Submitted:
10 October 2023
Posted:
11 October 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Participants
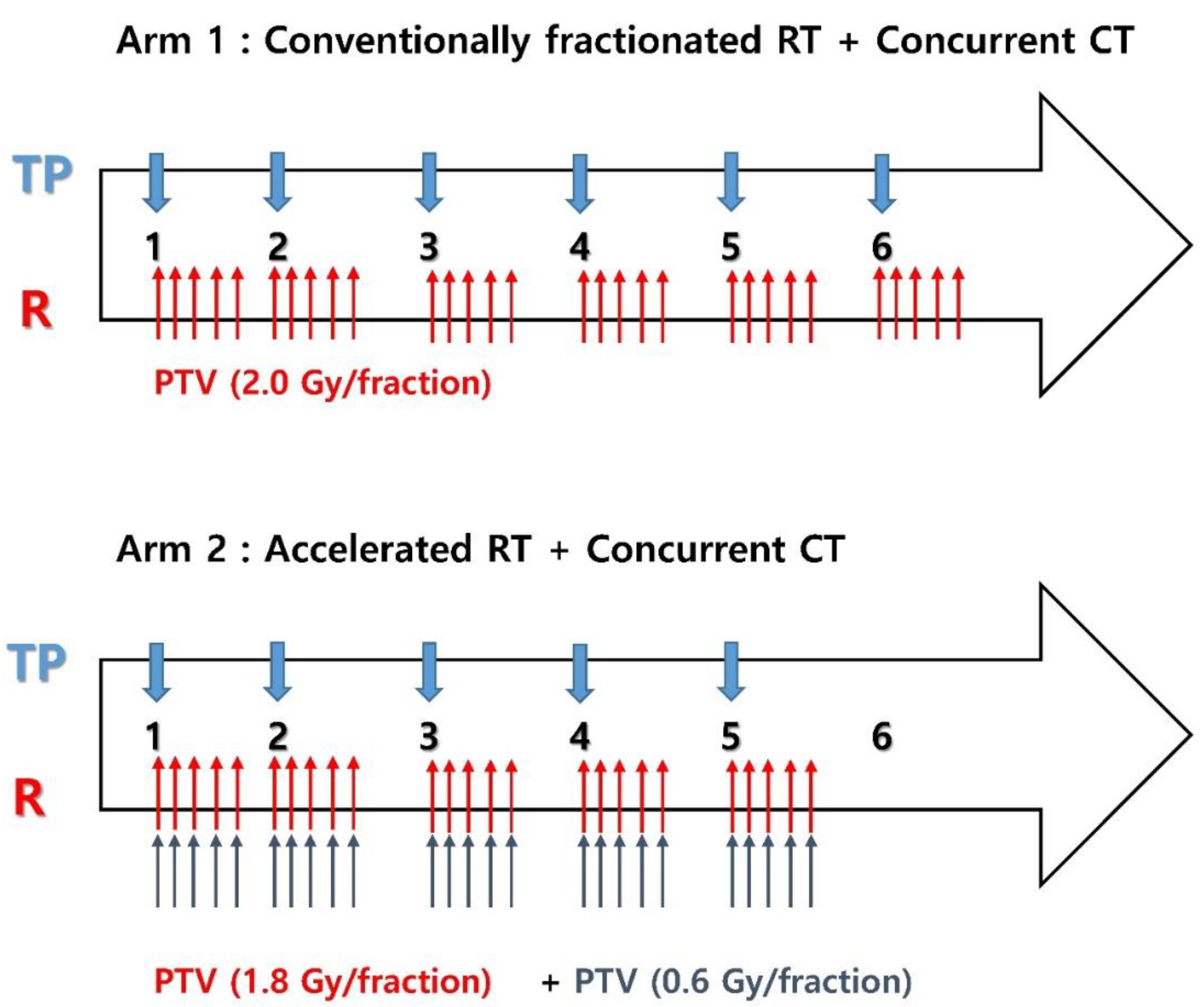
2.2. Dosimetric Analysis and Hematologic Evaluation
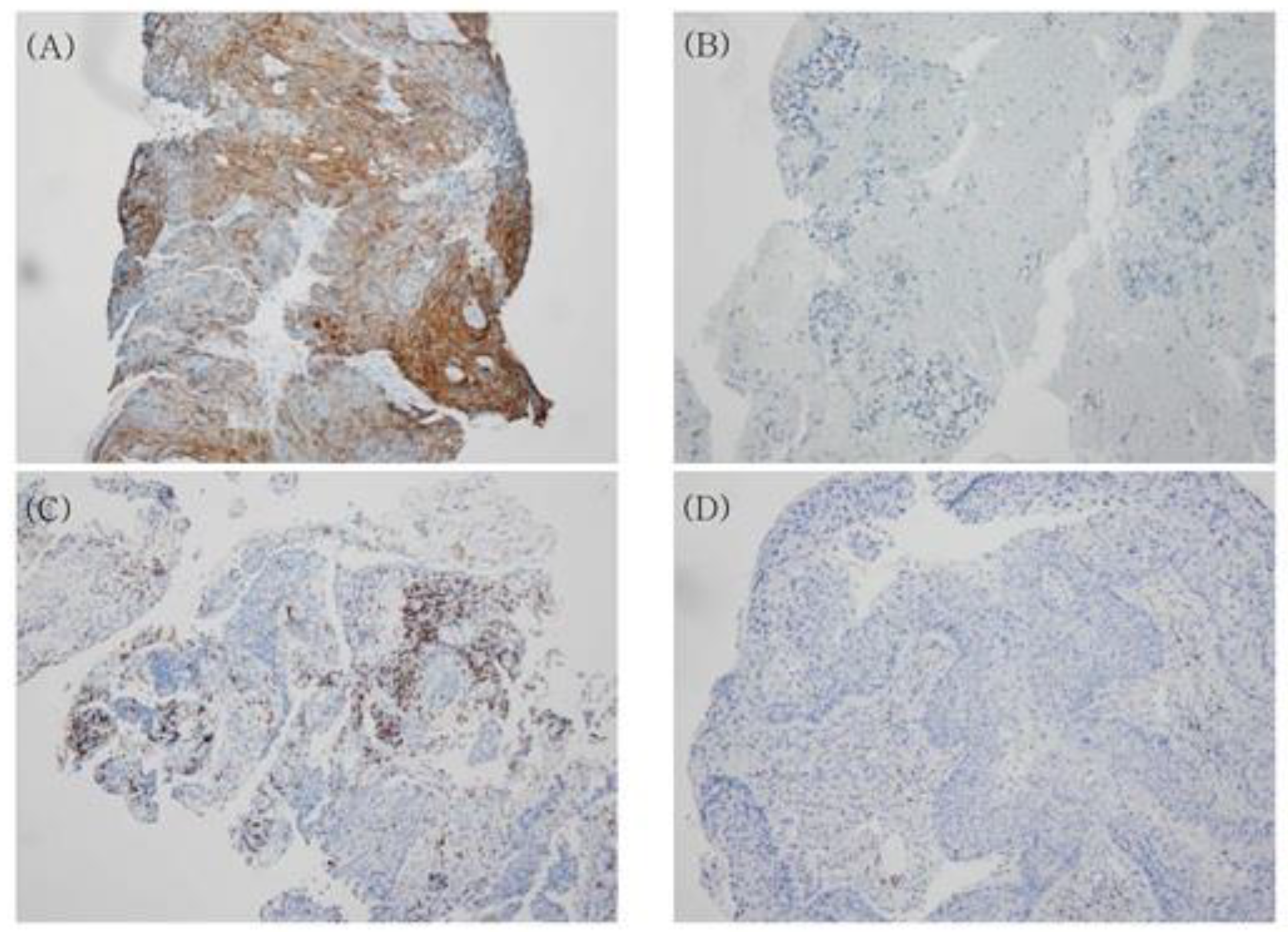
2.3. Immunohistochemistry for Programmed Death-Ligand 1 (PDL1) and CD8
2.3.1. Assessment of PDL1 Expression
2.3.2. Assessment of CD8 Expression on Tumor-Infiltrating Lymphocytes (TILs)
2.4. Follow-Up and Statistical Analyses
3. Results
3.1. Patient Characteristics
3.2. Survival Analysis and Prognostic Factor Determination
3.3. Association between Peripheral Blood Cells and Dosimetric Parameters
3.4. Toxicity Analysis
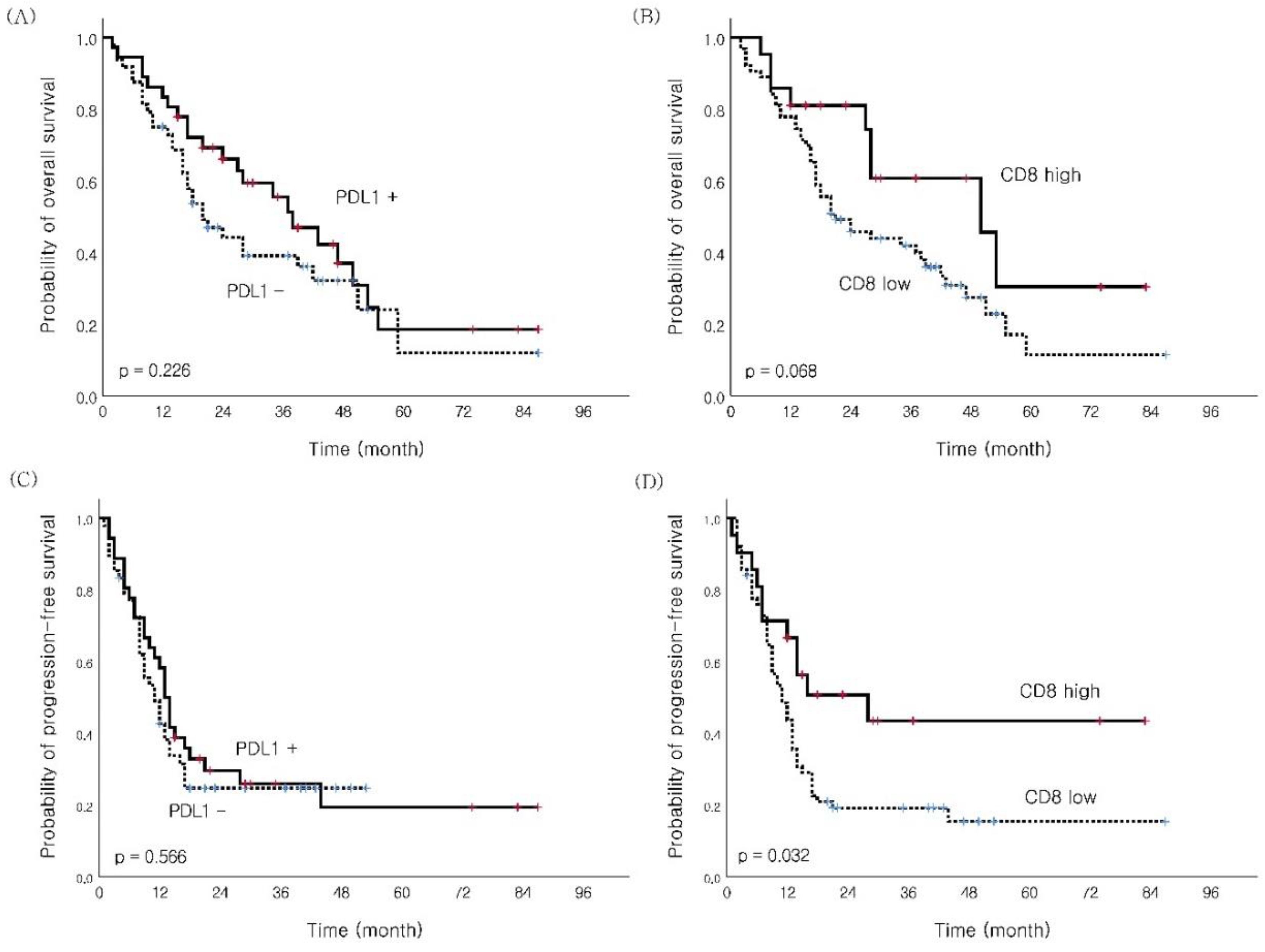
3.5. Subgroup Analysis for PD-L1 and CD8 TIL Expression
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- O'Callaghan, D.S.; O'Donnell, D.; O'Connell, F.; O'Byrne, K.J. The role of inflammation in the pathogenesis of non-small cell lung cancer. J. Thorac. Oncol. Off. Publ. Int. Assoc. Study Lung Cancer 2010, 5, 2024–2036. [Google Scholar] [CrossRef]
- Blank, C.U.; Haanen, J.B.; Ribas, A.; Schumacher, T.N. CANCER IMMUNOLOGY. The "cancer immunogram". Sci. (N. Y.) 2016, 352, 658–660. [Google Scholar] [CrossRef] [PubMed]
- Herbst, R.S.; Giaccone, G.; de Marinis, F.; Reinmuth, N.; Vergnenegre, A.; Barrios, C.H.; Morise, M.; Felip, E.; Andric, Z.; Geater, S.; et al. Atezolizumab for First-Line Treatment of PD-L1-Selected Patients with NSCLC. N. Engl. J. Med. 2020, 383, 1328–1339. [Google Scholar] [CrossRef] [PubMed]
- Mok, T.S.K.; Wu, Y.L.; Kudaba, I.; Kowalski, D.M.; Cho, B.C.; Turna, H.Z.; Castro, G., Jr.; Srimuninnimit, V.; Laktionov, K.K.; Bondarenko, I.; et al. Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): A randomised, open-label, controlled, phase 3 trial. Lancet 2019, 393, 1819–1830. [Google Scholar] [CrossRef]
- Grossman, S.A.; Ellsworth, S.; Campian, J.; Wild, A.T.; Herman, J.M.; Laheru, D.; Brock, M.; Balmanoukian, A.; Ye, X. Survival in Patients With Severe Lymphopenia Following Treatment With Radiation and Chemotherapy for Newly Diagnosed Solid Tumors. J. Natl. Compr. Cancer Netw. JNCCN 2015, 13, 1225–1231. [Google Scholar] [CrossRef] [PubMed]
- Campian, J.L.; Ye, X.; Brock, M.; Grossman, S.A. Treatment-related lymphopenia in patients with stage III non-small-cell lung cancer. Cancer Investig. 2013, 31, 183–188. [Google Scholar] [CrossRef]
- Choi, N.; Kim, J.H.; Chie, E.K.; Gim, J.; Kang, H.C. A meta-analysis of the impact of neutrophil-to-lymphocyte ratio on treatment outcomes after radiotherapy for solid tumors. Medicine 2019, 98, e15369. [Google Scholar] [CrossRef] [PubMed]
- Contreras, J.A.; Lin, A.J.; Weiner, A.; Speirs, C.; Samson, P.; Mullen, D.; Campian, J.; Bradley, J.; Roach, M.; Robinson, C. Cardiac dose is associated with immunosuppression and poor survival in locally advanced non-small cell lung cancer. Radiother. Oncol. J. Eur. Soc. Ther. Radiol. Oncol. 2018, 128, 498–504. [Google Scholar] [CrossRef] [PubMed]
- Ellsworth, S.G. Field size effects on the risk and severity of treatment-induced lymphopenia in patients undergoing radiation therapy for solid tumors. Adv. Radiat. Oncol. 2018, 3, 512–519. [Google Scholar] [CrossRef]
- Joseph, N.; McWilliam, A.; Kennedy, J.; Haslett, K.; Mahil, J.; Gavarraju, A.; Mistry, H.; Van Herk, M.; Faivre-Finn, C.; Choudhury, A. Post-treatment lymphocytopaenia, integral body dose and overall survival in lung cancer patients treated with radical radiotherapy. Radiother. Oncol. J. Eur. Soc. Ther. Radiol. Oncol. 2019, 135, 115–119. [Google Scholar] [CrossRef]
- Templeton, A.J.; McNamara, M.G.; Šeruga, B.; Vera-Badillo, F.E.; Aneja, P.; Ocaña, A.; Leibowitz-Amit, R.; Sonpavde, G.; Knox, J.J.; Tran, B.; et al. Prognostic role of neutrophil-to-lymphocyte ratio in solid tumors: A systematic review and meta-analysis. J. Natl. Cancer Inst. 2014, 106, dju124. [Google Scholar] [CrossRef] [PubMed]
- Yovino, S.; Kleinberg, L.; Grossman, S.A.; Narayanan, M.; Ford, E. The etiology of treatment-related lymphopenia in patients with malignant gliomas: Modeling radiation dose to circulating lymphocytes explains clinical observations and suggests methods of modifying the impact of radiation on immune cells. Cancer Investig. 2013, 31, 140–144. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Chen, G.; Ye, L.; Shi, S.; Du, S.; Zeng, Z.; He, J. Treatment-duration is related to changes in peripheral lymphocyte counts during definitive radiotherapy for unresectable stage III NSCLC. Radiat. Oncol. (Lond. Engl.) 2019, 14, 86. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.; Liao, Z.; Gomez, D.; Levy, L.; Zhuang, Y.; Gebremichael, R.A.; Hong, D.S.; Komaki, R.; Welsh, J.W. Lymphopenia association with gross tumor volume and lung V5 and its effects on non-small cell lung cancer patient outcomes. Int. J. Radiat. Oncol. Biol. Phys. 2014, 89, 1084–1091. [Google Scholar] [CrossRef]
- Pike, L.R.G.; Bang, A.; Mahal, B.A.; Taylor, A.; Krishnan, M.; Spektor, A.; Cagney, D.N.; Aizer, A.A.; Alexander, B.M.; Rahma, O.; et al. The Impact of Radiation Therapy on Lymphocyte Count and Survival in Metastatic Cancer Patients Receiving PD-1 Immune Checkpoint Inhibitors. Int. J. Radiat. Oncol. Biol. Phys. 2019, 103, 142–151. [Google Scholar] [CrossRef]
- Kim, Y.H.; Ahn, S.J.; Moon, S.H.; Kim, J.H.; Kim, Y.C.; Oh, I.J.; Park, C.K.; Jeong, J.U.; Yoon, M.S.; Song, J.Y.; et al. Randomized, Multicenter, Phase 3 Study of Accelerated Fraction Radiation Therapy With Concomitant Boost to the Gross Tumor Volume Compared With Conventional Fractionation in Concurrent Chemoradiation in Patients With Unresectable Stage III Non-Small Cell Lung Cancer: The Korean Radiation Oncology Group 09-03 Trial. Int. J. Radiat. Oncol. Biol. Phys. 2023, 115, 873–885. [Google Scholar] [CrossRef]
- Wheatley, M.; Gore, E.; Ad, V.B.; Robinson, C.; Bradley, J.J. Defining a novel cardiac contouring atlas for NSCLC using cadaveric anatomy. Int. J. Radiat. Oncol. Biol. Phys. 2014, 90, S658. [Google Scholar] [CrossRef]
- Antonia, S.J.; Villegas, A.; Daniel, D.; Vicente, D.; Murakami, S.; Hui, R.; Yokoi, T.; Chiappori, A.; Lee, K.H.; de Wit, M.; et al. Durvalumab after Chemoradiotherapy in Stage III Non-Small-Cell Lung Cancer. New Engl. J. Med. 2017, 377, 1919–1929. [Google Scholar] [CrossRef]
- Aupérin, A.; Le Péchoux, C.; Rolland, E.; Curran, W.J.; Furuse, K.; Fournel, P.; Belderbos, J.; Clamon, G.; Ulutin, H.C.; Paulus, R.; et al. Meta-analysis of concomitant versus sequential radiochemotherapy in locally advanced non-small-cell lung cancer. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2010, 28, 2181–2190. [Google Scholar] [CrossRef]
- Yoon, S.M.; Shaikh, T.; Hallman, M. Therapeutic management options for stage III non-small cell lung cancer. World J. Clin. Oncol. 2017, 8, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Bradley, J.D.; Paulus, R.; Komaki, R.; Masters, G.; Blumenschein, G.; Schild, S.; Bogart, J.; Hu, C.; Forster, K.; Magliocco, A.; et al. Standard-dose versus high-dose conformal radiotherapy with concurrent and consolidation carboplatin plus paclitaxel with or without cetuximab for patients with stage IIIA or IIIB non-small-cell lung cancer (RTOG 0617): A randomised, two-by-two factorial phase 3 study. Lancet. Oncol. 2015, 16, 187–199. [Google Scholar] [CrossRef]
- Jin, J.; Hu, C.; Xiao, Y.; Zhang, H.; Ellsworth, S.; Schild, S.; Bogart, J.; Dobelbower, M.; Kavadi, V.; Narayan, S.J. Higher radiation dose to immune system is correlated with poorer survival in patients with stage III non–small cell lung cancer: A secondary study of a phase 3 cooperative group trial (NRG Oncology RTOG 0617). Biol. Phys. 2017, 99, S151–S152. [Google Scholar] [CrossRef]
- Wang, S.J.; Haffty, B. Radiotherapy as a New Player in Immuno-Oncology. Cancers 2018, 10. [Google Scholar] [CrossRef] [PubMed]
- Sellins, K.S.; Cohen, J.J. Gene induction by gamma-irradiation leads to DNA fragmentation in lymphocytes. J. Immunol. 1987, 139, 3199–3206. [Google Scholar] [CrossRef] [PubMed]
- Coffelt, S.B.; Wellenstein, M.D.; de Visser, K.E. Neutrophils in cancer: Neutral no more. Nat. Rev. Cancer 2016, 16, 431–446. [Google Scholar] [CrossRef]
- Gridelli, C.; Ardizzoni, A.; Barberis, M.; Cappuzzo, F.; Casaluce, F.; Danesi, R.; Troncone, G.; De Marinis, F. Predictive biomarkers of immunotherapy for non-small cell lung cancer: Results from an Experts Panel Meeting of the Italian Association of Thoracic Oncology. Transl. Lung Cancer Res. 2017, 6, 373–386. [Google Scholar] [CrossRef]
- Reck, M.; Rodríguez-Abreu, D.; Robinson, A.G.; Hui, R.; Csőszi, T.; Fülöp, A.; Gottfried, M.; Peled, N.; Tafreshi, A.; Cuffe, S.; et al. Pembrolizumab versus Chemotherapy for PD-L1-Positive Non-Small-Cell Lung Cancer. New Engl. J. Med. 2016, 375, 1823–1833. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Li, G.; Wang, Y.; Wang, Y.; Zhao, S.; Haihong, P.; Zhao, H.; Wang, Y. PD-L1 expression in lung cancer and its correlation with driver mutations: A meta-analysis. Sci. Rep. 2017, 7, 10255. [Google Scholar] [CrossRef]
- Tokito, T.; Azuma, K.; Kawahara, A.; Ishii, H.; Yamada, K.; Matsuo, N.; Kinoshita, T.; Mizukami, N.; Ono, H.; Kage, M.; et al. Predictive relevance of PD-L1 expression combined with CD8+ TIL density in stage III non-small cell lung cancer patients receiving concurrent chemoradiotherapy. Eur. J. Cancer 2016, 55, 7–14. [Google Scholar] [CrossRef]
- Fujimoto, D.; Uehara, K.; Sato, Y.; Sakanoue, I.; Ito, M.; Teraoka, S.; Nagata, K.; Nakagawa, A.; Kosaka, Y.; Otsuka, K.; et al. Alteration of PD-L1 expression and its prognostic impact after concurrent chemoradiation therapy in non-small cell lung cancer patients. Sci. Rep. 2017, 7, 11373. [Google Scholar] [CrossRef]
- Gong, X.; Li, X.; Jiang, T.; Xie, H.; Zhu, Z.; Zhou, F.; Zhou, C. Combined Radiotherapy and Anti-PD-L1 Antibody Synergistically Enhances Antitumor Effect in Non-Small Cell Lung Cancer. J. Thorac. Oncol. Off. Publ. Int. Assoc. Study Lung Cancer 2017, 12, 1085–1097. [Google Scholar] [CrossRef] [PubMed]
- Zeng, D.Q.; Yu, Y.F.; Ou, Q.Y.; Li, X.Y.; Zhong, R.Z.; Xie, C.M.; Hu, Q.G. Prognostic and predictive value of tumor-infiltrating lymphocytes for clinical therapeutic research in patients with non-small cell lung cancer. Oncotarget 2016, 7, 13765–13781. [Google Scholar] [CrossRef]
- Teng, F.; Meng, X.; Wang, X.; Yuan, J.; Liu, S.; Mu, D.; Zhu, H.; Kong, L.; Yu, J. Expressions of CD8+TILs, PD-L1 and Foxp3+TILs in stage I NSCLC guiding adjuvant chemotherapy decisions. Oncotarget 2016, 7, 64318–64329. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.; Park, S.; Byun, H.K.; Lee, C.G.; Cho, J.; Hong, M.H.; Kim, H.R.; Cho, B.C.; Kim, S.; Park, J.; et al. Impact of Treatment-Related Lymphopenia on Immunotherapy for Advanced Non-Small Cell Lung Cancer. Int. J. Radiat. Oncol. Biol. Phys. 2019, 105, 1065–1073. [Google Scholar] [CrossRef] [PubMed]
| Characteristic | All Patients (Median) |
|---|---|
| Age, years | 40–75 (66) |
| Sex | |
| Male | 183 (93.4%) |
| Female | 13 (6.6%) |
| ECOG PS | |
| 0 | 25 (12.8%) |
| 1 | 171 (87.2%) |
| Weight loss (%) | 0–19 (0) |
| Smoking history | |
| Never | 16 (8.2%) |
| Ex-smoker | 53 (27.0%) |
| Current smoker (< 1 year quit) | 127 (64.8%) |
| Histology | |
| SqCC | 146 (74.5%) |
| Non-SqCC | 50 (25.5%) |
| Clinical stage | |
| IIIA | 141 (71.9%) |
| IIIB | 55 (28.1%) |
| GTV (cm3) | 20.2–869.0 (154.65) |
| Hematologic parameters (cells/μL) | |
| Pre-treatment WBC | 3400–32800 (8700) |
| Pre-treatment ALC | 860–4640 (2130) |
| Pre-treatment ANC | 1039–9760 (4985) |
| Pre-treatment monocyte | 150–1870 (680) |
| Pre-treatment NLR | 0.22–8.95 (2.33) |
| DLCO (%) | 27–193 (89) |
| RT duration (day) | 27–56 (41) |
| Chemotherapy cycle | 4–6 (6) |
| Variable | No. (%) | OS | PFS | LRFS | DRFS | ||||
|---|---|---|---|---|---|---|---|---|---|
| MS (months) | p-value | MS (months) | p-value | MS (months) | p-value | MS (months) | p-value | ||
|
Age (years) ≤65 > 65 |
91 (46.4) 105 (53.6) |
39 22 |
0.014 |
13 11 |
0.041 | 21 14 |
0.028 | 22 15 |
0.024 |
|
Clinical stage IIIA IIIB |
141 (71.9) 55 (28.1) |
33 27 |
0.592 |
13 10 |
0.473 | 15 14 |
0.625 | 17 17 |
0.527 |
|
Sex Male Female |
183 (93.4) 13 (6.6) |
29 36 |
0.532 |
12 11 |
0.233 | 15 14 |
0.950 | 17 16 |
0.489 |
|
ECOG PS 0 1 |
25 (12.8) 171 (87.2) |
43 28 |
0.124 |
13 12 |
0.336 | 14 15 |
0.364 | 14 17 |
0.341 |
|
Weight loss ≤ 5% > 5% |
140 (71.4) 56 (28.6) |
34 20 |
0.509 |
13 8 |
0.382 | 17 12 |
0.446 | 17 11 |
0.500 |
|
Current smoking No Yes (quit < 1 years) |
69 (35.2) 127 (64.8) |
29 29 |
0.813 | 12 13 |
0.595 |
18 14 |
0.997 | 18 16 |
0.740 |
|
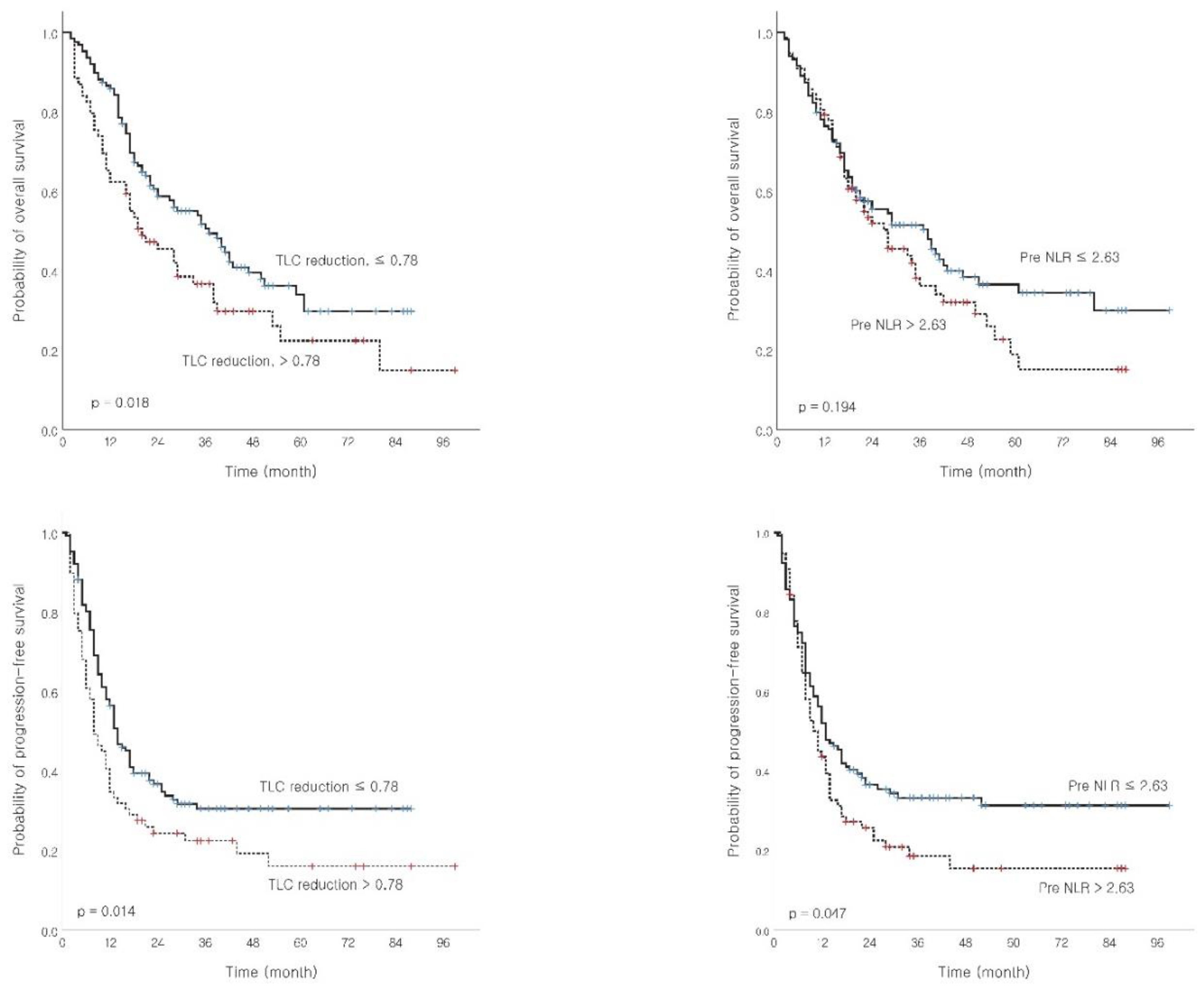
Pre NLR ≤2.63 > 2.63 |
119 (60.7) 77 (39.3) |
38 28 |
0.194 |
13 11 |
0.047 | 16 14 |
0.346 | 18 12 |
0.013 |
|
TLC reduction ≤0.78 > 0.78 |
127 (64.8) 69 (35.2) |
37 20 |
0.018 | 14 8 |
0.014 | 18 11 |
0.008 | 19 11 |
0.018 |
|
Nadir ALC (cell/µL) ≥ 500 < 500 |
113 (57.7) 83 (42.3) |
36 24 |
0.031 |
13 10 |
0.075 | 18 12 |
0.029 | 19 12 |
0.045 |
|
NLR during CCRT ≤ 9.25 > 9.25 |
163 (83.2) 33 (16.8) |
35 17 |
0.039 |
13 8 |
0.020 | 17 8 |
0.012 | 17 9 |
0.033 |
|
GTV (cc) ≤ 165 > 165 |
104 (53.1) 92 (46.9) |
38 19 |
0.032 |
14 10 |
0.022 | 22 11 |
0.010 | 22 12 |
0.088 |
|
RT duration (days) ≤42 > 43 |
128 (65.3) 68 (34.7) |
35 21 |
0.300 |
13 11 |
0.388 |
14 14 |
0.374 |
18 14 |
0.245 |
|
RT technique 3D CRT only IMRT* |
87 (44.4) 109 (55.6) |
28 36 |
0.867 | 11 13 |
0.567 | 14 16 |
0.728 | 16 18 |
0.804 |
| Variable | Prognostic Factor | HR (95% CI) | p-value |
|---|---|---|---|
| OS | Age (years), ≤ 65 vs. > 65 | 1.579 (1.097–2.274) | 0.014 |
| GTV (cc), ≤ 165 vs. > 165 | 1.482 (1.036–2.119) | 0.031 | |
| PFS | TLC reduction, ≤ 0.78 vs. > 0.78 | 1.510 (1.075–2.121) | 0.018 |
| Pre-treatment NLR ≤ 2.63 vs. > 2.63 | 1.561 (1.110–2.197) | 0.010 | |
| LRFS | TLC reduction, ≤ 0.78 vs. > 0.78 | 1.505 (1.060–2.136) | 0.022 |
| GTV (cc), ≤ 165 vs. > 165 | 1.467 (1.041–2.068) | 0.029 | |
| DRFS | Pre-treatment NLR ≤ 2.63 vs. > 2.63 | 1.578 (1.135–2.244) | 0.007 |
| TLC reduction, ≤ 0.78 vs. > 0.78 | 1.578 (1.118–2.228) | 0.010 |
| Adverse Event (n,%) | G0-1 | G2 | G3 | G4 | G5 |
|---|---|---|---|---|---|
| Hematologic toxicity | |||||
| Leukopenia | 152 (77.6) | 27 (13.8) | 15 (7.7) | 2 (1.0) | 0 (0.0) |
| Neutropenia | 147 (75.0) | 34 (17.3) | 11 (5.6) | 4 (2.0) | 0 (0.0) |
| Thrombocytopenia | 194 (99.0) | 2 (1.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Lymphopenia | 43 (21.9) | 70 (35.7) | 79 (40.3) | 4 (2.0) | 0 (0.0) |
| Non-hematologic toxicity | |||||
| Radiation pneumonitis | 171 (87.2) | 15 (7.7) | 4 (2.0) | 0 (0.0) | 6 (3.1) |
| Radiation esophagitis | 142 (72.5) | 47 (24.0) | 4 (2.0) | 2 (1.0) | 1 (0.5) |
| Variable | Univariate | Multivariate | |||
|---|---|---|---|---|---|
| OR | p-Value | OR | p-Value | 95% CI | |
| Age (years) | 1.016 | 0.445 | |||
| Treatment duration (day) | 0.999 | 0.984 | |||
| Pre-NLR | 0.764 | 0.043 | 0.714 | 0.018 | 0.540–0.945 |
| GTV (cm3) | 1.003 | 0.003 | 1.003 | 0.010 | 1.001–1.005 |
| Lung V2 (%) | 1.026 | 0.002 | |||
| Lung V20 (%) | 1.077 | 0.006 | |||
| Lung V50 (%) | 0.999 | 0.968 | |||
| Lung V60 (%) | 0.923 | 0.184 | |||
| Heart V2 (%) | 1.018 | 0.001 | |||
| Heart V20 (%) | 1.026 | 0.001 | 1.025 | 0.002 | 1.009–1.040 |
| Heart V50 (%) | 1.023 | 0.093 | |||
| Heart V60 (%) | 1.027 | 0.374 | |||
| Smoking (others vs. never) | 1.029 | 0.927 | |||
| RT technique (3D vs. IMRT) | 1.393 | 0.276 | |||
| Characteristic | n (%) | PDL1 Expression | p-Value | CD8 Expressiom | p-Value | ||
|---|---|---|---|---|---|---|---|
| No | Yes | Low | High | ||||
| Age (years) ≤ 65 > 65 |
46 (54.8) 38 (45.2) |
26 22 |
20 16 |
0.899 |
34 29 |
12 9 |
0.800 |
| Clinical stage IIIA IIIB |
60 (71.4) 24 (28.6) |
38 10 |
22 14 |
0.070 |
46 17 |
14 7 |
0.577 |
| Sex Male Female |
77 (91.7) 7 (8.3) |
44 4 |
33 3 |
1.000 |
58 5 |
19 2 |
0.820 |
| Smoking No Yes (quit < 1 year) |
28 (33.3) 56 (66.7) |
13 35 |
15 21 |
0.161 |
18 45 |
10 11 |
0.109 |
| Pathology SqCC Non-SqCC |
64 (76.2) 20 (23.8) |
37 11 |
27 9 |
0.824 |
48 15 |
16 5 |
1.000 |
| Pre NLR ≤2.63 >2.63 |
54 (64.3) 30 (35.7) |
32 16 |
22 14 |
0.599 |
40 19 |
10 11 |
0.066 |
| GTV (cc) ≤ 165 > 165 |
49 (58.3) 35 (41.7) |
27 21 |
22 14 |
0.655 |
38 25 |
11 10 |
0.523 |
| CD8 Low High |
63 (75.0) 21 (25.0) |
38 10 |
25 11 |
0.309 |
|||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





