Submitted:
10 October 2023
Posted:
10 October 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Electrochemical Measurements
2.2. Surface Characterization and Electrolyte Analysis
3. Results and Discussions
3.1. Electrochemical Evaluation of CoCrMoW Alloy
3.1.1. OCP evolution
3.1.2. Anodic Polarization
| Sample | icor. (µA cm-2) |
Ecor vs. Ag/AgCl (mV) | Corrosion rate (µm y-1) | |||
|---|---|---|---|---|---|---|
| 168 h | 1000 h | 168 h | 1000 h | 168 h | 1000 h | |
| Co21Cr8Mo7W | 0.038 | 0.049 | -61 | -59 | 1.09 | 2.81 |
| Co29Cr7W | 0.427 | 0.180 | -104 | -114 | 12.24 | 6.57 |
| Sample | Metal element | Quantity of ions released | |
|---|---|---|---|
| (mg L-1) | (µg cm-2) | ||
| Co21Cr8Mo7W | Co | 0.679 mg/L | 7.1 |
| Cr | < 0.02 mg/L | 0.15 | |
| Mo W |
21.8 µg/L undetectable |
0.22 | |
| Co29Cr7W | Co | 0.335 mg/L | 3.33 |
| Cr | 7.40 mg/L | 73.7 | |
| W | undetectable | ||
3.2. Electrochemical Impedance Spectroscopy
3.3. X-ray Photoelectron Spectroscopy Investigations
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Dobri, G.; Banu, A.; Donath, C.; Marcu, M. The Influence of the Tantalum Content on the Main Properties of the TixTa9Nb8Zr2Ag Alloy. Metals 2023, 13, 1294. https://doi.org/10.3390/met13071294. [CrossRef]
- Wanga, Q.; Eltit, F.; Fenge, R.; Garbuz, D.; Duncan, C.; Masri, B.A.; Greidanus, N.; Coxc, M.E.; Wanga, R. Nature of fretting corrosion products in CoCrMo hip implants from in vivo study to in vitro simulation. Materialia 2022, 22, 101433. https://doi.org/10.1016/j.mtla.2022.101433. [CrossRef]
- Acharya, S.; Soni, R.; Suwas, S.; Chatterjee, K. Additive manufacturing of Co–Cr alloys for biomedical applications: A concise review. J. of Mater. Res. 2021, 36, 3746-3760. https://doi.org/10.1557/s43578-021-00244-z. [CrossRef]
- Mazumder, S.; Man, K.; Radhakrishnan, M.; Pantawane, M.V.; Palaniappan, S.; Patil, S.M.; Yang, Y.; Dahotre, N.B. Microstructure enhanced biocompatibility in laser additively manufactured CoCrMo biomedical alloy, Biomater Adv. 150 (2023) 213415. https://doi.org/10.1016/j.bioadv.2023.213415 1. [CrossRef]
- Mace, A.; Khullar, P.; Bouknight, C.; Gilbert, J.L. Corrosion properties of low carbon CoCrMo and additively manufactured CoCr alloys for dental applications. Dent. Mater. 2022, 38 (7):1184-1193. https://doi.org/10.1016/j.dental.2022.06.021. [CrossRef]
- Yan, Y.; Neville, A.; Dowson, D.; Williams, S. Tribocorrosion in implants—Assessing high carbon and low carbon Co–Cr–Mo alloys by in situ electrochemical measurements. Tribol. Int. 2006, 39, 1509. https://doi.org/10.1016/j.triboint.2006.01.016. 1509. [CrossRef]
- España, F. A.; Balla, V.K.; Bose, S.; Bandyopadhyay, A. Design and fabrication of CoCrMo alloy based novel structures for load bearing implants using laser engineered net shaping. Mater. Sci. Eng. C 2010, 30, 50–57. https://doi.org/10.1016/j.msec.2009.08.006. [CrossRef]
- Yamanaka, K.; Mori, M.; Kuramoto, K.; Chiba, A. Development of new Co-Cr-E based biomedical alloys: Effects of micro alloying and thermo mechanical processing on microstructures and mechanical properties. Mater. Des. 2014, 55, 987-998. https://doi.org/10.1016/j.matdes.2013.10.052. [CrossRef]
- Igual, M.A.; Mischler, S. Interactive Effects of Albumin and Phosphate Ions on the Corrosion of CoCrMo Implant Alloy. J. Electrochem. Soc. 2007, 154 , 10, C562-C570. https://doi.org/10.1149/1.2764238. [CrossRef]
- Vidal,V.C.; Igual, M.A. Effect of physico-chemical properties of simulated body fluids on the electrochemical behaviour of CoCrMo alloy. Electrochim. Acta 2011, 56, 8239–8248. https://doi.org/10.1016/j.electacta.2011.06.068. [CrossRef]
- Banu, A.; Marcu, M.; Juganaru, C.; Osiceanu, P.; Anastasescu, M.; Capra, L. Corrosion behavior of CoCrMoW cast alloy in lactic environment. Arab. J. Chem. 2019,12, 2007-2016. https://doi.org/10.1016/j.arabjc.2017.06.003. [CrossRef]
- Metikos-Hukovic’, M.; Pilic´, Z.; Babic´, R.; Omanovic, D. Influence of alloying elements on the corrosion stability of CoCrMo implant alloy in Hank’s solution. Acta Biomater. 2006, 2, 93–700. https://doi.org/10.1016/j.actbio.2006.06.002. [CrossRef]
- Milosev, I.; Strehblow, H.-H. The composition of the surface passive film formed on CoCrMo alloy in simulated physiological solution. Electrochim. Acta 2003, 48, 2767-2774. https://doi.org/10.1016/S0013-4686(03)00396-7. [CrossRef]
- Garcia-Falcon, C.M.; Gil-Lopez, T.; Verdu-Vazquez, A.; Mirza-Rosca, J. Electrochemical characterization of some cobalt base alloys in Ringer solution, Mater. Chem. Phys. 2021, 260, 124164. https://doi.org/10.1016/j.matchemphys.2020.124164. [CrossRef]
- Wang, R.; Qin, G.; Zhang, E. Hot deformation characteristics and dynamic recrystallization of biomedical CoCrWCu alloy. Mater. Today Commun. 2022, 33, 104930. https://doi.org/10.1016/j.mtcomm.2022.104930. [CrossRef]
- Tian, W.-P.; Yang, H.-W.; Zhang, S.-De. Synergistic Effect of Mo, W, Mn and Cr on the Passivation Behavior of a Fe-Based Amorphous Alloy Coating. Acta Metall. Sin. (Engl. Lett.) 2018, 31, 308-320. https://doi.org/10.1007/s40195-017-0604-5. [CrossRef]
- Cwalina, K.L.; Demarest, C.R.; Gerard, A. Y.; Scully, J.R. Revisiting the effects of molybdenum and tungsten alloying on corrosion behavior of nickel-chromium alloys in aqueous corrosion. Curr. Opin. Solid State Mater. Sci. 2019, 23, 129-141. https://doi.org/10.1016/j.cossms.2019.03.002. [CrossRef]
- Gurel, S.; Nazarahari, A. Canadinc, D.; Gerstein, G.; Maier, H.J.; Cabuk, H.; Bukulmez, T.; Cananoglu, M.; Yagci, M.B.; Toker, S.M.; Gunes, S.; Soykan, M.N. From corrosion behavior to radiation response: A comprehensive biocompatibility assessment of a CoCrMo medium entropy alloy for utility in orthopedic and dental implants, Intermetallics 2022, 149, 107680. https://doi.org/10.1016/j.intermet.2022.107680. [CrossRef]
- Vidal, V.C.; Igual, M.A. Electrochemical characterisation of biomedical alloys for surgical implants in simulated body fluids. Corros. Sci. 2008, 50, 1954-1961. https://doi.org/10.1016/j.corsci.2008.04.002. [CrossRef]
- Milosev, I. The effect of biomolecules on the behaviour of CoCrMo alloy in various simulated physiological solutions. Electrochim. Acta 2012, 78, 259-273. https://doi.org/10.1016/j.electacta.2012.05.146. [CrossRef]
- Hodgson, A.W.E.; Kurz, S.; Virtanen, S.; Fervel, V.; Olsson, C-O. A.; Mischler, S. Passive and transpassive behaviour of CoCrMo in simulated biological solutions. Electrochim. Acta 2004, 49, 2167-2178. https://doi.org/10.1016/j.electacta.2003.12.043. [CrossRef]
- Tanawana, T.; Hiromoto, S.; Asami, K. Characterization on of the surface oxide film of a Co-Cr-Mo alloy after being located in quasi-biological environments using XPS. Appl. Surf. Sci. 2001, 183, 68-75. https://doi.org/10.1016/S0169-4332(01)00551-7. [CrossRef]
- Ouerd, A.; Alemany-Dumont, C.; Normand, B.; Szunerits, S. Reactivity of CoCrMo alloy in physiological medium: Electrochemical characterization of the metal/protein interface. Electrochim. Acta 2008, 53, 4461–4469. https://doi.org/10.1016/j.electacta.2008.01.025. [CrossRef]
- Girao, D.de C.; Beres, M.; Jardini, A.L.; Filho, R.M.; Silva, C.C.; Siero. A.; Gomes de Abreu, H.F.; Araujo, W.S. An assessment of biomedical CoCrMo alloy fabricated by direct metal laser sintering technique for implant applications. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 110305. https://doi.org/10.1016/j.msec.2019.110305. [CrossRef]
- Banu, A.; Radovici, O.; Marcu, M. The alloying influence on corrosion behavior of chromium surgical alloys. Rev. Roum. Chim. 2008, 53, 947-953.
- ISO 10271 Dentistry- Corrosion test methods for metallic materials. ISO: Geneva, Switzerland, 2011.
- Okazaki, Y.; Gotoh, E. Comparison of metal release from various metallic biomaterials in vitro. Biomaterials 2005, 26, 11–21. https://doi.org/10.1016/j.biomaterials.2004.02.005. [CrossRef]
- Spataru, T.; Preda, L.; Munteanu, C.; Caciuleanu, A.; Spataru, N.; Fujishima, A. Influence of boron-doped diamond surface termination on the characteristics of titanium dioxide anodically deposited in the presence of a surfactant. J. Electrochem. Soc. 2015, 162, H535-H540. https://doi.org/10.1149/2.0741508jes. [CrossRef]
- Marin, E.; Lanzutti, A.; Rondinella, A.; Sordetti, F.; Magnan, M.; Honma, T.; Yoshida, Y.; Zhu, W.; Pezzotti, G.; Fedrizzi, L. Multi-spectroscopic analysis of high temperature oxides formed on cobalt-chrome-molybdenum alloys. J. Mater.Res. Technol. 2022, 20, 3061-3073. https://doi.org/10.1016/j.jmrt.2022.08.071. [CrossRef]
- Spataru, T.; Mihai, M. A.; Preda, L.; Marcu, M.; Radu, M. M.; Becherescu, N. D.; Alin Velea, A.; Zaki, M. Y.; Udrea, R.; Satulu, V.; Spataru, N. Enhanced photoelectrochemical activity of WO3-decorated native titania films by mild laser treatment. Appl. Surf. Sci. 2022, 153682. https://doi.org/10.1016/j.apsusc.2022.153682. [CrossRef]
- Marcu, M.; Preda, L.; Vizireanu, S.; Bita, B.; Mihai, M. A.; Spataru, T.; Acsente, T.; Dinescu, G.; Spataru, N. Enhancement of the capacitive features of WO3 supported on pristine and functionalized graphite by appropriate adjustment of the electrodeposition regime. Mater. Sci. Eng. B 2022, 277, 115585. https://doi.org/10.1016/j.mseb.2021.115585. [CrossRef]
- 32. Vasilopoulou, M.; Soultati, A.; Georgiadou, D.G.; Stergiopoulos, T.; Palilis, L.C.; Kennou, S.; Stathopoulos, N.A.; Davazoglou, D.; Argitis, P. Hydrogenated understoichiometric tungsten oxide anode interlayers for efficient and stable organic photovoltaics. J. Mater. Chem. A 2014, 2,1738. doi.org/10.1039/ c3ta13975a. 101039/c3ta13975a.
- Alexander, M. R. ; Thompson, G. E.; Zhou, X.; Beamson, G.; Fairley, N. Quantification of oxide film thickness at the surface of aluminium using XPS. Surf. Interface Anal. 2002, 34, 485-489, https://doi.org/10.1002/sia.1344. [CrossRef]
- Preda, L.; Spataru, N.; Moreno, J. M. C.; Somacescu, S.; Marcu, M. Graphene Incorporation as a Propitious Approach for Improving the Oxygen Reduction Reaction (ORR) Activity of Self-assembled Polycrystalline NiCo2O4–NiO. Electrocatalysis 2020, 11, 443–453. https://doi.org/10.1007/s12678-020-00605-y. [CrossRef]
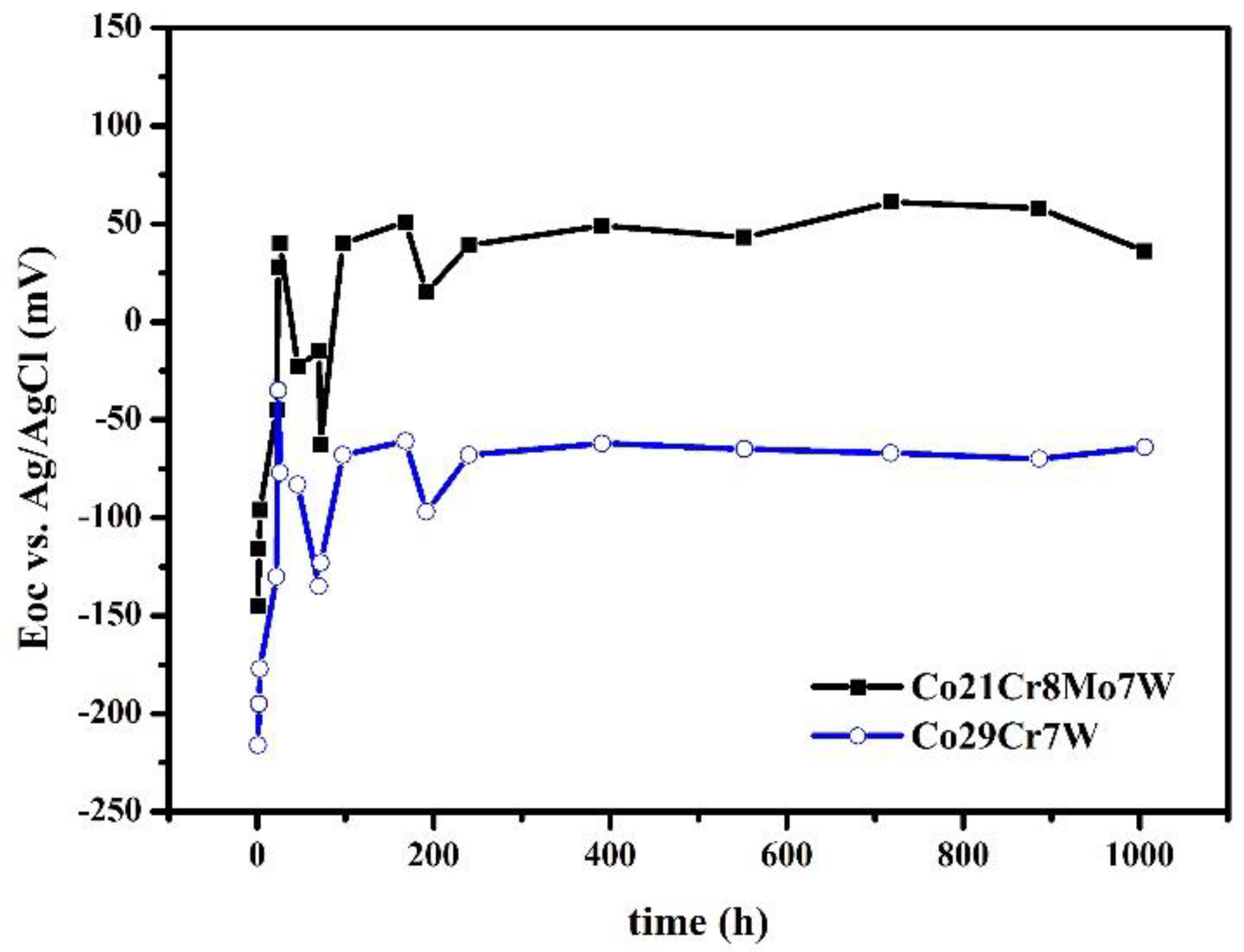
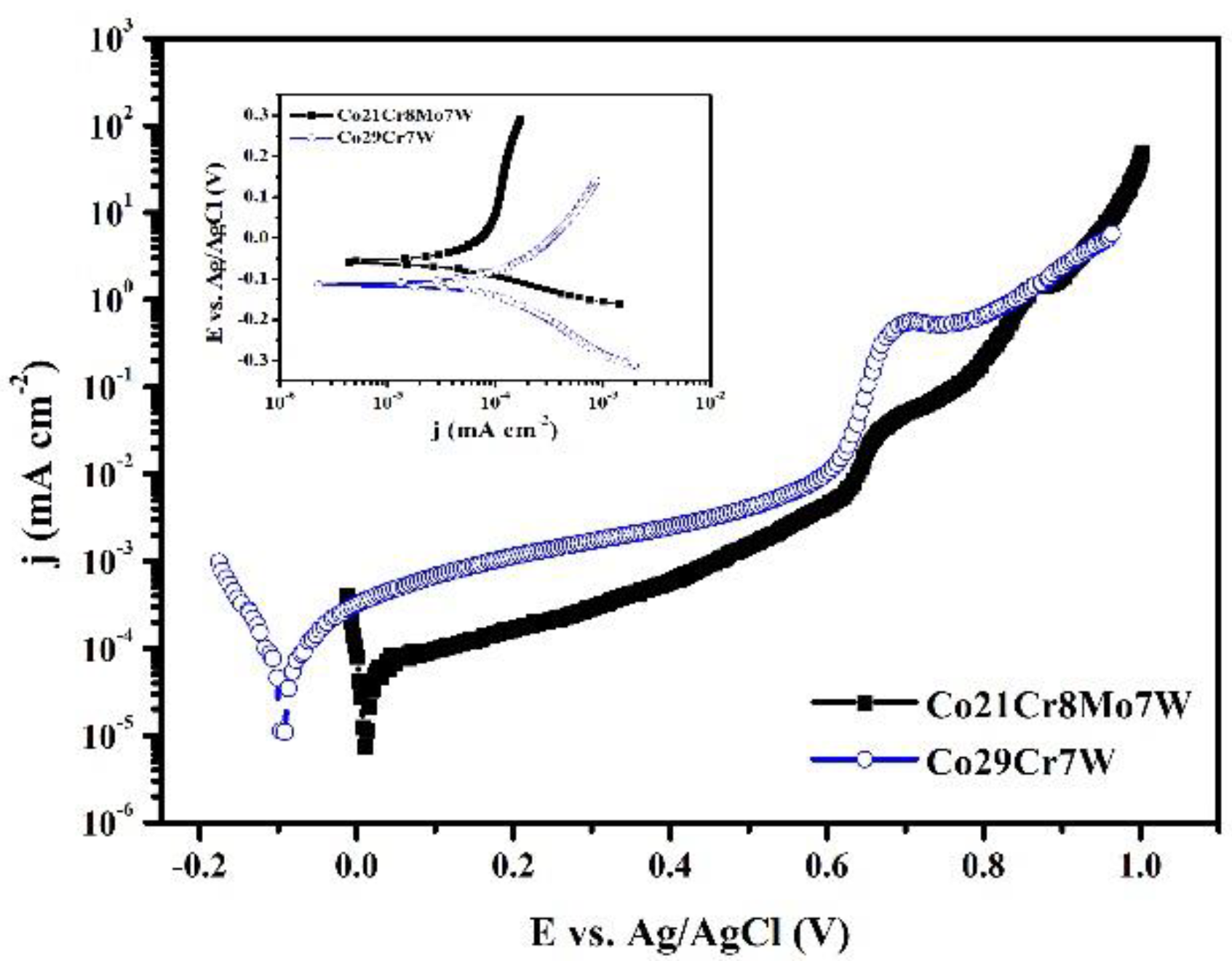
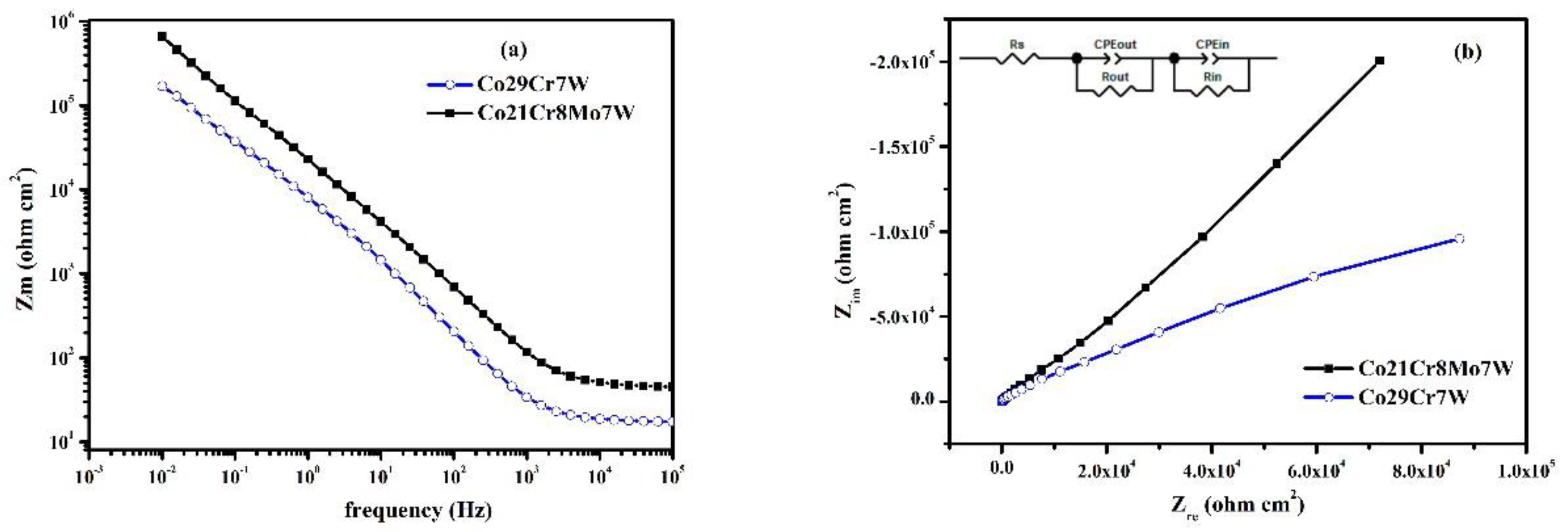

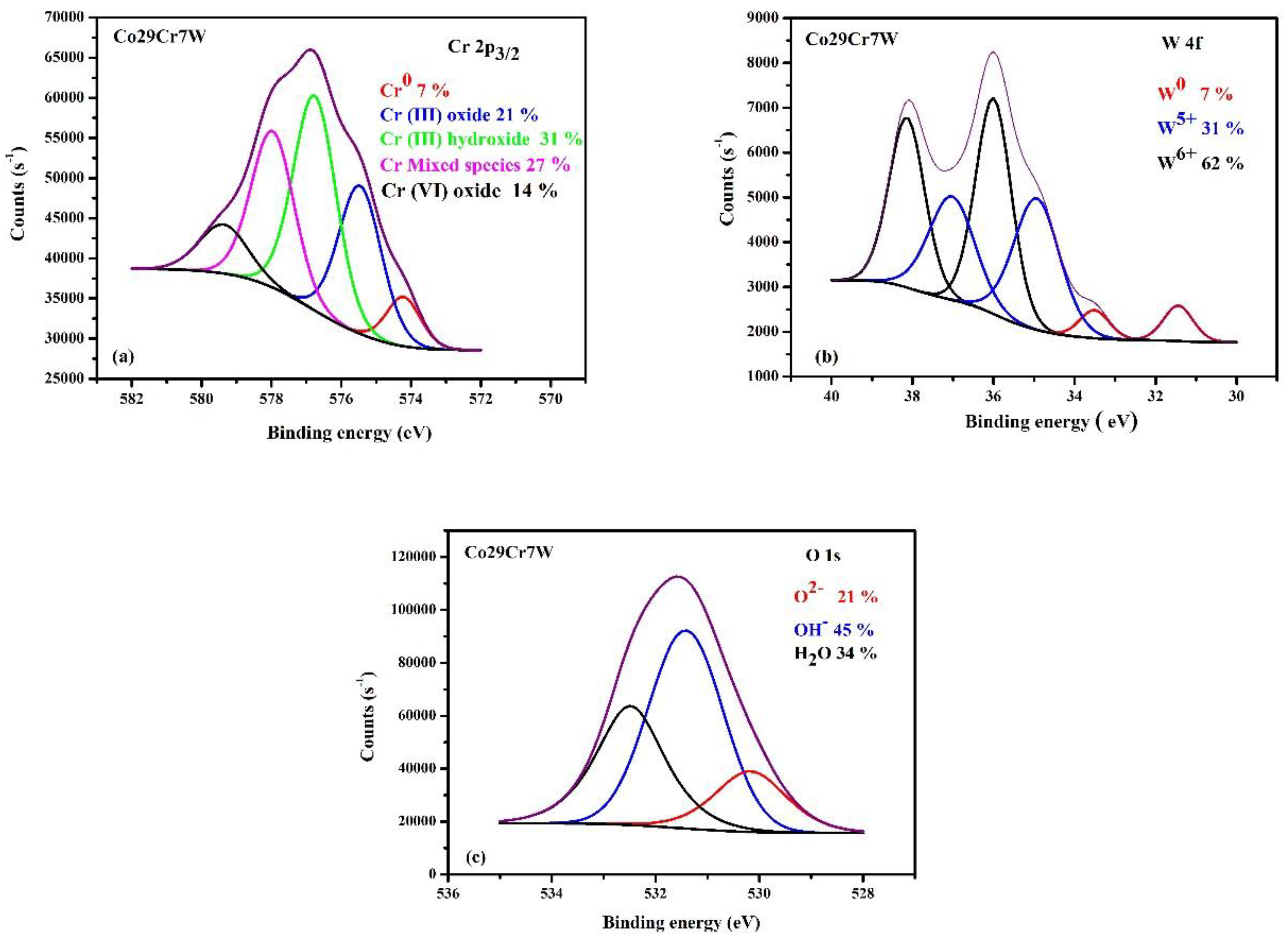
| Sample | % Co | % Cr |
|---|---|---|
| Co21Cr8Mo7W | 64 ± 0.54 | 21 ± 0.32 |
| Co29Cr7W | 64 ± 0.52 | 29 ± 0.35 |
| Sample | Rs (Ω cm2) |
CPEout (Sn Ω-1 cm-2) |
n | Rout (Ω cm2) |
CPEin (Sn Ω-1 cm-2) |
n | Rin (Ω cm2) |
|---|---|---|---|---|---|---|---|
| Co21Cr8Mo7W | 15.78 | 5.63 × 10-5 | 0.80 | 6461 | 3.85 × 10-5 | 0.81 | 8.6 × 106 |
| Co29Cr7W | 17.01 | 3.63 × 10-5 | 0.86 | 7540 | 4.36 × 10-5 | 0.84 | 2.3 × 106 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





