Submitted:
09 October 2023
Posted:
10 October 2023
You are already at the latest version
Abstract
Keywords:
INTRODUCTION
MATERIALS AND METHODS
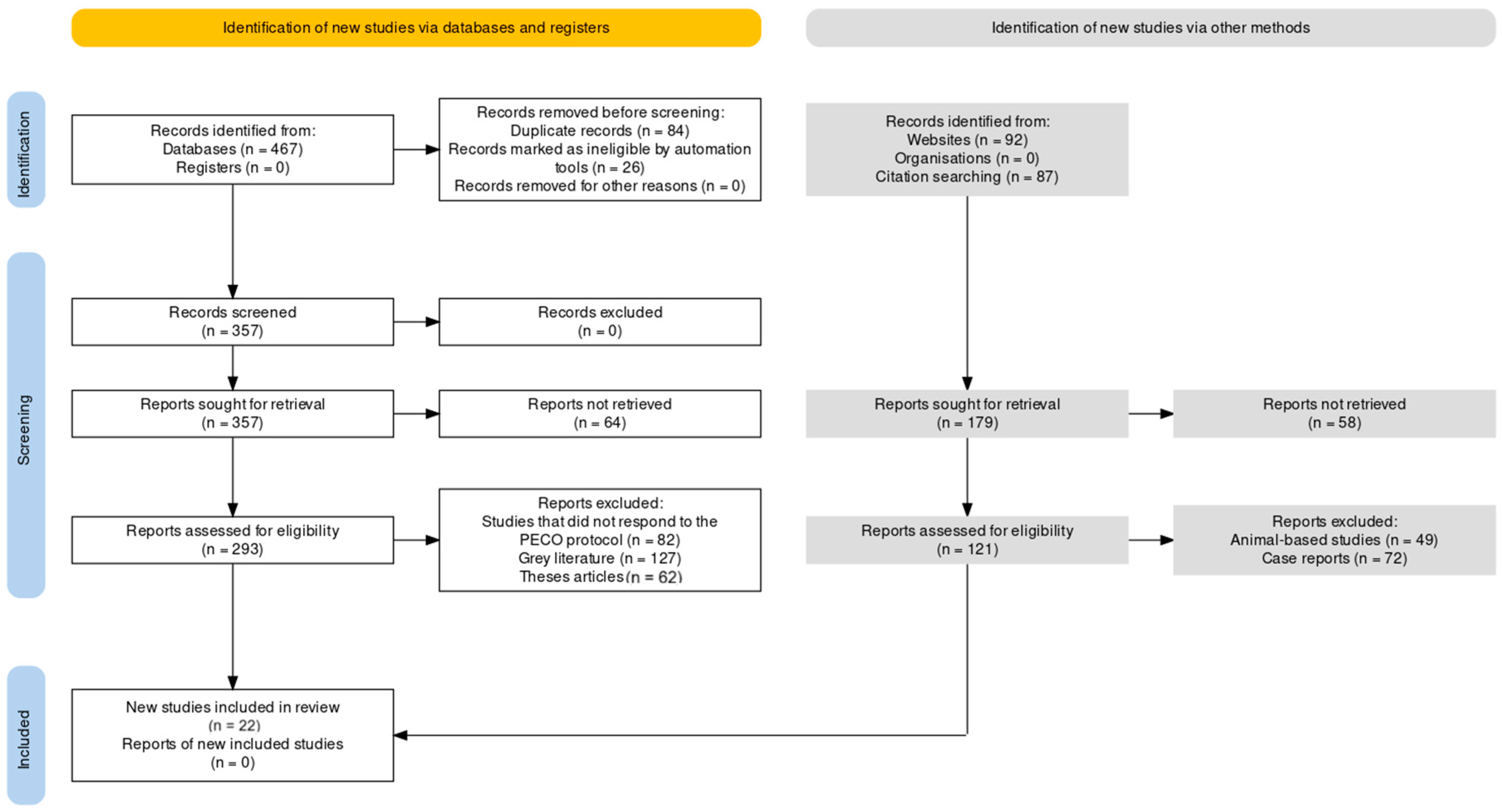
PRISMA and PECO for the review
Database search protocol
Selection criteria
Data extraction
Assessment of Bias
RESULTS
| Study | Aims | Study Design | Methodology Assessed | Type of Mitochondrial Intervention Assessed |
|---|---|---|---|---|
| Brouwers et al [26] | To assess the effect of a polynutrient supplement on fatigue and physical activity of patients with CFS. | Prospective randomized placebo-controlled, double-blind trial. | Fifty-three patients (16 males, 37 females) fulfilling the CDC criteria of CFS. | The intervention—a polynutrient supplement containing several vitamins, minerals and (co)enzymes, or placebo, twice daily for 10 weeks. |
| Castro et al [27] | We conducted an 8-week, randomized, double-blind placebo-controlled trial to evaluate the benefits of oral CoQ10 (200 mg/day) plus NADH (20 mg/day) supplementation on fatigue and biochemical parameters in 73 Spanish CFS patients. | Randomized, double-blind placebo-controlled trial. | This study was registered in ClinicalTrials.gov (NCT02063126). | The intervention was oral CoQ10 (200 mg/day) plus NADH (20 mg/day) supplementation for 8 weeks. |
| Hobday et al [28] | This study aims to determine the efficacy of dietary intervention on level of fatigue and quality of life (QoL) in individuals with CFS. | A 24-week randomized intervention study. | Conducted with 52 individuals diagnosed with CFS. | Patients were randomized to either a low sugar low yeast (LSLY) or healthy eating (HE) dietary interventions. |
| Joseph et al [29] | Research question: Does neurovascular dysregulation contribute to exercise intolerance in ME/CFS, and can its treatment improve exercise capacity? | Single-center, randomized, double-blind, placebo-controlled trial. | Forty-five subjects with ME/CFS were enrolled. | Subjects were assigned to receive a 60-mg dose of oral pyridostigmine or placebo after an invasive cardiopulmonary exercise test (iCPET). |
| Keller et al [30] | Investigate the difference between a first and second CPET in ME/CFS patients to identify individuals with ME/CFS, document their extent of disability, and provide a physiological basis for prescribing physical activity and a metric of functional impairment. | 22 subjects diagnosed with ME/CFS completed two repeat CPETs separated by 24 h. | Measures of oxygen consumption (VO2), heart rate (HR), minute ventilation (Ve), workload (Work), and respiratory exchange ratio (RER) were made at maximal (peak) and ventilatory threshold (VT) intensities. Data were analyzed using ANOVA and Wilcoxon’s Signed-Rank Test (for RER). | Repeat CPETs |
| Kujawski et al [31] | Explore the tolerability and effect of static stretching (SS) and whole body cryotherapy (WBC) upon fatigue, daytime sleepiness, cognitive functioning and objective and subjective autonomic nervous system functioning in those with Chronic Fatigue Syndrome (CFS) compared to a control population. | Thirty-two CFS and eighteen healthy controls (HC) participated in 2 weeks of a SS + WBC programme. | This programme was composed of five sessions per week, 10 sessions in total. | Static stretching (SS) and whole body cryotherapy (WBC) |
| Kujawski S et al [32] | Compare the functional interrelation of fatigue and cognitive, cardiovascular and autonomic nervous systems in a group of Chronic Fatigue Syndrome (CFS) patients with healthy individuals at different stages of analysis: at baseline and after changes induced by whole-body cryotherapy (WBC) combined with a static-stretching (SS) program. | The study included 32 patients (Fukuda criteria) and 18 healthy controls. | Fatigue, cognitive, cardiovascular and autonomic function and arterial stiffness were measured before and after 10 sessions of WBC with SS. | Whole-body cryotherapy (WBC) combined with a static-stretching (SS) program |
| Maes et al [33] | To examine the role of Coenzyme Q10 (CoQ10) in ME/CFS, assess its plasma levels in patients and normal controls, and explore the relationships between CoQ10 and the severity of ME/CFS as measured by the FibroFatigue (FF) scale. | Observational study | Plasma CoQ10 was assayed in patients with ME/CFS and in normal controls; the relationships between CoQ10 and the severity of ME/CFS were measured using the FF scale. | CoQ10 supplementation was suggested to normalize the low CoQ10 syndrome and the inflammation, oxidative & nitrosative stress (IO&NS) disorders. |
| Mandarano et al [34] | To investigate immune metabolism in ME/CFS, with a focus on T cell metabolism. | Observational study | Immune metabolism was investigated by isolating CD4+ and CD8+ T cells from patients with ME/CFS and healthy controls. Glycolysis and mitochondrial respiration in resting and activated T cells were analyzed, along with markers related to cellular metabolism and plasma cytokines. | Not specified (The study focused on assessing the metabolic alterations in T cells of ME/CFS patients rather than assessing a specific intervention). |
| McDermott et al [35] | To evaluate the effectiveness of BioBran MGN-3, a putative NK cell stimulant, in reducing fatigue in CFS patients. | Randomized, double-blind, placebo-controlled trial | Patients with CFS were given oral BioBran MGN-3 for 8 weeks or a placebo equivalent. The primary outcome measure was the Chalder physical fatigue score. Self-reported fatigue measures, self-assessment of improvement, change in key symptoms, quality of life, anxiety, and depression measures were also included. | BioBran MGN-3, a putative NK cell stimulant. |
| Moore et al [36] | To characterize the duration and severity of Post-Exertional Malaise (PEM) symptoms in ME/CFS subjects following two cardiopulmonary exercise tests (2-day CPET). | 2-day CPET study on 80 ME/CFS subjects and 64 controls. Symptom Severity Scale (SSS) scores were obtained at various time points. | Use of 2-day CPET and SSS to measure PEM in ME/CFS subjects. | Physical exercise as an intervention to assess mitochondrial function by examining post-exercise recovery time and symptom severity. |
| Nelson et al [37] | To establish cut-off values for differentiating between ME/CFS patients and healthy controls based on the onset of ventilatory threshold (VT) during consecutive-day CPET. | CPET on a cycle-ergometer on 2-consecutive days was carried out on 16 ME/CFS patients and 10 healthy controls. Various parameters were assessed on both days. | Use of consecutive-day CPET, HR, ventilation, RPE, and work rate measurements to establish VT onset differences. | Physical exercise as an intervention to assess mitochondrial function by examining changes in ventilatory threshold. |
| Rao et al [38] | To determine if orally administered probiotics could improve symptoms of depression and anxiety in adult patients with chronic fatigue syndrome. | A randomized pilot study with 39 CFS patients receiving either 24 billion colony forming units of Lactobacillus casei strain Shirota (LcS) or a placebo daily for two months. | Patients provided stool samples and completed the Beck Depression and Beck Anxiety Inventories before and after the intervention. | Oral administration of probiotics as an intervention to assess mitochondrial function by observing changes in gut microbiota and its relation to ME/CFS symptoms. |
| Rueda et al [39] | To understand how calcium regulates respiration and whether this is dependent on the increase in ATP demand or to Ca(2+) itself. | Experimental study on intact neurons exposed to different workloads in the absence and presence of Ca(2+). | Assessed [Na(+)]i, [Ca(2+)]i and [ATP]i dynamics. Investigated the role of aspartate-glutamate exchanger ARALAR/AGC1/Slc25a12 and ATP-Mg/Pi exchanger SCaMC-3/APC2/Slc25a23 in Ca(2+)-regulated mitochondrial metabolite transport. | Studied the role of Ca(2+) in regulating respiration and activating mitochondrial metabolite transport. |
| Sandvik et al [40] | To investigate large-vessel and small-vessel endothelial function in ME/CFS patients. | A substudy of the RituxME trial, a national, multicenter, randomized, double-blind, placebo-controlled phase III study on the effect of rituximab vs. placebo in ME/CFS patients in Norway. | Assessed Flow-mediated dilation (FMD) and post-occlusive reactive hyperemia (PORH) at baseline and after 18 months of treatment. Also measured symptom severity and various physical function measures. | Evaluated the effect of rituximab vs. placebo on endothelial function. |
| Sathyapalan et al [41] | To compare the effect of high cocoa liquor/polyphenol rich chocolate (HCL/PR) vs. simulated iso-calorific chocolate (cocoa liquor free/low polyphenols(CLF/LP)) on fatigue and residual function in subjects with chronic fatigue syndrome. | Double blinded, randomised, clinical pilot crossover study. | Assessed fatigue using the Chalder Fatigue Scale and residual function using the London Handicap scale. Also evaluated the Hospital Anxiety and Depression score. | Assessed the effect of high cocoa liquor/polyphenol rich chocolate (HCL/PR) vs. simulated iso-calorific chocolate (cocoa liquor free/low polyphenols(CLF/LP)) on fatigue and residual function. |
| Strayer et al [42] | To evaluate the effect of rintatolimod therapy based on disease duration in ME/CFS patients. | Phase II and Phase III double-blind, placebo-controlled, randomized, multi-site clinical trials. | The clinical activity of rintatolimod was evaluated by exercise treadmill tolerance (ETT) using a modified Bruce protocol. The ITT population (n = 208) was divided into two subsets of symptom duration. | The intervention assessed was rintatolimod, a selective TLR3 agonist. |
| Sullivan et al [43] | To evaluate the effect of Lactobacillus paracasei ssp. paracasei F19, Lactobacillus acidophilus NCFB 1748 and Bifidobacterium lactis Bb12 on fatigue and physical activity in CFS patients. | This was an observational study with a two-week baseline period, four weeks of probiotic intake, and a four-week follow-up period. | Fatigue, health, and physical activity were assessed by the use of the Visual Analogue Scales and the SF-12 Health Survey. Faecal samples were collected and the normal microflora was analysed. | The intervention assessed was the intake of a probiotic product containing Lactobacillus paracasei ssp. paracasei F19, Lactobacillus acidophilus NCFB 1748 and Bifidobacterium lactis Bb12. |
| Thambirajah et al [44] | To determine whether heat shock protein (HSP) expression is altered in CFS patients before and after exercise. | Observational study with exercise as the intervention. | HSP27, HSP60, HSP70 and HSP90 expression from 6 CFS patients and 7 age- and sex-matched controls were examined by western blot analysis of peripheral blood mononuclear cells immediately before, after, and at 1 day and 7 days following a standardized treadmill exercise. | |
| GK et al [45] | Measure the IGF1 and IGF binding protein (IGFBP) 3 status of CFS patients compared to age- and gender-matched neighborhood controls, and to assess the effect of Acclydine on fatigue severity, functional impairment, and biologically active IGF1 level (IGFBP3/IGF1 ratio). | A randomized, placebo-controlled, double-blind clinical trial. Fifty-seven adult patients who fulfilled the US Centers for Disease Control and Prevention criteria for CFS were studied. | IGF status of 22 CFS patients was compared to that of 22 healthy age- and gender-matched neighborhood control individuals. Outcome measures were fatigue severity (Checklist Individual Strength, subscale fatigue severity [CIS-fatigue]), functional impairment (Sickness Impact Profile-8 [SIP-8]), and biologically active IGF1 serum concentrations. Analyses were on an intention-to-treat basis. | Acclydine treatment for 14 weeks. |
| Wilshire et al [46] | Present results based on the original protocol-specified procedures and evaluate the conclusions from the trial as a whole. | Data from a recent Freedom of Information request were used to closely approximate these procedures. | The primary outcome measure was overall improvement rates. Secondary measures included rates of recovery and self-report measures. | Cognitive Behavioral Therapy (CBT) or Graded Exercise Therapy (GET). |
| Witham et al [47] | Test whether high-dose intermittent oral vitamin D therapy improved markers of vascular health and fatigue in patients with chronic fatigue syndrome. | Parallel-group, double-blind, randomised placebo-controlled trial. Patients with chronic fatigue syndrome according to the Fukuda (1994) and Canadian (2003) criteria were studied. | The primary outcome was arterial stiffness measured using carotid-femoral pulse wave velocity at 6 months. Secondary outcomes included flow-mediated dilatation of the brachial artery, blood pressure, cholesterol, insulin resistance, markers of inflammation and oxidative stress, and the Piper Fatigue scale. | High-dose intermittent oral vitamin D3 therapy (100,000 units every 2 months for 6 months). |
| Study | Parameters Assessed | Inferences Observed | Results Observed |
|---|---|---|---|
| Brouwers et al [26] | Effect of a polynutrient supplement on fatigue and physical activity of CFS patients. CIS fatigue score, number of CDC symptoms, and SIP8 score. | No significant differences were found between the placebo and the treated group on any of the outcome measures. | CIS fatigue +2.16 (95%CI -4.3 to +4.39, p=0.984); CDC symptoms +0.42 (95%CI -0.61 to +1.46, p=0.417); SIP8 +182 (95%CI -165 to +529, p=0.297). No patient reported full recovery. |
| Castro et al [27] | Benefits of oral CoQ10 (200 mg/day) plus NADH (20 mg/day) supplementation on fatigue and biochemical parameters in CFS patients. | A significant improvement of fatigue and a recovery of the biochemical parameters were reported in the treated group versus placebo. | NAD+/NADH (p<0.001), CoQ10 (p<0.05), ATP (p<0.05), and citrate synthase (p<0.05) were significantly higher, and lipoperoxides (p<0.05) were significantly lower in blood mononuclear cells of the treated group. |
| Hobday et al [28] | Efficacy of a low sugar low yeast (LSLY) diet or healthy eating (HE) dietary interventions on level of fatigue and QoL in CFS patients. | No statistically significant differences were observed on primary outcome measurements between the two diets. | In this randomized control trial, a LSLY diet appeared to be no more efficacious on levels of fatigue or QoL compared to HE. |
| Joseph et al [29] | Effect of oral pyridostigmine on exercise intolerance in ME/CFS patients. Peak exercise oxygen uptake (Vo2), exercise pulmonary and systemic hemodynamics, and gas exchange. | Pyridostigmine improves peak Vo2 in ME/CFS by increasing cardiac output and right ventricular filling pressures. | The peak Vo2 increased after pyridostigmine but decreased after placebo (13.3 ± 13.4 mL/min vs -40.2 ± 21.3 mL/min; P < .05). The treatment effect of pyridostigmine was 53.6 mL/min (95% CI, -105.2 to -2.0). |
| Keller et al [30] | Measures of oxygen consumption (VO2), heart rate (HR), minute ventilation (Ve), workload (Work), and respiratory exchange ratio (RER) at maximal (peak) and ventilatory threshold (VT) intensities in ME/CFS patients for two repeat CPETs separated by 24 h. | A disparity between a first and second CPET could serve to identify individuals with ME/CFS, document their extent of disability, and provide a physiological basis for prescribing physical activity as well as a metric of functional impairment. | Significant decreases from CPET1 to CPET2 in VO2peak (13.8%), HRpeak (9 bpm), Ve peak (14.7%), and Work@peak (12.5%). Decreases in VT measures included VO2@VT (15.8%), Ve@VT (7.4%), and Work@VT (21.3%). Peak RER was high (≥1.1) and did not differ between tests, indicating maximum effort by participants during both CPETs. |
| Kujawski et al [31] | Fatigue, daytime sleepiness, cognitive functioning, and objective and subjective autonomic nervous system functioning in Chronic Fatigue Syndrome (CFS) patients and healthy controls for 2 weeks of a SS + WBC programme. | The tolerability and effect of static stretching (SS) and whole body cryotherapy (WBC) upon aforementioned aspects in CFS patients compared to a control population. | A significant decrease in fatigue was noted in the CFS group in response to SS + WBC. Improvements in some domains of cognitive functioning (speed of processing visual information and set-shifting) were noted in both CFS and HC groups. WBC was well tolerated by those with CFS and led to symptomatic improvements associated with changes in cardiovascular and autonomic function. |
| Kujawski S et al [32] | Fatigue, cognitive, cardiovascular and autonomic function and arterial stiffness in CFS patients and healthy controls before and after 10 sessions of WBC with SS. | Comparison of the functional interrelation of fatigue and cognitive, cardiovascular and autonomic nervous systems in a group of CFS patients with healthy individuals at different stages of analysis. | Disturbance in homeostasis was observed in patients. Higher stress and eccentricity were observed in the CFS group. Increased fatigue was related to baroreceptor function, and baroreceptor function was in turn related to aortic stiffness in the CFS group but no such relationships were observed in the control group. Differences in the network structure underlying the interrelation among the four measured criteria were observed in both groups, before the intervention and after ten sessions of whole cryotherapy with a static stretching exercise. |
| Maes et al [33] | Plasma CoQ10 levels, severity of ME/CFS as measured by the FibroFatigue (FF) scale, CoQ10 relationship with total FF scale score, fatigue, autonomic symptoms, concentration, and memory disturbances. | Lowered levels of CoQ10 play a role in the pathophysiology of ME/CFS. Symptoms such as fatigue, autonomic and neurocognitive symptoms may be caused by CoQ10 depletion. Lower CoQ10 is an independent predictor of chronic heart failure (CHF) and mortality due to CHF. | Plasma CoQ10 was significantly lower in ME/CFS patients than in normal controls. Significant inverse relationships between CoQ10 and the total score on the FF scale, fatigue, and autonomic symptoms. Patients with very low CoQ10 suffered significantly more from concentration and memory disturbances. |
| Mandarano et al [34] | Metabolism of CD4+ and CD8+ T cells in ME/CFS patients and healthy controls, glycolysis and mitochondrial respiration in resting and activated T cells, markers related to cellular metabolism, plasma cytokines. | Patients have impaired T cell metabolism consistent with ongoing immune alterations in ME/CFS. Significant correlations between measures of T cell metabolism and plasma cytokine abundance in ME/CFS patients differ from those seen in healthy control subjects. | ME/CFS CD8+ T cells had reduced mitochondrial membrane potential compared with those from healthy controls. Both CD4+ and CD8+ T cells from patients with ME/CFS had reduced glycolysis at rest, whereas CD8+ T cells also had reduced glycolysis following activation. |
| McDermott et al [35] | Chalder physical fatigue score, self-reported fatigue measures, self-assessment of improvement, change in key symptoms, quality of life, anxiety, depression measures. | No significant difference observed between the effectiveness of BioBran MGN-3 and placebo in reducing fatigue in CFS patients, despite overall improvement in both groups over the study duration. | Both groups showed marked improvement over the study duration, but without significant differences. Mean improvement in the Chalder fatigue score (physical scale) was 0.3 lower in the BioBran group. |
| Moore et al [36] | Symptom Severity Scale (SSS), recovery time following 2-day CPET, PEM response. | ME/CFS subjects took an average of about two weeks to recover from a 2-day CPET, whereas sedentary controls needed only two days. | There was a highly significant difference in judged recovery time (ME/CFS = 12.7 ± 1.2 d; CTL = 2.1 ± 0.2 d, mean ± s.e.m., Chi2 = 90.1, p < 0.0001). The range of ME/CFS patient recovery was 1-64 days, while the range in CTL was 1-10 days. |
| Nelson et al [37] | Heart rate (HR), ventilation, ratings of perceived exertion (RPE), work rate (WR) at VT on two consecutive days of CPET. | The decrease in WR at VT of 6.3-9.8% on the 2nd day of consecutive-day CPET may represent an objective biomarker that can be used to assist with the diagnosis of ME/CFS. | WR at VT decreased from day 1 to day 2 and by a greater magnitude in ME/CFS patients (p < 0.01 group × time interaction). |
| Rao et al [38] | Beck Depression and Beck Anxiety Inventories, changes in gut microbiota, specifically Bifidobacteria and Lactobacillus levels. | Ingestion of the probiotic capsules contributed towards the predominance of bacteria that are associated with a healthy gastrointestinal system. | Compared to the placebo control group, the treatment group showed moderate increases in fecal total aerobes and anaerobes and significant increases in fecal total Bifidobacteria and Lactobacillus. |
| Rueda et al [39] | [Na(+)]i, [Ca(2+)]i and [ATP]i dynamics in intact neurons exposed to different workloads in the absence and presence of Ca(2+). Role of aspartate-glutamate exchanger ARALAR/AGC1/Slc25a12 and ATP-Mg/Pi exchanger SCaMC-3/APC2/Slc25a23 in Ca(2+)-regulated mitochondrial metabolite transport. | Ca(2+) might regulate respiration by activating metabolite transport in mitochondria. ARALAR-MAS is a major contributor of Ca(2+)-stimulated respiration in neurons by providing increased pyruvate supply to mitochondria. | The lack of SCaMC-3 resulted in a smaller Ca(2+)-dependent stimulation of respiration only at high workloads. The lack of ARALAR reduced basal OCR in intact neurons using glucose as energy source and completely suppressed the OCR responses to moderate and small workloads. |
| Sandvik et al [40] | Flow-mediated dilation (FMD) and post-occlusive reactive hyperemia (PORH) in ME/CFS patients vs healthy controls. Symptom severity and various physical function measures. | ME/CFS patients had markedly reduced FMD and significantly lower microvascular regulation measured by PORH than healthy controls. | ME/CFS patients had markedly reduced FMD compared to healthy controls at baseline, and significantly lower microvascular regulation measured by PORH than healthy controls. There were no differences between the treatment and placebo groups in symptom changes or vascular measures. PORH, but not FMD, was similarly improved. |
| Sathyapalan et al [41] | Fatigue and residual function in subjects with chronic fatigue syndrome consuming high cocoa liquor/polyphenol rich chocolate (HCL/PR) vs simulated iso-calorific chocolate (cocoa liquor free/low polyphenols(CLF/LP)). | Subjects with CFS showed improvement in fatigue and residual function when consuming high cocoa liquor/polyphenol rich chocolate. | The Chalder Fatigue Scale score improved significantly after 8 weeks of the HCL/PR chocolate arm, but deteriorated significantly when subjects were given simulated iso-calorific chocolate. Residual function, as assessed by the London Handicap scale, also improved significantly after the HCL/PR arm and deteriorated after iso-calorific chocolate. |
| Strayer et al [42] | The clinical activity of rintatolimod, exercise treadmill tolerance (ETT) using a modified Bruce protocol; Symptom duration. | The study aimed to identify a demographic subset of ME/CFS patients that respond better to rintatolimod therapy, focusing on symptom duration. | The Target Subset, with a symptom duration of 2-8 years, showed more than twice the placebo-adjusted percentage improvements in exercise duration and vertical rise than the ITT population. The Non-Target Subset showed no significant ETT response to rintatolimod compared to placebo. Within the Target Subset, 51.2% of rintatolimod-treated patients improved their exercise duration by ≥25% (p = 0.003). |
| Sullivan et al [43] | The effect of a probiotic product on fatigue and physical activity; Fatigue and health were assessed through the Visual Analogue Scales and the SF-12 Health Survey; Analyses of faecal samples. | The study aimed to evaluate the effect of specific probiotic strains on fatigue and physical activity in CFS patients. | Neurocognitive functions improved during the study period while there were no significant changes in fatigue and physical activity scores. No major changes occurred in the gastrointestinal microflora. At the end of the study, 6 of 15 patients reported that they had improved according to the assessment described. |
| Thambirajah et al [44] | HSP27, HSP60, HSP70 and HSP90 expression from 6 CFS patients and 7 age- and sex-matched controls were examined by western blot analysis of peripheral blood mononuclear cells before, after, and 1 and 7 days following a standardized treadmill exercise. | The study sought to determine whether heat shock protein expression is altered in CFS patients before and after exercise. | Basal HSP27 was higher among CFS patients than in controls. These levels in CFS patients decreased immediately post-exercise and remained below basal levels at day 1 post-exercise. Similar patterns of declining HSP levels in CFS patients were also observed for HSP60 and HSP90 at day 7 post-exercise compared with basal levels. In contrast, HSP60 levels in control subjects increased at day 1 and day 7 post-exercise compared to levels immediately post-exercise. |
| GK et al [45] | IGF1 and IGFBP3 status, fatigue severity, functional impairment, and biologically active IGF1 level | No difference in IGF status between CFS patients and healthy controls. Acclydine treatment did not result in significant differences compared to placebo across measures. | CIS-fatigue +1.1 (95% CI −4.4 to +6.5, p = 0.70), SIP-8 +59.1 (95% CI −201.7 to +319.8, p = 0.65), and IGFBP3/IGF1 ratio −0.5 (95% CI −2.8 to +1.7, p = 0.63) |
| Wilshire et al [46] | Overall improvement rates, rates of recovery, and secondary self-report measures | Significant effects of treatment group on primary outcome measure, but CBT or GET groups did not significantly outperform control after correcting for multiple comparisons. Modest treatment effects on self-reported measures that did not endure beyond 2 years. | Low and non-significant recovery rates across treatment groups. Self-report measure effects did not endure beyond 2 years. |
| Witham et al [47] | Arterial stiffness, flow-mediated dilatation of the brachial artery, blood pressure, cholesterol, insulin resistance, markers of inflammation and oxidative stress, and the Piper Fatigue scale | No effect of high-dose intermittent oral vitamin D therapy on pulse wave velocity, other vascular and metabolic outcomes, or Piper Fatigue scale. | At 6 months, adjusted treatment effect on pulse wave velocity 0.0 m/s (95% CI -0.6 to 0.6; p = 0.93), no improvement in other vascular and metabolic outcomes, Piper Fatigue scale 0.2 points (95% CI -0.8 to 1.2; p = 0.73) |
DISCUSSION
CONCLUSION
References
- Carruthers BM, Van de Sande MI, De Meirleir KL, Klimas NG, Broderick G, Mitchell T, et al. Myalgic encephalomyelitis: international Consensus Criteria. J Intern Med. 2011;270(4):327–338. [CrossRef]
- Fukuda K, Straus SE, Hickie I, Sharpe MC, Dobbins JG, Komaroff A. The chronic fatigue syndrome: a comprehensive approach to its definition and study. International Chronic Fatigue Syndrome Study Group. Ann Intern Med. 1994;121(12):953–959. [CrossRef]
- Committee on the Diagnostic Criteria for Myalgic Encephalomyelitis/Chronic Fatigue Syndrome, Board on the Health of Select Populations, Institute of Medicine. Beyond Myalgic Encephalomyelitis/Chronic Fatigue Syndrome: Redefining an Illness [Internet]. Washington (DC): National Academies Press (US); 2015 [cited 2023 October 05]. (The National Academies Collection: Reports funded by National Institutes of Health).
- Carruthers BM, Jain AK, Meirleir KLD, Peterson DL, Klimas NG, Lerner AM, et al. Myalgic Encephalomyelitis/Chronic Fatigue Syndrome. J Chronic Fatigue Syndrome. 2003;11(1):7–115.
- Armstrong CW, McGregor NR, Lewis DP, Butt HL, Gooley PR. Metabolic profiling reveals anomalous energy metabolism and oxidative stress pathways in chronic fatigue syndrome patients. Metabolomics. 2015;11(6):1626–1639. [CrossRef]
- Billing-Ross P, Germain A, Ye K, Keinan A, Gu Z, Hanson MR. Mitochondrial DNA variants correlate with symptoms in myalgic encephalomyelitis/chronic fatigue syndrome. J Transl Med. 2016;14(1):19. [CrossRef]
- Booth NE, Myhill S, McLaren-Howard J. Mitochondrial dysfunction and the pathophysiology of myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) Int J Clin Exp Med. 2012;5(3):208–220.
- Liu Q, Zhang D, Hu D, Zhou X, Zhou Y. The role of mitochondria in NLRP3 inflammasome activation. Mol Immunol. 2018;1(103):115–124. [CrossRef]
- Castro-Marrero J, Cordero MD, Sáez-Francas N, Jimenez-Gutierrez C, Aguilar-Montilla FJ, Aliste L, et al. Could mitochondrial dysfunction be a differentiating marker between chronic fatigue syndrome and fibromyalgia? Antioxid Redox Signal. 2013;19(15):1855–1860.
- Germain A, Ruppert D, Levine SM, Hanson MR. Metabolic profiling of a myalgic encephalomyelitis/chronic fatigue syndrome discovery cohort reveals disturbances in fatty acid and lipid metabolism. Mol BioSyst. 2017;13(2):371–379. [CrossRef]
- Light KC, Agarwal N, Iacob E, White AT, Kinney AY, VanHaitsma TA, et al. Differing leukocyte gene expression profiles associated with fatigue in patients with prostate cancer versus chronic fatigue syndrome. Psychoneuroendocrinology. 2013;38(12):2983–2995. [CrossRef]
- Mandarano AH, Maya J, Giloteaux L, Peterson DL, Maynard M, Gottschalk CG, et al. Myalgic encephalomyelitis/chronic fatigue syndrome patients exhibit altered T cell metabolism and cytokine associations. J Clin Invest. 2019;130(3):1491–1505. [CrossRef]
- Naviaux RK, Naviaux JC, Li K, Bright AT, Alaynick WA, Wang L, et al. Metabolic features of chronic fatigue syndrome. Proc Natl Acad Sci USA. 2016;113(37):E5472–E5480. [CrossRef]
- Nguyen T, Staines D, Nilius B, Smith P, Marshall-Gradisnik S. Novel identification and characterisation of Transient receptor potential melastatin 3 ion channels on Natural Killer cells and B lymphocytes: effects on cell signalling in Chronic fatigue syndrome/Myalgic encephalomyelitis patients. Biol Res. 2016;49(1):1–8. [CrossRef]
- Nguyen T, Staines D, Johnston S, Marshall-Gradisnik S. Reduced glycolytic reserve in isolated natural killer cells from myalgic encephalomyelitis/chronic fatigue syndrome patients: a preliminary investigation. Asian Pac J Allergy Immunol. 2019;37(2):102–108. [CrossRef]
- Plioplys AV, Plioplys S. Electron-microscopic investigation of muscle mitochondria in chronic fatigue syndrome. Neuropsychobiology. 1995;32(4):175–181. [CrossRef]
- Shungu DC, Weiduschat N, Murrough JW, Mao X, Pillemer S, Dyke JP, et al. Increased ventricular lactate in chronic fatigue syndrome. III. Relationships to cortical glutathione and clinical symptoms implicate oxidative stress in disorder pathophysiology. NMR Biomed. 2012;25(9):1073–1087. [CrossRef]
- Sweetman E, Ryan M, Edgar C, Mackay A, Vallings R, Tate W. Changes in the transcriptome of circulating immune cells of a New Zealand cohort with myalgic encephalomyelitis/chronic fatigue syndrome. Int J Immunopathol Pharmacol. 2019;33:2058738418820402. [CrossRef]
- Tomas C, Brown A, Strassheim V, Elson J, Newton J, Manning P. Cellular bioenergetics is impaired in patients with chronic fatigue syndrome. PLoS ONE. 2017;12(10):e0186802. [CrossRef]
- Venter M, Tomas C, Pienaar IS, Strassheim V, Erasmus E, Ng W-F, et al. MtDNA population variation in Myalgic encephalomyelitis/Chronic fatigue syndrome in two populations: a study of mildly deleterious variants. Sci Rep. 2019;9(1):1–8. [CrossRef]
- Yamano E, Sugimoto M, Hirayama A, Kume S, Yamato M, Jin G, et al. Index markers of chronic fatigue syndrome with dysfunction of TCA and urea cycles. Sci Rep. 2016;6:1–9.
- Missailidis D, Annesley SJ, Allan CY, Sanislav O, Lidbury BA, Lewis DP, et al. An isolated complex V inefficiency and dysregulated mitochondrial function in immortalized lymphocytes from ME/CFS patients. Int J Mol Sci. 2020;21(3):1074.
- Missailidis D, Sanislav O, Allan CY, Annesley SJ, Fisher PR. Cell-based blood biomarkers for myalgic encephalomyelitis/chronic fatigue syndrome. Int J Mol Sci. 2020;21(3):1142.
- Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, Chou R, Glanville J, Grimshaw JM, Hróbjartsson A, Lalu MM, Li T, Loder EW, Mayo-Wilson E, McDonald S, McGuinness LA, Stewart LA, Thomas J, Tricco AC, Welch VA, Whiting P, Moher D. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021 Mar 29;372:n71. [CrossRef]
- Lo, C.KL., Mertz, D. & Loeb, M. Newcastle-Ottawa Scale: comparing reviewers’ to authors’ assessments. BMC Med Res Methodol 14, 45 (2014). [CrossRef]
- Brouwers FM, Van Der Werf S, Bleijenberg G, Van Der Zee L, Van Der Meer JW. The effect of a polynutrient supplement on fatigue and physical activity of patients with chronic fatigue syndrome: a double-blind randomized controlled trial. QJM. 2002 Oct;95(10):677-83. [CrossRef]
- Castro-Marrero J, Cordero MD, Segundo MJ, Sáez-Francàs N, Calvo N, Román-Malo L, Aliste L, Fernández de Sevilla T, Alegre J. Does oral coenzyme Q10 plus NADH supplementation improve fatigue and biochemical parameters in chronic fatigue syndrome? Antioxid Redox Signal. 2015 Mar 10;22(8):679-85. [CrossRef]
- Hobday RA, Thomas S, O'Donovan A, Murphy M, Pinching AJ. Dietary intervention in chronic fatigue syndrome. J Hum Nutr Diet. 2008 Apr;21(2):141-9. [CrossRef]
- Joseph P, Pari R, Miller S, Warren A, Stovall MC, Squires J, Chang CJ, Xiao W, Waxman AB, Systrom DM. Neurovascular Dysregulation and Acute Exercise Intolerance in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome: A Randomized, Placebo-Controlled Trial of Pyridostigmine. Chest. 2022 Nov;162(5):1116-1126. [CrossRef]
- Keller, B.A., Pryor, J.L. & Giloteaux, L. Inability of myalgic encephalomyelitis/chronic fatigue syndrome patients to reproduce VO2peak indicates functional impairment. J Transl Med 12, 104 (2014). [CrossRef]
- Kujawski, S., Słomko, J., Godlewska, B.R. et al. Combination of whole body cryotherapy with static stretching exercises reduces fatigue and improves functioning of the autonomic nervous system in Chronic Fatigue Syndrome. J Transl Med 20, 273 (2022). [CrossRef]
- Kujawski, S.; Bach, A.M.; Słomko, J.; Pheby, D.F.H.; Murovska, M.; Newton, J.L.; Zalewski, P. Changes in the Allostatic Response to Whole-Body Cryotherapy and Static-Stretching Exercises in Chronic Fatigue Syndrome Patients vs. Healthy Individuals. J. Clin. Med. 2021, 10, 2795. [CrossRef]
- Maes M, Mihaylova I, Kubera M, Uytterhoeven M, Vrydags N, Bosmans E. Coenzyme Q10 deficiency in myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) is related to fatigue, autonomic and neurocognitive symptoms and is another risk factor explaining the early mortality in ME/CFS due to cardiovascular disorder. Neuro Endocrinol Lett. 2009;30(4):470-6.
- Mandarano AH, Maya J, Giloteaux L, Peterson DL, Maynard M, Gottschalk CG, Hanson MR. Myalgic encephalomyelitis/chronic fatigue syndrome patients exhibit altered T cell metabolism and cytokine associations. J Clin Invest. 2020 Mar 2;130(3):1491-1505. [CrossRef]
- McDermott C, Richards SC, Thomas PW, Montgomery J, Lewith G. A placebo-controlled, double-blind, randomized controlled trial of a natural killer cell stimulant (BioBran MGN-3) in chronic fatigue syndrome. QJM. 2006 Jul;99(7):461-8. [CrossRef] [PubMed]
- Moore GE, Keller BA, Stevens J, Mao X, Stevens SR, Chia JK, Levine SM, Franconi CJ, Hanson MR. Recovery from Exercise in Persons with Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS). Medicina (Kaunas). 2023 Mar 15;59(3):571. [CrossRef]
- Nelson MJ, Buckley JD, Thomson RL, Clark D, Kwiatek R, Davison K. Diagnostic sensitivity of 2-day cardiopulmonary exercise testing in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome. J Transl Med. 2019 Mar 14;17(1):80. [CrossRef]
- Rao AV, Bested AC, Beaulne TM, Katzman MA, Iorio C, Berardi JM, Logan AC. A randomized, double-blind, placebo-controlled pilot study of a probiotic in emotional symptoms of chronic fatigue syndrome. Gut Pathog. 2009 Mar 19;1(1):6. [CrossRef]
- Rueda CB, Llorente-Folch I, Amigo I, Contreras L, González-Sánchez P, Martínez-Valero P, Juaristi I, Pardo B, del Arco A, Satrústegui J. Ca(2+) regulation of mitochondrial function in neurons. Biochim Biophys Acta. 2014 Oct;1837(10):1617-24. [CrossRef]
- Sandvik MK, Sørland K, Leirgul E, Rekeland IG, Stavland CS, et al. (2023) Endothelial dysfunction in ME/CFS patients. PLOS ONE 18(2): e0280942. [CrossRef]
- Sathyapalan T, Beckett S, Rigby AS, Mellor DD, Atkin SL. High cocoa polyphenol rich chocolate may reduce the burden of the symptoms in chronic fatigue syndrome. Nutr J. 2010 Nov 22;9:55. [CrossRef]
- Strayer DR, Young D, Mitchell WM. Effect of disease duration in a randomized Phase III trial of rintatolimod, an immune modulator for Myalgic Encephalomyelitis/Chronic Fatigue Syndrome. PLoS One. 2020 Oct 29;15(10):e0240403. [CrossRef]
- Sullivan A, Nord CE, Evengård B. Effect of supplement with lactic-acid producing bacteria on fatigue and physical activity in patients with chronic fatigue syndrome. Nutr J. 2009 Jan 26;8:4. [CrossRef]
- Thambirajah AA, Sleigh K, Stiver HG, Chow AW. Differential heat shock protein responses to strenuous standardized exercise in chronic fatigue syndrome patients and matched healthy controls. Clin Invest Med. 2008 Dec 1;31(6):E319-27. [CrossRef]
- The GK, Bleijenberg G, van der Meer JW. The effect of acclydine in chronic fatigue syndrome: a randomized controlled trial. PLoS Clin Trials. 2007 May 18;2(5):e19. [CrossRef]
- Wilshire CE, Kindlon T, Courtney R, Matthees A, Tuller D, Geraghty K, Levin B. Rethinking the treatment of chronic fatigue syndrome-a reanalysis and evaluation of findings from a recent major trial of graded exercise and CBT. BMC Psychol. 2018 Mar 22;6(1):6. [CrossRef]
- Witham MD, Adams F, McSwiggan S, Kennedy G, Kabir G, Belch JJ, Khan F. Effect of intermittent vitamin D3 on vascular function and symptoms in chronic fatigue syndrome--a randomised controlled trial. Nutr Metab Cardiovasc Dis. 2015 Mar;25(3):287-94. [CrossRef]
- Pivovarova NB, Andrews SB. Calcium-dependent mitochondrial function and dysfunction in neurons. FEBS J. 2010;277(18):3622–3636. [CrossRef]
- Nguyen T, Johnston S, Clarke L, Smith P, Staines D, Marshall-Gradisnik S. Impaired calcium mobilization in natural killer cells from chronic fatigue syndrome/myalgic encephalomyelitis patients is associated with transient receptor potential melastatin 3 ion channels. Clin Exp Immunol. 2017;187(2):284–293. [CrossRef]
- Cabanas H, Muraki K, Eaton N, Balinas C, Staines D, Marshall-Gradisnik S. Loss of Transient Receptor Potential Melastatin 3 ion channel function in natural killer cells from Chronic Fatigue Syndrome/Myalgic Encephalomyelitis patients. Mol Med. 2018;24(1):44. [CrossRef]
- Kennedy G, Spence VA, McLaren M, Hill A, Underwood C, Belch JJF. Oxidative stress levels are raised in chronic fatigue syndrome and are associated with clinical symptoms. Free Radical Biol Med. 2005;39(5):584–589. [CrossRef]
- Eaton-Fitch N, DuPreez S, Cabanas H, Staines D, Marshall-Gradisnik S. A systematic review of natural killer cells profile and cytotoxic function in myalgic encephalomyelitis/chronic fatigue syndrome. Systematic Reviews. 2019;8(1):279. [CrossRef]
- Lawson N, Hsieh C-H, March D, Wang X. Elevated energy production in chronic fatigue syndrome patients. J Nat Sci. 2016;2(10):e221.
- Huth TK, Eaton-Fitch N, Staines D, Marshall-Gradisnik S. A systematic review of metabolomic dysregulation in chronic fatigue syndrome/myalgic encephalomyelitis/systemic exertion intolerance disease (CFS/ME/SEID) J Transl Med. 2020;18(1):198.
- Gorman GS, Elson JL, Newman J, Payne B, McFarland R, Newton JL, et al. Perceived fatigue is highly prevalent and debilitating in patients with mitochondrial disease. Neuromuscul Disord. 2015;25(7):563–566. [CrossRef]
- Smits B, van den Heuvel L, Knoop H, Küsters B, Janssen A, Borm G, et al. Mitochondrial enzymes discriminate between mitochondrial disorders and chronic fatigue syndrome. Mitochondrion. 2011;11(5):735–738. [CrossRef]
- Nilsson I, Palmer J, Apostolou E, Gottfries C-G, Rizwan M, Dahle C, et al. Metabolic dysfunction in myalgic encephalomyelitis/chronic fatigue syndrome not due to anti-mitochondrial antibodies. Front Med. 2020;7:108. [CrossRef]
| Database | Intermittent Cold Exposure | Intermittent Heat Exposure | Evolutionary Based Foods | Intermittent Fasting | Circadian-Based Interventions | Fermented Drinks | Fermented Foods | Intermittent Hypercapnia | Intermittent Hypoxia | Intermittent Exercise |
|---|---|---|---|---|---|---|---|---|---|---|
| PubMed | ("ice bath" OR "cold plunge" OR "whole body cryotherapy" OR "cryochamber") AND ("ME/CFS" OR "ME/CFS") | ("sauna" OR "infrared sauna") AND ("ME/CFS" OR "ME/CFS") | ("paleo diet" OR "paleolithic diet" OR "ketogenic diet" OR "carnivore diet") AND ("ME/CFS" OR "ME/CFS") | ("intermittent fasting" OR "caloric restriction" OR "fasting") AND ("ME/CFS" OR "ME/CFS") | ("bluelight therapy" OR "melatonin" OR "bright light therapy" OR "light therapy" OR "blue light blocker") AND ("ME/CFS" OR "ME/CFS") | ("probiotic drinks" OR "kefir" OR "kombucha" OR "ayran" OR "buttermilk") AND ("ME/CFS" OR "ME/CFS") | ("fermented foods" OR "miso" OR "natto" OR "Tempeh" OR "skyr" OR "strained yoghurt" OR "greek yoghurt") AND ("ME/CFS" OR "ME/CFS") | ("breath holding" OR "hypercapnia") AND ("ME/CFS" OR "ME/CFS") | ("ihht" OR "altitude training" OR "breath holding") AND ("ME/CFS" OR "ME/CFS") | ("hiit" OR "high intensity interval training" OR "tabata" OR "interval training") AND ("ME/CFS" OR "ME/CFS") |
| ScienceDirect | ("ice bath" OR "cold plunge" OR "whole body cryotherapy" OR "cryochamber") AND ("ME/CFS" OR "ME/CFS") | ("sauna" OR "infrared sauna") AND ("ME/CFS" OR "ME/CFS") | ("paleo diet" OR "paleolithic diet" OR "ketogenic diet" OR "carnivore diet") AND ("ME/CFS" OR "ME/CFS") | ("intermittent fasting" OR "caloric restriction" OR "fasting") AND ("ME/CFS" OR "ME/CFS") | ("bluelight therapy" OR "melatonin" OR "bright light therapy" OR "light therapy" OR "blue light blocker") AND ("ME/CFS" OR "ME/CFS") | ("probiotic drinks" OR "kefir" OR "kombucha" OR "ayran" OR "buttermilk") AND ("ME/CFS" OR "ME/CFS") | ("fermented foods" OR "miso" OR "natto" OR "Tempeh" OR "skyr" OR "strained yoghurt" OR "greek yoghurt") AND ("ME/CFS" OR "ME/CFS") | ("breath holding" OR "hypercapnia") AND ("ME/CFS" OR "ME/CFS") | ("ihht" OR "altitude training" OR "breath holding") AND ("ME/CFS" OR "ME/CFS") | ("hiit" OR "high intensity interval training" OR "tabata" OR "interval training") AND ("ME/CFS" OR "ME/CFS") |
| IEEE Xplore | ("ice bath" OR "cold plunge" OR "whole body cryotherapy" OR "cryochamber") AND ("ME/CFS" OR "ME/CFS") | ("sauna" OR "infrared sauna") AND ("ME/CFS" OR "ME/CFS") | ("paleo diet" OR "paleolithic diet" OR "ketogenic diet" OR "carnivore diet") AND ("ME/CFS" OR "ME/CFS") | ("intermittent fasting" OR "caloric restriction" OR "fasting") AND ("ME/CFS" OR "ME/CFS") | ("bluelight therapy" OR "melatonin" OR "bright light therapy" OR "light therapy" OR "blue light blocker") AND ("ME/CFS" OR "ME/CFS") | ("probiotic drinks" OR "kefir" OR "kombucha" OR "ayran" OR "buttermilk") AND ("ME/CFS" OR "ME/CFS") | ("fermented foods" OR "miso" OR "natto" OR "Tempeh" OR "skyr" OR "strained yoghurt" OR "greek yoghurt") AND ("ME/CFS" OR "ME/CFS") | ("breath holding" OR "hypercapnia") AND ("ME/CFS" OR "ME/CFS") | ("ihht" OR "altitude training" OR "breath holding") AND ("ME/CFS" OR "ME/CFS") | ("hiit" OR "high intensity interval training" OR "tabata" OR "interval training") AND ("ME/CFS" OR "ME/CFS") |
| PsycINFO | ("ice bath" OR "cold plunge" OR "whole body cryotherapy" OR "cryochamber") AND ("ME/CFS" OR "ME/CFS") | ("sauna" OR "infrared sauna") AND ("ME/CFS" OR "ME/CFS") | ("paleo diet" OR "paleolithic diet" OR "ketogenic diet" OR "carnivore diet") AND ("ME/CFS" OR "ME/CFS") | ("intermittent fasting" OR "caloric restriction" OR "fasting") AND ("ME/CFS" OR "ME/CFS") | ("bluelight therapy" OR "melatonin" OR "bright light therapy" OR "light therapy" OR "blue light blocker") AND ("ME/CFS" OR "ME/CFS") | ("probiotic drinks" OR "kefir" OR "kombucha" OR "ayran" OR "buttermilk") AND ("ME/CFS" OR "ME/CFS") | ("fermented foods" OR "miso" OR "natto" OR "Tempeh" OR "skyr" OR "strained yoghurt" OR "greek yoghurt") AND ("ME/CFS" OR "ME/CFS") | ("breath holding" OR "hypercapnia") AND ("ME/CFS" OR "ME/CFS") | ("ihht" OR "altitude training" OR "breath holding") AND ("ME/CFS" OR "ME/CFS") | ("hiit" OR "high intensity interval training" OR "tabata" OR "interval training") AND ("ME/CFS" OR "ME/CFS") |
| Web of Science | ("ice bath" OR "cold plunge" OR "whole body cryotherapy" OR "cryochamber") AND ("ME/CFS" OR "ME/CFS") | ("sauna" OR "infrared sauna") AND ("ME/CFS" OR "ME/CFS") | ("paleo diet" OR "paleolithic diet" OR "ketogenic diet" OR "carnivore diet") AND ("ME/CFS" OR "ME/CFS") | ("intermittent fasting" OR "caloric restriction" OR "fasting") AND ("ME/CFS" OR "ME/CFS") | ("bluelight therapy" OR "melatonin" OR "bright light therapy" OR "light therapy" OR "blue light blocker") AND ("ME/CFS" OR "ME/CFS") | ("probiotic drinks" OR "kefir" OR "kombucha" OR "ayran" OR "buttermilk") AND ("ME/CFS" OR "ME/CFS") | ("fermented foods" OR "miso" OR "natto" OR "Tempeh" OR "skyr" OR "strained yoghurt" OR "greek yoghurt") AND ("ME/CFS" OR "ME/CFS") | ("breath holding" OR "hypercapnia") AND ("ME/CFS" OR "ME/CFS") | ("ihht" OR "altitude training" OR "breath holding") AND ("ME/CFS" OR "ME/CFS") | ("hiit" OR "high intensity interval training" OR "tabata" OR "interval training") AND ("ME/CFS" OR "ME/CFS") |
| Embase | ("ice bath" OR "cold plunge" OR "whole body cryotherapy" OR "cryochamber") AND ("ME/CFS" OR "ME/CFS") | ("sauna" OR "infrared sauna") AND ("ME/CFS" OR "ME/CFS") | ("paleo diet" OR "paleolithic diet" OR "ketogenic diet" OR "carnivore diet") AND ("ME/CFS" OR "ME/CFS") | ("intermittent fasting" OR "caloric restriction" OR "fasting") AND ("ME/CFS" OR "ME/CFS") | ("bluelight therapy" OR "melatonin" OR "bright light therapy" OR "light therapy" OR "blue light blocker") AND ("ME/CFS" OR "ME/CFS") | ("probiotic drinks" OR "kefir" OR "kombucha" OR "ayran" OR "buttermilk") AND ("ME/CFS" OR "ME/CFS") | ("fermented foods" OR "miso" OR "natto" OR "Tempeh" OR "skyr" OR "strained yoghurt" OR "greek yoghurt") AND ("ME/CFS" OR "ME/CFS") | ("breath holding" OR "hypercapnia") AND ("ME/CFS" OR "ME/CFS") | ("ihht" OR "altitude training" OR "breath holding") AND ("ME/CFS" OR "ME/CFS") | ("hiit" OR "high intensity interval training" OR "tabata" OR "interval training") AND ("ME/CFS" OR "ME/CFS") |
| CINAHL | ("ice bath" OR "cold plunge" OR "whole body cryotherapy" OR "cryochamber") AND ("ME/CFS" OR "ME/CFS") | ("sauna" OR "infrared sauna") AND ("ME/CFS" OR "ME/CFS") | ("paleo diet" OR "paleolithic diet" OR "ketogenic diet" OR "carnivore diet") AND ("ME/CFS" OR "ME/CFS") | ("intermittent fasting" OR "caloric restriction" OR "fasting") AND ("ME/CFS" OR "ME/CFS") | ("bluelight therapy" OR "melatonin" OR "bright light therapy" OR "light therapy" OR "blue light blocker") AND ("ME/CFS" OR "ME/CFS") | ("probiotic drinks" OR "kefir" OR "kombucha" OR "ayran" OR "buttermilk") AND ("ME/CFS" OR "ME/CFS") | ("fermented foods" OR "miso" OR "natto" OR "Tempeh" OR "skyr" OR "strained yoghurt" OR "greek yoghurt") AND ("ME/CFS" OR "ME/CFS") | ("breath holding" OR "hypercapnia") AND ("ME/CFS" OR "ME/CFS") | ("ihht" OR "altitude training" OR "breath holding") AND ("ME/CFS" OR "ME/CFS") | ("hiit" OR "high intensity interval training" OR "tabata" OR "interval training") AND ("ME/CFS" OR "ME/CFS") |
| Scopus | ("ice bath" OR "cold plunge" OR "whole body cryotherapy" OR "cryochamber") AND ("ME/CFS" OR "ME/CFS") | ("sauna" OR "infrared sauna") AND ("ME/CFS" OR "ME/CFS") | ("paleo diet" OR "paleolithic diet" OR "ketogenic diet" OR "carnivore diet") AND ("ME/CFS" OR "ME/CFS") | ("intermittent fasting" OR "caloric restriction" OR "fasting") AND ("ME/CFS" OR "ME/CFS") | ("bluelight therapy" OR "melatonin" OR "bright light therapy" OR "light therapy" OR "blue light blocker") AND ("ME/CFS" OR "ME/CFS") | ("probiotic drinks" OR "kefir" OR "kombucha" OR "ayran" OR "buttermilk") AND ("ME/CFS" OR "ME/CFS") | ("fermented foods" OR "miso" OR "natto" OR "Tempeh" OR "skyr" OR "strained yoghurt" OR "greek yoghurt") AND ("ME/CFS" OR "ME/CFS") | ("breath holding" OR "hypercapnia") AND ("ME/CFS" OR "ME/CFS") | ("ihht" OR "altitude training" OR "breath holding") AND ("ME/CFS" OR "ME/CFS") | ("hiit" OR "high intensity interval training" OR "tabata" OR "interval training") AND ("ME/CFS" OR "ME/CFS") |
| Google Scholar | ("ice bath" OR "cold plunge" OR "whole body cryotherapy" OR "cryochamber") AND ("ME/CFS" OR "ME/CFS") | ("sauna" OR "infrared sauna") AND ("ME/CFS" OR "ME/CFS") | ("paleo diet" OR "paleolithic diet" OR "ketogenic diet" OR "carnivore diet") AND ("ME/CFS" OR "ME/CFS") | ("intermittent fasting" OR "caloric restriction" OR "fasting") AND ("ME/CFS" OR "ME/CFS") | ("bluelight therapy" OR "melatonin" OR "bright light therapy" OR "light therapy" OR "blue light blocker") AND ("ME/CFS" OR "ME/CFS") | ("probiotic drinks" OR "kefir" OR "kombucha" OR "ayran" OR "buttermilk") AND ("ME/CFS" OR "ME/CFS") | ("fermented foods" OR "miso" OR "natto" OR "Tempeh" OR "skyr" OR "strained yoghurt" OR "greek yoghurt") AND ("ME/CFS" OR "ME/CFS") | ("breath holding" OR "hypercapnia") AND ("ME/CFS" OR "ME/CFS") | ("ihht" OR "altitude training" OR "breath holding") AND ("ME/CFS" OR "ME/CFS") | ("hiit" OR "high intensity interval training" OR "tabata" OR "interval training") AND ("ME/CFS" OR "ME/CFS") |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




