3.1. Distribution of Patients
In the current study, 108 neonates with GA ≤ 37 weeks and BW < 2500 g were included to study the effects EPO administration in first 7 days of life, iron administration during 7th to 21st days of life, and other perinatal characteristics, including GA, BW, Apgar scores at 1 and 5 minutes, and LDH, Hb, HCT, RBC count, aPTT, PT, and serum EPO at 1 day of life, have on various outcomes, including AOP (any severity), AOP severity, RBC count, Hb, HCT, and serum EPO, ferritin, and iron levels at 21 days of life and transfusion administration within the first 21 days of life.
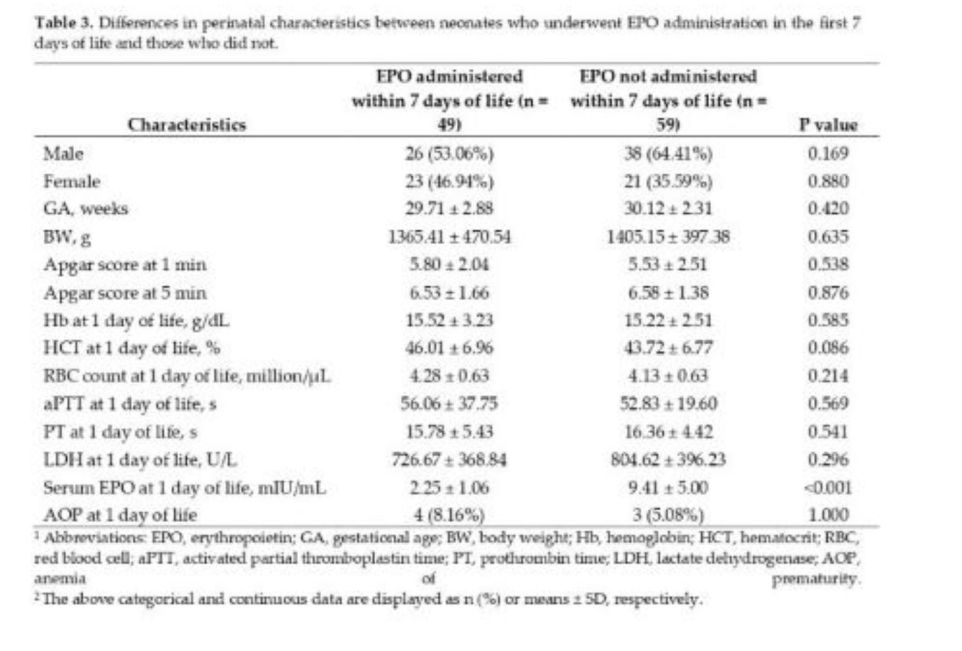
The male to female ratio was 1.45 (64 males and 44 females), with no statistically significant difference being found between sexes in regard to GA (p=0.33), BW (p=0.79), 1- and 5-minutes Apgar scores (p=0.098 and p=0.171, respectively), RBC count (p=0.078), aPTT (p=0.96), PT (p=0.18), LDH (p=0.72), and serum EPO (p=0.53) at 1 day of life. The only baseline characteristics which significantly differed were day-1 Hb and HCT levels (p<0.01 and p<0.05, respectively).
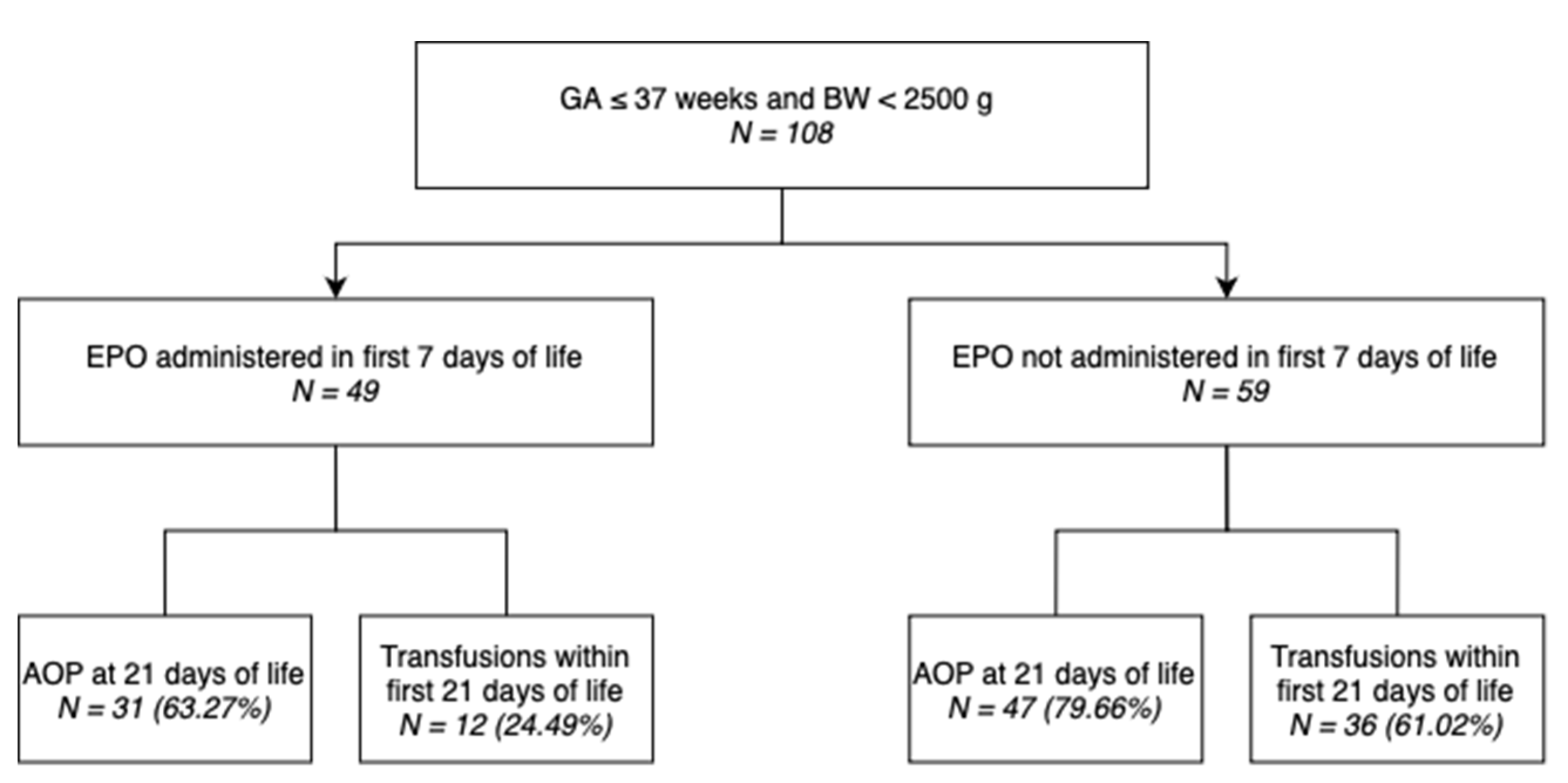
Out of 108 neonates included in this study, 49 (45.37%) underwent EPO administration within the first 7 days of life, while 60 (55.56%) underwent iron administration in the 7
th to 21
st day of life, as shown in the schematic diagram above (
Figure 1).
Table 1 showcases the incidence of AOP (any severity) and specific stages in different subgroups of our study population.
The newborns were grouped based on various perinatal characteristics, such as sex, GA category, BW category, 1- and 5-minute Apgar score categories, LDH within range, aPTT within range, PT within range, day-1 AOP, and iron and EPO treatment.
Most of the males (78.13%) and females (63.64%) were diagnosed with some AOP stage (mild, moderate, or severe). The incidence of AOP (any severity) gradually decreased as GA and BA increased: 88.89%, 76.36%, and 57.14% for extremely preterm (<28 weeks), very preterm (28–31 weeks), and moderate to late preterm (32–37 weeks), and 85.71%, 74.47%, and 62.50% for extremely low birth weight (<1000 g), very low birth weight (1000–1499 g), and low birth weight (1500–2499 g) neonates, respectively. Similarly, there was a gradual decrease in the incidence of AOP (any severity) as 1- and 5-minute Apgar scores increased: 81.82%, 76.47%, and 65.38% for 0–3, 4–6, and 7–10 1-minute Apgar scores, and 100%, 75.56%, and 69.35% for 0–3, 4–6, and 7–10 5-minute Apgar scores, respectively.
Neonates with pathological LDH, PT, and serum EPO levels on day 1 of life showed higher AOP (any severity) incidence rates compared to those presenting levels within the reference ranges (85.87%, 78.52%, and 83.18%, respectively).
Surprisingly, less neonates with pathological aPTT levels on day 1 of life presented AOP (any severity) compared to those with normal aPTT levels (69.47% vs. 92.31%, respectively).
Neonates that were administered iron during the 7th to 21st days of life showed a higher AOP (any severity) incidence rate compared to those that did not undergo iron treatment (80.00% vs. 62.50%), while neonates that were administered EPO during the first 7 days of life showed a lower AOP (any severity) incidence rate compared to those that did not undergo EPO treatment (63.27% vs. 79.66%).
Table 2 showcases the incidence of RBC transfusions in different subgroups of the study population.
Half of the male neonates required transfusions within the first 21 days of life, compared to only 36.36% of female neonates.
The need for transfusions within the first 21 days of life also gradually decreased as GA and BA increased: 77.78%, 47.27%, and 22.86% for extremely preterm, very preterm, and moderate to late preterm, and 76.19%, 51.06%, and 20.00% for extremely low birth weight, very low birth weight, and low birth weight neonates, respectively.
Neonates with pathological PT levels at 1 day of life required transfusions within the first 21 days of life compared to those with normal PT levels (46.97% vs. 40.48%, respectively).
Surprisingly, less neonates with pathological aPTT levels on day 1 of life required transfusions within the first 21 days of life (42.11% vs. 61.54%, respectively).
Neonates that were administered iron during the 7th to 21st days of life showed a higher transfusion incidence rate compared to those that did not undergo iron treatment (60.00% vs. 25.00%, respectively), while neonates that were administered EPO during the first 7 days of life showed a lower transfusion incidence rate compared to those that did not undergo EPO treatment (24.49% vs. 61.02%, respectively).
3.5. Multivariate Analysis of Risk Factors
3.5.1. Multivariate Analysis of Risk Factors and Their Correlation to AOP (any severity)
Factors with a statistically significant correlation to AOP in the previous analysis, namely GA, BW, Hb at 1 day of life, HCT at 1 day of life, RBC count at 1 day of life, and iron administration were analyzed by multivariate binomial logistic regression analysis, and the AORs were calculated. The analysis was performed following the same variable classification used in the above tests.
As indicated in
Table 8, unlike in univariate logistic regression analysis, the only factor that remains statistically significant is iron administration between the 7
th and 21
st day of life, with p<0.05. Therefore, iron administration is shown to increase the risk of neonates developing AOP at 21 days of age 2.7-fold, having an AOR of 2.75 (95% CI, 1.06–7.11). However, this wide confidence interval warrants further research involving larger data sets.
3.5.2. Multinomial Analysis of Risk Factors and Their Correlation to Specific AOP Stages
Factors with a statistically significant correlation to AOP above were then analyzed by multivariate multinomial (ordinal/ranked dependent variable) logistic regression analysis, and the ORs were calculated. The analysis was performed following the variable classification used in above, but instead of using the dichotomous any-severity AOP variable, the AOP category variable (ranked) was used to determine which correlation between multiple factors and AOP stages/levels was the highest.
As presented in
Table 9, the first set of coefficients represents comparisons between neonates with Absence of AOP at 21 days of life (coded 0) and those with Mild AOP at 21 days of life (coded 1 in this portion of the output). None of the above factors were significant predictors in the model, as all p-values >0.05.
The second set of coefficients represents comparisons between neonates with Absence of AOP at 21 days of life (coded 0) and those with Moderate AOP at 21 days of life (coded 2 in this portion of the output). Only iron administration in the 7th to 21st day of life was a significant predictor (b=1.27, s.e.=0.52, p<0.05) in the model, as neonates that underwent iron administration were more 3.5-fold more likely of having moderate AOP that those who did not undergo iron administration, with an OR of 3.56 (95% CI, 1.28–9.93).
The final set of coefficients represents comparisons between neonates with Absence of AOP at 21 days of life (coded 0) and those with Severe AOP at 21 days of life (coded 3 in this portion of the output). Due to only 1 case of Severe AOP, there was insufficient data to test this set.
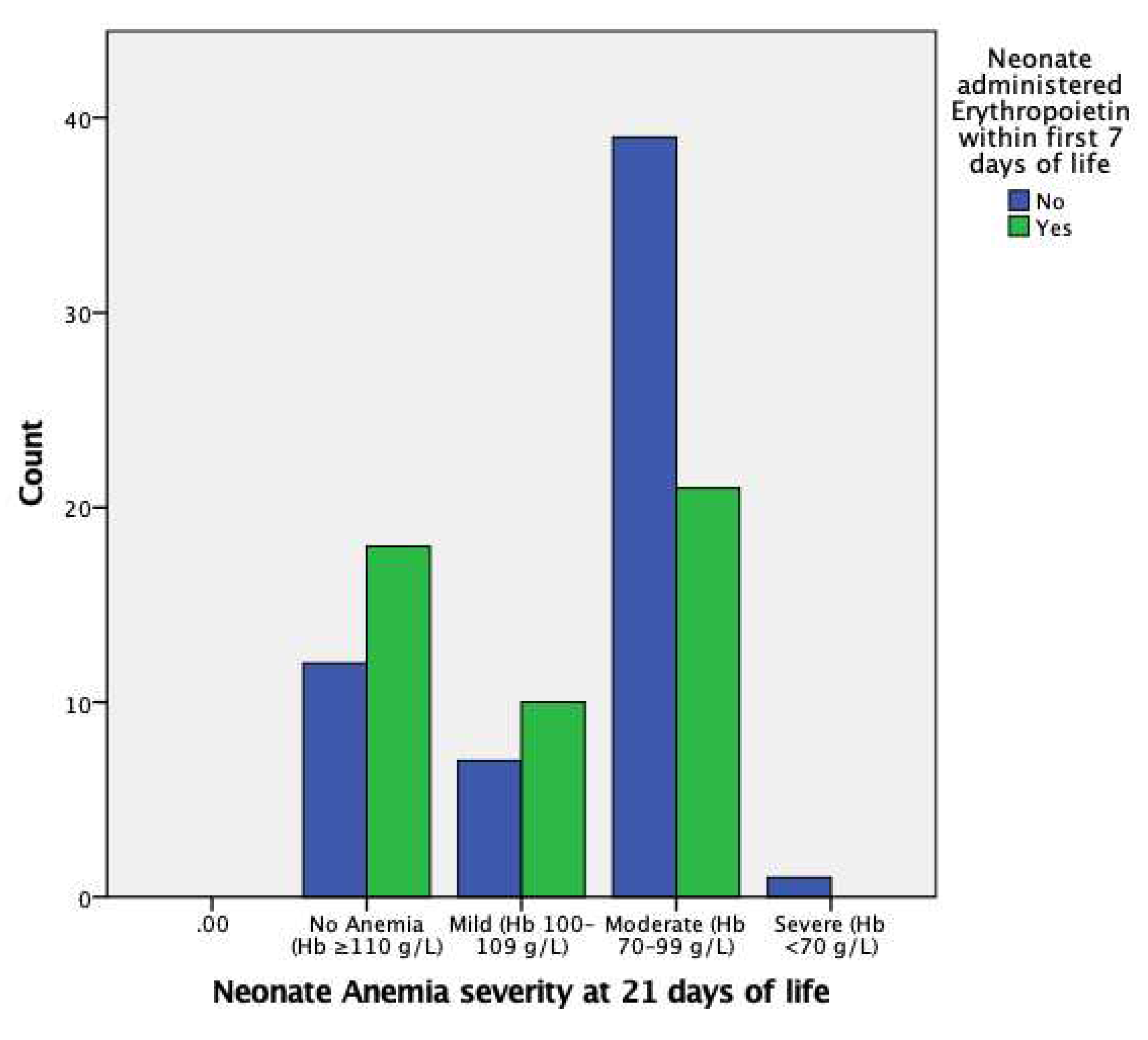
EPO administration in the first 7 days of life is insignificant in regard to mild AOP at 21 days of life; however, it is significant in reducing the likelihood of moderate AOP at 21 days of life, with an OR of 0.36 (95% CI, 0.15–0.89) and p<0.05, as also indicated in
Figure 2. There were insufficient data for severe AOP at 21 days of life.
3.5.3. Spearman Ranked Correlations to Specific AOP Stages
The relationship between particular AOP stages and either rank-transformed GA (32–37, 28–31, and <28 weeks), BW (1500–2499, 1000–1499, and <1000 g), Apgar score at 5 minutes (7–10, 4–6, and 0–3) and dichotomized data (Sex, Normal LDH, Normal aPTT, Normal PT, Iron administration, and EPO administration) was further studied using either the Spearman ranked and rank-biserial correlation coefficients, respectively.
In regard to GA category, there was a strong (ρ = 0.67), statistically significant (p<0.001) correlation between GA category and BW category (p<0.001), a “near strong” (ρ=0.42), statistically significant (p<0.001) correlation between GA category and 5-minute Apgar score category, and a moderate (ρ=-0.32), statistically significant (p = 0.001) negative correlation (-0.314) between GA category and AOP severity at 21 days of life.
In regard to BW category, there was a strong (ρ=0.67), statistically significant (p<0.001) correlation between BW category and GA category, a moderate (ρ=0.36), statistically significant (p<0.001) correlation between BW category and 5-minute Apgar score category, and a moderate (ρ=-0.28), statistically significant (p<0.01) negative correlation between BW category and AOP severity at 21 days of life.
In regard to 5-minute Apgar score category, there was a “near strong” (ρ=0.42), statistically significant (p<0.001) correlation between 5-minute Apgar score category and GA category, a moderate (ρ=0.36), statistically significant (p<0.001) correlation between 5-minute Apgar score category and BW category, and a weak negative correlation (ρ=-0.10), statistically unsignificant (p>0.05) negative correlation between 5-minute Apgar score category and AOP severity at 21 days of life.
Therefore, following the Spearman Ranked analysis, it was demonstrated that GA had a stronger negative correlation with AOP stage/level than BW (ρ=-0.31 vs. ρ=-0.28) but that it was only a moderate correlation.
3.5.4. Multivariate Analysis of Risk Factors and Their Correlation to Transfusion Administration
Factors with a statistically significant correlation to transfusions in the previous Univariate Binary Logistic Regression Analysis, i.e., GA, BW, Hb at 1 day of life, HCT at 1 day of life, RBC count at 1 day of life, serum EPO at 1 day of life and EPO administration, were analyzed using multivariate binomial logistic regression, and the AORs were calculated. The analysis was performed following the same variable classification used in the above tests.
As indicated in
Table 10, unlike in univariate logistic regression analysis, none of the factors remain statistically significant (p<0.05), with BW being the only “near significant” factor, with a p-value of exactly 0.05 and an AOR of 0.998 (95% CI, 0.996–1.000).