Submitted:
10 October 2023
Posted:
11 October 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
3. Results
3.1. Features of Plasma Synthesis of Oxide Powders
3.2. ZnO Powder Characterization
3.3. Photocatalytic Properties of ZnO Powder
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ren, G.; Han, H.; Wang, Y.; Liu, S.; Zhao, J.; Meng, X.; Li, Z. Recent Advances of Photocatalytic Application in Water Treatment: A Review. Nanomaterials 2021, 11, 1804. [Google Scholar] [CrossRef]
- Byrne, J.A.; Dunlop, P.S.M.; Hamilton, J.W.J.; Fernández-Ibáñez, P.; Polo-López, I.; Sharma, P.K.; Vennard, A.S.M. A Review of Heterogeneous Photocatalysis for Water and Surface Disinfection. Molecules 2015, 20, 5574–5615. [Google Scholar] [CrossRef]
- Herrmann, J.-M. Heterogeneous photocatalysis: Fundamentals and applications to the removal of various types of aqueous pollutants. Catalysis Today 1999, 53, 115–129. [Google Scholar] [CrossRef]
- Matthews, R.W.; McEvoy, S.R. Destruction of phenol in water with sun, sand, and photocatalysis. Solar Energy. 1992, 49, 507–513. [Google Scholar] [CrossRef]
- Mugumo, R.; Ichipi, E.; Tichapondwa, S.M.; Chirwa, E.M.N. Visible-Light-Induced Photocatalytic Degradation of Rhodamine B Dye Using a CuS/ZnS p-n Heterojunction Nanocomposite under Visible-Light Irradiation. Catalysts 2023, 13, 1184. [Google Scholar] [CrossRef]
- Chen, H.; Hu, Y.; Ying, Z.; Xia, Y.; Ye, J.; Zhao, J.; Zhang, S. BiOI-SnO2 Heterojunction Design to Boost Visible-Light-Driven Photocatalytic NO Purification. Int. J. Environ. Res. Public Health 2023, 20, 4009. [Google Scholar] [CrossRef]
- Chowdhury, A.; Balu, S.; Lan, K.-W.; Wei-Chih Lee, L.; Yang, T.C.-K. Synergistic Effect of BiVO4/P-g-C3N4 Heterojunction with Enhanced Optoelectronic Properties on Synthetic Colorants under Visible Light. Colorants 2023, 2, 426–442. [Google Scholar] [CrossRef]
- Yan, X.; Ning, G.; Zhao, P. Enhanced Visible Light Photocatalytic Reduction of Cr(VI) over a Novel Square Nanotube Poly (Triazine Imide)/TiO2 Heterojunction. Catalysts 2019, 9, 55. [Google Scholar] [CrossRef]
- Wang, W.-Y.; Sang, T.; Zhong, Y.; Hu, C.-H.; Wang, D.-H.; Ye, J.-C.; Wei, N.-N.; Liu, H. Surfactant-Modified CdS/CdCO3 Composite Photocatalyst Morphology Enhances Visible-Light-Driven Cr(VI) Reduction Performance. Nanomaterials 2022, 12, 3923. [Google Scholar] [CrossRef] [PubMed]
- Yadav, A.A.; Hunge, Y.M.; Kang, S.-W. Visible Light-Responsive CeO2/MoS2 Composite for Photocatalytic Hydrogen Production. Catalysts 2022, 12, 1185. [Google Scholar] [CrossRef]
- Mohamed, K.M.; Benitto, J.J.; Vijaya, J.J.; Bououdina, M. Recent Advances in ZnO-Based Nanostructures for the Photocatalytic Degradation of Hazardous, Non-Biodegradable Medicines. Crystals 2023, 13, 329. [Google Scholar] [CrossRef]
- Ma, S.; Huang, Y.; Hong, R.; Lu, X.; Li, J.; Zheng, Y. Enhancing Photocatalytic Activity of ZnO Nanoparticles in a Circulating Fluidized Bed with Plasma Jets. Catalysts 2021, 11, 77. [Google Scholar] [CrossRef]
- Alkauskas, A.; Pasquarello, A. Band-edge problem in the theoretical determination of defect energy levels: The O vacancy in ZnO as a benchmark case. Physical Review B 2011, 84. [Google Scholar] [CrossRef]
- Hanif, M.A.; Kim, Y.S.; Ameen, S.; Kim, H.G.; Kwac, L.K. Boosting the Visible Light Photocatalytic Activity of ZnO through the Incorporation of N-Doped for Wastewater Treatment. Coatings 2022, 12, 579. [Google Scholar] [CrossRef]
- Heo, S.-G.; Jo, S.-I.; Jeong, G.-H. Revealing the enhanced photocatalytic properties of ZnO tetrapods produced by atmospheric-pressure microwave plasma jet system. Current Applied Physics 2023, 46, 46–54. [Google Scholar] [CrossRef]
- Fortov, V.E.; Ivlev, A.V.; Khrapak, S.A.; Khrapak, A.G.; Morfill, G.E. Complex (dusty) plasmas: Current status, open issues, perspectives. Phys. Rep. 2005, 421, 1–103. [Google Scholar] [CrossRef]
- Ishihara, O. Complex plasma: Dusts in plasma. J. Phys. D Appl. Phys. 2007, 40, R121. [Google Scholar] [CrossRef]
- Koohgard, M.; Masoudi Sarvestani, A.; Hosseini-Sarvari, M. Photocatalytic synthesis of unsymmetrical thiourea derivatives via visible-light irradiation using nitrogen-doped ZnO nanorods. New J. Chem. 2020, 44, 14505–14512. [Google Scholar] [CrossRef]
- Moisan, M.; Sauve, G.; Zakrzewski, Z.; Hubert, J. Plasma Sources Sci. Technol. 1994, 3, 584–592.
- Baltin, L.; Batenin, V.M.; Lebedeva, V.; Tsemko, N.; Devyatkin, I. Stationary microwave discharge in nitrogen at atmospheric pressure. High Temp. 1971, 9, 1019. [Google Scholar]
- Lavand, A.B.; Malghe, Y.S. Synthesis, characterization and visible light photocatalytic activity of nitrogen-doped zinc oxide nanospheres. J. Asian Ceram. Soc. 2015, 3, 305–310. [Google Scholar] [CrossRef]
- García, J.F.; Sánchez, S.; Metz, R. Complete Oxidation of Zinc Powder. Validation of Kinetics Models. Oxid. Met. 2008, 69, 317–325. [Google Scholar] [CrossRef]
- Hassan, N.; Jalil, A.; Fei, I.; Razak, M.; Khusnun, N.; Bahari, M.; Riwayati, Y.; Suprapto, S.; Prasetyoko, D.; Firmansyah, M.; et al. Vanadia as an electron-hole recombination inhibitor on fibrous silica-titania for selective hole oxidation of ciprofloxacin and Congo red photodegradation. Chemosphere 2023, 338, 139502. [Google Scholar] [CrossRef]
- Van Thuan, D.; Nguyen, T.B.; Pham, T.H.; Kim, J.; Chu, T.T.H.; Nguyen, M.V.; Nguyen, K.D.; Al-Onazi, W.A.; Elshikh, M.S. Photodegradation of ciprofloxacin antibiotic in water by using ZnO-doped g-C3N4 photocatalyst. Chemosphere 2022, 308, 136408. [Google Scholar] [CrossRef]
- Mukherjee, I.; Cilamkoti, V.; Dutta, R.K. Sunlight-Driven Photocatalytic Degradation of Ciprofloxacin by Carbon Dots Embedded in ZnO Nanostructures. ACS Appl. Nano Mater. 2021, 4, 7686–7697. [Google Scholar] [CrossRef]
- Costa, L.N.; Nobre, F.X.; Lobo, A.d.O.; de Matos, J.M.E. Photodegradation of ciprofloxacin using Z-scheme TiO2/SnO2 nanostructures as photocatalyst. Environ. Nanotechnology, Monit. Manag. 2021, 16, 100466. [Google Scholar] [CrossRef]
- Park, S.J.; Das, G.S.; Schütt, F.; Adelung, R.; Mishra, Y.K.; Tripathi, K.; Kim, T.M. Visible-light photocatalysis by carbon-nano-onion-functionalized ZnO tetrapods: Degradation of 2,4-dinitrophenol and a plant-model-based ecological assessment. NPG Asia Mater. 2019, 11, 8. [Google Scholar] [CrossRef]
- Mao, L.; Shen, J.; Ma, X.; Lan, Z.; Zhang, X. Effects of operational parameters on the photodegradation of 2,4-dinitrophenol in TiO2dispersion. Desalination Water Treat. 2014, 56, 744–751. [Google Scholar] [CrossRef]
- Das, A.; S.K., N.; Nair, R.G. Influence of surface morphology on photocatalytic performance of zinc oxide: A review. Nano-Struct. Nano-Objects 2019, 19, 100353. [Google Scholar] [CrossRef]
- Wang, M.; Zhang, Y.; Zhou, Y.; Yang, F.; Kim, E.J.; Hahn, S.H.; Seong, S.G. Rapid room-temperature synthesis of nanosheet-assembled ZnO mesocrystals with excellent photocatalytic activity. CrystEngComm 2013, 15, 754–763. [Google Scholar] [CrossRef]
- Kislov, N.; Lahiri, J.; Verma, H.; Goswami, D.Y.; Stefanakos, E.; Batzill, M. Photocatalytic Degradation of Methyl Orange over Single Crystalline ZnO: Orientation Dependence of Photoactivity and Photostability of ZnO. Langmuir 2009, 25, 3310–3315. [Google Scholar] [CrossRef]
- Vempati, S.; Mitra, J.; Dawson, P. One-step synthesis of ZnO nanosheets: A blue-white fluorophore. Nanoscale Res. Lett. 2012, 7, 470. [Google Scholar] [CrossRef] [PubMed]
- I. V. Rogozin, A.N. Georgobiani, M.B. Kotlyarevsky, and A. V. Marakhovskii, Inorg. Mater. 2009, 45, 391.
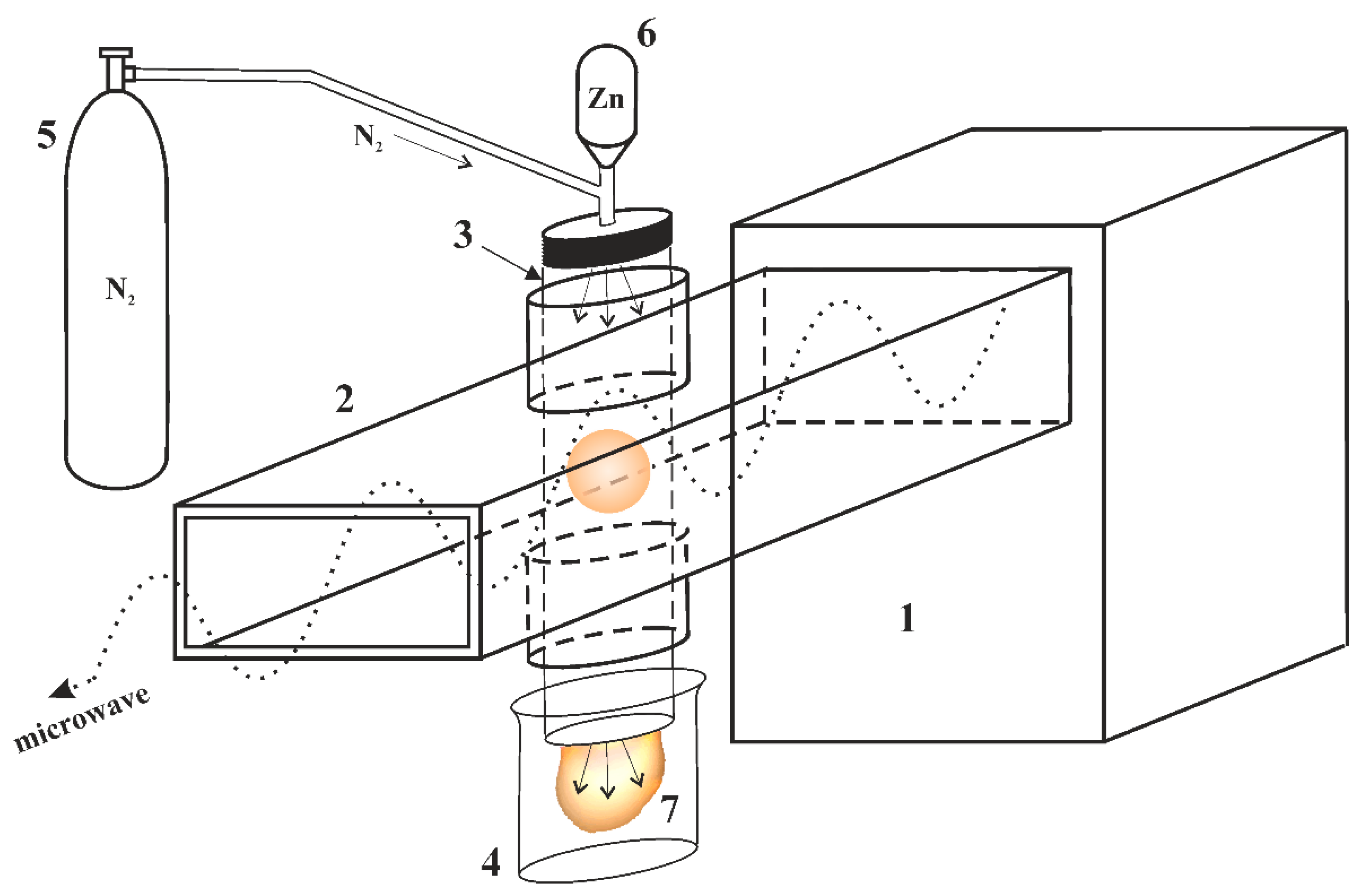
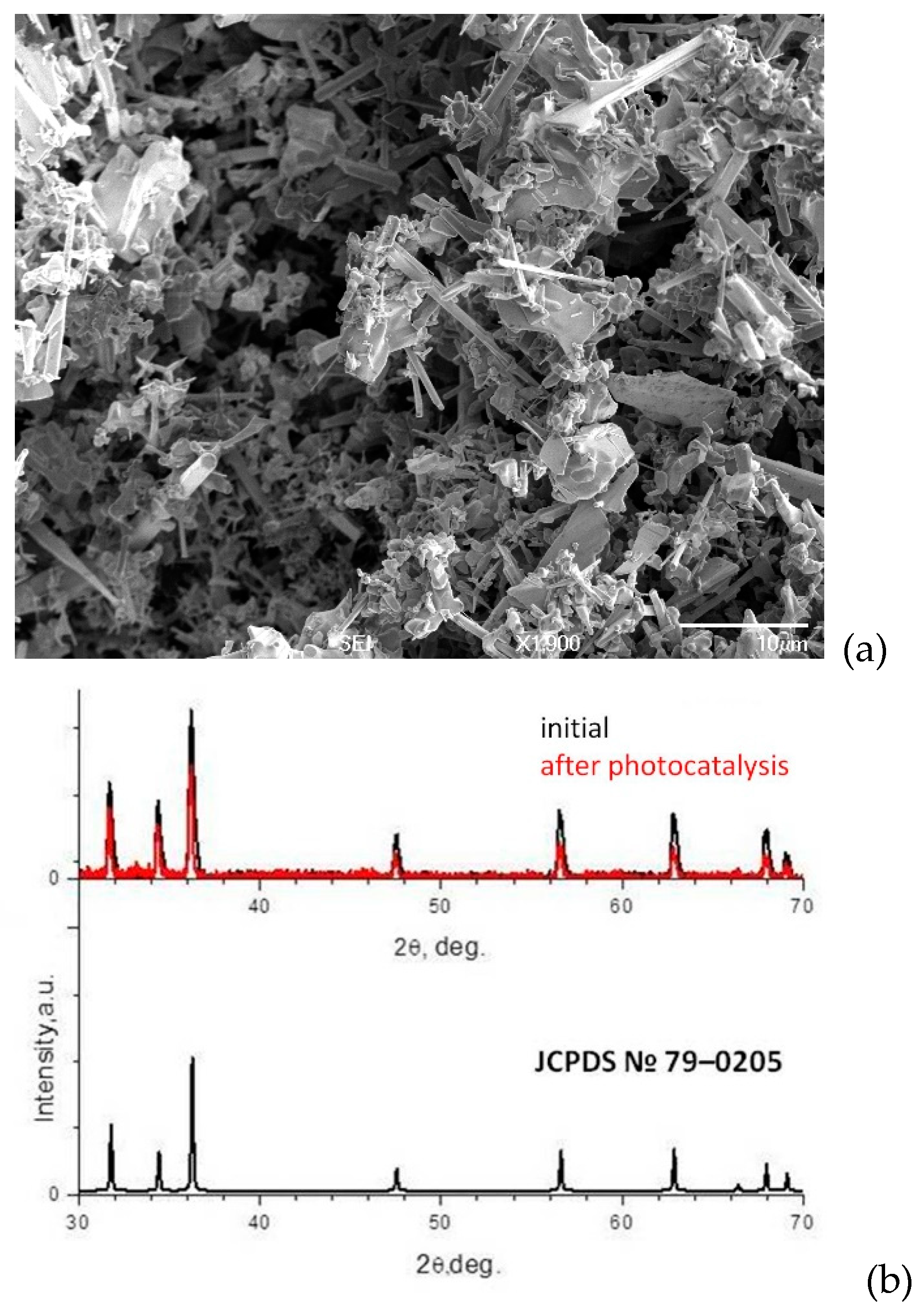
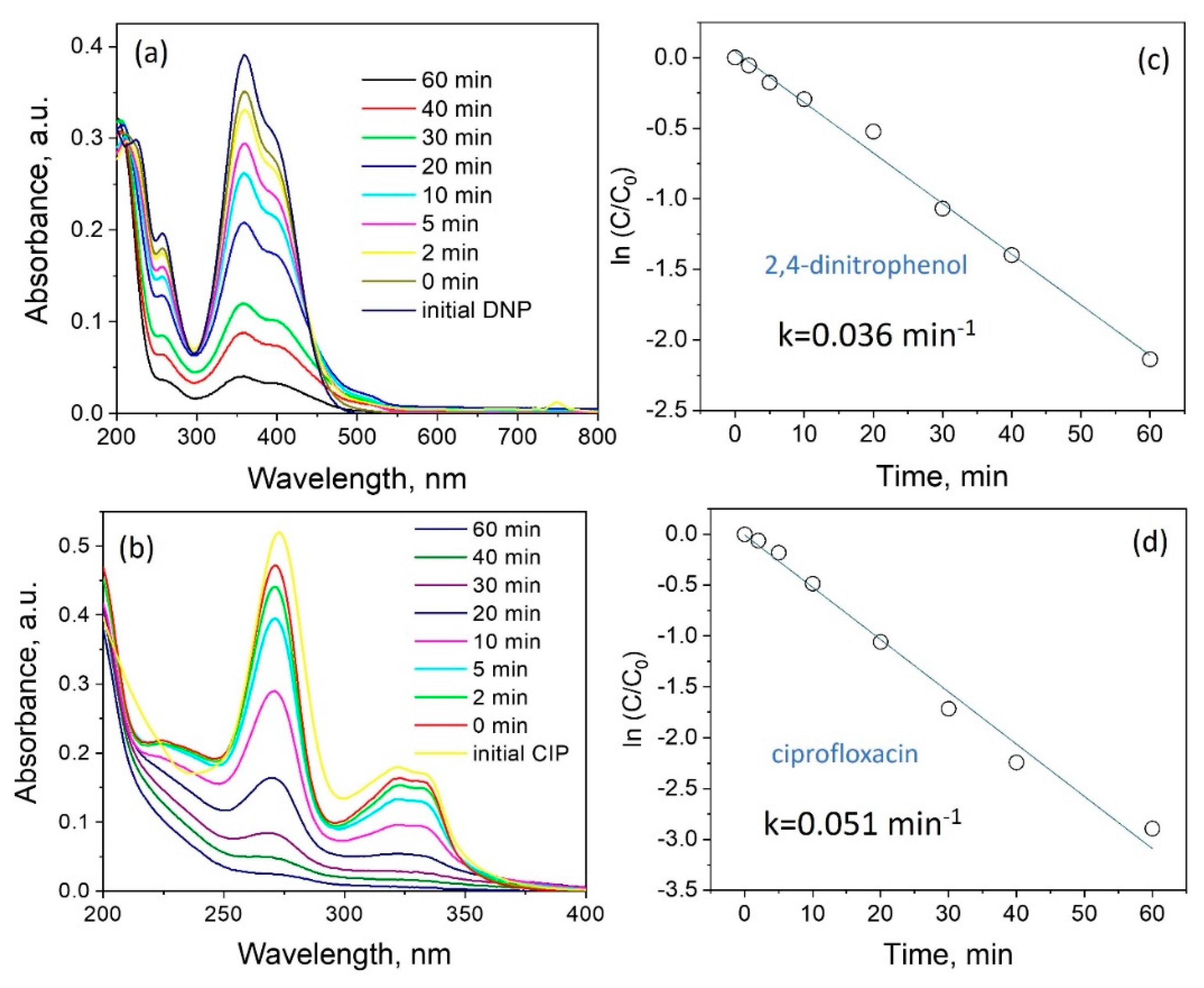
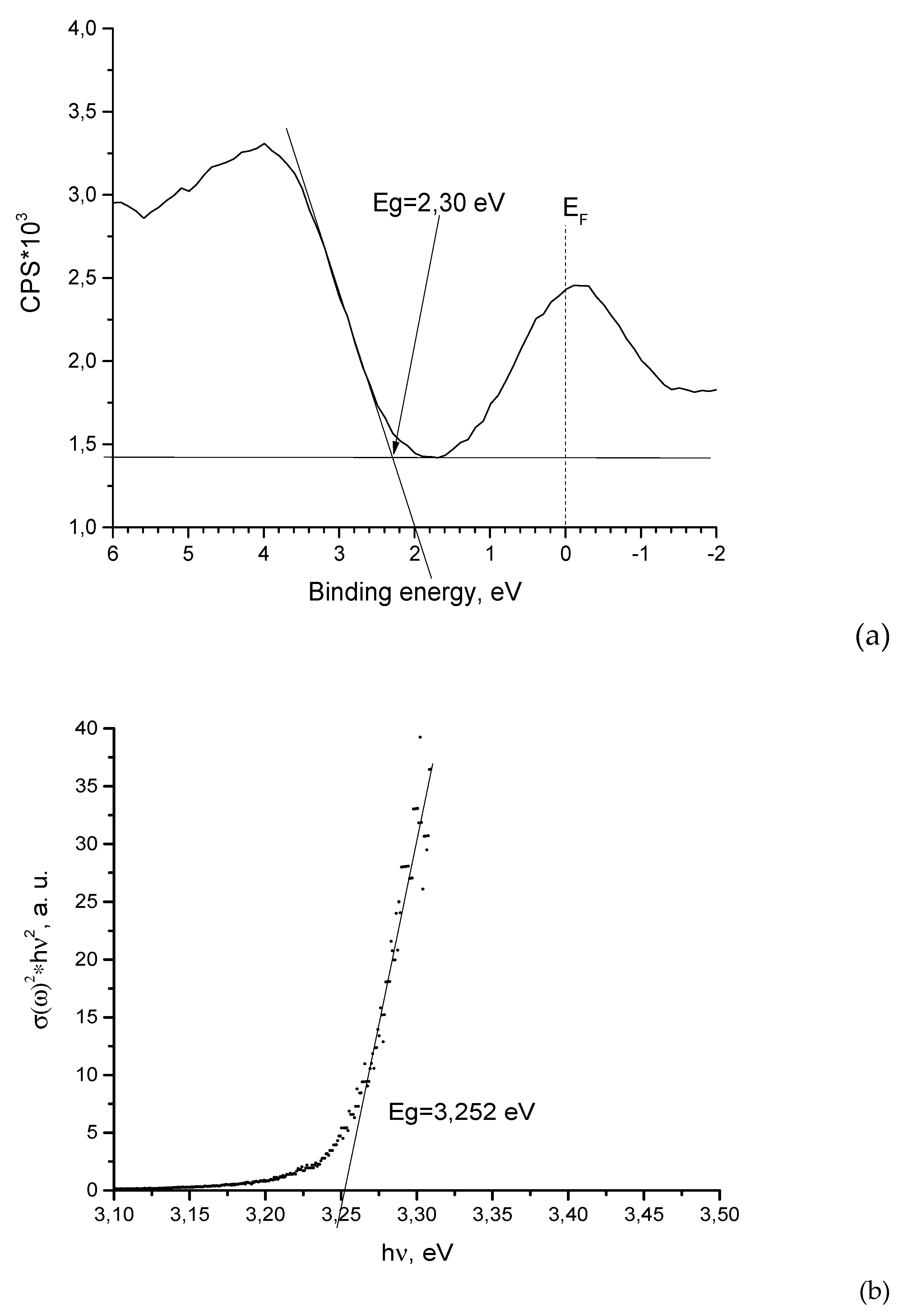
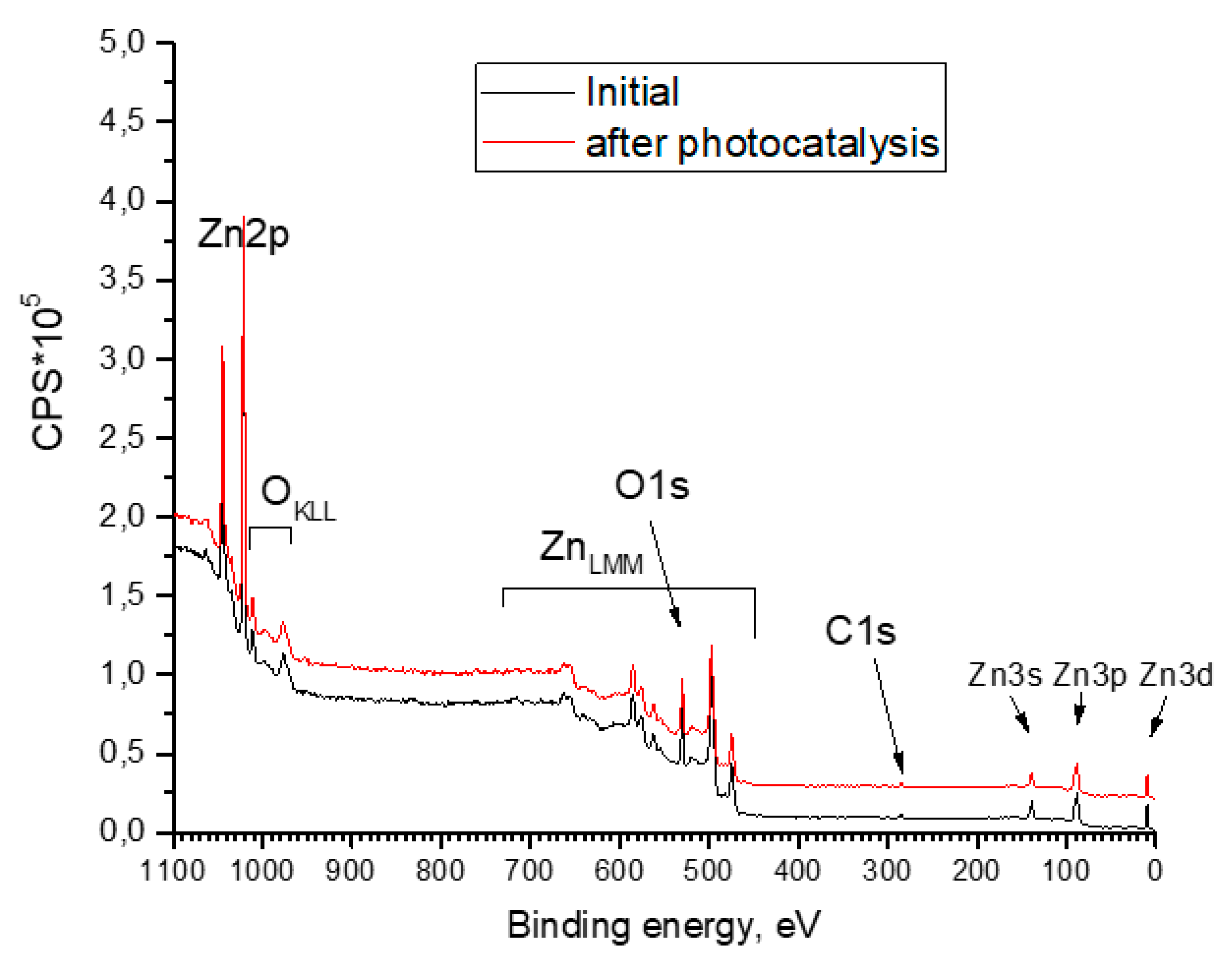
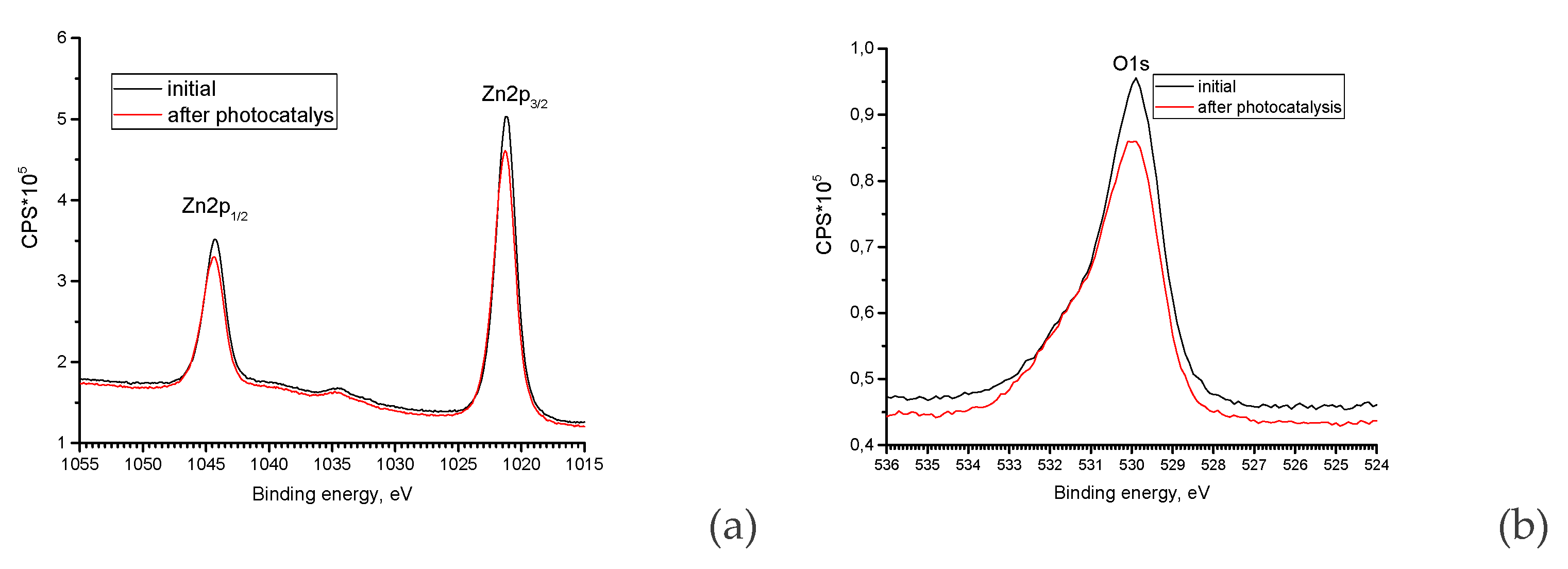
| Element | Zn | O | N |
|---|---|---|---|
| Quantity,% | 27.56 | 68.32 | 4.12 |
| Sample | Zn | O | C | N |
|---|---|---|---|---|
| Initial | 49.6 | 40.8 | 9.6 | 0 |
| After photocatalysis | 49.5 | 41.3 | 9.2 | 0 |
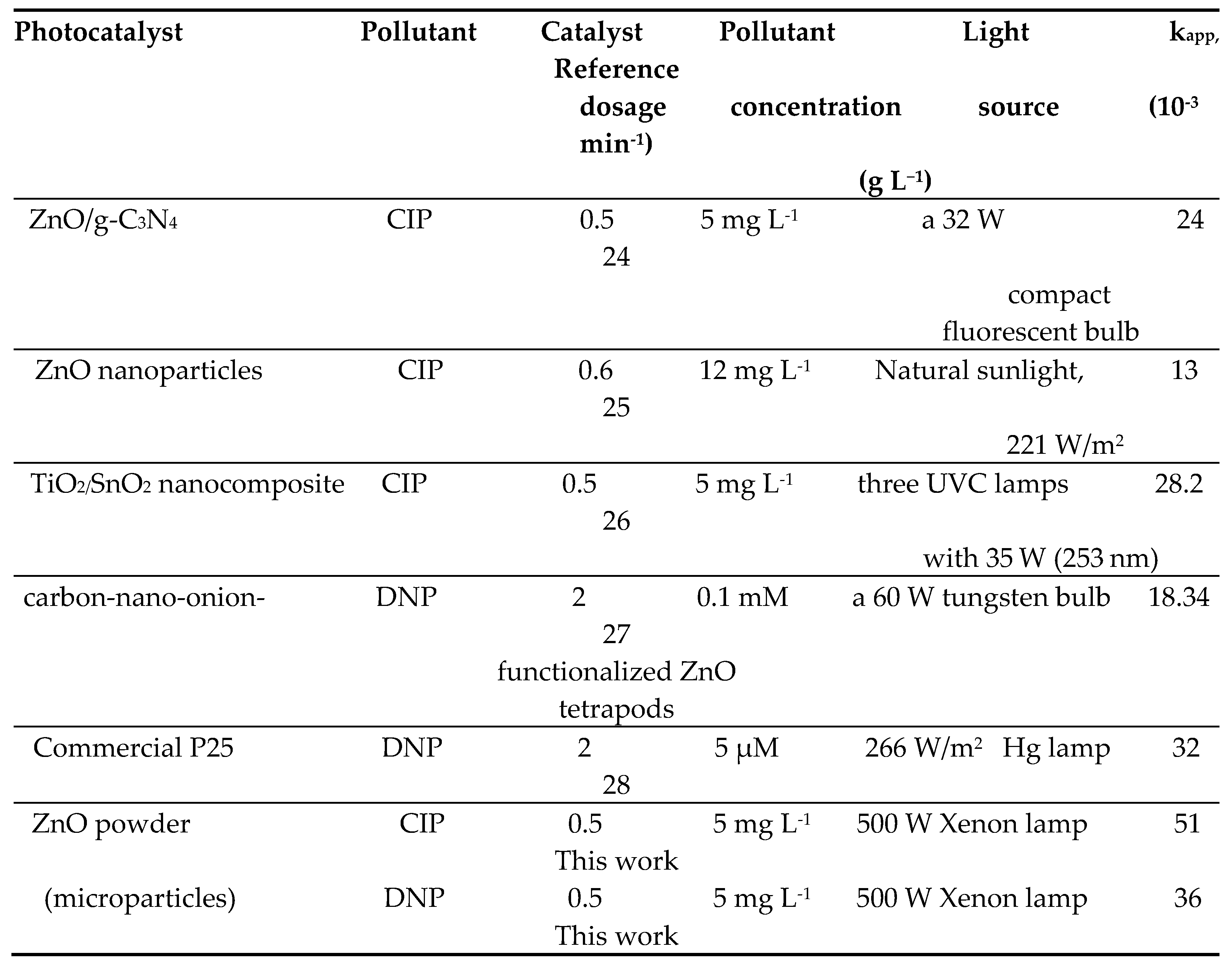 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





