychas, G.-J. E.; Skandamis, P.N.; Tassou, C.C.; Koutsoumanis, K.P. Meat spoilage during distribution. Meat science 2008, 78, 77–89. [Google Scholar] [CrossRef]
- Przemieniecki, S.W.; Kurowski, T.P.; Kotlarz, K.; Krawczyk, K.; Damszel, M.; Karwowska, A. Plant growth promoting properties of Serratia fonticola ART-8 and Pseudomonas putida ART-9 and their effect on the growth of spring wheat (Triticum aestivum L.). Environmental Biotechnology 2016, 12, 35--39.
- Ranasinghe, R.; Satharasinghe, D.; Anwarama, P.; Parakatawella, P.; Jayasooriya, L.; Ranasinghe, R. . . . Nakaguchi, Y. Prevalence and Antimicrobial Resistance of Escherichia coli in Chicken Meat and Edible Poultry Organs Collected from Retail Shops and Supermarkets of North Western Province in Sri Lanka. Journal of Food Quality 2022, 2022. [Google Scholar]
- Sallam, K.I.; Mohammed, M.A.; Hassan, M.A.; Tamura, T. Prevalence, molecular identification and antimicrobial resistance profile of Salmonella serovars isolated from retail beef products in Mansoura, Egypt. Food control 2014, 38, 209–214. [Google Scholar] [CrossRef]
- Sanderson, K. E., Liu, S.-L., Tang, L., Johnston, R. N. (2015). Salmonella Typhi and Salmonella Paratyphi A. In
Molecular medical microbiology (pp. 1275-1306): Elsevier.
- Terentjeva, M.; Avsejenko, J.; Streikiša, M.; Utināne, A.; Kovaļenko, K.; Bērziņš, A. Prevalence and antimicrobial resistance of Salmonella in meat and meat products in Latvia. Annals of Agricultural and Environmental Medicine 2017, 24. [Google Scholar] [CrossRef] [PubMed]
- Todd, E.C.; Greig, J.D.; Bartleson, C.A.; Michaels, B.S. Outbreaks where food workers have been implicated in the spread of foodborne disease. Part 6. Transmission and survival of pathogens in the food processing and preparation environment. Journal of food protection 2009, 72, 202–219. [Google Scholar] [CrossRef]
- Vogt, R.L.; Dippold, L. Escherichia coli O157: H7 outbreak associated with consumption of ground beef, June–July 2002. Public health reports 2005, 120, 174–178. [Google Scholar] [CrossRef] [PubMed]
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. PCR protocols: a guide to methods and applications 1990, 18, 315–322. [Google Scholar]
- Zerabruk, K.; Retta, N.; Muleta, D.; Tefera, A.T. (2019). Assessment of microbiological safety and quality of minced meat and meat contact surfaces in selected butcher shops of Addis Ababa, Ethiopia. Journal of Food Quality, 2019.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).