Submitted:
11 October 2023
Posted:
11 October 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results
2.1. Pigment Purification
2.2. Pigment Characterization
2.2.1. Pigment Characterization by Thin Layer Chromatography (TLC)
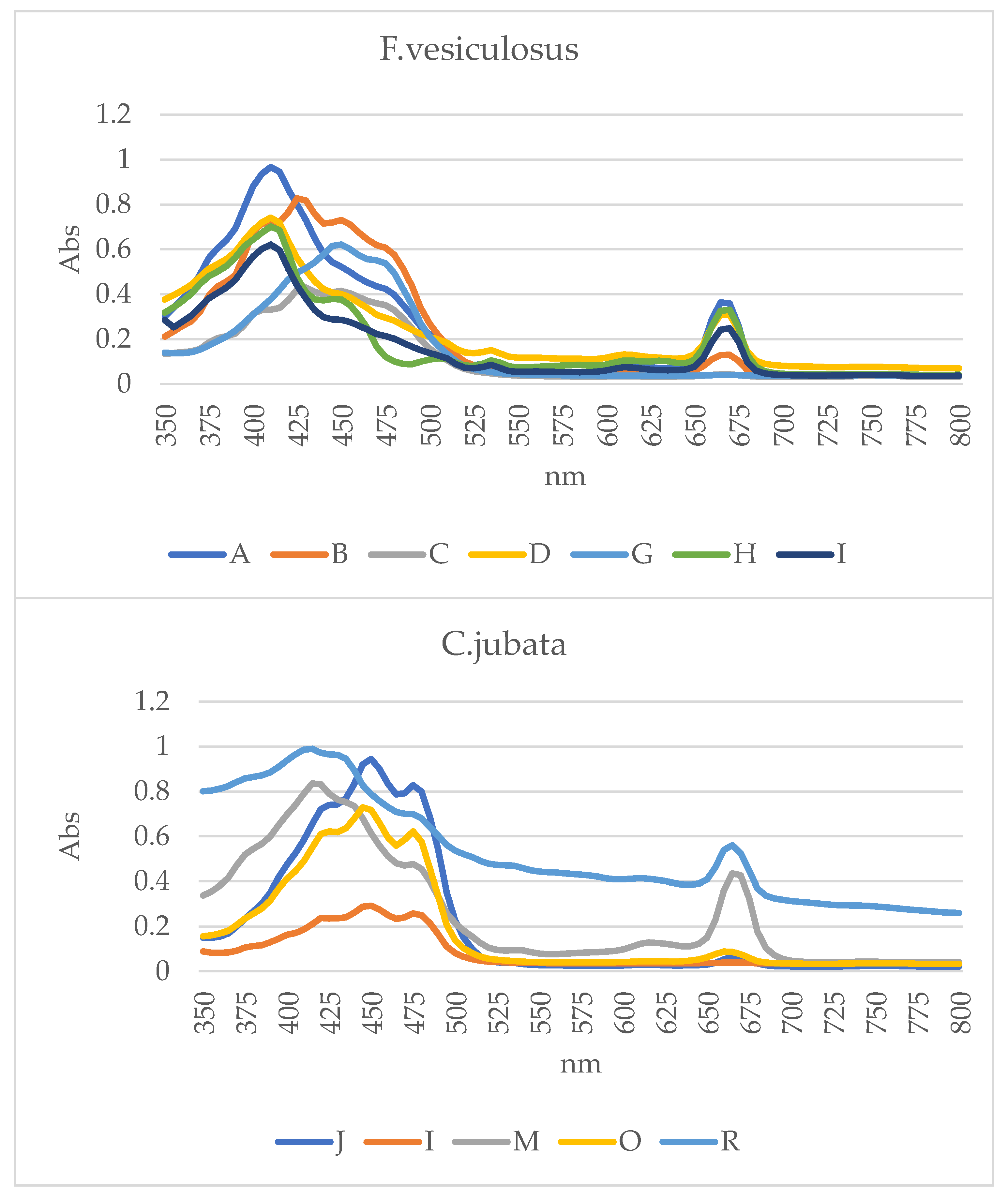
2.2.2. Ultraviolet and Visible (UV-Vis) Spectrophotometry
2.2.3. FTIR-ATR Analysis

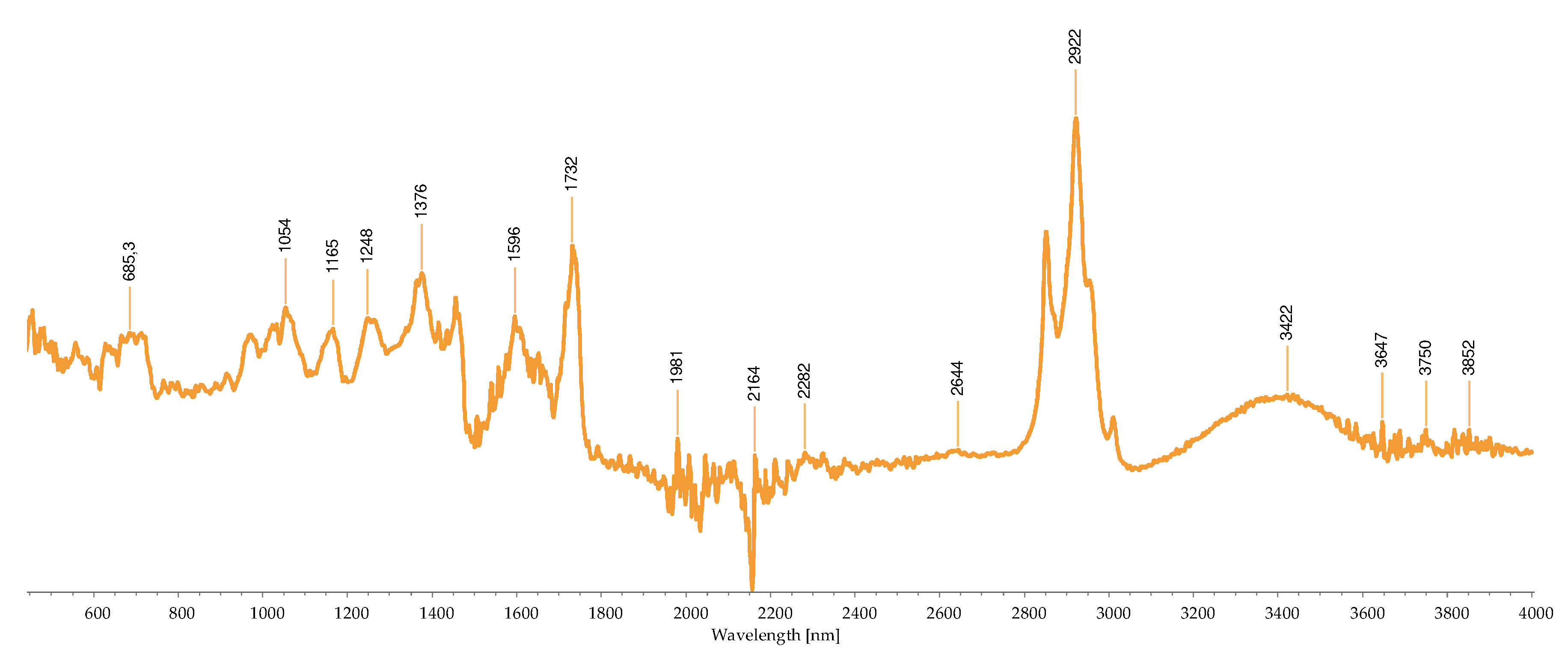
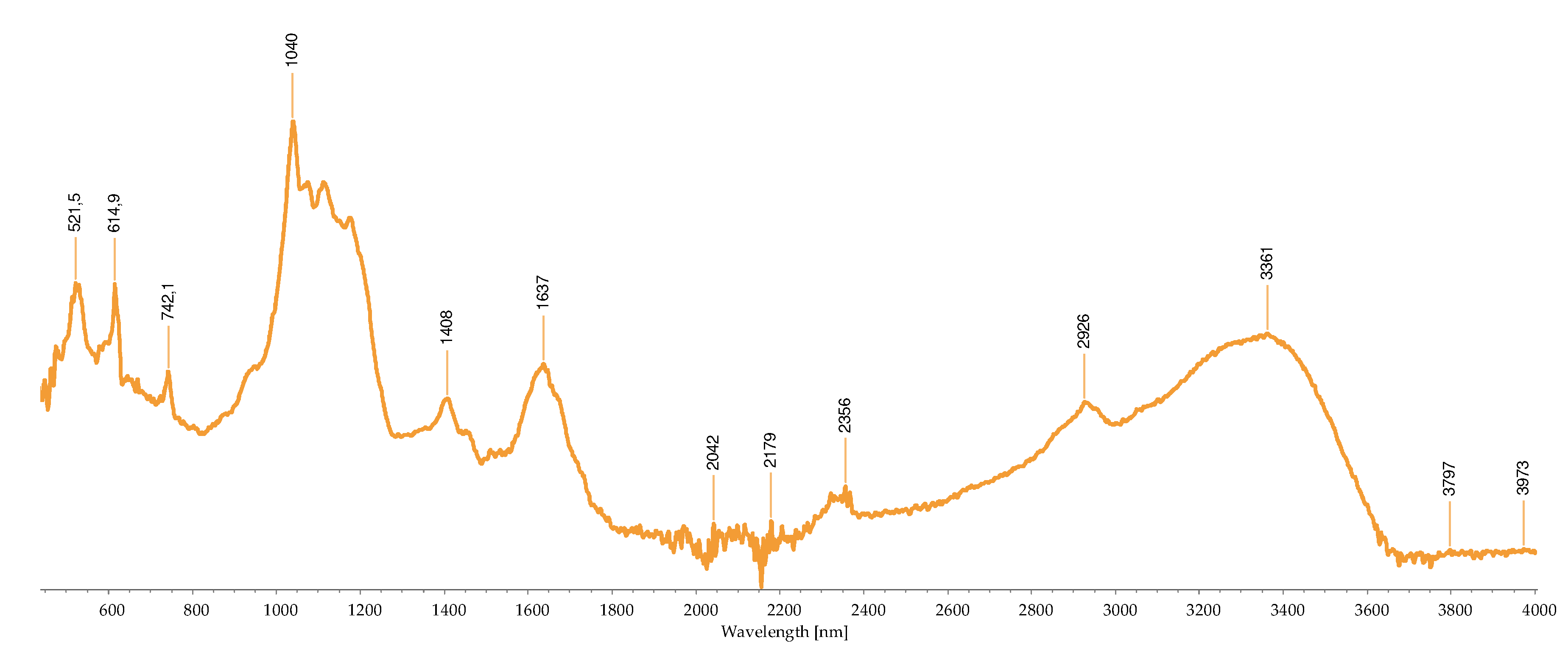
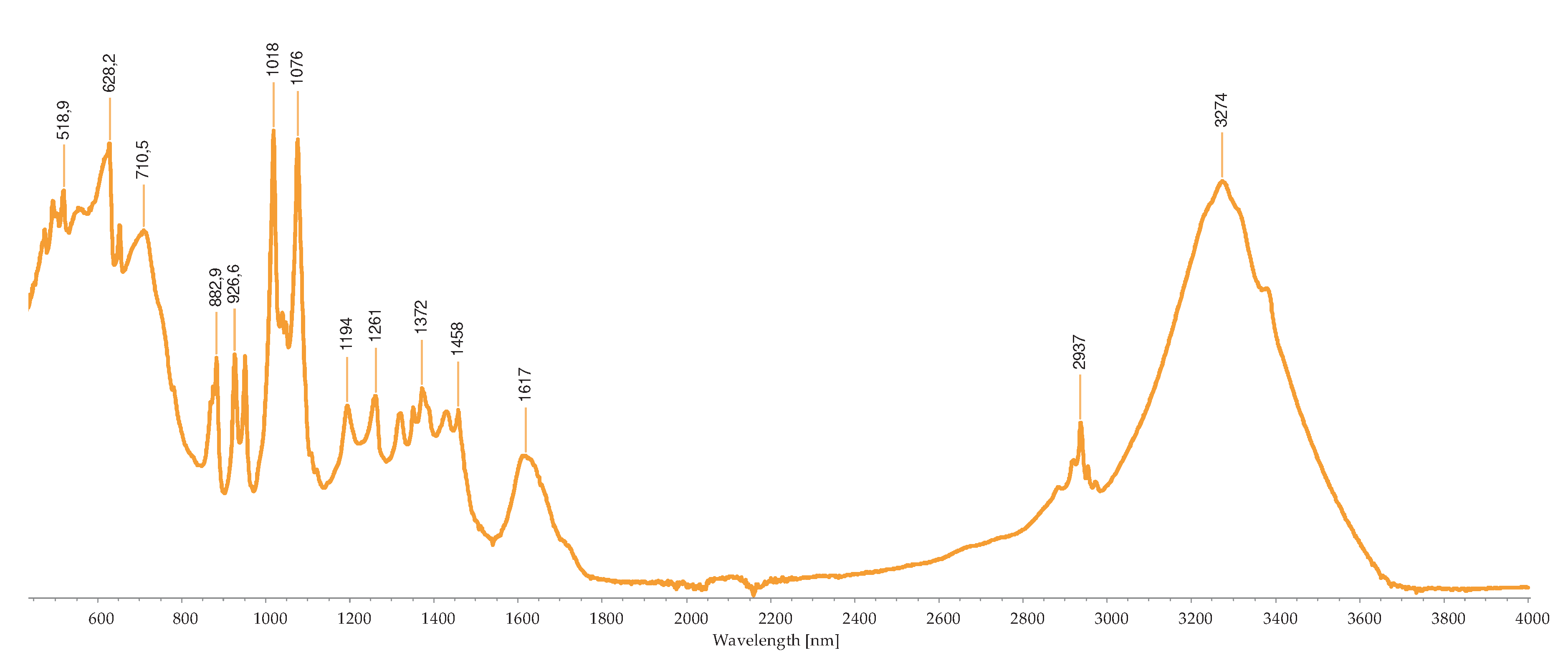
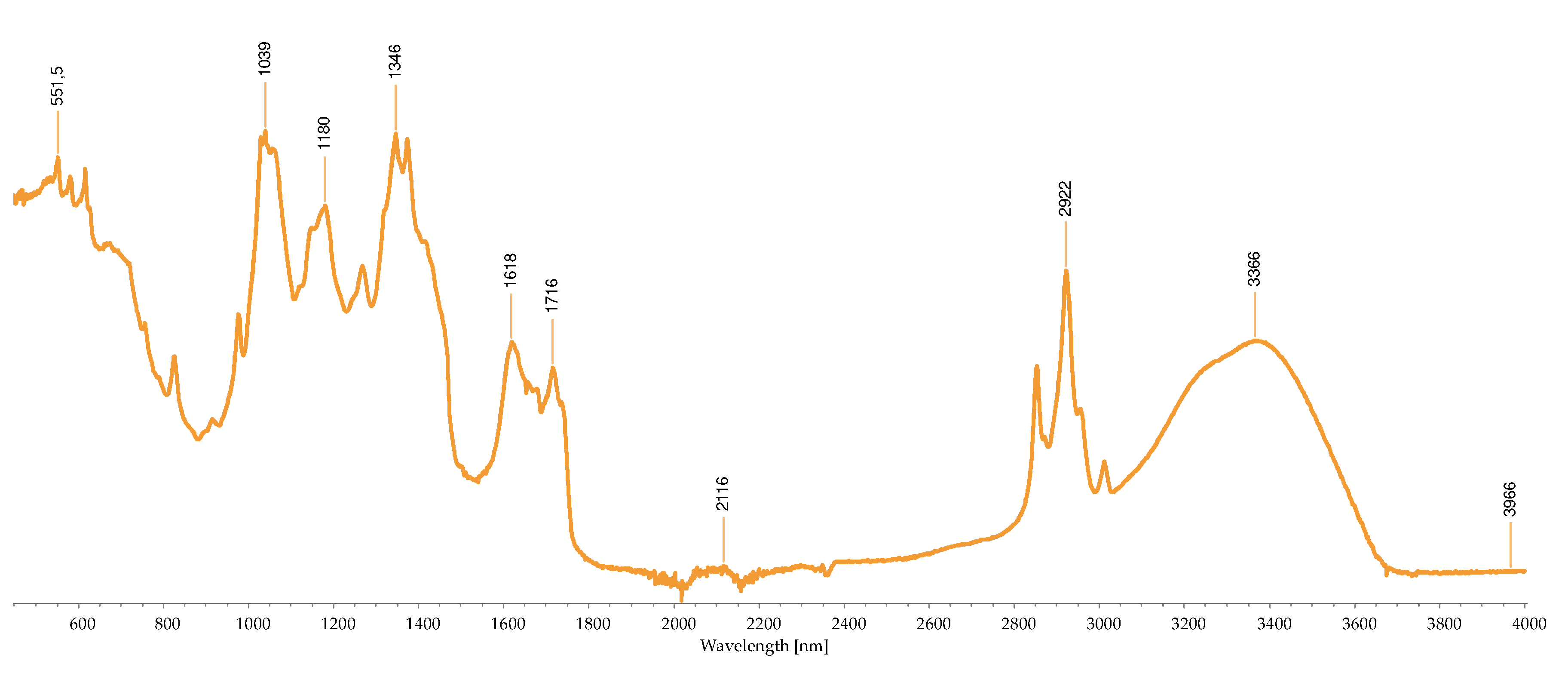
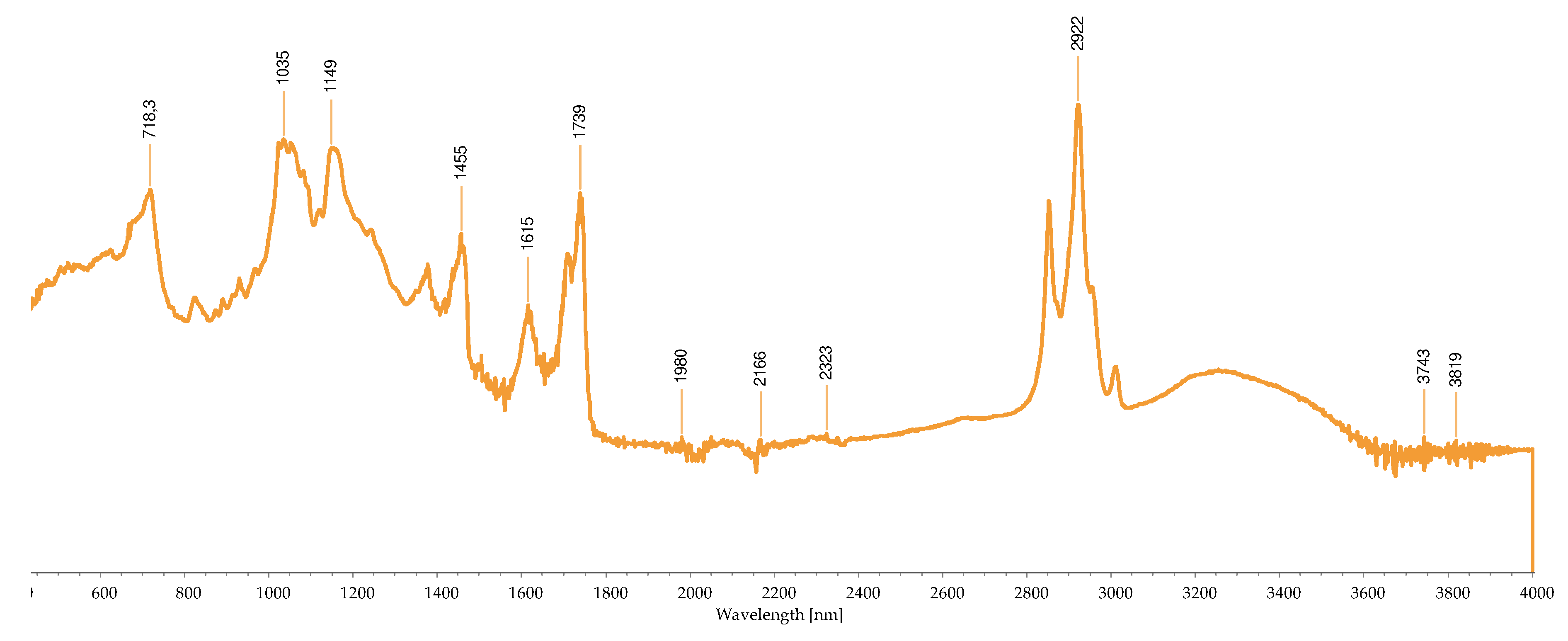
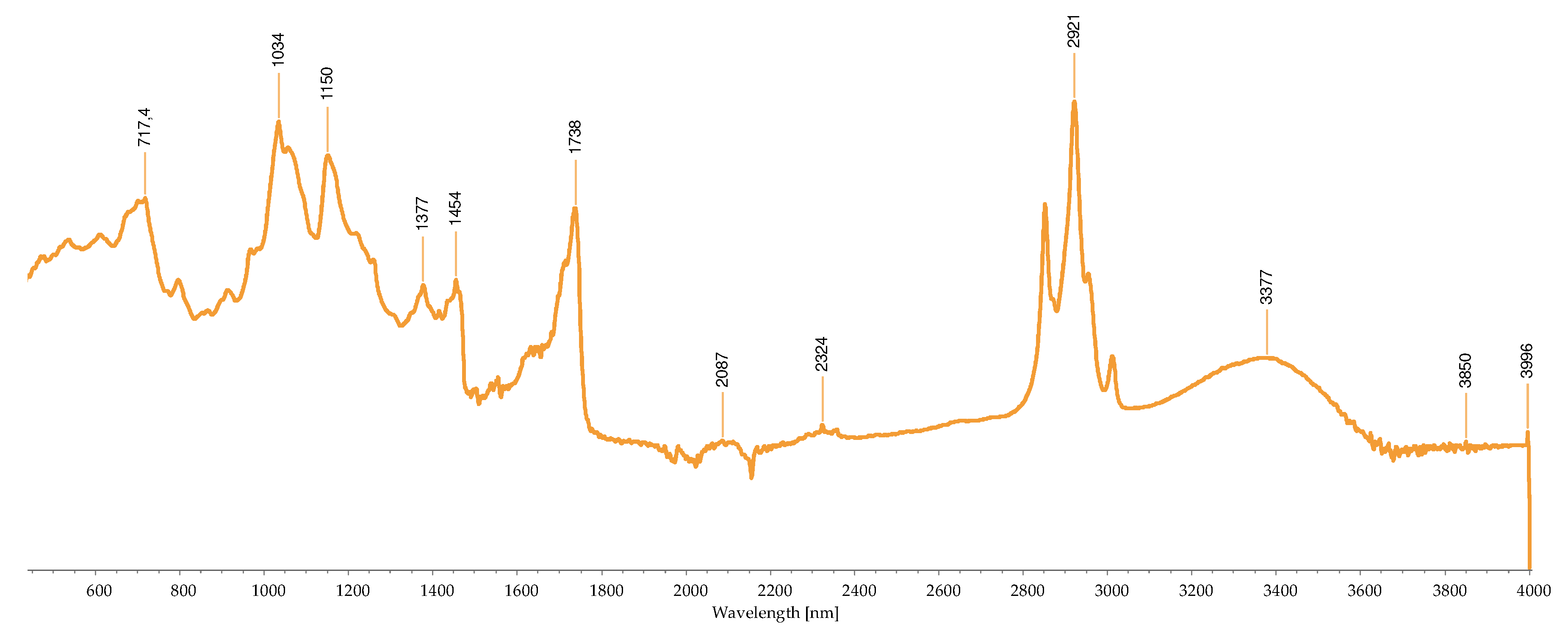
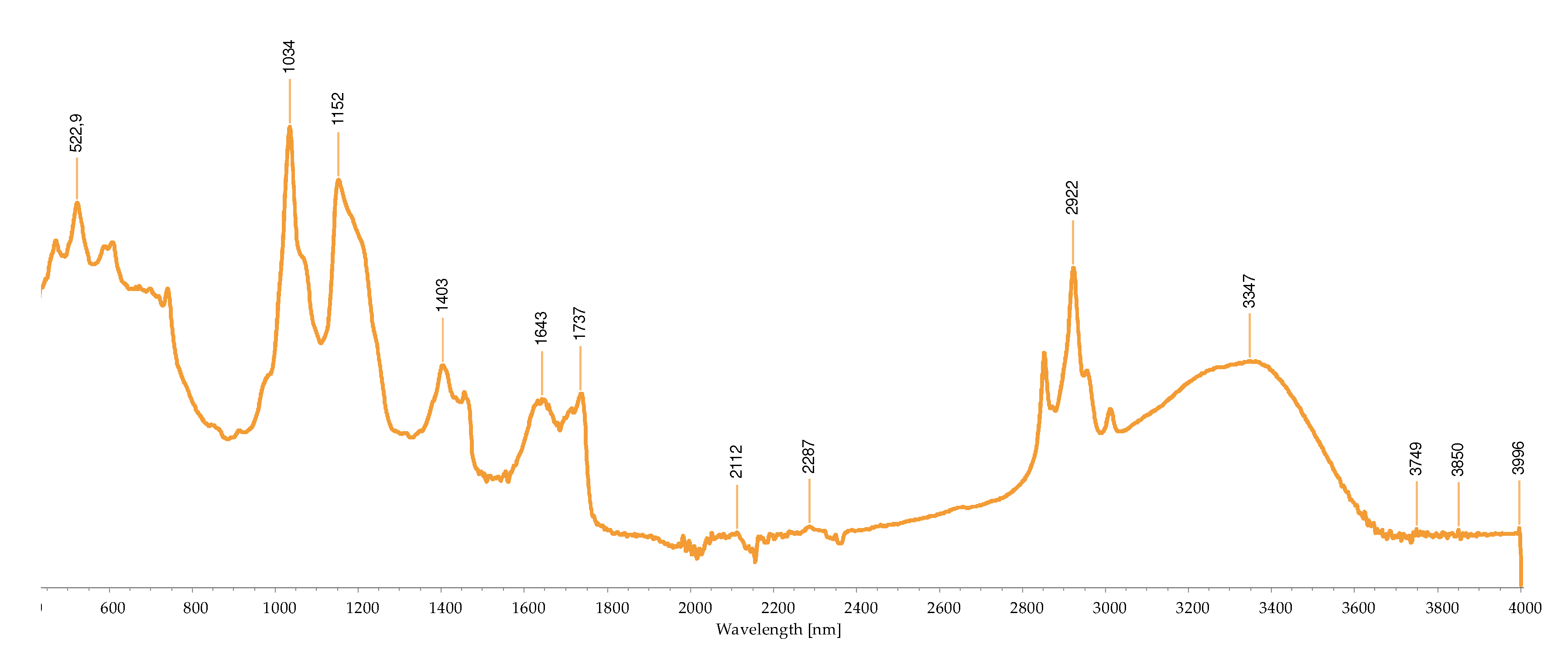
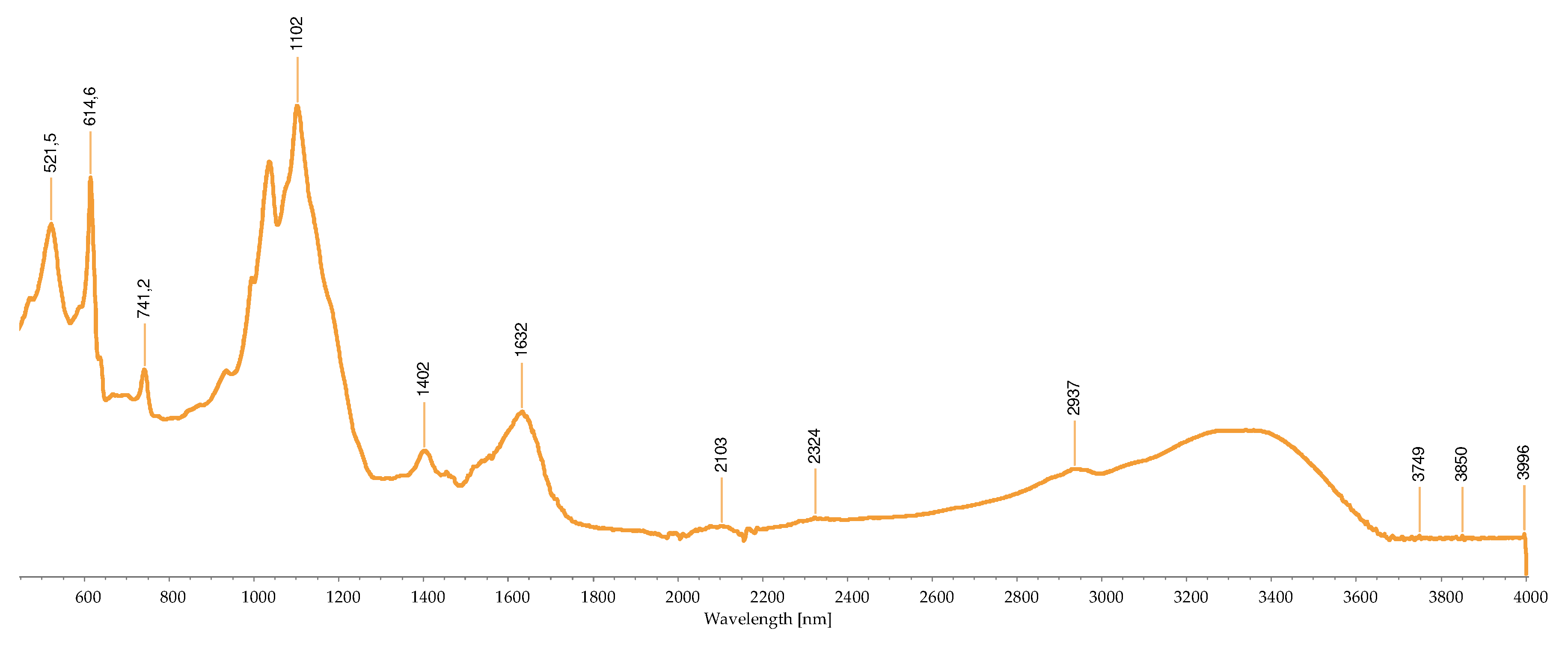
2.3. Concentration of the Isolated Pigments
2.4. Antifungal Activity
3. Discussion
4. Materials and Methods
4.1. Seaweed Harvesting Location
4.2. Biomass Treatment for the Pigment Extraction
4.3. Preparation of the Sample for the Extraction of Pigments
4.4. Isolation and Characterization of Pigments
4.4.1. Chromatography Methods
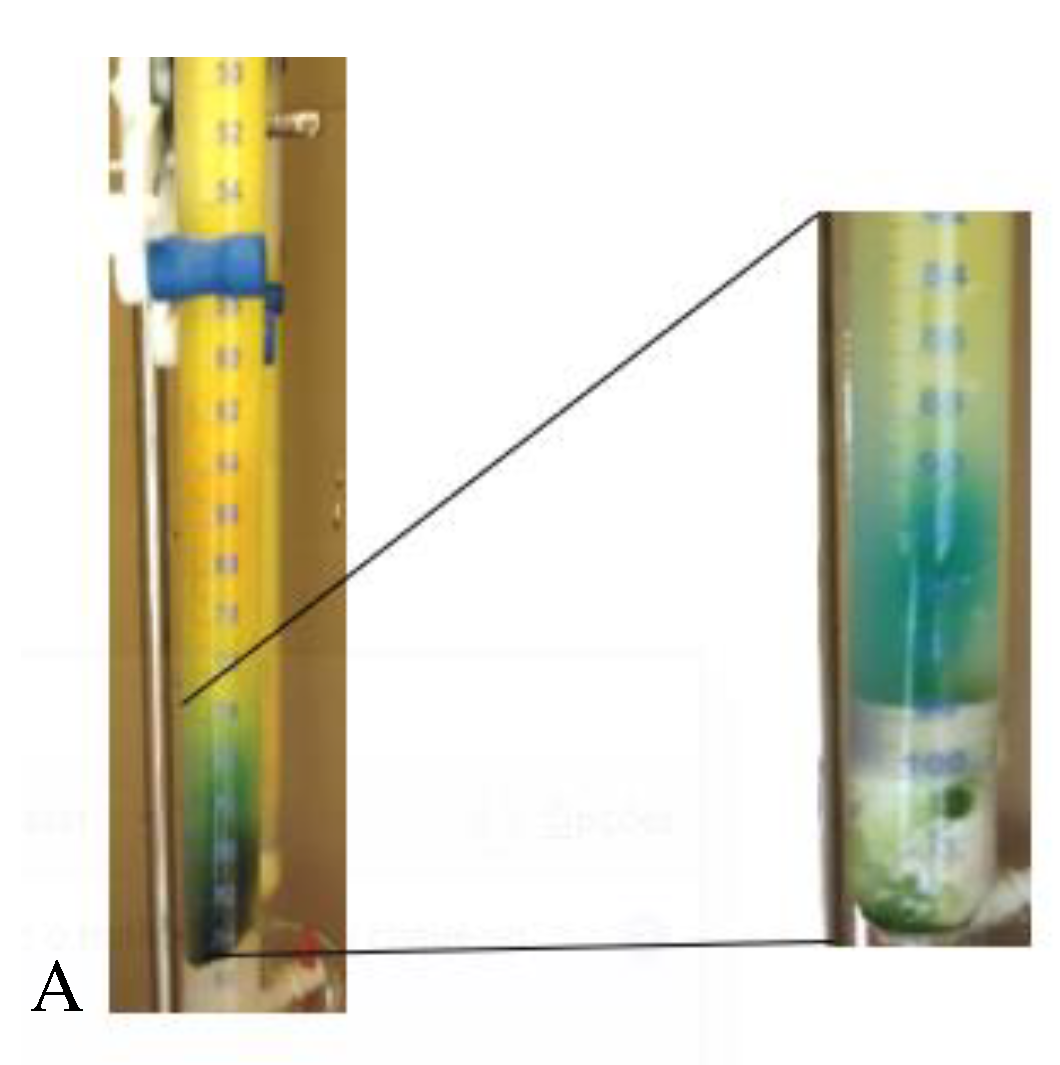
4.4.2. Thin Layer Chromatography (TLC)
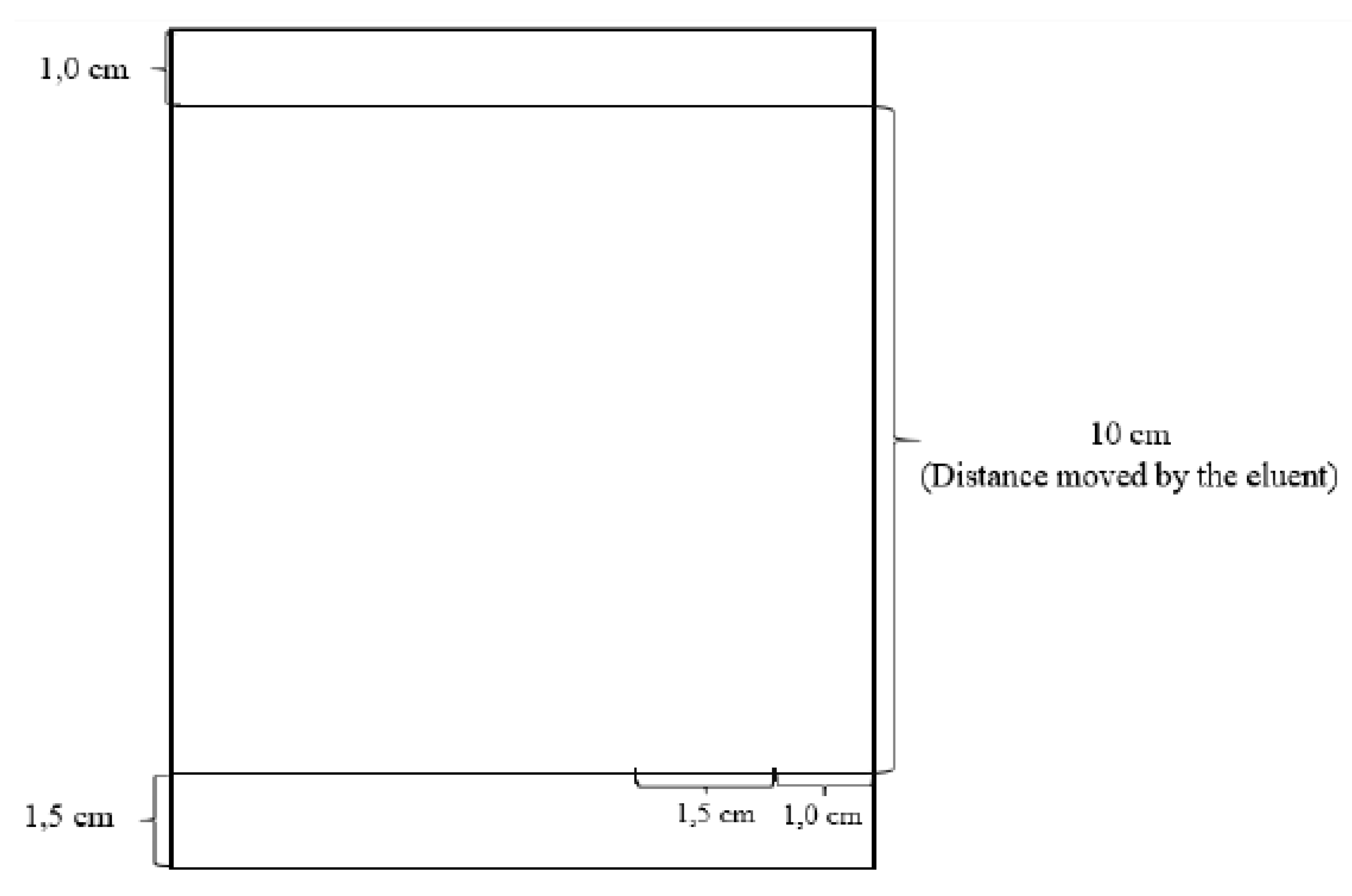
4.4.3. Ultraviolet and Visible (UV-Vis) Spectrophotometry
4.4.4. Fourier-Transform Infrared Spectroscopy (FTIR)
4.5. Resuspension of the Pigment Fractions in Water
4.6. Determination of the Concentration
4.7. Sterility Assays
4.8. Antifungal Assays
4.8.1. Microorganism Culture and Growth Conditions
4.8.2. Quantification of the Antifungal Activity
4.8.3. Minimum Fungicidal Concentration
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jiménez, E.; Dorta, F.; Medina, C.; Ramírez, A.; Ramírez, I.; Peña-Cortés, H. Anti-Phytopathogenic Activities of Macro-Algae Extracts. Mar. Drugs 2011, 9, 739–756. [Google Scholar] [CrossRef]
- Déléris, P.; Nazih, H.; Bard, J.-M. Seaweeds in Human Health. In Seaweed in Health and Disease Prevention; Elsevier: Amsterdam, The Netherlands, 2016; pp. 319–367. [Google Scholar]
- Pádua, D.; Rocha, E.; Gargiulo, D.; Ramos, A.A. Bioactive Compounds from Brown Seaweeds: Phloroglucinol, Fucoxanthin and Fucoidan as Promising Therapeutic Agents against Breast Cancer. Phytochem. Lett. 2015, 14, 91–98. [Google Scholar] [CrossRef]
- Pinheiro, M.S.; Aguiar, P.F. de; Quaresma, C.H.; Garcia, S. Validation of Impregnation Process for Homeopathic Globules by Spectrophotometric-UV Method. Brazilian J. Pharm. Sci. 2014, 50, 137–146. [Google Scholar] [CrossRef]
- Jesumani, V.; Du, H.; Aslam, M.; Pei, P.; Huang, N. Potential Use of Seaweed Bioactive Compounds in Skincare—A Review. Mar. Drugs 2019, 17, 688. [Google Scholar] [CrossRef] [PubMed]
- Gomes, L.; Monteiro, P.; Cotas, J.; Gonçalves, A.M.M.; Fernandes, C.; Gonçalves, T.; Pereira, L. Seaweeds’ Pigments and Phenolic Compounds with Antimicrobial Potential. Biomol. Concepts 2022, 13, 89–102. [Google Scholar] [CrossRef] [PubMed]
- Janarthanan, M.; Senthil Kumar, M. The Properties of Bioactive Substances Obtained from Seaweeds and Their Applications in Textile Industries. J. Ind. Text. 2018, 48, 361–401. [Google Scholar] [CrossRef]
- Pereira, L. Macroalgae. Encyclopedia 2021, 1, 177–188. [Google Scholar] [CrossRef]
- Sustainable Global Resources of Seaweeds Volume 2; Ranga Rao, A., Ravishankar, G.A., Eds.; Springer International Publishing: Cham, 2022; ISBN 978-3-030-92173-6.
- Aryee, A.N.; Agyei, D.; Akanbi, T.O. Recovery and Utilization of Seaweed Pigments in Food Processing. Curr. Opin. Food Sci. 2018, 19, 113–119. [Google Scholar] [CrossRef]
- Pereira, L. Calliblepharis jubata Available online:. Available online: http://www.flordeutopia.pt/macoi/ (accessed on 8 July 2022).
- Araujo, G.S.; Cotas, J.; Morais, T.; Leandro, A.; García-Poza, S.; Gonçalves, A.M.M.; Pereira, L. Calliblepharis Jubata Cultivation Potential—A Comparative Study between Controlled and Semi-Controlled Aquaculture. Appl. Sci. 2020, 10, 7553. [Google Scholar] [CrossRef]
- Balina, K.; Romagnoli, F.; Blumberga, D. Chemical Composition and Potential Use of Fucus vesiculosus from Gulf of Riga. Energy Procedia 2016, 95, 43–49. [Google Scholar] [CrossRef]
- Maehre, H.K.; Malde, M.K.; Eilertsen, K.-E.; Elvevoll, E.O. Characterization of Protein, Lipid and Mineral Contents in Common Norwegian Seaweeds and Evaluation of Their Potential as Food and Feed. J. Sci. Food Agric. 2014, 94, 3281–3290. [Google Scholar] [CrossRef] [PubMed]
- Freitas, M. V.; Pacheco, D.; Cotas, J.; Mouga, T.; Afonso, C.; Pereira, L. Red Seaweed Pigments from a Biotechnological Perspective. Phycology 2021, 2, 1–29. [Google Scholar] [CrossRef]
- Cserháti, T. Liquid Chromatography of Natural Pigments and Synthetic Dyes; Elsevier Science Ltd, 2006.
- Morais, T.; Cotas, J.; Pacheco, D.; Pereira, L. Seaweeds Compounds: An Ecosustainable Source of Cosmetic Ingredients? Cosmetics 2021, 8, 8. [Google Scholar] [CrossRef]
- Kalasariya, H.S.; Yadav, V.K.; Yadav, K.K.; Tirth, V.; Algahtani, A.; Islam, S.; Gupta, N.; Jeon, B.-H. Seaweed-Based Molecules and Their Potential Biological Activities: An Eco-Sustainable Cosmetics. Molecules 2021, 26, 5313. [Google Scholar] [CrossRef] [PubMed]
- Pereira, A.G.; Otero, P.; Echave, J.; Carreira-Casais, A.; Chamorro, F.; Collazo, N.; Jaboui, A.; Lourenço-Lopes, C.; Simal-Gandara, J.; Prieto, M.A. Xanthophylls from the Sea: Algae as Source of Bioactive Carotenoids. Mar. Drugs 2021, 19, 188. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Gálvez, A.; Viera, I.; Roca, M. Carotenoids and Chlorophylls as Antioxidants. Antioxidants 2020, 9, 505. [Google Scholar] [CrossRef]
- Silva, A.; Silva, S.A.; Carpena, M.; Garcia-Oliveira, P.; Gullón, P.; Barroso, M.F.; Prieto, M.A.; Simal-Gandara, J. Macroalgae as a Source of Valuable Antimicrobial Compounds: Extraction and Applications. Antibiotics 2020, 9, 642. [Google Scholar] [CrossRef]
- Cabral, E.M.; Oliveira, M.; Mondala, J.R.M.; Curtin, J.; Tiwari, B.K.; Garcia-Vaquero, M. Antimicrobials from Seaweeds for Food Applications. Mar. Drugs 2021, 19, 211. [Google Scholar] [CrossRef]
- Tamilarasu, A.; Nethaji, M.; Bharathi, S.; Lloyd, C.C.; Somu, S.L.R. Seaweeds: A Potent Source of Antimicrobial Drugs for Aquaculture Industry. Med. Plants - Int. J. Phytomedicines Relat. Ind. 2021, 13, 33–44. [Google Scholar] [CrossRef]
- Pang, R.; Tao, J.-Y.; Zhang, S.-L.; Zhao, L.; Yue, X.; Wang, Y.-F.; Ye, P.; Dong, J.-H.; Zhu, Y.; Wu, J.-G. In Vitro Antiviral Activity of Lutein against Hepatitis B Virus. Phyther. Res. 2010, 24, 1627–1630. [Google Scholar] [CrossRef] [PubMed]
- Fatima, K.; Mathew, S.; Suhail, M.; Ali, A.; Damanhouri, G.; Azhar, E.; Qadri, I. Docking Studies of Pakistani HCV NS3 Helicase: A Possible Antiviral Drug Target. PLoS One 2014, 9, e106339. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.K.; Venkataraman, M.; Renaud, H.J.; Summerbell, R.; Shear, N.H.; Piguet, V. The Increasing Problem of Treatment-resistant Fungal Infections: A Call for Antifungal Stewardship Programs. Int. J. Dermatol. 2021, 60. [Google Scholar] [CrossRef]
- Su, H.; Han, L.; Huang, X. Potential Targets for the Development of New Antifungal Drugs. J. Antibiot. (Tokyo). 2018, 71, 978–991. [Google Scholar] [CrossRef]
- Rokas, A. Evolution of the Human Pathogenic Lifestyle in Fungi. Nat. Microbiol. 2022, 7, 607–619. [Google Scholar] [CrossRef]
- Lopes, G.; Pinto, E.; Salgueiro, L. Natural Products: An Alternative to Conventional Therapy for Dermatophytosis? Mycopathologia 2017, 182, 143–167. [Google Scholar] [CrossRef]
- Martinez-Rossi, N.M.; Peres, N.T.A.; Rossi, A. Antifungal Resistance Mechanisms in Dermatophytes. Mycopathologia 2008, 166, 369–383. [Google Scholar] [CrossRef]
- Dhayanithi, N.; Kumar, T.A.; Kalaiselvam, M.; Balasubramanian, T.; Sivakumar, N. Anti-Dermatophytic Activity of Marine Sponge, Sigmadocia carnosa (Dendy) on Clinically Isolated Fungi. Asian Pac. J. Trop. Biomed. 2012, 2, 635–639. [Google Scholar] [CrossRef]
- Bhatia, S.; Nagpal, K.; Bera, T.; Sharma, A.; Sharma, K. Evaluation of Pharmacognostical, Phytochemical and Anti-Microbial Properties of Porphyra vietnamensis. Int. J. Green Pharm. 2015, 9, 131. [Google Scholar] [CrossRef]
- Forgacs, E.; Cserhati, T. Thin-Layer Chromatography of Natural Pigments: New Advances. J. Liq. Chromatogr. Relat. Technol. 2002, 25, 1521–1541. [Google Scholar] [CrossRef]
- Johnston, A.; Scaggs, J.; Mallory, C.; Haskett, A.; Warner, D.; Brown, E.; Hammond, K.; McCormick, M.M.; McDougal, O.M. A Green Approach To Separate Spinach Pigments by Column Chromatography. J. Chem. Educ. 2013, 90, 796–798. [Google Scholar] [CrossRef]
- Koizumi, J.; Takatani, N.; Kobayashi, N.; Mikami, K.; Miyashita, K.; Yamano, Y.; Wada, A.; Maoka, T.; Hosokawa, M. Carotenoid Profiling of a Red Seaweed Pyropia Yezoensis: Insights into Biosynthetic Pathways in the Order Bangiales. Mar. Drugs 2018, 16, 426. [Google Scholar] [CrossRef] [PubMed]
- Robertson, D. Algal Pigments. Available online: https://wordpress.clarku.edu/debrobertson/laboratory-protocols/algal-pigments/ (accessed on 25 November 2022).
- Rajauria, G.; Abu-Ghannam, N. Isolation and Partial Characterization of Bioactive Fucoxanthin from Himanthalia elongata Brown Seaweed: A TLC-Based Approach. Int. J. Anal. Chem. 2013, 2013, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Savira, A.D.R.; Amin, M.N.G.; Alamsjah, M.A. The Effect of Different Type of Solvents on the Antioxidant Activity of Fucoxanthin Extract from Brown Seaweed Sargassum duplicatum. IOP Conf. Ser. Earth Environ. Sci. 2021, 718, 012010. [Google Scholar] [CrossRef]
- Dumay, J.; Morançais, M. Proteins and Pigments. In Seaweed in Health and Disease Prevention; Elsevier, 2016; pp. 275–318.
- Cotas, J.; Figueirinha, A.; Pereira, L.; Batista, T. The Effect of Salinity on Fucus ceranoides (Ochrophyta, Phaeophyceae) in the Mondego River (Portugal). J. Oceanol. Limnol. 2019, 37, 881–891. [Google Scholar] [CrossRef]
- Clementson, L.A.; Wojtasiewicz, B. Dataset on the Absorption Characteristics of Extracted Phytoplankton Pigments. Data Br. 2019, 24, 103875. [Google Scholar] [CrossRef]
- Merdekawati, W.; Limantara, L.; Susanto, A. TThe Content and Pigment Composition of Sargassum crassifolium J. Agardh on Several Drying Treatments. Ina. Pap. 2018. [Google Scholar] [CrossRef]
- Latsos, C.; van Houcke, J.; Blommaert, L.; Verbeeke, G.P.; Kromkamp, J.; Timmermans, K.R. Effect of Light Quality and Quantity on Productivity and Phycoerythrin Concentration in the Cryptophyte Rhodomonas Sp. J. Appl. Phycol. 2021, 33, 729–741. [Google Scholar] [CrossRef]
- Meng, D.; Zhang, L.; Wang, Q.; Zhang, Y.; Sun, Y.; Zhang, H.; Wang, Z.; Zhou, Z.; Yang, R. Self-Assembly of Phycoerythrin with Oligochitosan by Electrostatic Interaction for Stabilization of Phycoerythrin. J. Agric. Food Chem. 2021, 69, 12818–12827. [Google Scholar] [CrossRef]
- MubarakAli, D.; Gopinath, V.; Rameshbabu, N.; Thajuddin, N. Synthesis and Characterization of CdS Nanoparticles Using C-Phycoerythrin from the Marine Cyanobacteria. Mater. Lett. 2012, 74, 8–11. [Google Scholar] [CrossRef]
- Mudunkotuwa, I.A.; Minshid, A. Al; Grassian, V.H. ATR-FTIR Spectroscopy as a Tool to Probe Surface Adsorption on Nanoparticles at the Liquid–Solid Interface in Environmentally and Biologically Relevant Media. Analyst 2014, 139, 870–881. [Google Scholar] [CrossRef] [PubMed]
- Righini, H.; Francioso, O.; Di Foggia, M.; Quintana, A.M.; Roberti, R. Preliminary Study on the Activity of Phycobiliproteins against Botrytis cinerea. Mar. Drugs 2020, 18, 600. [Google Scholar] [CrossRef]
- Setyawati, H.; Darmokoesoemo, H.; Ningtyas, A.T.A.; Kadmi, Y.; Elmsellem, H.; Kusuma, H.S. Effect of Metal Ion Fe(III) on the Performance of Chlorophyll as Photosensitizers on Dye Sensitized Solar Cell. Results Phys. 2017, 7, 2907–2918. [Google Scholar] [CrossRef]
- Suica-bunghez, I.R.; Sorescu, A.A.; Doncea, S.M.; Constantin, M.; Raut, I.; Ion, R.M. Phytochemical, Antioxidant and Antimicrobial Characterization of Hedera Helix l. Extract. J. Plant Dev. 2020, 27, 47–53. [Google Scholar] [CrossRef]
- Aneke, C.I.; Rhimi, W.; Otranto, D.; Cafarchia, C. Synergistic Effects of Efflux Pump Modulators on the Azole Antifungal Susceptibility of Microsporum Canis. Mycopathologia 2020. [Google Scholar] [CrossRef]
- Bhatia, V.; Sharma, P. Determination of Minimum Inhibitory Concentrations of Itraconazole, Terbinafine and Ketoconazole against Dermatophyte Species by Broth Microdilution Method. Indian J. Med. Microbiol. 2015, 33, 533–537. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.K.; Kohli, Y.; Batra, R. In Vitro Activities of Posaconazole, Ravuconazole, Terbinafine, Itraconazole and Fluconazole against Dermatophyte, Yeast and Non-Dermatophyte Species. Med. Mycol. 2005, 43, 179–185. [Google Scholar] [CrossRef]
- Indira, K.; Balakrishnan, S.; Srinivasan, M.; Bragadeeswaran, S.; Balasubramanian, T. Evaluation of in Vitro Antimicrobial Property of Seaweed (Halimeda tuna) from Tuticorin Coast, Tamil Nadu, Southeast Coast of India. African J. Biotechnol. 2013, 12, 284–289. [Google Scholar] [CrossRef]
- Pérez, M.J.; Falqué, E.; Domínguez, H. Antimicrobial Action of Compounds from Marine Seaweed. Mar. Drugs 2016, 14. [Google Scholar] [CrossRef] [PubMed]
- Thakur, R.; Kalsi, A.S. Outbreaks And Epidemics Of Superficial Dermatophytosis Due To Trichophyton Mentagrophytes Complex And Microsporum Canis: Global And Indian Scenario. Clin. Cosmet. Investig. Dermatol. 2019, Volume 12, 887–893. [Google Scholar] [CrossRef]
- Pardilhó, S.L.; Machado, S.; F. Bessada, S.M.; F. Almeida, M.; Oliveira, M.B.; M. Dias, J. Marine Macroalgae Waste from Northern Portugal: A Potential Source of Natural Pigments? Waste and Biomass Valorization 2021, 12, 239–249. [Google Scholar] [CrossRef]
- Silva, A.; Abreu, H.; Silva, A.; Cardoso, S. Effect of Oven-Drying on the Recovery of Valuable Compounds from Ulva Rigida, Gracilaria Sp. and Fucus Vesiculosus. Mar. Drugs 2019, 17, 90. [Google Scholar] [CrossRef] [PubMed]
- Warkoyo, W.; Saati, E.A. The Solvent Effectiveness on Extraction Process of Seaweed Pigment. Makara J. Technol. 2011, 15, 5–8. [Google Scholar] [CrossRef]
- Yang, L.; Li, P.; Fan, S. The Extraction of Pigments from Fresh Laminaria japonica. Chinese J. Oceanol. Limnol. 2008, 26, 193–196. [Google Scholar] [CrossRef]
- Sudhakar, M.P.; Ananthalakshmi, J.S.; Nair, B.B. Extraction, Purification and Study on Antioxidant Properties of Fucoxanthin from Brown Seaweeds. J. Chem. Pharm. Res. 2013, 5, 169–175. [Google Scholar]
- Kannaujiya, V.K.; Kumar, D.; Singh, V.; Sinha, R.P. Advances in Phycobiliproteins Research: Innovations and Commercialization. In Natural Bioactive Compounds; Elsevier, 2021; pp. 57–81.
- Li, W.; Su, H.-N.; Pu, Y.; Chen, J.; Liu, L.-N.; Liu, Q.; Qin, S. Phycobiliproteins: Molecular Structure, Production, Applications, and Prospects. Biotechnol. Adv. 2019, 37, 340–353. [Google Scholar] [CrossRef]
- Hemlata; Afreen, S. ; Fatma, T. Extraction, Purification and Characterization of Phycoerythrin from Michrochaete and Its Biological Activities. Biocatal. Agric. Biotechnol. 2018, 13, 84–89. [Google Scholar] [CrossRef]
- Najdenski, H.M.; Gigova, L.G.; Iliev, I.I.; Pilarski, P.S.; Lukavský, J.; Tsvetkova, I. V.; Ninova, M.S.; Kussovski, V.K. Antibacterial and Antifungal Activities of Selected Microalgae and Cyanobacteria. Int. J. Food Sci. Technol. 2013, 48, 1533–1540. [Google Scholar] [CrossRef]
- Ngamwonglumlert, L.; Devahastin, S.; Chiewchan, N. Natural Colorants: Pigment Stability and Extraction Yield Enhancement via Utilization of Appropriate Pretreatment and Extraction Methods. Crit. Rev. Food Sci. Nutr. 2017, 57, 3243–3259. [Google Scholar] [CrossRef] [PubMed]
- Özkan, G.; Ersus Bilek, S. Enzyme-Assisted Extraction of Stabilized Chlorophyll from Spinach. Food Chem. 2015, 176, 152–157. [Google Scholar] [CrossRef] [PubMed]
- Delgado-Vargas, F.; Paredes-Lopez, O. Natural Colorants for Food and Nutraceutical Uses; CRC Press, 2002; ISBN 9781420031713.
- Boon, C.S.; McClements, D.J.; Weiss, J.; Decker, E.A. Factors Influencing the Chemical Stability of Carotenoids in Foods. Crit. Rev. Food Sci. Nutr. 2010, 50, 515–532. [Google Scholar] [CrossRef] [PubMed]
- Galetović, A.; Seura, F.; Gallardo, V.; Graves, R.; Cortés, J.; Valdivia, C.; Núñez, J.; Tapia, C.; Neira, I.; Sanzana, S.; et al. Use of Phycobiliproteins from Atacama Cyanobacteria as Food Colorants in a Dairy Beverage Prototype. Foods 2020, 9, 244. [Google Scholar] [CrossRef] [PubMed]
- Sonani, R.R. Recent Advances in Production, Purification and Applications of Phycobiliproteins. World J. Biol. Chem. 2016, 7, 100. [Google Scholar] [CrossRef] [PubMed]
- Nowruzi, B.; Konur, O.; Anvar, S.A.A. The Stability of the Phycobiliproteins in the Adverse Environmental Conditions Relevant to the Food Storage. Food Bioprocess Technol. 2022, 15, 2646–2663. [Google Scholar] [CrossRef]
- Numan, M.; Bashir, S.; Mumtaz, R.; Tayyab, S.; Rehman, N.U.; Khan, A.L.; Shinwari, Z.K.; Al-Harrasi, A. Therapeutic Applications of Bacterial Pigments: A Review of Current Status and Future Opportunities. 3 Biotech 2018, 8, 207. [Google Scholar] [CrossRef]
- Tuli, H.S.; Chaudhary, P.; Beniwal, V.; Sharma, A.K. Microbial Pigments as Natural Color Sources: Current Trends and Future Perspectives. J. Food Sci. Technol. 2015, 52, 4669–4678. [Google Scholar] [CrossRef]
- Polat, S.; Trif, M.; Rusu, A.; Šimat, V.; Čagalj, M.; Alak, G.; Meral, R.; Özogul, Y.; Polat, A.; Özogul, F. Recent Advances in Industrial Applications of Seaweeds. Crit. Rev. Food Sci. Nutr. 2021, 1–30. [Google Scholar] [CrossRef]
- Cotas, J.; Pacheco, D.; Araujo, G.S.; Valado, A.; Critchley, A.T.; Pereira, L. On the Health Benefits vs. Risks of Seaweeds and Their Constituents: The Curious Case of the Polymer Paradigm. Mar. Drugs 2021, 19, 164. [Google Scholar] [CrossRef] [PubMed]
- Cotas, J. Fucus ceranoides (Ochrophyta, Phaeophyceae): Bioatividades Dependentes do Gradiente Salino, MSC Thesis, University of Coimbra, Coimbra, Portugal, 2015. [Google Scholar]
- Sudhakar, M.P.; Jagatheesan, A.; Perumal, K.; Arunkumar, K. Methods of Phycobiliprotein Extraction from Gracilaria Crassa and Its Applications in Food Colourants. Algal Res. 2015, 8, 115–120. [Google Scholar] [CrossRef]
- Uju; Dewi, N. P.S.U.K.; Santoso, J.; Setyaningsih, I.; Hardingtyas, S.D.; Yopi Extraction of Phycoerythrin from Kappaphycus alvarezii Seaweed Using Ultrasonication. IOP Conf. Ser. Earth Environ. Sci. 2020, 414, 012028. [Google Scholar] [CrossRef]
- Monteiro, P.; Cotas, J.; Pacheco, D.; Figueirinha, A.; da Silva, G.J.; Pereira, L.; Gonçalves, A.M.M. Seaweed as Food: How to Guarantee Their Quality. In Sustainable Global Resources of Seaweeds Volume 2; Springer International Publishing: Cham, 2022; pp. 309–321. [Google Scholar]
- Srivastava, N.; Singh, A.; Kumari, P.; Nishad, J.H.; Gautam, V.S.; Yadav, M.; Bharti, R.; Kumar, D.; Kharwar, R.N. Advances in Extraction Technologies: Isolation and Purification of Bioactive Compounds from Biological Materials. In Natural Bioactive Compounds; Elsevier, 2021; pp. 409–433.
- Dias, A.M.; Ferreira, M.L.S. “Supermarket Column Chromatography of Leaf Pigments” Revisited: Simple and Ecofriendly Separation of Plant Carotenoids, Chlorophylls, and Flavonoids from Green and Red Leaves. J. Chem. Educ. 2015, 92, 189–192. [Google Scholar] [CrossRef]
- Poole, C.F. Thin-Layer Chromatography: Challenges and Opportunities. J. Chromatogr. A 2003, 1000, 963–984. [Google Scholar] [CrossRef]
- Misra, N.N.; Rai, D.K.; Hossain, M. Analytical Techniques for Bioactives from Seaweed. In Seaweed Sustainability; Elsevier: : Amsterdam, The Netherlands, 2015; pp. 271–287. [Google Scholar]
- Mujahid, A.; Dickert, F.L. Molecularly Imprinted Polymers for Sensors. In Molecularly Imprinted Sensors; Elsevier, 2012; pp. 125–159.
- Roberts, J.; Power, A.; Chapman, J.; Chandra, S.; Cozzolino, D. The Use of UV-Vis Spectroscopy in Bioprocess and Fermentation Monitoring. Fermentation 2018, 4, 18. [Google Scholar] [CrossRef]
- Mokrane, H.; Ghaliaoui, N.; Hazzit, M.; Hadjadj, M.; Otmani, F.-S.; Touati, S.; Seridi, H. Impact of Freezing and Drying Preprocessing on Pigments Extraction from the Brown Seaweed Phyllaria reniformis Collected in Algerian Coast. Carpathian J. Food Sci. Technol. 2020, 81–94. [Google Scholar] [CrossRef]
- Arendrup, M.C.; Kahlmeter, G.; Guinea, J.; Meletiadis, J. How to: Perform Antifungal Susceptibility Testing of Microconidia-Forming Dermatophytes Following the New Reference EUCAST Method E.Def 11.0, Exemplified by Trichophyton. Clin. Microbiol. Infect. 2021, 27, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Espinel-Ingroff, A.; Chaturvedi, V.; Fothergill, A.; Rinaldi, M.G. Optimal Testing Conditions for Determining MICs and Minimum Fungicidal Concentrations of New and Established Antifungal Agents for Uncommon Molds: NCCLS Collaborative Study. J. Clin. Microbiol. 2002, 40, 3776–3781. [Google Scholar] [CrossRef] [PubMed]
| Phylum | Chl | PBPs | Carotenoids | References |
|---|---|---|---|---|
| Chlorophyta | a, b | β-Carotene, lutein, neoxanthin, violaxanthin, and zeaxanthin | [5,6,7,8,10] | |
| Ochrophyta | a, c | β-Carotene, fucoxanthin, and violaxanthin | ||
| Rhodophyta | a | Phycocyanin Phycoerythrin |
β-Carotene, lutein, and zeaxanthin |
| Nomenclature of the pigments | Retention Factor (Rf) | Pigments | References | |
|---|---|---|---|---|
| Ethanol extracts | A | 0,54 | Chlorophyll b | [32,33,34,35,36,37,38] |
| 0,85 | β-carotene | |||
| B | 0,48 | Fucoxanthin | ||
| C | 0,48 | Fucoxanthin | ||
| D Crude Extract |
0,7 | *NA | ||
| 0,13 | Neoxanthin | |||
| 0,19 | *NA | |||
| 0,65 | *NA | |||
| 0,9 | β-carotene | |||
| 0,95 | β-carotene | |||
| Acetone Extracts | G | 0,38 | *NA | |
| H | 0,06 | Neoxanthin | ||
| 0,11 | Neoxanthin | |||
| I Crude Extract |
0,38 | *NA | ||
| 0,45 | Lutein | |||
| 0,02 | Chlorophyll c | |||
| 0,59 | Fucoxanthin | |||
| 0,85 | *NA |
| Nomenclature of the pigments | Retention Factor (Rf) | Pigments | References | |
|---|---|---|---|---|
| Acetone Extracts | J | 0,82 | *NA | [32,33,34,35,36] |
| L | 0,53 | Chlorophyll b | ||
| M (Crude Extract) |
0,04 | Chlorophyll c | ||
| 0,42 | Lutein | |||
| 0,62 | *NA | |||
| 0,68 | Chlorophyll a | |||
| 0,74 | Zeaxanthin | |||
| 0,81 | *NA | |||
| 0,84 | *NA | |||
| 0,86 | *NA | |||
| 0,91 | β-carotene | |||
| Ethanol extracts | O | 0,53 | Chlorophyll b | |
| 0,7 | Violaxanthin | |||
| R (Crude Extract) |
0,75 | *NA | ||
| 0,86 | *NA | |||
| 0,91 | β-carotene | |||
| Extract | Peaks (nm) | Pigment | Reference |
|---|---|---|---|
| A | 415, 665 | Chl a | [36,37,38,39,40,41,42] |
| B | 410,430,455 | Fucoxanthin | |
| C | 410,430,455, 670 | Fucoxanthin enriched with Chl a | |
| D | 415, 670 | Chl a | |
| G | 455, 475 | β-carotene | |
| H | 415, 450, 500, 535, 670 | Enriched Chl a | |
| I | 415, 670 | Chl a | |
| J | 425, 450, 475 ,670 | Enriched Extract | |
| L | 420 ,450, 480 | Enriched Extract | |
| M | 420, 475, 675 | Enriched Extract | |
| O | 425, 450, 475, 665 | Violaxanthin enriched with Chl a | |
| R | 415, 435, 480, 665 | Enriched Extract | |
| S | 495, 630 | Mixture of PBS |
| Wave number (cm-1) |
Bond | Possible compound | A | B | C | D | H | I | M | R | S | References |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1034 | C-N | Phycoerythrin/ Chlorophyll |
- | - | - | - | - | - | + | - | - | [40,43,44,45,46,47,48,49] |
| 1035 | C-N | Phycoerythrin/ Chlorophyll |
- | - | - | - | - | - | - | + | - | |
| 1037 | C-N | Phycoerythrin/ Chlorophyll |
- | - | - | - | - | - | - | - | + | |
| 1039 | C-N | Phycoerythrin/ Chlorophyll |
- | - | + | - | + | - | - | - | - | |
| 1057 | C-O | Common bond present in the compounds | + | - | - | - | - | - | - | - | - | |
| 1076 | (>P=O) stretching | Phosphated compound | - | - | - | + | - | - | - | - | - | |
| 1149 | C-O stretch | Common bond present in the compounds | - | - | - | - | - | + | + | - | - | |
| 1152 | C-O stretch | Common bond present in the compounds | - | - | - | - | - | - | - | + | - | |
| 1159 | C-O stretch | Common bond present in the compounds | + | - | - | - | - | - | - | - | - | |
| 1176 | CO-C-O | Fucoxanthin | - | - | + | - | - | - | - | - | - | |
| 1180 | CO-C-O | Fucoxanthin | - | - | - | - | + | - | - | - | - | |
| 1372 | C-N | Phycoerythrin/ Chlorophyll |
- | - | - | + | - | - | - | - | - | |
| 1377 | C-N | Phycoerythrin/ Chlorophyll |
+ | - | - | - | - | + | + | - | - | |
| 1403 | -COO- | Chlorophyll/ Phycoerythrin |
- | - | + | - | - | - | - | + | + | |
| 1455 | CH2 scissoring | Carotenoid | - | - | - | - | - | + | + | - | - | |
| 1457 | CH2 scissoring | Carotenoid | + | - | - | - | - | - | - | - | - | |
| 1458 | CH2 scissoring | Carotenoid | - | - | - | + | - | - | - | - | - | |
| 1615 | C=C aromatic | Chlorophyll | - | - | - | - | - | + | - | - | - | |
| 1617 | C=C aromatic | Chlorophyll | - | - | - | + | - | - | - | - | - | |
| 1618 | C=C aromatic | Chlorophyll | - | - | - | - | + | - | - | - | - | |
| 1634 | C=O | Phycoerythrin | - | - | - | - | - | - | - | - | + | |
| 1636 | C=O | Carotenoid | - | - | + | - | - | - | - | - | - | |
| 1651 | C=O | Phycoerythrin | - | - | - | - | - | - | - | + | - | |
| 1733 | - C=O | Caro/chl | - | + | - | - | - | - | - | - | - | |
| 1738 | - C=O | Caro/chl | - | - | - | - | - | - | + | + | - | |
| 1739 | - C=O | Caro/chl | - | - | - | - | - | + | - | - | - | |
| 1742 | - C=O | Caro/chl | + | - | - | - | - | - | - | - | - | |
| 2852 | O-CH3 | Carotenoids | - | + | - | - | - | + | + | + | - | |
| 2853 | O-CH3 | Carotenoids | + | - | - | - | + | - | - | - | - | |
| 2921 | CH2 stretch | Carotenoids | - | - | - | - | - | - | + | - | - | |
| 2922 | CH2 stretch | Carotenoids | + | + | - | - | + | + | - | + | - | |
| 2926 | CH2 stretch | Carotenoids | - | - | + | - | - | - | - | - | - | |
| 2936 | CH3 stretch | Carotenoids / Phycoerythrin | - | - | - | - | - | - | - | - | + | |
| 2937 | CH3 stretch | Carotenoids / Phycoerythrin | + | - | - | - | - | - | - | - | - | |
| 3010 | Chlorophyll | - | - | - | - | - | + | - | - | - | ||
| 3011 | Chlorophyll | - | - | - | - | + | - | + | + | - |
| F. vesiculosus Pigments | Concentration (µg/mL) | C. jubata Pigments |
Concentration (µg/mL) |
|---|---|---|---|
| A | 266.66 | J | 133.33 |
| B | 233.33 | L | 100 |
| C | 166.66 | M | 766.67 |
| D | 1666.7 | O | 100 |
| G | 166.67 | R | 6900 |
| H | 200 | S | 11133 |
| I | 1833.3 |
| Concentration | μg/mL | |||||||
|---|---|---|---|---|---|---|---|---|
| Extracts | R | S | Itra. | |||||
| Weeks | W=1 | W=2 | W=3 | W=1 | W=2 | W=3 | W=1,2,3 | |
| T. mentagrophytes | MIC | 3450 | >3450 | >3450 | 2783.25 | 5566.5 | 5566.5 | 1 |
| MIC50 | 1725 | >3450 | >3450 | >5566.5 | 2783.25 | 2783.25 | 0.5 | |
| MFC | 3450 | >3450 | >3450 | 2783.25 | 5566.5 | >5566.5 | >1 | |
| T. rubrum | MIC | 3450 | 3450 | 3450 | 2783.25 | 2783.25 | 5566.5 | 1 |
| MIC50 | 1725 | 1725 | 1725 | Nd* | Nd* | 2783.25 | 0.5 | |
| MFC | 3450 | >3450 | >3450 | 2783.25 | 2783.25 | >5566.5 | >1r | |
| M. canis | MIC | 3450 | >3450 | >3450 | 2783.25 | 2783.25 | 2783.25 | 1 |
| MIC50 | 862,5 | >3450 | >3450 | 1391.625 | 1391.63 | 1391.63 | 0.5 | |
| MFC | >3450 | >3450 | >3450 | 2783.25 | 5566.5 | 5566.5 | >1 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





