Submitted:
10 October 2023
Posted:
11 October 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Design
2.2. Subjects
2.3. Contact Lenses
2.4. Procedure
2.5. Data Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dupps Jr, W.J., Wilson, S.E. (2006). Biomechanics and wound healing in the cornea. Experimental eye research, 83(4), 709-720.
- Torres R, Merayo-Lloves J, Jaramillo, M., Galvis, V. Biomecánica de la córnea. Arch Soc Esp Oftalmol. 2005;80(4):215-223. https://scielo.isciii.es/scielo.php?script=sci_arttext&pid=S0365-66912005000400004&lng=es&nrm=iso&tlng=es. Accessed February 8, 2022.
- Kling, S., & Hafezi, F. (2017). Corneal biomechanics–a review. Ophthalmic and Physiological Optics, 37(3), 240-252.
- Ambrósio Jr, R., Ramos, I., Luz, A., Faria, F.C., Steinmueller, A., Krug, M., ... & Roberts, C.J. (2013). Dynamic ultra high speed Scheimpflug imaging for assessing corneal biomechanical properties. Revista Brasileira de Oftalmologia, 72, 99-102.
- Huseynova, T., Waring IV, G.O., Roberts, C., Krueger, R.R., & Tomita, M. (2014). Corneal biomechanics as a function of intraocular pressure and pachymetry by dynamic infrared signal and Scheimpflug imaging analysis in normal eyes. American journal of ophthalmology, 157(4), 885-893. 4.
- Elsheikh, A., Alhasso, D., & Rama, P. (2008). Assessment of the epithelium's contribution to corneal biomechanics. Experimental eye research, 86(2), 445-451.
- Ma, J.; Wang, Y.; Wei, P.; Jhanji, V. Biomechanics and structure of the cornea: implications and association with corneal disorders. Surv. Ophthalmol. 2018, 63, 851–861. [Google Scholar] [CrossRef] [PubMed]
- Esporcatte, L.P.G.; Salomão, M.Q.; Lopes, B.T.; Vinciguerra, P.; Vinciguerra, R.; Roberts, C.; Elsheikh, A.; Dawson, D.G.; Ambrósio, R. Biomechanical diagnostics of the cornea. Eye Vis. 2020, 7, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Elsheikh, A.; Alhasso, D.; Kotecha, A.; Garway-Heath, D. Assessment of the Ocular Response Analyzer as a Tool for Intraocular Pressure Measurement. J. Biomech. Eng. 2009, 131, 081010–081010. [Google Scholar] [CrossRef] [PubMed]
- Pepose, J.S.; Feigenbaum, S.K.; Qazi, M.A.; Sanderson, J.P.; Roberts, C.J. Changes in Corneal Biomechanics and Intraocular Pressure Following LASIK Using Static, Dynamic, and Noncontact Tonometry. Arch. Ophthalmol. 2007, 143, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Ambrósio Jr, R., Correia, F.F., Lopes, B., Salomão, M.Q., Luz, A., Dawson, D.G., ... & Roberts, C.J. (2017). Suppl-1, M2: Corneal biomechanics in ectatic diseases: Refractive surgery implications. The open ophthalmology journal, 11, 176.
- Goldich, Y., Marcovich, A.L., Barkana, Y., Mandel, Y., Hirsh, A., Morad, Y., ... & Zadok, D. (2012). Clinical and corneal biomechanical changes after collagen cross-linking with riboflavin and UV irradiation in patients with progressive keratoconus: Results after 2 years of follow-up. Cornea, 31(6), 609-614.
- Eliasy, A.; Chen, K.-J.; Vinciguerra, R.; Lopes, B.T.; Abass, A.; Vinciguerra, P.; Ambrósio, R., Jr.; Roberts, C.J.; Elsheikh, A. Determination of Corneal Biomechanical Behavior in-vivo for Healthy Eyes Using CorVis ST Tonometry: Stress-Strain Index. Front. Bioeng. Biotechnol. 2019, 7, 105. [Google Scholar] [CrossRef]
- Piñero, D.P.; Alcón, N. Corneal biomechanics: a review. Clin. Exp. Optom. 2015, 98, 107–116. [Google Scholar] [CrossRef]
- Garcia-Porta, N.; Fernandes, P.; Queiros, A.; Salgado-Borges, J.; Parafita-Mato, M.; González-Méijome, J.M. Corneal Biomechanical Properties in Different Ocular Conditions and New Measurement Techniques. ISRN Ophthalmol. 2014, 2014, 1–19. [Google Scholar] [CrossRef]
- Vinciguerra, R., Elsheikh, A., Roberts, C.J., Ambrósio Jr, R., Kang, D.S.Y., Lopes, B.T., ... & Vinciguerra, P. (2016). Influence of pachymetry and intraocular pressure on dynamic corneal response parameters in healthy patients. Journal of refractive surgery, 32(8), 550-561. 8.
- Roberts, C.J., & Dupps Jr, W.J. (2014). Biomechanics of corneal ectasia and biomechanical treatments. Journal of Cataract & Refractive Surgery, 40(6), 991-998.
- Ambrósio, J.R., Jr.; Correia, F.F.; Lopes, B.; Salomão, M.Q.; Luz, A.; Dawson, D.G.; Elsheikh, A.; Vinciguerra, R.; Vinciguerra, P.; Roberts, C.J. Corneal Biomechanics in Ectatic Diseases: Refractive Surgery Implications. Open Ophthalmol. J. 2017, 11, 176–193. [Google Scholar] [CrossRef]
- Lopes, B.T.; Roberts, C.J.; Elsheikh, A.; Vinciguerra, R.; Vinciguerra, P.; Reisdorf, S.; Berger, S.; Koprowski, R.; Ambrósio, R. Repeatability and Reproducibility of Intraocular Pressure and Dynamic Corneal Response Parameters Assessed by the Corvis ST. J. Ophthalmol. 2017, 2017, 1–4. [Google Scholar] [CrossRef]
- Luce, D.A. Determining in vivo biomechanical properties of the cornea with an ocular response analyzer. J. Cataract. Refract. Surg. 2005, 31, 156–162. [Google Scholar] [CrossRef]
- Piñero, D.P.; Alcón, N. In vivo characterization of corneal biomechanics. J. Cataract. Refract. Surg. 2014, 40, 870–887. [Google Scholar] [CrossRef] [PubMed]
- Hashemi, H.; Asgari, S.; Mortazavi, M.; Ghaffari, R. Evaluation of Corneal Biomechanics After Excimer Laser Corneal Refractive Surgery in High Myopic Patients Using Dynamic Scheimpflug Technology. Eye Contact Lens: Sci. Clin. Pr. 2017, 43, 371–377. [Google Scholar] [CrossRef] [PubMed]
- Kling, S.; Hafezi, F. Corneal biomechanics – a review. Ophthalmic Physiol. Opt. 2017, 37, 240–252. [Google Scholar] [CrossRef]
- Darlen Rodríguez Rivero D, Dra Silvia María López Hernández I, Yoanner Martín Perera, I.; et al. Corneal ulcers in contact lens wearers. Rev Cuba Oftalmol. 2015;28(2):220-227. http://scielo.sld.cu.
- Alba-Bueno, F.; Beltran-Masgoret, A.; Sanjuan, C.; Biarnés, M.; Marín, J. Corneal shape changes induced by first and second generation silicone hydrogel contact lenses in daily wear. Contact Lens Anterior Eye 2009, 32, 88–92. [Google Scholar] [CrossRef] [PubMed]
- Tyagi, G.; Collins, M.; Read, S.; Davis, B. Regional Changes in Corneal Thickness and Shape with Soft Contact Lenses. Optom. Vis. Sci. 2010, 87, 567–575. [Google Scholar] [CrossRef] [PubMed]
- Braun, D.A.; Penno, E.E.A. Effect of contact lens wear on central corneal thickness measurements. J. Cataract. Refract. Surg. 2003, 29, 1319–1322. [Google Scholar] [CrossRef]
- Sánchez-Tena, M. .; Martinez-Perez, C.; Villa-Collar, C.; Alvarez-Peregrina, C. Long-term effect of contact lens wear: A citation network study. Contact Lens Anterior Eye 2022, 45, 101527. [Google Scholar] [CrossRef]
- Alipour, F.; Khaheshi, S.; Soleimanzadeh, M.; Heidarzadeh, S.; Heydarzadeh, S. Contact Lens-related Complications: A Review. Journal of ophthalmic vision research, 2017, 12, 193–204. [CrossRef]
- Peyman, A.; Ghoreishi, M.; Hashemi-Estabragh, S.-S.; Mirmohammadkhani, M.; Mohammadinia, M.; Pourazizi, M. Corneal biomechanical properties after soft contact lens wear measured on a dynamic Scheimpflug analyzer: A before and after study. Journal Français d’Ophtalmologie2021, 44, 391–396. [CrossRef]
- Armstrong, R.A. Statistical guidelines for the analysis of data obtained from one or both eyes. Ophthalmic Physiol. Opt. 2012, 33, 7–14. [Google Scholar] [CrossRef]
- Jaisankar D, Leube A, Gifford KL, Schmid KL, Atchison DA. Effects of eye rotation and contact lens decentration on horizontal peripheral refraction. Ophthalmic Physiol Opt. 2019;39(5):370-377.
- Hassan Z, Modis L, Szalai E, Berta, A., Nemeth, G. Author ’ s personal copy Contact Lens & Anterior Eye Examination of ocular biomechanics with a new Scheimpflug technology after corneal refractive surgery.
- Hashemi, H.; Asgari, S.; Mortazavi, M.; Ghaffari, R. Evaluation of Corneal Biomechanics After Excimer Laser Corneal Refractive Surgery in High Myopic Patients Using Dynamic Scheimpflug Technology. Eye Contact Lens: Sci. Clin. Pr. 2017, 43, 371–377. [Google Scholar] [CrossRef]
- Yang, K.; Xu, L.; Fan, Q.; Gu, Y.; Song, P.; Zhang, B.; Zhao, D.; Pang, C.; Ren, S. Evaluation of new Corvis ST parameters in normal, Post-LASIK, Post-LASIK keratectasia and keratoconus eyes. Sci. Rep. 2020, 10, 1–9. [Google Scholar] [CrossRef]
- Chen, D.; Lam, A.K.C.; Cho, P. A pilot study on the corneal biomechanical changes in short-term orthokeratology. Ophthalmic Physiol. Opt. 2009, 29, 464–471. [Google Scholar] [CrossRef] [PubMed]
- González-Méijome, J.M.; Villa-Collar, C.; Queirós, A.; Jorge, J.; A Parafita, M. Pilot Study on the Influence of Corneal Biomechanical Properties Over the Short Term in Response to Corneal Refractive Therapy for Myopia. Cornea 2008, 27, 421–426. [Google Scholar] [CrossRef] [PubMed]
- Cankaya, A.B.; Beyazyildiz, E.; Ileri, D.; Ozturk, F. The Effect of Contact Lens Usage on Corneal Biomechanical Parameters in Myopic Patients. Cornea 2012, 31, 764–769. [Google Scholar] [CrossRef] [PubMed]
- Jafarzadehpur, E.; Radaie-Moghadam, S.; Hashemi, H.; Yekta, A.; Khabazkhoob, M. Corneal biomechanical changes following toric soft contact lens wear. 2016, 11, 131–5. [CrossRef]
- Lau, W.; Pye, D. Changes in Corneal Biomechanics and Applanation Tonometry with Induced Corneal Swelling. Investig. Opthalmology Vis. Sci. 2011, 52, 3207–3214. [Google Scholar] [CrossRef]
- Braun, D.A.; Penno, E.E.A. Effect of contact lens wear on central corneal thickness measurements. J. Cataract. Refract. Surg. 2003, 29, 1319–1322. [Google Scholar] [CrossRef]
- Çavdarlı, C.; Topçu-Yılmaz, P. Does Long-term Soft Contact Lens Wear Affect Corneal and Anterior Chamber Parameters? Turk. J. Ophthalmol. 2018, 48, 166–170. [Google Scholar] [CrossRef]
- Yeniad, Barş, M.D.ı; Yiğit, Bülent, M.D.; İşsever, Halim PH.D.; Bilgin, Lale Közer, M.D.. Effects of Contact Lenses on Corneal Thickness and Corneal Curvature During Usage. Eye & Contact Lens: Science & Clinical Practice: October 2003 - Volume 29 - Issue 4 - p 223-229. 20 October. [CrossRef]
- Sapkota, K.; Franco, S.; Lira, M. Intraocular pressure measurement with ocular response analyzer over soft contact lens. Contact Lens Anterior Eye 2014, 37, 415–419. [Google Scholar] [CrossRef]
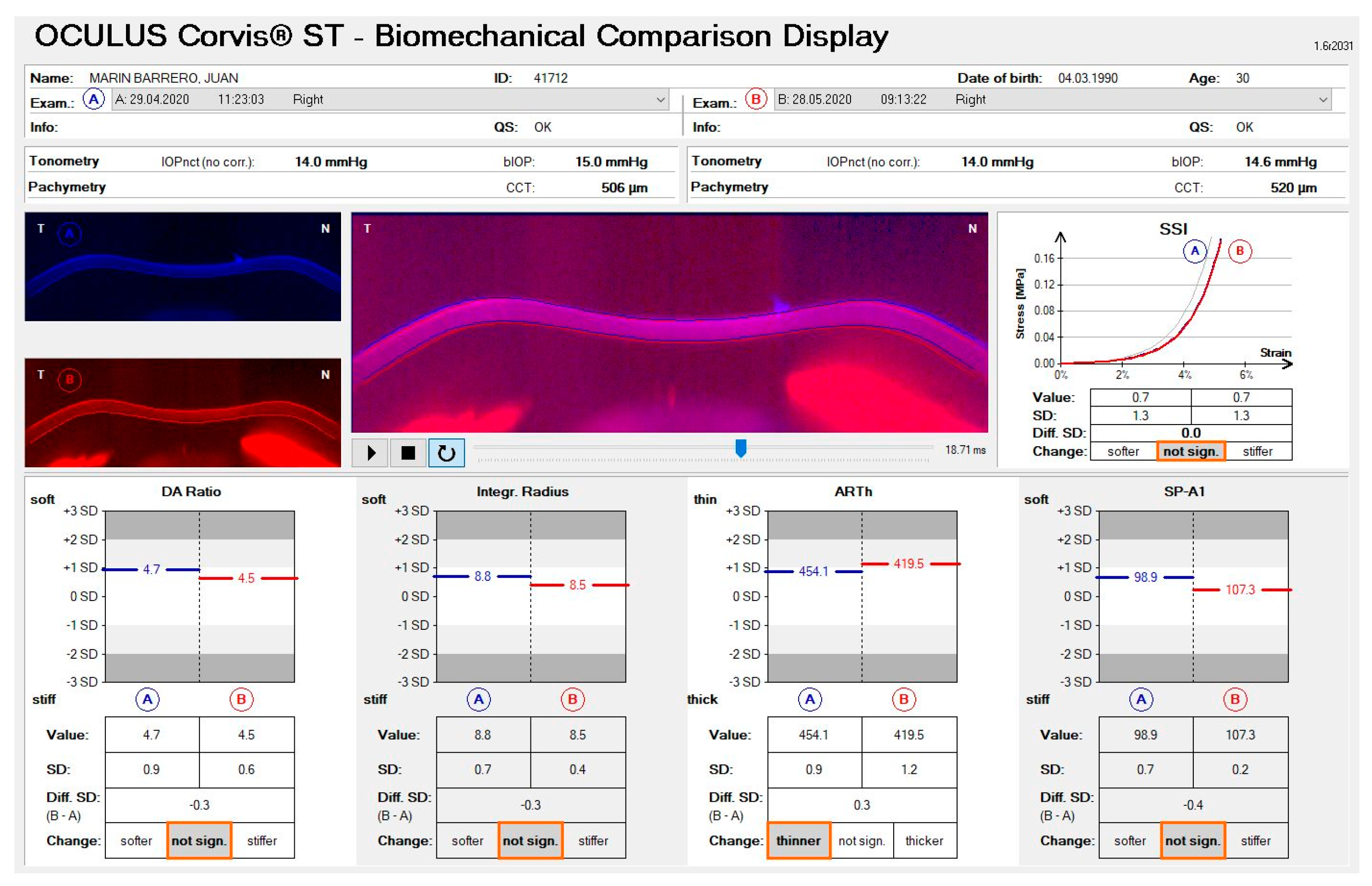
| B-A Not Significant | B-A Stiffer | B-A Softer | |
|---|---|---|---|
| DAR | ± 1.0 | > -1.0 | > +1.0 |
| Int. Radius | ± 0.7 | > -0.7 | > +0.7 |
| SP-A1 | ± 0.8 | > -0.8 | > +0.8 |
| SSI | ± 0.4 | > -0.4 | > +0.4 |
| B-A Not Significant | B-A Thicker | B-A Thinner | |
| ARTh | ± 0.3 | > -0.3 | > +0.3 |
| Variables | |||
|---|---|---|---|
| Mean ± SD | Mean ± SD | ||
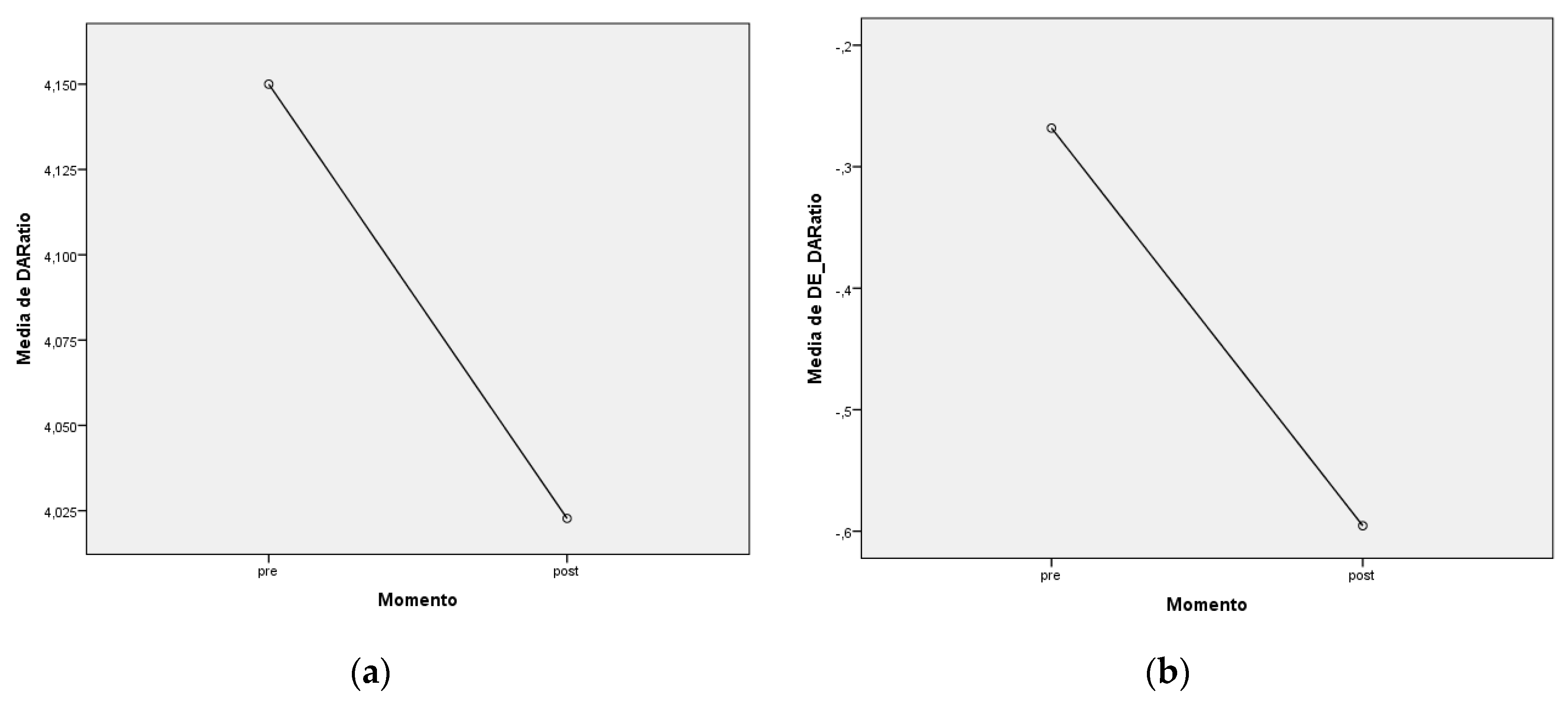
| DAR |
Pre 4,15 ± 0,32 Post 4,02 ± 0,29 |
SD_DAR |
Pre -0,27 ± 0,78 Post -0,60 ± 0,68 |
| Int. Radius |
Pre 7,57 ± 1,05 Post 7,38 ± 0,83 |
SD_Int. Ratius |
Pre -0,43 ± 0,93 Post -0,59 ± 0,75 |
| ARTh |
Pre 521,14 ± 81,60 Post 528,77 ± 106,75 |
SD_ARTh | Pre 0,33 ± 0,66 Post 0,29 ± 0,85 |
| SP-A1 |
Pre 112,77 ± 15,50 Post 108,82 ± 12,69 |
SD_SP-A1 | Pre -0,04 ± 0,82 Post 0,16 ± 0,69 |
| SSI |
Pre 0,97 ± 0,14 Post 0,95 ± 0,14 |
SD_SSI | Pre 0,20 ± 0,58 Post 0,17 ± 0,66 |
| bIOP |
Pre 16,14 ± 2,67 Post 15,61 ± 1,85 |
CCT | Pre 556,00 ± 91,38 Post 545,23 ± 21,75 |
| Variables | p-valor | IC (µpre - µpost) | Resultado | Clasificación |
|---|---|---|---|---|
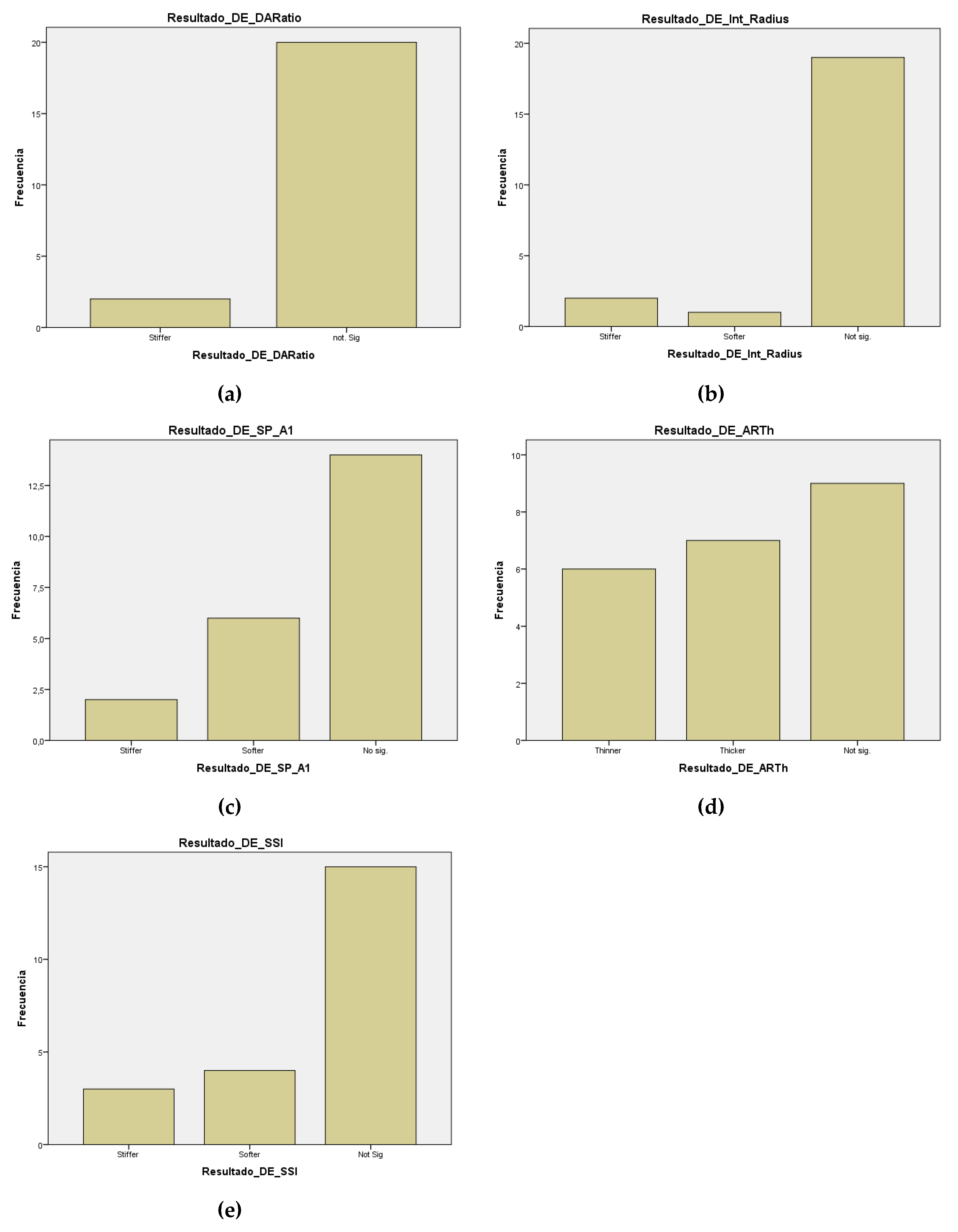
| DAR (SD) | 0,002 | (0,1361;0,5184) | pre > post | |post-pre|<1 |
| Int. Radius (SD) | 0,1 | No normalidad | No sig. | |post-pre|<0,7 |
| SP-A1 (SD) | 0,129 | (-0,46355; 0,06355) | No sig. | |post-pre|<0,8 |
| SSI (SD) | 0,779 | (-0,20054; 0,26418) | No sig. | |post-pre|<0,4 |
| ARTh (SD) | 0,986 | No normalidad | No sig. | |post-pre|<0,3 |
| bIOP | 0,135 | No normalidad | No sig. | |
| CCT | 0,013 | No normalidad | Pre < post |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





