1. Introduction
Bell pepper (
Capsicum annuum L.) is a commercially important fruit vegetable widely grown in the world because of its economic value and consumer demand [
1]. However, marketing is constrained by the relatively short shelf life of bell pepper due to water loss, shriveling, and decay during prolonged storage [
2,
3]. Extending its shelf life is concomitant with a plethora of benefits including taste, and natural antioxidants, such as phenolic compounds [
4,
5,
6].
Bell pepper, as an emerging export crop, its production is divided into summer and winter cultivation in Korea. Summer cultivation begins in early March and harvested in June until November, while winter cultivation starts in early September and harvested in early December until June.
Bell pepper postharvest quality is associated with multiple traits, including visual appearance, flavor, chemical composition, and nutritional value. The maturity of bell pepper solely depends on appearance, firmness, and shelf-life, which marketers, exporters, or consumers consider at the spell of the initial purchase [
7]. A major constraint in its production is determining the appropriate harvesting stage [
5,
8]. Immature fruits (onset of turning color) may have unacceptable qualities, while whole-colored fruits lose their attractiveness and firmness, becoming soft in texture within a short time [
9].
Postharvest losses hitherto are a major constraint of bell pepper estimated as 25–35% of the total production [
10]. Temperature has various physiological effects on horticultural crops during shipping, marketing, and storage [
11,
12]. Metabolic activities such as respiration, transpiration, ethylene production, and shriveling after harvesting of horticultural crops are inclined by temperature which affect the postharvest quality [
13,
14]. Postharvest deterioration of fruits and vegetables elevates due to high air temperature and relative humidity especially during the summer cultivation [
15]. Therefore, meticulous handling and adequate care are required to maintain postharvest quality.
Forced-air precooling (FAP) is a process of quickly removing field heat from freshly harvested produce by creating air through the produce [
16,
17]. Immediate precooling of fruit vegetables after harvest lowers the rate of respiration, minimizes water loss, slows enzymatic activity, and shriveling [
13,
18] maintaining shelf life and postharvest quality. [
16] reported that forced-air precooling preserved postharvest quality (mass loss and firmness) of bell pepper during storage.
Modified atmosphere packaging (MAP) is a technology used to extend the quality and shelf life of horticultural produce of high commercial value [
7,
19,
20]. The packaging of bell pepper in perforated plastic films has been confirmed to reduce water loss, delay softening, and extend shelf life during storage [
3,
21]. Suitable packaging material such as polyethylene (PE) liner can inhibit biochemical processes and prolong the shelf life of pepper during storage [
14,
22].
This study assessed the effects of forced-air precooling and modified atmosphere packaging treatments on the sensory (weight loss, firmness, and color), physicochemical (soluble solids content and soluble sugars), and health-promoting properties (total phenolic content, total flavonoid content, and antioxidants activities) of bell pepper cv. Nagano harvested at 90% and 50% coloring stages during cold storage.
2. Materials and Methods
2.1. Plant Material and Weather Condition
Bell peppers (
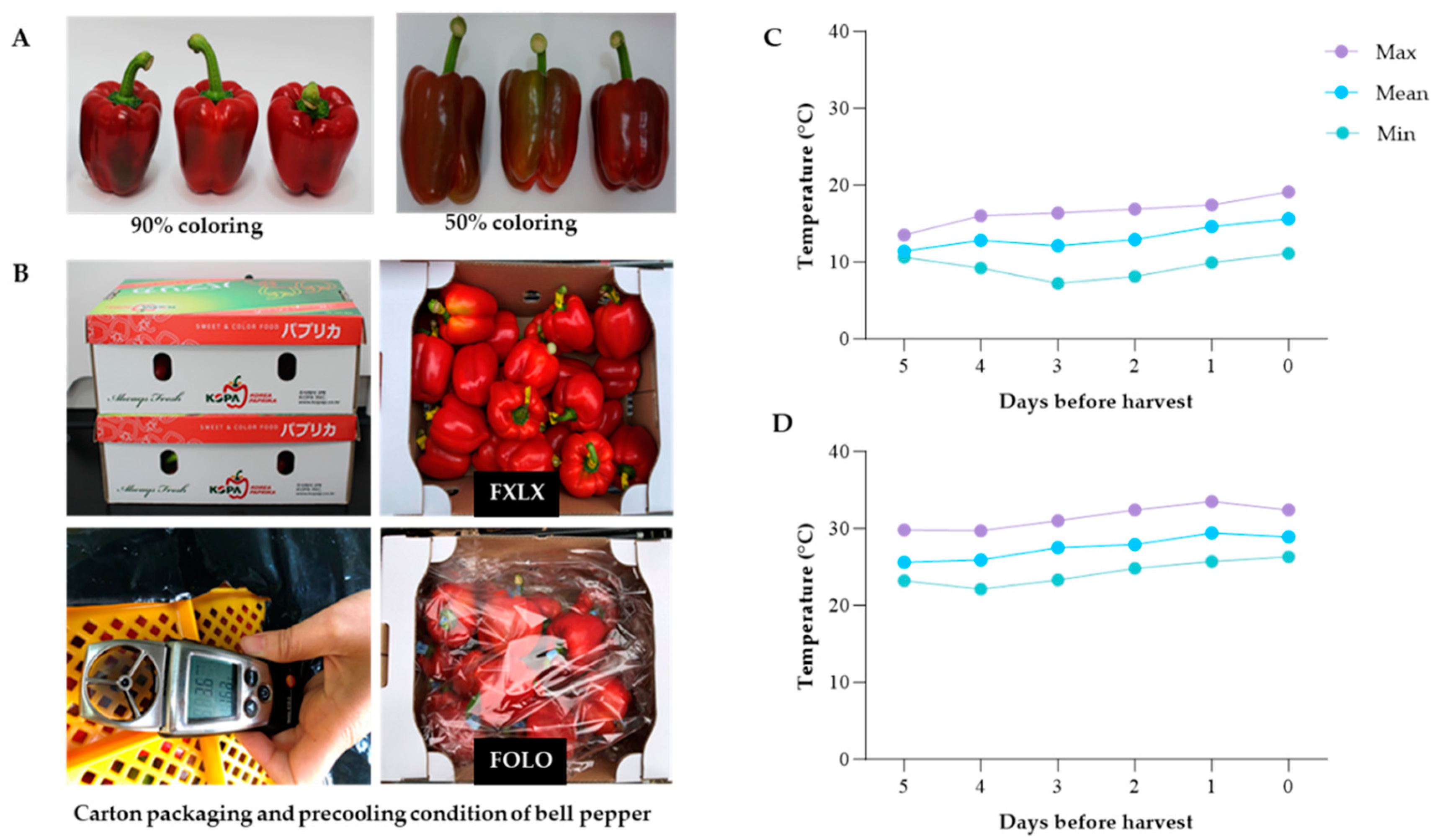
Capsicum annuum L. cv Nagano) were procured from Samcheok, Korea. Fruits were harvested at 90% and 50% based on fruit pericarp (
Figure 1A) in May and July, respectively. The fruits were packed in cartons and transferred to Gangneung-Wonju National University Postharvest Biology and Technology Laboratory. Fruits cartons (52 × 37 × 32 cm) weighing about 10 kg were palletized (1 × 2) and precooled at 8
oC for 6 h using (FOX-S1004, DSFOX, Korea) at a wind speed of 3.2–3.6 m/s. Fruits were weighed and subjected to four treatments; Forced-air precooling + 30 μm polyethylene (PE) liner (FOLO); forced-air precooling (FOLX); 30 μm polyethylene (PE) liner (FXLO); and Control (FXLX) were stored at 11
oC and 95% relative humidity for 15 and 16 days for domestic and export market, respectively (
Figure 1B).
The air temperature at Samcheok cultivation area monitored 5 days before harvesting is shown (
Figure 1C and 1D). The daily average temperature in May, 5 days before harvesting 90% coloring fruits was maintained at 11.4–14.6
oC, the maximum temperature was 13.5–17.4
oC, and the internal temperature of the plastic greenhouse on the day of harvesting was 23.3
oC. On the other hand, 50% coloring fruits daily average temperature ranged from 25.6–29.4
oC, the maximum temperature was 29.7–33.5
oC and the internal temperature of the plastic greenhouse increased to 30.3
oC.
2.2. Chemicals and Reagents Used for the Study
All chemicals including solvents used were of analytical grade. Glucose (99.5%), fructose (contains < 0.05 mole % glucose by enzymatic assay) and sucrose (98%), DPPH free radical, ABTS (98%), Folin-Ciocalteu’s phenol reagents, ethyl alcohol, sodium carbonate (99.9%), aluminum nitrate (98%), quercetin 95%, gallic acid (97.5–102.5% titration), potassium acetate (99%), and ascorbic acid were purchased from Sigma-Aldrich (St. Louis, MO, USA).
2.3. Quality Assessment of Bell Pepper cv. Nagano
Fruit weight loss was measured by the method of [
21]. The weight change was expressed as a percentage of the differences between the initial weight and the weight after the storage period.
Firmness was assessed using a texture analyzer (EZ Test/CE-500N, Shimadzu, Kyoto, Japan) at the equator of the fruit with a 5 mm probe at 120 mm/min crosshead speed and expressed as Newton (N).
The chromaticity of the fruit skin was measured with a colorimeter (CR-400, Minolta, Osaka, Japan) and expressed as Chroma, Hue angle, Hunter L, a, and b values. Hunter L value represents lightness (0 = black, 100 = white), Hunter a value represents green (-) to red (+), Hunter b value represents blue (-) to yellow (+). Using Hunter a and b values, the Chroma value was calculated as (C = √ (a2 + b2)1/2), and the hue angle (H = tan-1 (b/a)). The hue angle of the color wheel represents red-purple, yellow, bluish-green, and blue at 0o, 90o,180o, and 270o respectively.
The soluble solids content was measured with a refractometer (PAL-1, Atago, Tokyo, Japan) after the flesh tissue was juiced and expressed as oBrix.
2.4. Extraction Procedure and HPLC Analysis of Soluble Sugars
Finely ground powder (0.2 g) of lyophilized fruit samples were extracted with 12 mL of 50% methanol. Samples were vortexed, sonicated at 30oC for 5 min, and then centrifuged at 3,075×g at 10oC for 5 min. An aliquot (1.0 mL) of the supernatants were filtered (0.45 μm syringe filter), followed by an injection of a volume of 5 µL into high-performance liquid chromatography (YL 9100 HPLC, Youngin, Anyang, Korea), and each elution was performed for 30 min. The separation of sugars was carried out using the Sugar-Pak column (6.5 x 300 mm, 10 μm, Waters, USA) operated at 30oC, with 3rd distilled water as a mobile phase, and the flow rate was 0.5 mL/min. A refractive index detector was used to monitor the eluted carbohydrates. The separated sugars were monitored by refractive index and identified by comparing their retention time with standards (fructose, glucose, and sucrose).
2.5. Extraction Procedure and Spectrophotometric Analysis of Total Phenolic Content, Total Flavonoid Content, and Antioxidant Activities
Lyophilized samples (0.1 g) were ground into a fine powder and extracted with 70% ethanol, vortexed, and kept in darkness for a day at room temperature. It was vortexed again and then centrifuge at 3,075×g for 5 min at 10oC and finally filtered (0.45 μm syringe filter) for spectrophotometric (Thermo Fischer Scientific Inc., Waltham, MA, USA) analysis.
Total phenolic content (TPC) was determined according to the Folin-Ciocalteu spectrophotometric method described by [
23] and expressed as mg of GAE/g dry weight (DW).
Total flavonoid content (TFC) was determined by [
24] method and expressed as mg of QE/g DW.
The DPPH and ABTS radical scavenging assays were performed using a method previously described by [
25] method with some modifications.
2.6. Experimental Design and Statistical Analysis
The experiment was laid out in a factorial (FAP and MAP treatments) completely randomized block design with six replications. Data were subjected to an analysis of variance (ANOVA) in SAS version 9.1.3 statistical software. Means separation was performed using Duncan Multiple Range Test (DMRT) (p < 0.05) and results were expressed as means ± standard deviation. To assess the relationship among metabolites and antioxidants, and different treatments groups, sparse partial least squares-discriminant analysis (sPLS-DA) and heatmaps were performed in MetaboAnalyst (version 5.0), GraphPad Prism (version 10.0) and R (version 4.2.1).
4. Discussion
Weight loss is one of the paramount factors that adversely affect bell pepper fruit quality during shipment, storage, and marketing [
14,
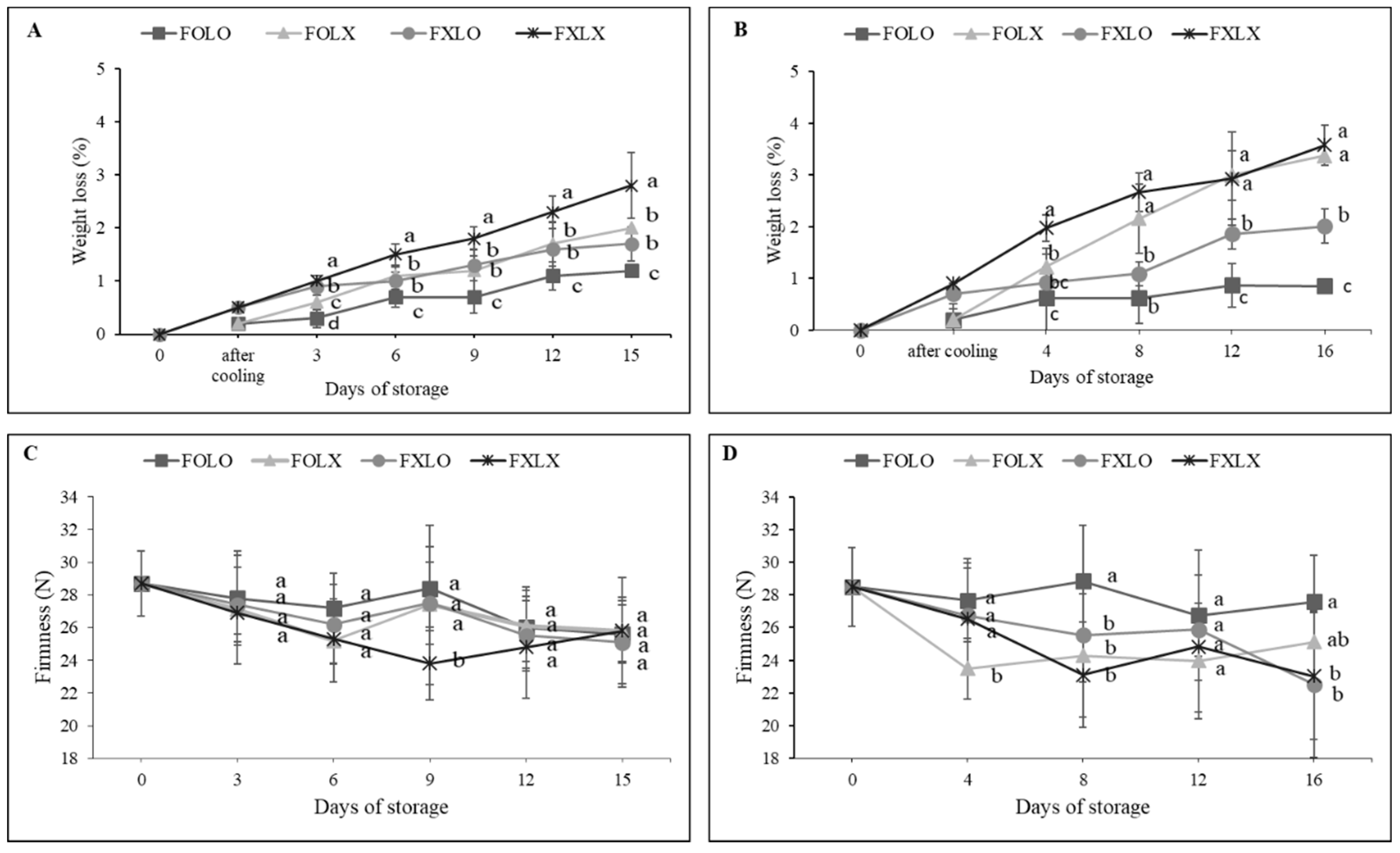
26]. In this study, FOLO rapidly reduced weight loss as compared to FXLX samples at the end of the storage period (
Figure 2A and B), confirming the efficacy of forced-air precooling and PE liner treatments on bell pepper fruit quality during shipment and storage. This result corroborated with [
27] who observed a reduced weight loss (1.46%) in bell pepper when precooled and immediately stored in a controlled chamber. The increased weight loss of FXLX might be due to changes in the permeability of cell membranes that made them more sensitive to water loss [
28] since the fruits were not precooled and wrapped in PE liners during storage. FOLO treated samples significantly negated weight loss during the entire storage period and this is because the water vapor permeability was lower than other treatments, hence preventing more water vapor transfer [
29].
The cell wall and cell membrane play an important role in maintaining the quality and visual appeal of detached fruits from the mother plant [
10]. The authors highlighted that the fruit surface is a protective barrier preventing water loss and leakage of solutes. Forced-air precooling and PE liner treatments significantly maintained fruit firmness as compared to the control (
Figure 2). The gaseous equilibrium between the produce and the sealed atmosphere in the treated samples resulted in low oxygen and high carbon dioxide concentrations, and thus reduced the activation of the cell wall or tissue softening enzymes allowing retention of firmness during storage as confirmed by [
30]. On the contrary, the decrease in firmness observed in the control fruits could be influenced by a high respiration rate and weight loss [
14]. Consequently, the cell wall softening of fruits is enhanced by enzymes such as pectin methylesterase which directly induced the level of firmness [
29,
31]. The gradual increase in liquid exudation observed in FXLO treated fruit after 8 days of storage (
Figure 2 B), induced its firmness at 16 days of storage and this might be due to cell wall autolysis.
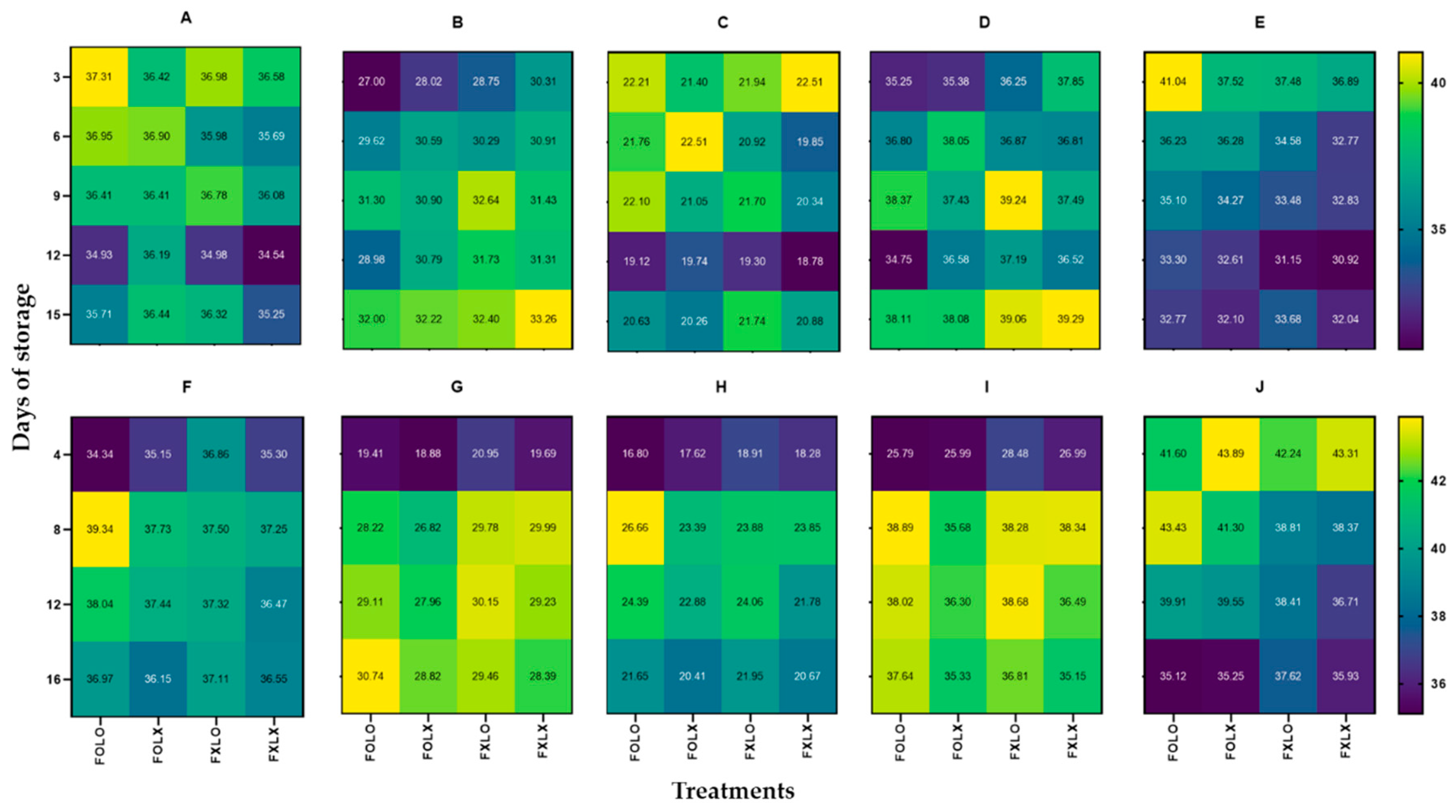
Studies have shown that the color change of fresh produce packaged under a high CO
2 and low O
2 atmosphere was delayed due to reducing ethylene synthesis [
19]. The FOLO treatment was the most effective in preserving the color of bell pepper and maintained its visual quality compared to other treatments.
There was a tendency of increase and decrease in SSC of 50% coloring fruit, which was relatively huge compared to the fruits harvested at 90% coloring. The rate of metabolism in the 50% coloring fruit progressed as the SSC increased. This is because 90% coloring fruits harvested at low temperature in May developed while the required period for each growth stage elapsed, whereas, in the high temperature in July, the period required for each growth stage was shortened due to the high temperature of the growing season [
12]. As a result, carbohydrates, which are photosynthetic products including glucose, and starch, could not be efficiently accumulated [
32]. The increase in sugar may be due to the breakdown of other complex sugars such as pectin, which is broken down by either autolysis, microbial enzymes, or both [
5,
21]. It has been reported that sucrose metabolism-related sugars in horticultural crops mainly include glucose, fructose, and sucrose [
4,
31,
32], which is further explained [
33,
34]. Sucrose is the most important and the primary form of transported sugars in fruits [
35]. However, they only account for a smaller proportion of the water-soluble sugars, while the reducing sugars in approximately equal contents reach over 50% [
32,
36]. After most fruits are separated from the parent plant prior to maturity, various biochemical changes occur during cold storage. Degradation of pectin polysaccharides and starch cause dynamic changes in soluble sugars mainly the inversion of sucrose into reducing sugars, as well as starch degradation have a major effect on fruit flavor and nutrition since sucrose, glucose, and fructose are the predominant soluble sugars [
33,
34]. During fruit ripening, the biochemical changes of carbohydrates are highly coordinated and a good equilibrium through synergy and antagonism systems is maintained. The reducing sugars in 90% were higher than in 50% coloring fruits, however, the sucrose content was lower, and this is because the low temperature in May when fruit was harvested at 90% coloring inhibited the activity of enzymes and the resultant consumption of soluble sugars as substrate and energy were delayed [
2,
37]. Inverse sucrose content was observed, and this is because sugar accumulation was induced by a series of complex enzymatic reactions, which depend on maturity, and environmental conditions, sugar content varies from one bell pepper sample to another [
31]. Therefore, it can be deduced that; the less the fruit (50%) color, the more accumulation of sucrose. As maturation progresses sucrose will be broken down probably leading to an increase in reducing sugars [
33,
34].
The increment of bioactive compounds of most vegetables has been ascribed to dehydration, destruction of the tissue cell, and inactivation of enzymes such as polyphenol oxidase, as reported by [
38]. However, high CO
2 concentration can induce abiotic stress which in turn increase phenolic compounds in horticultural plants, and the high CO
2 or low oxygen atmosphere might have resulted in minimal changes in phenolic and flavonoids [
24]. In this study, the average outside and inside air temperature of 90% coloring fruits harvested in May, 5 days before, and on the day of harvest was lower compared to temperatures recorded in July (
Figure 1C and D). We assumed that higher temperatures recorded in July when the fruits were harvested at 50% coloring might have affected the level of bioactive compounds and this corresponds with the findings of [
4,
25]
The assessment of antioxidant activity by DPPH and ABTS radical assay provides important information about the functional quality of plants. Free radical scavenging is one of the ways through which antioxidants inhibit oxidation that is resultant from the free radicals. In the current study, antioxidant activity was higher irrespective of the treatments. The antioxidant capacities have been shown to be related to their phytochemical constituents. Hence, the increase in antioxidant activities in 50% coloring could be attributed to the presence of total phenolic content in the fruit [
17,
37]. A strong correlation between TPC and DPPH (r = 0.7266, and r = 0.8761) of fruits harvested at 90% and 50% coloring were observed in our current study respectively, which confirmed [
25] findings of nine peppers DPPH antioxidant activity that correlated well with their TPC (r = 0.8504). Although, [
23] reported a higher amount of antioxidant activity in whole-colored bell pepper than in turning color fruit. Our study showed opposite results where 50 % colored fruit recorded the highest antioxidant activity than 90% colored fruit. The rate of ripening in 90% colored fruit binds phenols to protein and the change in chemical structure of the fruit resulted in low antioxidant activity [
17]. The high antioxidant activity observed in 50% coloring fruits could be due to the strong intensity of solar radiation in the Samcheok cultivation area when the fruit was harvested in July. Our results corroborated with the theories of [
10,
38] who stated that fruit antioxidant properties are associated with temperature.
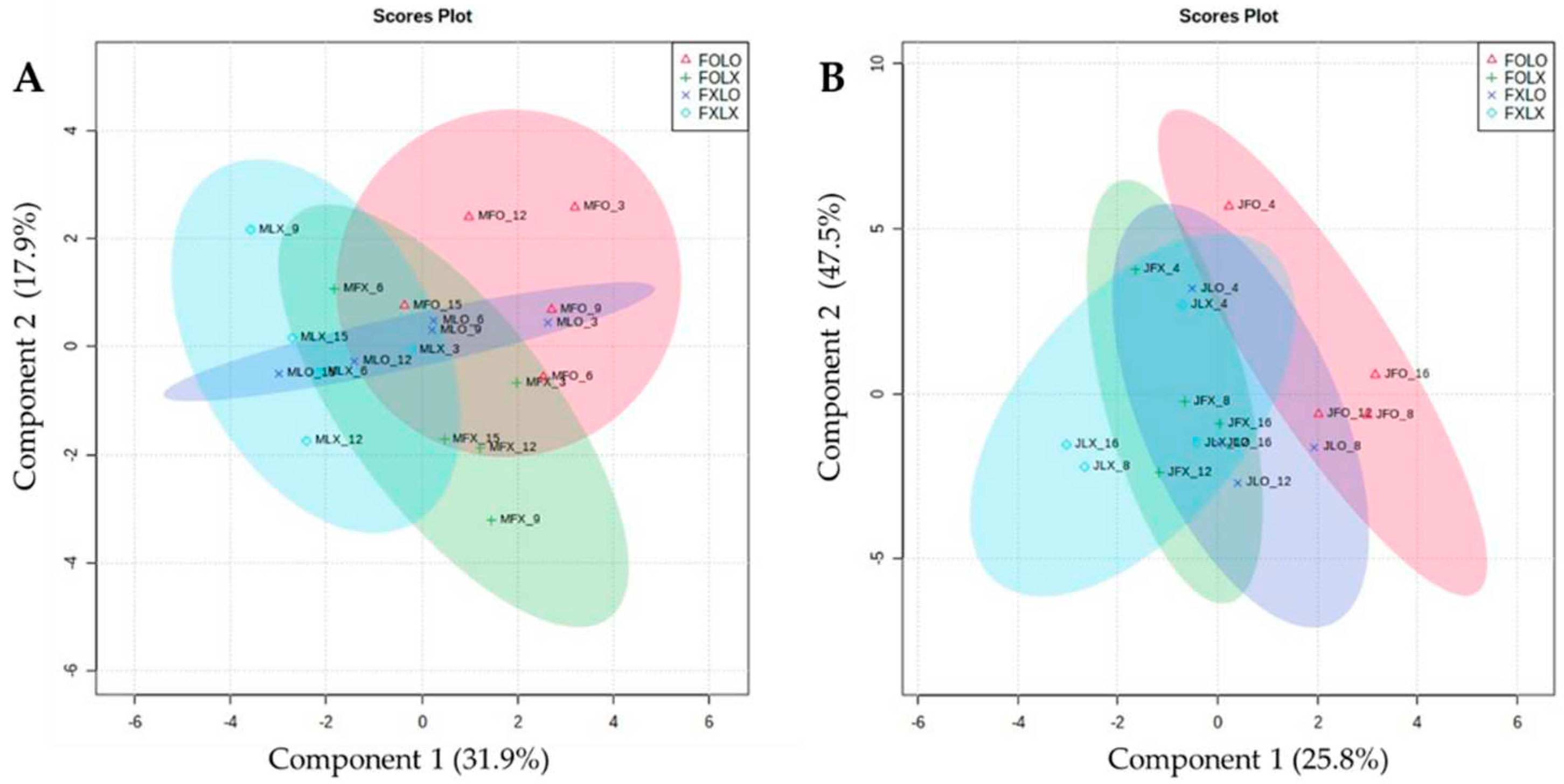
Multivariate analysis has been useful in studies regarding bioactive compounds and functional properties in the food industry. To further elucidate our current research, we used spare partial squares-discriminant analysis (sPLS-DA) and heatmaps to assess the segregation among different fruit samples and the relationship among variables. sPLS-DA was conducted to reduce the number of variables to produce robust and easy-to-interpret models. The sPLS-DA score plots showed the treatment’s effect on the studied variables in two harvest stages (90% and 50% coloring) (
Figure 4). The sPLS-DA models identified components 1 and 2 in a two-dimensional figure to differentiate between treatment groups according to the parameters, which indicates that each treatment in both harvest times has distinct separation, accounting for 31.9% and 17.9% for fruits harvested at 90% coloring and 25.8% and 47.5% at 50% coloring in May and July respectively. A synchronized three-dimensional figure clearly separates FOLO treatment from other studied treatments in the supplementary data (
Figure S1).
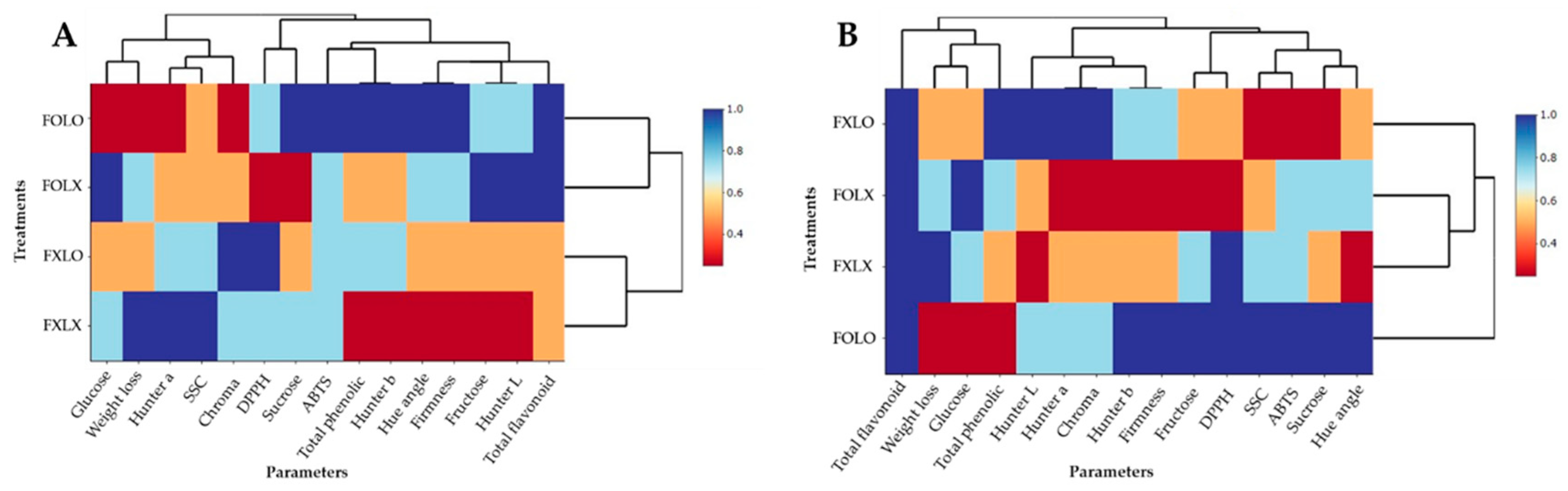
Furthermore, the heatmaps constructed to examine the data structure and look for similarities between treatments and the studied variables (
Figure 5) show the effect of treatment on the mean abundance of the studied parameters. FOLO treated fruits at 90% and 50% coloring showed higher mean abundance especially in bioactive compounds, antioxidant activities, and sugars, while effectively preserving postharvest quality and shelf life of bell pepper than other treatments.
Figure 1.
(A) The degree of coloring; (B) Carton packaging and precooling condition; Temperature five days before harvesting bell pepper cv. Nagano: (C) 90% coloring in May and (D) 50% coloring in July (D) respectively.
Figure 1.
(A) The degree of coloring; (B) Carton packaging and precooling condition; Temperature five days before harvesting bell pepper cv. Nagano: (C) 90% coloring in May and (D) 50% coloring in July (D) respectively.
Figure 2.
(A and B) Changes in weight loss; (C and D) Firmness of bell pepper cv. Nagano during storage at 11oC, 95% RH. Fruit at 90% coloring (A and C); 50% coloring (B and D) were treated with forced-air precooling or 30 μm PE liner. FOLO, forced-air precooling + 30 μm PE liner; FOLX, forced-air precooling; FXLO, 30 μm PE liner; FXLX, control. Means with different superscripts in the same column are significantly different at p < 0.05 (Duncan multiple range test) (n = 6).
Figure 2.
(A and B) Changes in weight loss; (C and D) Firmness of bell pepper cv. Nagano during storage at 11oC, 95% RH. Fruit at 90% coloring (A and C); 50% coloring (B and D) were treated with forced-air precooling or 30 μm PE liner. FOLO, forced-air precooling + 30 μm PE liner; FOLX, forced-air precooling; FXLO, 30 μm PE liner; FXLX, control. Means with different superscripts in the same column are significantly different at p < 0.05 (Duncan multiple range test) (n = 6).
Figure 3.
Heatmaps (A, B, C, D, and E) and (F, G, H, I, and J) represent Hunter L, a, b, Chroma, and Hue angle of bell pepper cv. Nagano harvested at 90% coloring and 50% coloring, stored at 11oC 95% RH for 15 and 16 days respectively. FOLO, forced-air precooling + 30 μm PE liner; FOLX, forced-air precooling; FXLO, 30 μm PE liner; FXLX, control. The color key indicates an increase or decrease in color change (n = 6).
Figure 3.
Heatmaps (A, B, C, D, and E) and (F, G, H, I, and J) represent Hunter L, a, b, Chroma, and Hue angle of bell pepper cv. Nagano harvested at 90% coloring and 50% coloring, stored at 11oC 95% RH for 15 and 16 days respectively. FOLO, forced-air precooling + 30 μm PE liner; FOLX, forced-air precooling; FXLO, 30 μm PE liner; FXLX, control. The color key indicates an increase or decrease in color change (n = 6).
Figure 4.
Two-dimensional (2D) sparse partial least squares-discriminant analysis (sPLSDA) scores plot representing treatments of observed parameters of bell pepper cv. Nagano harvested at 90% coloring (A) and 50% coloring (B), stored at 11oC 95% RH for 15 and 16 days respectively. FOLO, forced-air precooling + 30 μm PE liner; FOLX, forced-air precooling; FXLO, 30 μm PE liner; FXLX, control.
Figure 4.
Two-dimensional (2D) sparse partial least squares-discriminant analysis (sPLSDA) scores plot representing treatments of observed parameters of bell pepper cv. Nagano harvested at 90% coloring (A) and 50% coloring (B), stored at 11oC 95% RH for 15 and 16 days respectively. FOLO, forced-air precooling + 30 μm PE liner; FOLX, forced-air precooling; FXLO, 30 μm PE liner; FXLX, control.
Figure 5.
Heatmaps representing treatments and parameters of bell pepper cv. Nagano harvested at 90% coloring (A) and 50% coloring (B), stored at 11oC 95% RH for 15 and 16 days respectively. FOLO, forced-air precooling + 30 μm PE liner; FOLX, forced-air precooling; FXLO, 30 μm PE liner; FXLX, control.
Figure 5.
Heatmaps representing treatments and parameters of bell pepper cv. Nagano harvested at 90% coloring (A) and 50% coloring (B), stored at 11oC 95% RH for 15 and 16 days respectively. FOLO, forced-air precooling + 30 μm PE liner; FOLX, forced-air precooling; FXLO, 30 μm PE liner; FXLX, control.
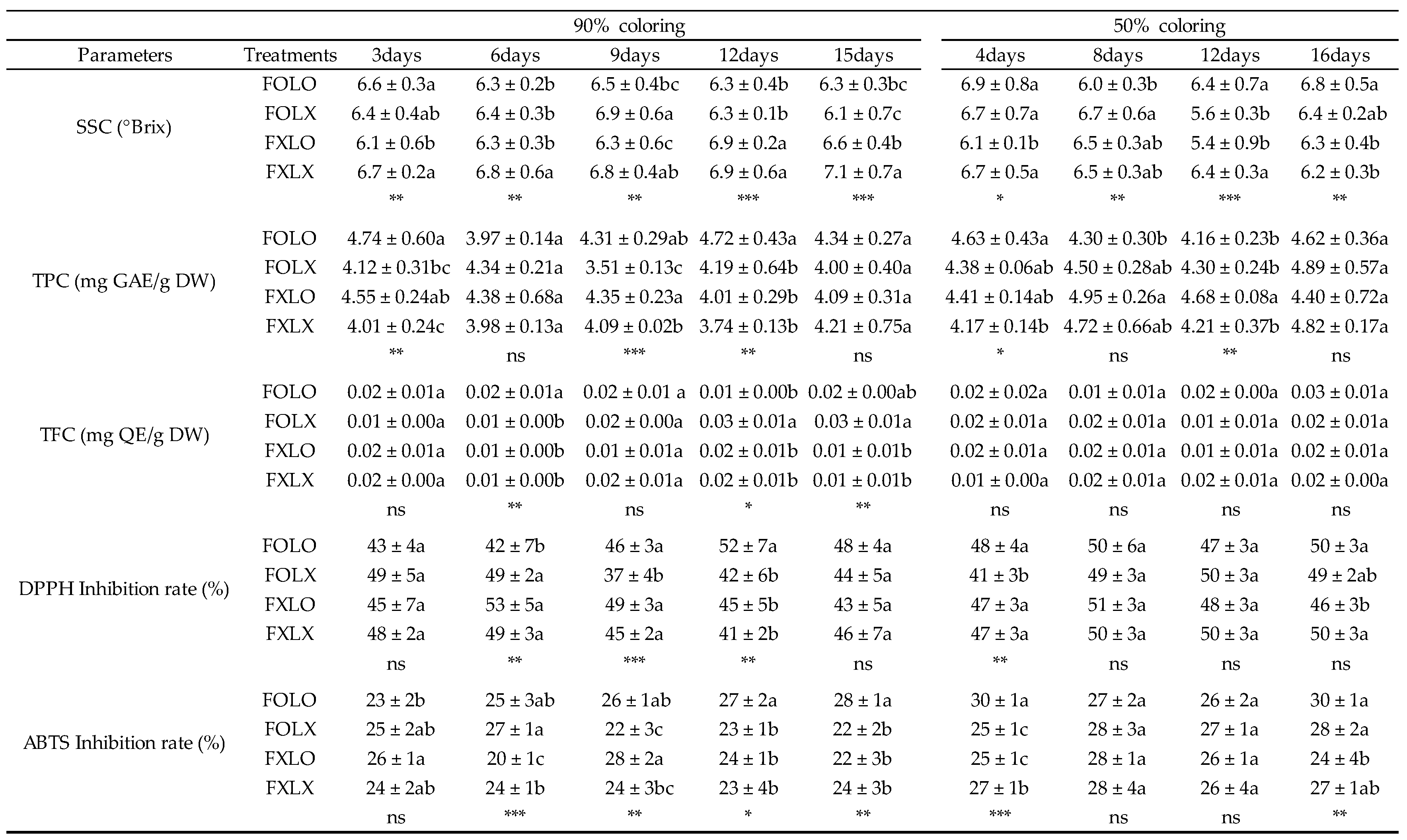
Table 1.
Soluble solids content (SSC), total phenolic content (TPC), total flavonoid content (TFC), DPPH, and ABTS of bell pepper cv. Nagano during storage at 11 °C 95% RH. FOLO, forced-air precooling + 30 μm PE liner; FOLX, forced-air precooling; FXLO, 30 μm PE liner; FXLX, control. zMean separation within columns by Duncan’s multiple range test (DMRT) at p < 0.05. ns, *, **, *** not significant or significant at p < 0.05, p < 0.01, p < 0.001, and p < 0.0001, respectively. DW (dry weight), SSC (n = 6), TPC, TFC, DPPH, and ABTS (n = 3).
Table 1.
Soluble solids content (SSC), total phenolic content (TPC), total flavonoid content (TFC), DPPH, and ABTS of bell pepper cv. Nagano during storage at 11 °C 95% RH. FOLO, forced-air precooling + 30 μm PE liner; FOLX, forced-air precooling; FXLO, 30 μm PE liner; FXLX, control. zMean separation within columns by Duncan’s multiple range test (DMRT) at p < 0.05. ns, *, **, *** not significant or significant at p < 0.05, p < 0.01, p < 0.001, and p < 0.0001, respectively. DW (dry weight), SSC (n = 6), TPC, TFC, DPPH, and ABTS (n = 3).