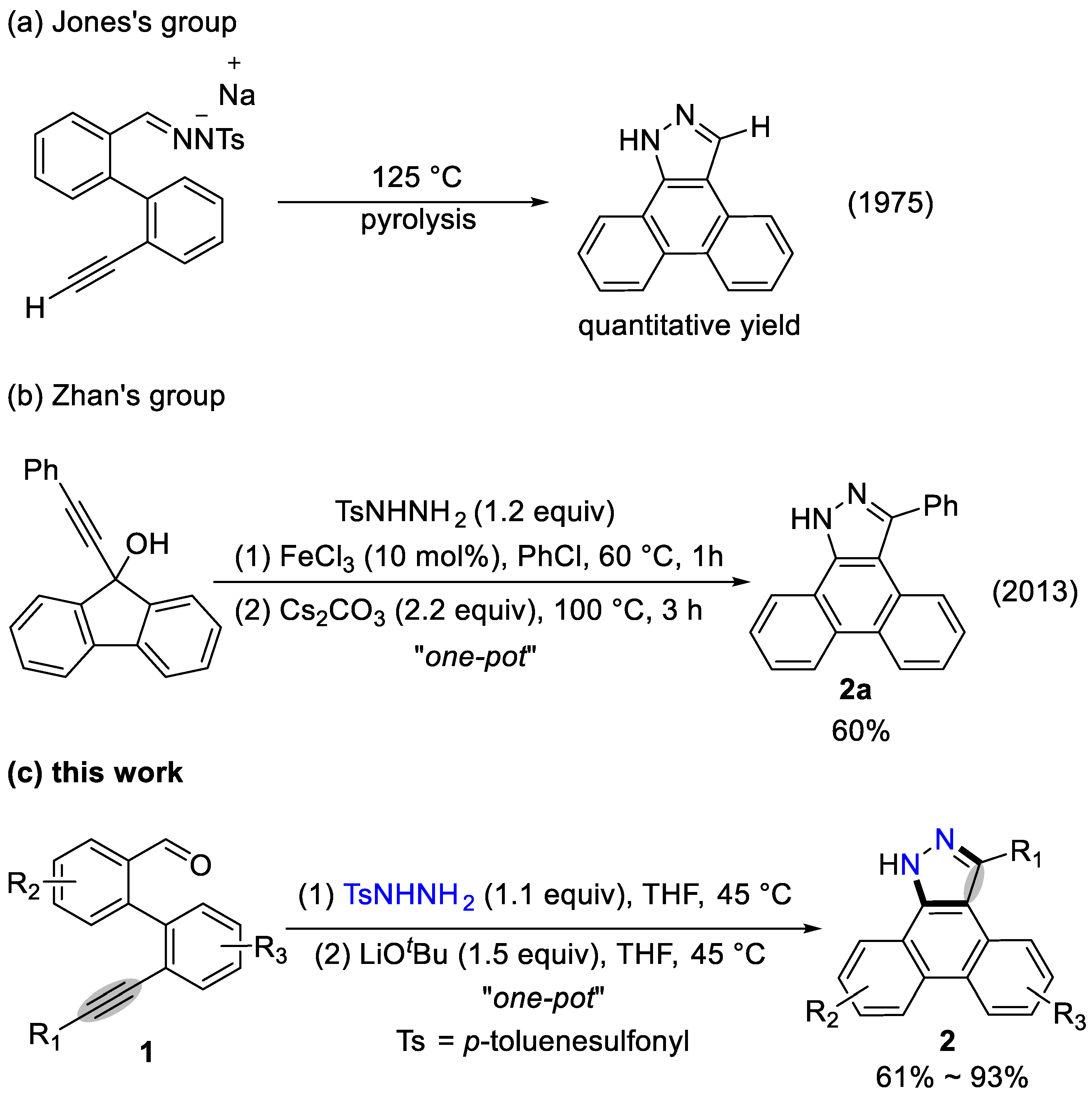
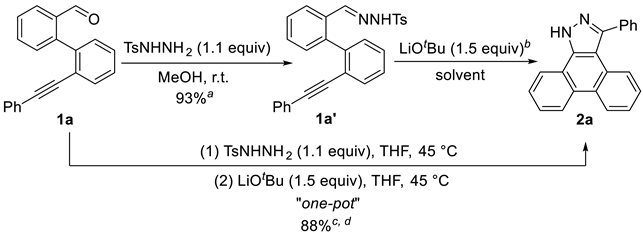
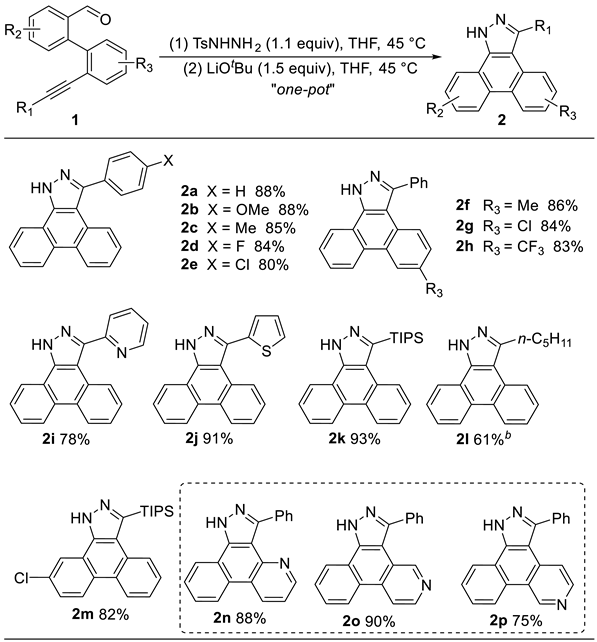
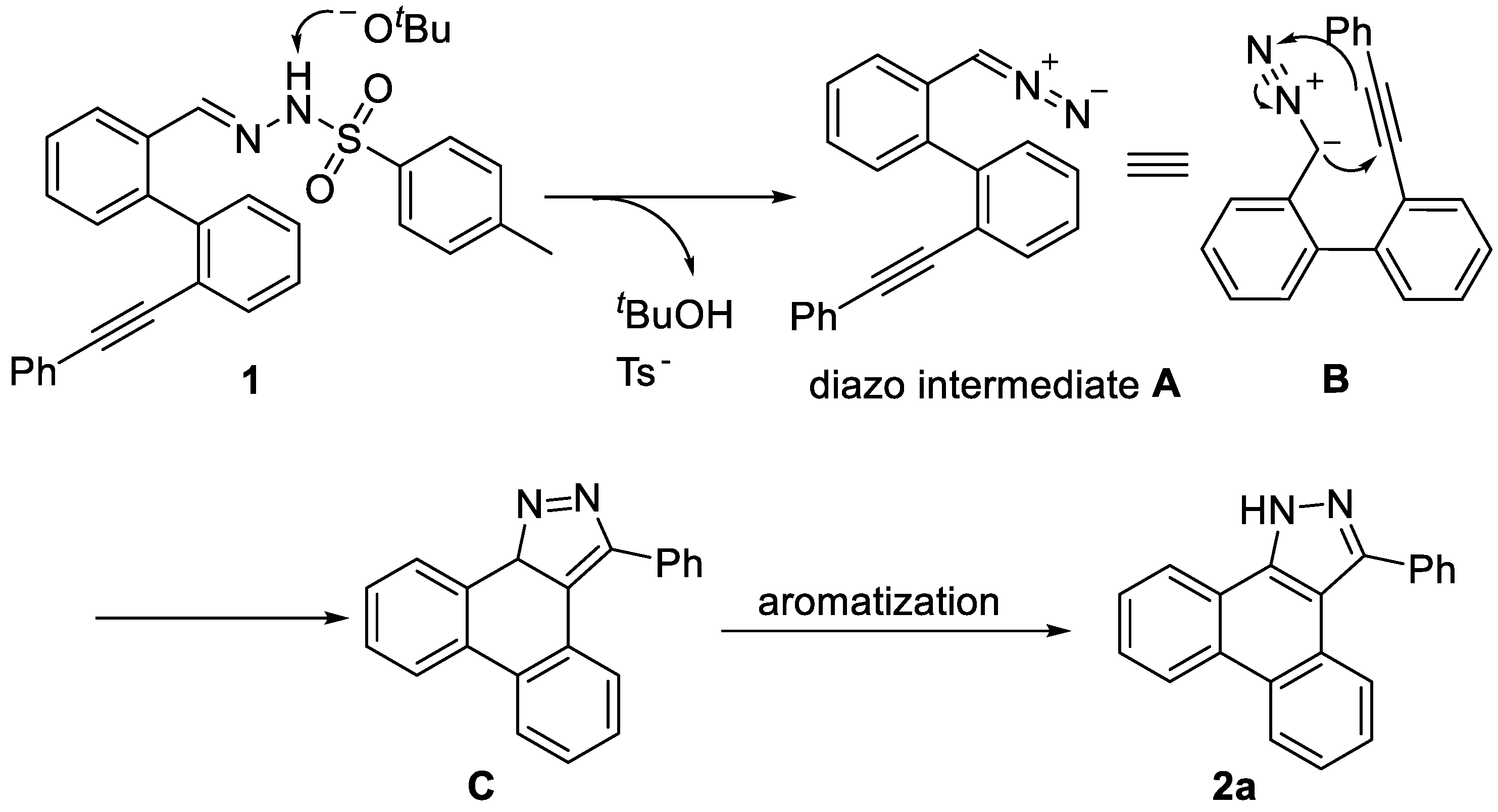
3.2 Characterization Data of Substrates
2’-(Phenylethynyl)-[1,1’-biphenyl]-2-carbaldehyde (1a). Pale yellow oil (355 mg, 1.26 mmol, 84%). Rf = 0.40 (PE/EA = 10/1). 1H NMR (400 MHz, Chloroform-d) δ 9.94 (s, 1H), 8.09 (dd, J = 7.8, 1.5 Hz, 1H), 7.68 – 7.64 (m, 2H), 7.54 (t, J = 7.6 Hz, 1H), 7.46 – 7.38 (m, 4H), 7.25 – 7.22 (m, 4H), 7.17 – 7.15 (m, 2H). 13C NMR (101 MHz, Chloroform-d) δ 191.95, 144.42, 140.40, 134.34, 133.59, 132.10, 131.40, 130.37, 128.55, 128.37, 128.34, 126.98, 123.83, 122.79, 93.90, 88.30.
2’-((4-Methoxyphenyl)ethynyl)-[1,1’-biphenyl]-2-carbaldehyde (1b). Yellow oil (332 mg, 1.07 mmol, 71%). Rf = 0.55 (PE/EA = 10/1). 1H NMR (400 MHz, Chloroform-d) δ 9.93 (s, 1H), 8.08 (dd, J = 7.9, 1.5 Hz, 1H), 7.66 – 7.59 (m, 2H), 7.52 (t, J = 7.5 Hz, 1H), 7.43 – 7.36 (m, 4H), 7.12 – 7.08 (m, 2H), 6.77 – 6.74 (m, 2H), 3.74 (s, 3H). 13C NMR (101 MHz, Chloroform-d) δ 191.92, 159.83, 144.50, 140.08, 134.32, 133.54, 132.83, 131.80, 131.38, 130.27, 128.27, 128.23, 128.18, 126.82, 124.14, 114.85, 114.02, 94.04, 87.12, 55.32. HRMS (ESI IT-TOF) m/z [M + H]+ Calcd for C22H17O2 313.1223, found 313.1223.
2’-(p-Tolylethynyl)-[1,1’-biphenyl]-2-carbaldehyde (1c). Pale yellow oil (417 mg, 1.41 mmol, 94%). Rf = 0.50 (PE/EA = 10/1). 1H NMR (400 MHz, Chloroform-d) δ 9.93 (s, 1H), 8.08 (dd, J = 7.7, 1.5 Hz, 1H), 7.66 – 7.61 (m, 2H), 7.52 (t, J = 7.6 Hz, 1H), 7.44 – 7.36 (m, 4H), 7.07 – 7.02 (m, 4H), 2.29 (s, 3H). 13C NMR (101 MHz, Chloroform-d) δ 191.91, 144.44, 140.25, 138.71, 134.31, 133.53, 131.96, 131.38, 131.26, 130.31, 129.12, 128.34, 128.27, 126.89, 123.99, 119.68, 94.14, 87.70, 21.59.
2’-((4-Fluorophenyl)ethynyl)-[1,1’-biphenyl]-2-carbaldehyde (1d). Pale yellow oil (333 mg, 1.11 mmol, 74%). Rf = 0.40 (PE/EA = 10/1). 1H NMR (400 MHz, Chloroform-d) δ 9.93 (s, 1H), 8.08 (dd, J = 7.8, 1.5 Hz, 1H), 7.67 – 7.61 (m, 2H), 7.53 (t, J = 7.6 Hz, 1H), 7.46 – 7.38 (m, 4H), 7.16 – 7.11 (m, 2H), 6.95 – 6.90 (m, 2H). 13C NMR (101 MHz, Chloroform-d) δ 191.86, 162.65 (d, J = 249.6 Hz), 144.29, 140.33, 134.31, 133.60, 133.27 (d, J = 8.3 Hz), 131.95, 131.36, 130.29, 128.63, 128.34, 126.88, 123.64, 118.84 (d, J = 3.6 Hz), 115.67 (d, J = 22.2 Hz), 92.80, 88.01. HRMS (ESI IT-TOF) m/z [M + H]+ Calcd for C21H14FO 301.1023, found 301.1023.
2’-((4-Chlorophenyl)ethynyl)-[1,1’-biphenyl]-2-carbaldehyde (1e). Pale yellow oil (436 mg, 1.38 mmol, 92%). Rf = 0.40 (PE/EA = 10/1). 1H NMR (400 MHz, Chloroform-d) δ 9.92 (s, 1H), 8.08 (dd, J = 7.8, 1.5 Hz, 1H), 7.69 – 7.62 (m, 2H), 7.55 (t, J = 7.5 Hz, 1H), 7.49 – 7.40 (m, 4H), 7.22 – 7.19 (m, 2H), 7.09 – 7.07 (m, 2H). 13C NMR (101 MHz, Chloroform-d) δ 191.90, 144.27, 140.47, 134.60, 134.33, 133.64, 132.58, 132.06, 131.39, 130.37, 128.82, 128.75, 128.42, 128.40, 126.97, 123.52, 121.26, 92.73, 89.25.
5’-Methyl-2’-(phenylethynyl)-[1,1’-biphenyl]-2-carbaldehyde (1f). Pale yellow oil (404 mg, 1.36 mmol, 91%). Rf = 0.40 (PE/EA = 10/1). 1H NMR (400 MHz, Chloroform-d) δ 9.94 (s, 1H), 8.08 (dd, J = 7.9, 1.6 Hz, 1H), 7.66 – 7.61 (m, 1H), 7.53 – 7.49 (m, 2H), 7.42 (d, J = 7.5 Hz, 1H), 7.23 – 7.20 (m, 5H), 7.16 – 7.14 (m, 2H), 2.41 (s, 3H). 13C NMR (101 MHz, Chloroform-d) δ 192.00, 144.53, 140.25, 138.78, 134.31, 133.50, 131.94, 131.33, 131.28, 131.11, 129.12, 128.30, 128.20, 126.84, 122.97, 120.82, 93.10, 88.45, 21.57.
5’-Chloro-2’-(phenylethynyl)-[1,1’-biphenyl]-2-carbaldehyde (1g). Pale yellow solid (374 mg, 1.18 mmol, 79%). Rf = 0.40 (PE/EA = 10/1). m.p. 83.7 – 84.2 °C. 1H NMR (400 MHz, Chloroform-d) δ 9.93 (s, 1H), 8.09 (dd, J = 7.8, 1.5 Hz, 1H), 7.67 (td, J = 7.4, 1.5 Hz, 1H), 7.58 – 7.54 (m, 2H), 7.42 – 7.39 (m, 3H), 7.26 – 7.21(m, 4H), 7.16 – 7.13 (m, 2H). 13C NMR (101 MHz, Chloroform-d) δ 191.35, 142.86, 142.06, 134.50, 134.25, 133.77, 133.12, 131.39, 131.17, 130.27, 128.83, 128.59, 128.42, 127.30, 122.45, 94.77, 87.27.
2’-(Phenylethynyl)-5’-(trifluoromethyl)-[1,1’-biphenyl]-2-carbaldehyde (1h). Pale yellow solid (483 mg, 1.38 mmol, 92%). Rf = 0.40 (PE/EA = 10/1). m.p. 79.5 – 80.1 °C. 1H NMR (400 MHz, Chloroform-d) δ 9.92 (s, 1H), 8.11 (dd, J = 7.8, 1.5 Hz, 1H), 7.75 – 7.67 (m, 4H), 7.58 (t, J = 7.6 Hz, 1H), 7.41 (d, J = 7.8 Hz, 1H), 7.28 – 7.26 (m, 3H), 7.18 – 7.15 (m, 2H). 13C NMR (101 MHz, Chloroform-d) δ 191.09, 142.68, 141.21, 134.30, 133.87, 132.36, 131.56, 131.27, 130.33 (q, J = 32.7 Hz), 129.14, 128.99, 128.46, 127.60, 127.54, 126.88 (q, J = 3.8 Hz), 125.09 (q, J = 3.5 Hz), 123.85 (q, J = 273.7 Hz), 122.06, 96.35, 87.08. 19F NMR (376 MHz, Chloroform-d) δ -62.53. HRMS (ESI IT-TOF) m/z [M + H]+ Calcd for C22H14F3O 351.0991, found 351.0991.
2’-(Pyridin-2-ylethynyl)-[1,1’-biphenyl]-2-carbaldehyde (1i). Pale yellow solid (378 mg, 1.34 mmol, 89%). Rf = 0.40 (PE/EA = 10/1). m.p. 104.6 – 104.9 °C. 1H NMR (400 MHz, Chloroform-d) δ 9.95 (s, 1H), 8.50 (d, J = 3.3 Hz, 1H), 8.09 (d, J = 7.7 Hz, 1H), 7.74 (dd, J = 7.3, 1.7 Hz, 1H), 7.66 (td, J = 7.5, 1.4 Hz, 1H), 7.55 – 7.39 (m, 6H), 7.15 – 7.11 (m, 1H), 7.00 (d, J = 7.8 Hz, 1H). 13C NMR (101 MHz, Chloroform-d) δ 191.60, 149.85, 143.88, 142.79, 140.57, 136.03, 134.09, 133.50, 132.57, 131.31, 130.26, 129.12, 128.29, 128.26, 127.06, 126.81, 122.83, 122.60, 92.68, 87.76. HRMS (ESI IT-TOF) m/z [M + H]+ Calcd for C20H14NO 284.1070, found 284.1069.
2’-(Thiophen-2-ylethynyl)-[1,1’-biphenyl]-2-carbaldehyde (1j). Pale yellow oil (363 mg, 1.26 mmol, 84%). Rf = 0.40 (PE/EA = 10/1). 1H NMR (400 MHz, Chloroform-d) δ 9.91 (s, 1H), 8.08 (dd, J = 7.7, 1.5 Hz, 1H), 7.67 – 7.59 (m, 2H), 7.52 (t, J = 7.6 Hz, 1H), 7.45 – 7.36 (m, 4H), 7.18 (dd, J = 5.1, 1.2 Hz, 1H), 6.98 (dd, J = 3.7, 1.2 Hz, 1H), 6.88 (dd, J = 5.2, 3.6 Hz, 1H). 13C NMR (101 MHz, Chloroform-d) δ 191.72, 144.11, 140.17, 134.24, 133.56, 132.06, 131.68, 131.32, 130.38, 128.59, 128.33, 128.29, 127.71, 127.14, 127.08, 123.47, 122.63, 92.02, 87.32. HRMS (ESI IT-TOF) m/z [M + H]+ Calcd for C19H13OS 289.0682, found 289.0682.
2’-((Triisopropylsilyl)ethynyl)-[1,1’-biphenyl]-2-carbaldehyde (1k). White solid (391 mg, 1.08 mmol, 72%). Rf = 0.50 (PE/EA = 10/1). m.p. 68.0 – 68.3 °C. 1H NMR (400 MHz, Chloroform-d) δ 9.85 (s, 1H), 8.01 (d, J = 7.7 Hz, 1H), 7.61 – 7.58 (m, 2H), 7.46 (t, J = 7.6 Hz, 1H), 7.41 – 7.34 (m, 3H), 7.31 – 7.28 (m, 1H), 0.91 (s, 21H). 13C NMR (101 MHz, Chloroform-d) δ 191.69, 144.63, 140.73, 134.10, 133.48, 132.78, 131.09, 130.19, 128.36, 128.10, 128.04, 127.07, 123.94, 105.21, 95.72, 18.52, 11.16. HRMS (ESI IT-TOF) m/z [M + H]+ Calcd for C24H31OSi 363.2139, found 363.2138.
2’-(Hept-1-yn-1-yl)-[1,1’-biphenyl]-2-carbaldehyde (1l). Pale yellow oil (99 mg, 0.36 mmol, 24%). Rf = 0.50 (PE/EA = 10/1). 1H NMR (400 MHz, Chloroform-d) δ 9.85 (s, 1H), 8.03 (d, J = 7.7 Hz, 1H), 7.64 – 7.60 (m, 1H), 7.50 – 7.49 (m, 2H), 7.35 – 7.32 (m, 4H), 7.25 (s, 1H), 2.17 – 2.13 (s, 2H), 1.33 – 1.26 (m, 2H), 1.21 – 1.16 (m, 2H), 1.11 – 1.05 (m, 2H), 0.83 – 0.79 (m, 3H). 13C NMR (101 MHz, Chloroform-d) δ 192.01, 144.75, 140.20, 134.19, 133.48, 132.08, 131.21, 130.18, 128.14, 128.04, 127.75, 126.76, 124.57, 95.57, 79.50, 30.83, 27.86, 22.23, 19.34, 14.02. HRMS (ESI IT-TOF) m/z [M + H]+ Calcd for C20H21O 277.1587, found 277.1587.
4-Chloro-2’-((triisopropylsilyl)ethynyl)-[1,1’-biphenyl]-2-carbaldehyde (1m). Pale yellow oil (422 mg, 1.07 mmol, 71%). Rf = 0.50 (PE/EA = 10/1). 1H NMR (400 MHz, Chloroform-d) δ 9.77 (s, 1H), 7.98 (d, J = 2.6 Hz, 1H), 7.62 – 7.56 (m, 2H), 7.44 – 7.37 (m, 2H), 7.34 (d, J = 8.2 Hz, 1H), 7.30 – 7.28 (m, 1H), 0.92 (s, 21H). 13C NMR (101 MHz, Chloroform-d) δ 190.42, 142.86, 139.53, 135.30, 134.74, 133.38, 132.91, 132.64, 130.08, 128.57, 128.46, 126.95, 124.06, 104.90, 96.42, 18.51, 11.19. HRMS (ESI IT-TOF) m/z [M + H]+ Calcd for C21H24ClOSi 355.1279, found 355.1278.
2-(2-(Phenylethynyl)pyridin-3-yl)benzaldehyde (1n). Pale yellow oil (365 mg, 1.29 mmol, 86%). Rf = 0.40 (PE/EA = 10/1). 1H NMR (400 MHz, Chloroform-d) δ 9.96 (s, 1H), 8.69 (dd, J = 4.7, 1.8 Hz, 1H), 8.11 (dd, J = 7.8, 1.4 Hz, 1H), 7.73 – 7.67 (m, 2H), 7.59 (t, J = 7.5 Hz, 1H), 7.43 – 7.36 (m, 2H), 7.30 – 7.19 (m, 5H). 13C NMR (101 MHz, Chloroform-d) δ 190.85, 149.76, 142.85, 141.49, 137.52, 136.68, 134.22, 133.75, 131.74, 131.39, 129.18, 128.95, 128.31, 127.74, 122.65, 121.66, 93.72, 87.72. HRMS (ESI IT-TOF) m/z [M + H]+ Calcd for C20H14NO 284.1070, found 284.1069.
2-(3-(Phenylethynyl)pyridin-4-yl)benzaldehyde (1o). Pale yellow oil (386 mg, 1.36 mmol, 91%). Rf = 0.40 (PE/EA = 10/1). 1H NMR (400 MHz, Chloroform-d) δ 9.93 (s, 1H), 8.86 (s, 1H), 8.65 (d, J = 5.1 Hz, 1H), 8.11 (dd, J = 7.9, 1.5 Hz, 1H), 7.71 (td, J = 7.5, 1.5 Hz, 1H), 7.61 (t, J = 7.7 Hz, 1H), 7.43 – 7.40 (m, 1H), 7.34 (d, J = 5.1 Hz, 1H), 7.31 – 7.20 (m, 6H). 13C NMR (101 MHz, Chloroform-d) δ 190.69, 152.52, 148.75, 147.84, 141.12, 133.85, 131.47, 130.73, 129.36, 129.05, 128.43, 127.84, 124.25, 122.04, 120.65, 96.70, 84.94. HRMS (ESI IT-TOF) m/z [M + H]+ Calcd for C20H14NO 284.1070, found 284.1069.
2-(4-(Phenylethynyl)pyridin-3-yl)benzaldehyde (1p). Pale yellow oil (378 mg, 1.33 mmol, 89%). Rf = 0.40 (PE/EA = 10/1). 1H NMR (400 MHz, Chloroform-d) δ 9.95 (s, 1H), 8.67 – 8.66 (m, 2H), 8.12 (d, J = 7.8 Hz, 1H), 7.71 (td, J = 7.4, 1.4 Hz, 1H), 7.60 (t, J = 7.6 Hz, 1H), 7.49 (d, J = 5.1 Hz, 1H), 7.44 (d, J = 7.6 Hz, 1H), 7.32 – 7.19 (m, 5H). 13C NMR (101 MHz, Chloroform-d) δ 190.88, 150.23, 149.34, 140.16, 135.03, 134.48, 133.81, 131.67, 131.63, 131.42, 129.48, 129.07, 128.45, 127.78, 125.13, 121.52, 98.27, 85.70. HRMS (ESI IT-TOF) m/z [M + H]+ Calcd for C20H14NO 284.1070, found 284.1069.
3.4 Characterization Data of Products
3-Phenyl-1H-dibenzo[e,g]indazole (2a). White solid (259 mg, 0.88 mmol, 88%). Rf = 0.40 (PE/EA = 1/1). m.p. 260.4 – 260.8 °C. 1H NMR (400 MHz, DMSO-d6) δ 14.24 – 13.95 (s, 1H), 8.78 – 8.71 (m, 2H), 8.55 (d, J = 7.8 Hz, 1H), 8.02 (d, J = 8.1 Hz, 1H), 7.74 – 7.69 (m, 4H), 7.59 – 7.54 (m, 3H), 7.50 – 7.40 (m, 2H). 13C NMR (101 MHz, DMSO-d6) δ 147.37, 137.25, 135.45, 129.62, 128.61, 128.32, 127.51, 127.41, 127.13, 124.95, 124.18, 124.07, 122.63, 122.34, 121.00, 112.55.
3-(4-Methoxyphenyl)-1H-dibenzo[e,g]indazole (2b). White solid (285 mg, 0.88 mmol, 88%). Rf = 0.40 (PE/EA = 1/1). m.p. 204.2 – 204.7 °C. 1H NMR (400 MHz, DMSO-d6) δ 13.97 (s, 1H), 8.71 (dd, J = 16.9, 8.1 Hz, 2H), 8.56 (d, J = 7.7 Hz, 1H), 8.05 (d, J = 7.3 Hz, 1H), 7.74 – 7.63 (m, 4H), 7.49 – 7.40 (m, 2H), 7.14 (d, J = 8.4 Hz, 2H), 3.84 (s, 3H). 13C NMR (101 MHz, DMSO-d6) δ 159.34, 147.25, 137.27, 130.91, 129.68, 127.45, 127.31, 127.12, 124.87, 124.09, 123.99, 122.67, 122.40, 121.14, 114.02, 112.64, 55.14. HRMS (ESI IT-TOF) m/z [M + H]+ Calcd for C22H17N2O 325.1335, found 325.1334.
3-(p-Tolyl)-1H-dibenzo[e,g]indazole (2c). White solid (262 mg, 0.85 mmol, 85%). Rf = 0.40 (PE/EA = 1/1). m.p. 199.6 – 200.0 °C. 1H NMR (400 MHz, DMSO-d6) δ 11.79 (s, 1H), 8.71 (dd, J = 17.1, 8.1 Hz, 2H), 8.57 (d, J = 7.7 Hz, 1H), 8.06 (d, J = 7.7 Hz, 1H), 7.73 (t, J = 7.5 Hz, 1H), 7.66 (t, J = 7.5 Hz, 1H), 7.61 (d, J = 7.8 Hz, 2H), 7.48 – 7.36 (m, 4H), 2.40 (s, 3H). 13C NMR (101 MHz, DMSO-d6) δ 145.18, 139.07, 137.85, 131.46, 129.70, 129.49, 129.26, 127.59, 127.49, 127.36, 127.12, 125.64, 124.99, 124.15, 123.99, 122.67, 122.38, 112.36, 20.95. HRMS (ESI IT-TOF) m/z [M + H]+ Calcd for C22H17N2 309.1386, found 309.1385.
3-(4-Fluorophenyl)-1H-dibenzo[e,g]indazole (2d). White solid (262 mg, 0.84 mmol, 84%). Rf = 0.40 (PE/EA = 1/1). m.p. 235.7 – 236.2 °C. 1H NMR (400 MHz, DMSO-d6) δ 14.31 – 14.03 (s, 1H), 8.70 – 8.58 (m, 3H), 8.00 (s, 1H), 7.80 – 7.62 (m, 4H), 7.43 – 7.39 (m, 4H). 13C NMR (101 MHz, DMSO-d6) δ 162.26 (d, J = 245.1 Hz), 146.41, 137.38, 131.89, 131.77, 131.69, 129.66, 127.48, 127.39, 127.17, 124.93, 124.13, 124.01, 122.60, 122.40, 121.04, 115.55 (d, J = 21.5 Hz), 112.66. HRMS (ESI IT-TOF) m/z [M + H]+ Calcd for C21H14FN2 313.1136, found 313.1134.
3-(4-Chlorophenyl)-1H-dibenzo[e,g]indazole (2e). White solid (262 mg, 0.80 mmol, 80%). Rf = 0.40 (PE/EA = 1/1). m.p. 265.3 – 265.9 °C. 1H NMR (400 MHz, DMSO-d6) δ 14.18 (s, 1H), 8.76 (dd, J = 17.0, 8.0 Hz, 2H), 8.53 (dd, J = 7.7, 1.7 Hz, 1H), 7.96 (d, J = 7.5 Hz, 1H), 7.76 – 7.68 (m, 4H), 7.66 – 7.64 (m, 2H), 7.53 – 7.44 (m, 2H). 13C NMR (101 MHz, DMSO-d6) δ 144.83, 138.58, 133.68, 133.32, 131.39, 129.69, 128.73, 127.61, 127.47, 127.40, 127.19, 127.12, 125.05, 124.13, 123.95, 122.64, 122.40, 121.72, 112.52. HRMS (ESI IT-TOF) m/z [M + H]+ Calcd for C21H14ClN2 329.0840, found 329.0838.
6-Methyl-3-phenyl-1H-dibenzo[e,g]indazole (2f). White solid (265 mg, 0.86 mmol, 86%). Rf = 0.40 (PE/EA = 1/1). m.p. 235.6 – 235.9 °C. 1H NMR (400 MHz, DMSO-d6) δ 14.22 – 13.93 (s, 1H), 8.76 – 8.52 (m, 3H), 7.94 (d, J = 8.3 Hz, 1H), 7.76 – 7.52 (m, 7H), 7.19 (d, J = 8.4 Hz, 1H), 2.44 (s, 3H). 13C NMR (101 MHz, DMSO-d6) δ 147.12, 137.04, 135.60, 134.02, 129.61, 129.51, 128.55, 128.40, 128.23, 127.53, 127.30, 127.19, 124.88, 123.99, 122.59, 122.34, 121.16, 112.64, 21.26. HRMS (ESI IT-TOF) m/z [M + H]+ Calcd for C22H17N2 309.1386, found 309.1385.
6-Chloro-3-phenyl-1H-dibenzo[e,g]indazole (2g). White solid (276 mg, 0.84 mmol, 84%). Rf = 0.40 (PE/EA = 1/1). m.p. 286.9 – 287.3 °C. 1H NMR (400 MHz, DMSO-d6) δ 13.77 (s, 1H), 8.73 – 8.69 (m, 2H), 8.51 (d, J = 7.6 Hz, 1H), 7.92 (d, J = 8.6 Hz, 1H), 7.75 – 7.64 (m, 4H), 7.60 – 7.52 (m, 3H), 7.40 (dd, J = 8.6, 2.1 Hz, 1H). 13C NMR (101 MHz, DMSO-d6) δ 129.86, 129.55, 129.22, 128.74, 128.59, 128.12, 127.49, 127.08, 125.86, 124.32, 124.20, 123.65, 122.33, 111.74. HRMS (ESI IT-TOF) m/z [M + H]+ Calcd for C21H14ClN2 329.0840, found 329.0838.
3-Phenyl-6-(trifluoromethyl)-1H-dibenzo[e,g]indazole (2h). White solid (300 mg, 0.83 mmol, 83%). Rf = 0.40 (PE/EA = 1/1). m.p. 276.9 – 277.3 °C. 1H NMR (400 MHz, DMSO-d6) δ 14.29 – 14.10 (s, 1H), 8.88 – 8.84 (m, 1H), 8.72 – 8.65 (m, 1H), 8.55 – 8.47 (m, 1H), 8.07 – 8.02 (m, 1H), 7.71 – 7.51 (m, 8H). 13C NMR (101 MHz, DMSO-d6) δ 147.73, 138.05, 135.04, 129.87, 129.58, 128.80, 128.64, 128.45, 128.15, 127.61, 127.08, 126.02, 125.06 (q, J = 31.7 Hz), 124.14, 123.35 (d, J = 6.7 Hz), 122.84, 122.39, 121.13 (d, J = 15.7 Hz), 111.73. 19F NMR (376 MHz, DMSO-d6) δ -60.08. HRMS (ESI IT-TOF) m/z [M + H]+ Calcd for C22H14F3N2 363.1104, found 363.1102.
3-(Pyridin-2-yl)-1H-dibenzo[e,g]indazole (2i). White solid (230 mg, 0.78 mmol, 78%). Rf = 0.40 (PE/EA = 1/1). m.p. 209.3 – 209.7 °C. 1H NMR (400 MHz, DMSO-d6) δ 14.44 – 14.28 (s, 1H), 9.04 – 9.02 (m, 1H), 8.86 (d, J = 4.9 Hz, 1H), 8.79 – 8.58 (m, 3H), 8.06 – 7.97 (m, 2H), 7.78 – 7.67 (m, 2H), 7.52 – 7.49 (m, 3H). 13C NMR (101 MHz, DMSO-d6) δ 154.35, 148.81, 147.16, 137.80, 137.04, 129.72, 127.58, 127.54, 127.48, 127.30, 127.05, 125.65, 125.24, 124.34, 124.04, 123.71, 123.15, 122.30, 120.92, 113.53. HRMS (ESI IT-TOF) m/z [M + H]+ Calcd for C20H14N3 296.1182, found 296.1181.
3-(Thiophen-2-yl)-1H-dibenzo[e,g]indazole (2j). White solid (273 mg, 0.91 mmol, 91%). Rf = 0.40 (PE/EA = 1/1). m.p. 255.4 – 255.9 °C. 1H NMR (400 MHz, DMSO-d6) δ 14.40 – 14.17 (s, 1H), 8.71 – 8.56 (m, 3H), 8.34 – 8.31 (m, 1H), 7.76 – 7.73 (m, 2H), 7.66 (t, J = 7.7 Hz, 1H), 7.56 (d, J = 3.6 Hz, 1H), 7.52 – 7.46 (m, 2H), 7.32 – 7.30 (m, 1H). 13C NMR (101 MHz, DMSO-d6) δ 140.47, 137.44, 136.12, 129.61, 128.07, 127.72, 127.55, 127.30, 127.10, 126.97, 125.19, 124.11, 124.03, 122.65, 122.36, 120.86, 113.25. HRMS (ESI IT-TOF) m/z [M + H]+ Calcd for C19H13N2S 301.0794, found 301.0793.
3-(Triisopropylsilyl)-1H-dibenzo[e,g]indazole (2k). White solid (348 mg, 0.93 mmol, 93%). Rf = 0.40 (PE/EA = 1/1). m.p. 87.8 – 88.3 °C. 1H NMR (400 MHz, Chloroform-d) δ 12.12 (s, 1H), 8.75 (d, J = 7.6 Hz, 1H), 8.58 – 8.53 (m, 2H), 8.27 (d, J = 7.8 Hz, 1H), 7.64 – 7.46 (m, 4H), 1.78 (hept, J = 7.5 Hz, 3H), 1.12 (d, J = 7.7 Hz, 18H). 13C NMR (101 MHz, Chloroform-d) δ 130.58, 129.10, 128.79, 127.31, 127.26, 126.54, 126.04, 125.39, 124.01, 123.62, 123.40, 123.21, 18.89, 12.74. HRMS (ESI IT-TOF) m/z [M + H]+ Calcd for C24H31N2Si 375.2251, found 375.2251.
3-Pentyl-1H-dibenzo[e,g]indazole (2l). White solid (176 mg, 0.61 mmol, 61%). Rf = 0.40 (PE/EA = 1/1). m.p. 188.5 – 188.9 °C. 1H NMR (400 MHz, DMSO-d6) δ 13.69 – 13.49 (s, 1H), 8.78 – 8.67 (m, 2H), 8.44 (d, J = 7.5 Hz, 1H), 8.24 – 8.15 (m, 1H), 7.69 – 7.52 (m, 4H), 3.19 (t, J = 7.6 Hz, 2H), 1.82 – 1.80 (m, 2H), 1.42 – 1.32 (m, 4H), 0.87 (t, J = 7.1 Hz, 3H). 13C NMR (101 MHz, DMSO-d6) δ 147.37, 137.15, 129.46, 127.67, 127.32, 127.11, 124.40, 124.08, 123.17, 122.21, 121.17, 112.43, 31.20, 28.96, 27.75, 21.97, 13.94. HRMS (ESI IT-TOF) m/z [M + H]+ Calcd for C20H21N2 289.1699, found 289.1698.
10-Chloro-3-(triisopropylsilyl)-1H-dibenzo[e,g]indazole (2m). White solid (335 mg, 0.82 mmol, 82%). Rf = 0.40 (PE/EA = 1/1). m.p. 191.1 – 191.7 °C. 1H NMR (400 MHz, Chloroform-d) δ 11.96 (s, 1H), 8.68 (s, 1H), 8.52 (d, J = 7.9 Hz, 1H), 8.47 (d, J = 9.0 Hz, 1H), 8.23 (d, J = 7.7 Hz, 1H), 7.58 – 7.50 (m, 3H), 1.76 (hept, J = 7.5 Hz, 3H), 1.15 (d, J = 7.6 Hz, 18H). 13C NMR (101 MHz, Chloroform-d) δ 133.34, 129.03, 128.72, 128.55, 127.65, 126.94, 125.72, 125.52, 125.10, 124.01, 123.97, 122.83, 18.93, 12.78. HRMS (ESI IT-TOF) m/z [M + H]+ Calcd for C24H30ClN2Si 409.1861, found 409.1860.
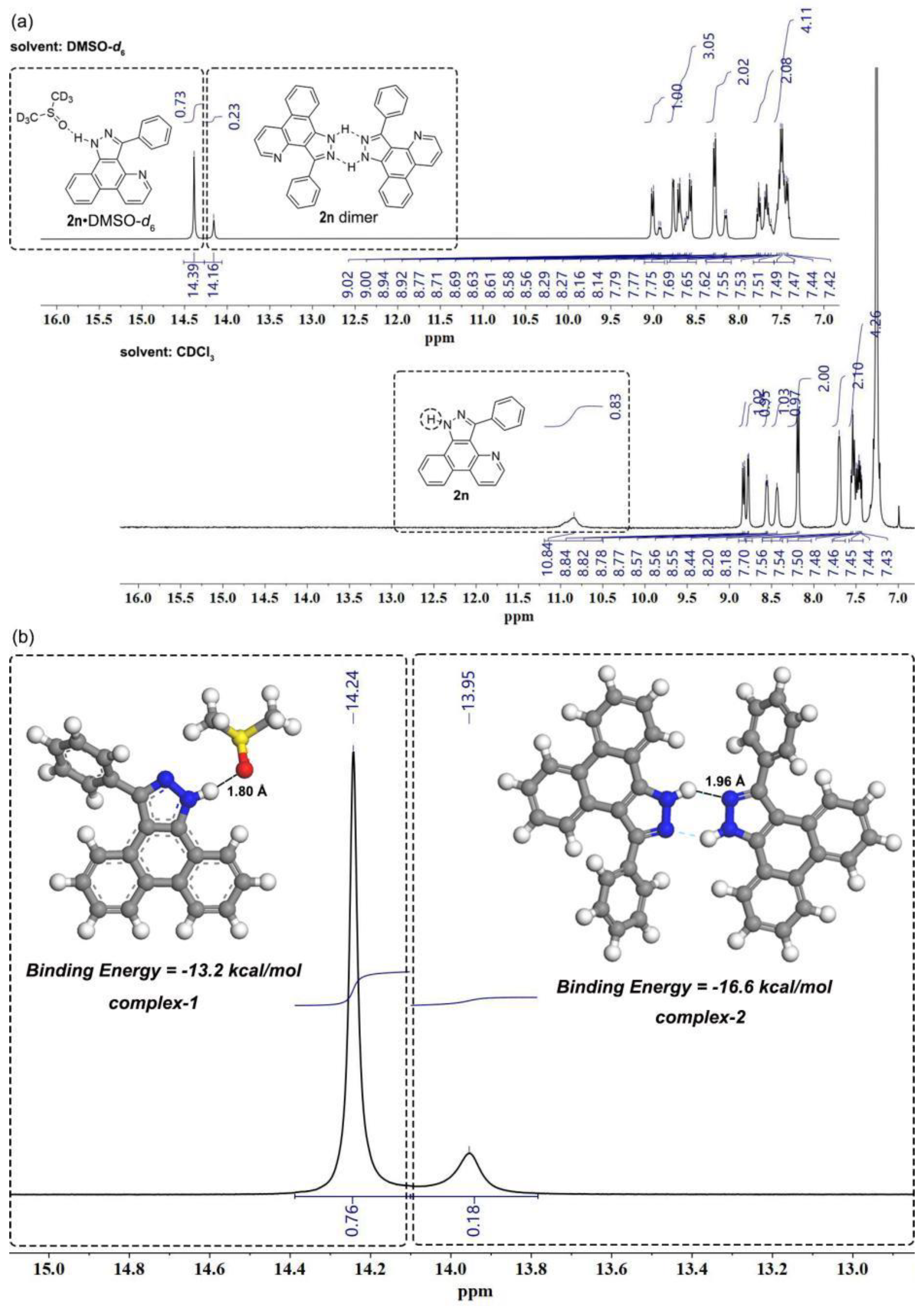
3-Phenyl-1H-benzo[f]pyrazolo[3,4-h]quinoline (2n). White solid (260 mg, 0.88 mmol, 88%). Rf = 0.40 (PE/EA = 1/1). m.p. 265.7 – 266.2 °C. 1H NMR (400 MHz, DMSO-d6) δ 14.39 – 14.16 (s, 1H), 9.02 – 8.92 (m, 1H), 8.78 – 8.56 (m, 3H), 8.29 – 8.14 (m, 2H), 7.79 – 7.62 (m, 2H), 7.55 – 7.42 (m, 4H). 13C NMR (101 MHz, DMSO-d6) δ 148.60, 148.23, 147.43, 145.91, 144.68, 139.95, 139.70, 134.63, 131.70, 130.10, 129.96, 129.66, 128.92, 128.57, 128.08, 127.98, 127.63, 127.49, 126.03, 124.19, 123.89, 123.45, 122.44, 122.31, 120.87, 120.59, 120.16, 113.43, 112.53. 1H NMR (400 MHz, Chloroform-d) δ 10.84 (s, 1H), 8.83 (d, J = 8.3 Hz, 1H), 8.78 (d, J = 4.2 Hz, 1H), 8.57 – 8.55 (m, 1H), 8.44 (s, 1H), 8.19 (d, J = 7.9 Hz, 2H), 7.70 (s, 2H), 7.56 – 7.43 (m, 4H) b. HRMS (ESI IT-TOF) m/z [M + H]+ Calcd for C20H14N3 296.1182, found 296.1181.
3-Phenyl-1H-benzo[f]pyrazolo[3,4-h]isoquinoline (2o). White solid (266 mg, 0.90 mmol, 90%). Rf = 0.40 (PE/EA = 1/1). m.p. 277.9 – 278.4 °C. 1H NMR (400 MHz, DMSO-d6) δ 14.37 – 14.12 (s, 1H), 9.22 (s, 1H), 8.82 – 8.72 (m, 1H), 8.56 – 8.52 (m, 3H), 7.85 – 7.59 (m, 7H). 13C NMR (101 MHz, DMSO-d6) δ 146.96, 145.14, 144.19, 137.71, 135.14, 132.51, 129.63, 129.48, 128.76, 128.57, 127.74, 127.52, 124.76, 122.43, 117.54, 110.61. HRMS (ESI IT-TOF) m/z [M + H]+ Calcd for C20H14N3 296.1182, found 296.1181.
3-Phenyl-1H-benzo[h]pyrazolo[4,3-f]isoquinoline (2p). White solid (221 mg, 0.75 mmol, 75%). Rf = 0.40 (PE/EA = 1/1). m.p. 324.3 – 324.8 °C. 1H NMR (400 MHz, DMSO-d6) δ 14.46 – 14.20 (s, 1H), 10.06 – 9.95 (m, 1H), 9.04 – 8.87 (m, 1H), 8.56 – 8.48 (m, 2H), 7.86 – 7.55 (m, 8H). HRMS (ESI IT-TOF) m/z [M + H]+ Calcd for C20H14N3 296.1182, found 296.1181. The 13C NMR spectroscopic data could not be recorded due to the poor solubility in deuterated solvents, such as DMSO-d6, CDCl3.