Submitted:
10 October 2023
Posted:
12 October 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Collection and emergence of parasitoids.
2.2. Identification of the parasitoids.
2.3. Infestation and Parasitism Rate.
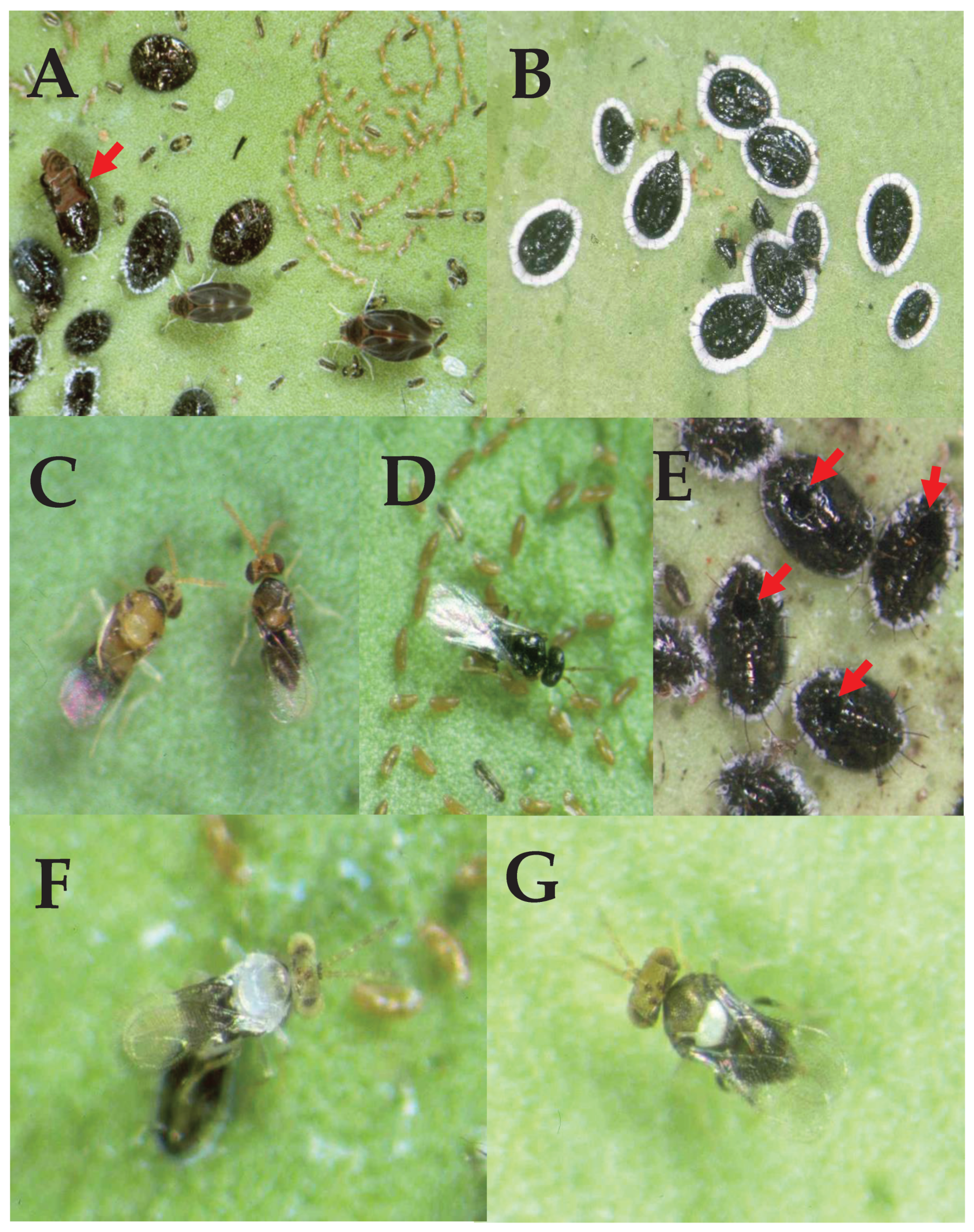
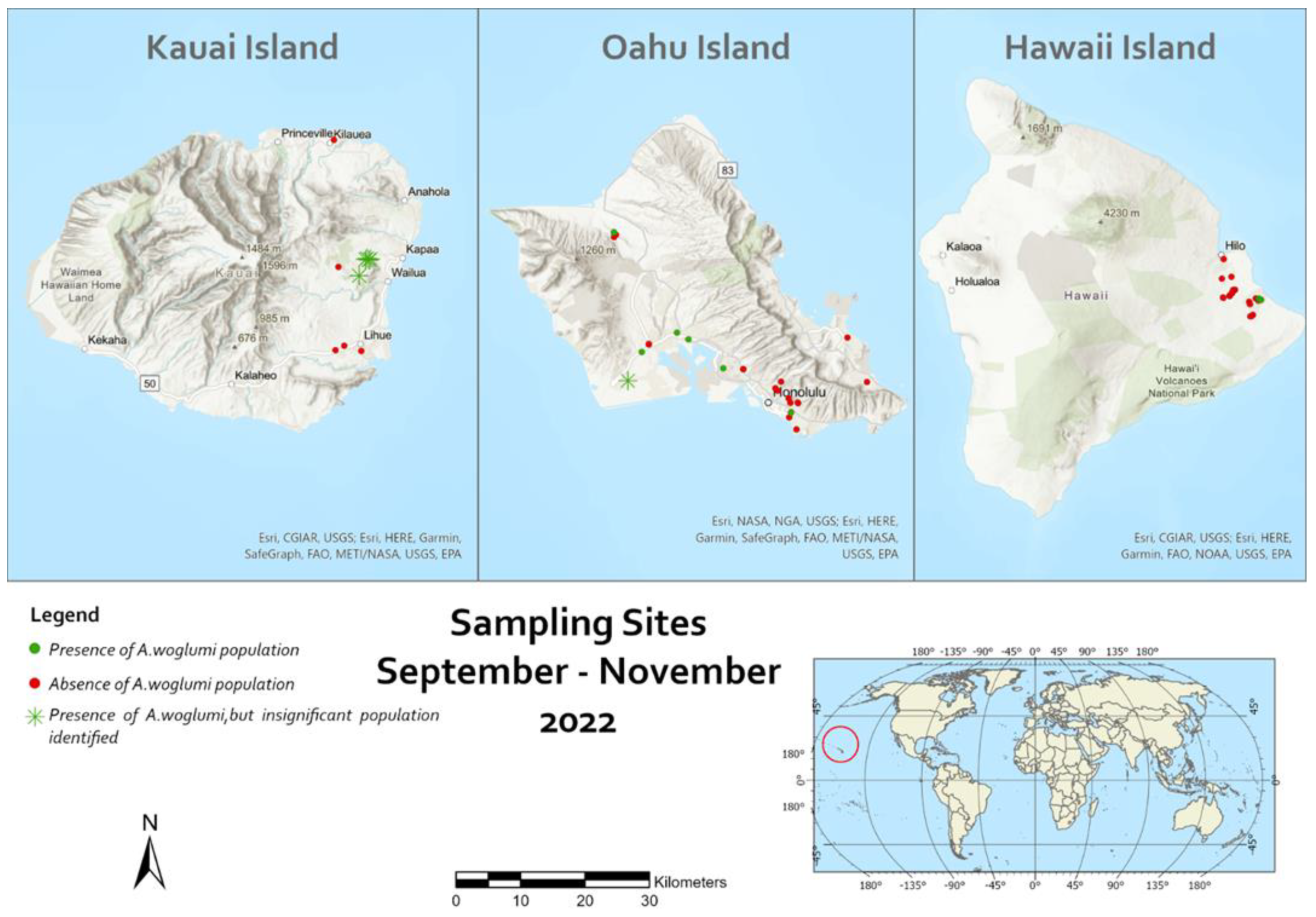
3. Results & Discussion
Acknowledgments
References
- Kapantaidaki, D., E., Antonatos, S., Kontodimas, D., Milonas, P., Papachristos, D., P. Presence of the invasive whitefly Aleurocanthus spiniferus (Hemiptera: Aleyrodidae) in Greece. Bulletin OEPP/EPPO Bulletin. 2019, 49 (1), 127–131.
- Ramadan, M., M., Kaufman, L., V., Wright, M., G. Insect and weed biological control in Hawaii: Recent case studies and trends. Biol. Control. 2023, 179, 105170. [CrossRef]
- EFSA Plant Health Panel (EFSA PLH Panel), Bragard, C., Dehnen-Schmutz, K., Di Serio, F., Gonthier, P., Jacques, M-A., Jaques Miret, J., A., Justesen, A., F., Magnusson, C., S., Milonas, P., Navas-Cortes, J., A., Parnell, S., Potting, R., Reignault, P., L., Thulke, H.,-H., Van der Werf, W., Vicent Civera, A., Yuen, J., Zappalà, L., Navarro, M., N., Kertesz, V., Czwienczek, E., MacLeod, A. 2018. Scientific Opinion on the pest categorisation of Aleurocanthus spp. EFSA Journal. 2018, 16 (10) :5436, 31 pp. [CrossRef]
- Akrivou, A., Georgopoulou, I., Papachristos, D., P., Milonas, P., G., Kriticos, D., J. Potential global distribution of Aleurocanthus woglumi considering climate change and irrigation. PLoS ONE, 2021, 16(12): e0261626. [CrossRef]
- Porcelli, F. First record of Aleurocanthus spiniferus (Homoptera: Aleyrodidae) in Puglia, Southern Italy. EPPO Bulletin, 2008, 38, 516– 518.
- Nugnes, F., Laudonia, S., Jesu, G., Jansen, M., G., M., Bernardo, U., Porcelli, F. Aleurocanthus spiniferus (Hemiptera: Aleyrodidae) in Some European Countries: Diffusion, Hosts, Molecular Characterization, and Natural Enemies. Insects, 2020, 11, 42. [CrossRef]
- Šimala, M., Masten Milek, T. First record of the orange spiny whitefly, Aleurocanthus spiniferus Quaintance, 1903 (Hemiptera: Aleyrodidae), in Croatia. Plant Zbornik predavanj in referatov 11. Slovenskega posvetovanja o varstvu rastlin z mednarodno udeležbo Bled, d. (Plant Protection Society of Slovenia). 2013, 5.–6.
- Radonjic, S., Hrncic, S., Malumphy, C. First Record of Aleurocanthus spiniferus (Quaintance) (Hemiptera: Aleyrodidae) in Montenegro. CRA-Research Centre for Agrobiology and Pedology, 2014, 97, 141– 154.
- EFSA (European Food Safety Authority), Schrader G, Camilleri M, Ciubotaru RM, Diakaki M and Vos S, Pest survey card on Aleurocanthus spiniferus and Aleurocanthus woglumi. EFSA supporting publication, 2019, EN-1565. 17pp. [CrossRef]
- Uesugi, R., Yara, K., Sato, Y. Changes in population density of Aleurocanthus camelliae (Hemiptera: Aleyrodidae) and parasitism rate of Encarsia smithi (Hymenoptera: Aphelinidae) during the early invasion stages. Appl. Entomol. Zool. 2016, 51, 581–588, . [CrossRef]
- Nguyen, R., Brasil, J., R., Poucher, C. Population density of the citrus blackfly, Aleurocanthus woglumi Ashby (Homoptera: Aleyrodidae), and its parasites in urban Florida in 1979-81. Environmental Entomology, 1983, 12: 878-884.
- Dowell, R.V. Factors affecting the field effectiveness of Encarsia opulenta (Hymenoptera: Aphelinidae), a parasitoid of citrus blackfly, Aleurocanthus woglumi (Homoptera: Aleyrodidae). Trop. Agr. 1989, 66: 110–112.
- Lopez, V., F., Kairo, M., T., Pollard, G., V., Pierre, C., Commodore, N., Dominique, D., 2009. Post-release survey to assess impact and potential host range expansion by Amitus hesperidum and Encarsia perplexa, two parasitoids introduced for the biological control of the citrus blackfly, Aleurocanthus woglumi in Dominica. BioControl, 2009, 54: 497–503. [CrossRef]
- Yamashita, K., Kasai, A., Suzuki, Y., Yoshiyasu, Y. Population dynamics of the camellia spiny whitefly, Aleurocanthus camelliae (Hemiptera: Aleyrodidae), in tea fields during the early phase of invasion into Kyoto, Japan. Applied Entomology and Zoology, 2016, 51, 117– 124.
- Anonymous, 1974. New States records orange spiny black fly, Aleurocanthus spiniferus (Quaintance). Hawaii Co-operative Economic Insect Report 24, 585 (R.A.E. Services A, 1974, (1975) 63 (781).
- Culliney, T., W., Nagamine, W., T., Teramoto, K., K. Introductions for Biological Control in Hawaii 1997-2001. Proc. Hawaii. Entomol. Soc. 2003, 36, 145-153. https://scholarspace.manoa.hawaii.edu/bitstream/10125/112/1/36_145-153.pdf.
- Nakao, H., K., Funasaki, G., Y. Introductions for biological control in Hawaii: 1975 and 1976. Proc. Hawaii. Entomol. Soc. 1979, 23:125–128.
- Heu, R., A., Nagamine, W., T. Citrus Blackfly Aleurocanthus woglumi Ashby (Homoptera: Aleyrodidae). New Pest Advisory, 2001, No. 99-03.
- Rossato, V. Ocorrência de parasitóides de Aleurocanthus woglumi Ashby, 1903 (Hemiptera: Aleyrodidae) e seu parasitismo por Cales noacki Howard, 1907 (Hymenoptera: Aphelinidae) nos municípios de Belém, Capitão Poço e Irituia no estado do Pará. Dissertação de Mestrado, Universidade Federal Rural da Amazônia, Belém/PA. 2007, 40 pp.
- MacGown, M., W., Nebeker, T., E. Taxonomic review of Amitus (Hymenoptera: Proctotrupoidae, Platygastridae). Can. Ent. 1978, 110: 275-283.
- Viggiani, G., Mazzone, P. The Amitus Hald. (Hym. Platygastridae) of Italy, with description of three new species. Bollettino de Laboratorio di entomologia agrarian “Filippo Silvestri” Portici. 1978, (39):59-69.
- Huang, J., Polaszek, A. A revision of the Chinese species of Encarsia Forster (Hymenoptera: Aphelinidae): parasitoids of whiteflies, scale insects and aphids (Hemiptera: Aleyrodidae, Diaspididae, Aphidoidea). Journal of Natural History, 1998, 32: 1825-1966.
- Nguyen, R. Amitus hesperidum (Hymenoptera: Platygasteridae), a parasite of the citrus blackfly (Aleurocanthus woglumi). Florida Department of Agricultural Consumer Services, Division Plant Industry, Entomolgy Circular.1988, 311:1–2. https://entnemdept.ufl.edu/creatures/beneficial/amitus_hesperidum.htm.
- Noyes, J.S. 2019. Universal Chalcidoidea Database. World Wide Web electronic publication. http://www.nhm.ac.uk/chalcidoids.
- Noyes, J., S. Collecting and preserving chalcid wasps (Hymenoptera: Chalcidoidea). Journal of Natural History, 1982, 16 (3): 315-334.
- Noyes, J., S. Chapter 2.7.2.5. Chalcid parasitoids. (In: Rosen, D. [editor] - The Armored Scale Insects. Their Biology, Naural Enemies and Control.) World Crop Pests, 1990, 4B:247-262.
- Hebert, P.D., Cywinska, A., Ball, S.L., deWaard, J.R. Biological identifications through DNA barcodes. Proc. Biol. Sci. 2003, 270, 313–321. [CrossRef]
- Pentinsaari, M., Salmela, H., Mutanen, M., Roslin, T. Molecular evolution of a widely-adopted taxonomic marker (COI) across the animal tree of life. Sci. Rep. 2016, 6, 35275. [CrossRef]
- Folmer, O.; Black, M.; Hoeh, W.; Lutz, R.; Vrijenhoek, R. DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Mol Mar Biol Biotechnol, 3(5), 294-299. PMID: 1994, 7881515.
- Dowton, M., Austin. A.D., Simultaneous analysis of 16S, 28S, COI and morphology in the Hymenoptera: Apocrita–evolutionary transitions among parasitic wasps. Biol. J. Linn. Soc. 2001, 74, 87- 111. [CrossRef]
| Target whitefly (Hemiptera: Aleyrodidiae) |
Introduced and adventive natural enemies | ||||||
|---|---|---|---|---|---|---|---|
| Family | species | Origin of introduction |
Year of introduction and release period | Colonization records (total numbers released per island) |
Establishment records |
Release records |
|
|
Aleurocanthus spiniferus (Quaintance), citrus orange spiny whitefly |
Platygastridae |
Amitus hesperidum Silvestri |
Mexico | 1974 | Recorded on Oahu | Only Oahu Island | |
| Platygastridae |
Amitus spiniferus (Brethes) |
California | 1979-1982 | Oahu (200) | Introduced for Aleurothrixus floccosus (Maskell). Established on Oahu Island. |
Only on Oahu Island in 1982. |
|
| Aphelinidae |
Cales noacki Howard |
California | Feb. 1981 - Aug. 1982 | Lanai (275) Molokai (300) Oahu (12149) |
Introduced for Aleurothrixus floccosus (Maskell) Hawaii 1992 Kauai 1997 Oahu 1982 |
Hawaii 1992 Lanai 1982 Molokai 1982 Oahu 1982 |
|
| Aphelinidae |
Encarsia clypealis (Silvestri) |
Texas | 1975 | No recovery | Only on Oahu Island. |
||
| Aphelinidae |
Encarsia perplexa Huang & Polaszek |
Texas | 1975 | Oahu (165) | No recovery | Only on Oahu, released as Encarsia opulenta (Silvestri). |
|
| Aphelinidae |
Encarsia smithi (Silvestri) |
Japan (Nagasaki) and Guam |
September 1974 | Oahu (5330) | Established on Oahu Sep. 1975, reported 1981, 1984, 1999 on Oahu. |
Only on Oahu Island, some of the colony from Guam 1984 record (25 adults). |
|
|
Aleurocanthus woglumi Ashby, citrus blackfly |
Platygastridae |
Amitus hesperidum Silvestri |
Guatemala | Introduced 1998, released May 1999 -August 2000 |
Hawaii (20), Kauai (112), Maui (82), Oahu (8595) |
Established | Hawaii, Kauai, Maui, Molokai, Oahu Islands |
| Aphelinidae |
Cales noacki Howard |
California | Feb. 1981 - Aug. 1982 | Molokai (300) Oahu (12149) |
Introduced for Aleurothrixus floccosus (Maskell) |
Only on Oahu Island in 1982. Recorded established on Oahu Island. |
|
| Aphelinidae |
Encarsia perplexa Huang & Polaszek |
Guatemala | Introduced 1998, released April 1999 – June 2002 |
Hawaii (49190) Kauai (17090) Maui (47025) Molokai (27165) Oahu (5740) |
Established | Hawaii, Kauai, Maui, Molokai, Oahu Islands. First identified as Encarsia opulenta (Silvestri) |
|
| Aphelinidae | Encarsia nipponica Silvestri | Adventive, native to Japan and China |
- | - | Hawaii 2000, Kauai 2001 Oahu 1997, 1999 |
Fortuitous species | |
| Aphelinidae | Encarsia smithi (Silvestri) | Japan (Nagasaki) and Guam |
September 1974 | Reported on Oahu 1989, 1999 on citrus | Established on Oahu Sep. 1975 Reported in 1999 on Oahu |
Only on Oahu Island. |
|
| Survey area and habitata |
Citrus Plant | Coordinate and elevation |
Date | Total Number of leaves collected | Total Number of nymphs |
Level of infestation |
% parasitism |
|---|---|---|---|---|---|---|---|
| University of Hawaii at Manoa |
Sour orange | 21º 18’ 17.98” N 157º 48’ 51.87” W, 40 m |
26/9/2022 | 0 | 0 | 0 | - |
| University of Hawaii at Manoa |
Sweet lime | 21º 18’ 15.16” N 157º 48’ 48.99” W, 38 m |
“ | 0 | 0 | 0 | - |
| Ala Wai Community Garden |
lime | 21º 17’ 02.50” N 157º 49’ 37.15” W, 1.5 m |
“ | 0 | 0 | 0 | - |
| Waimanalo Research Station |
Citron, | 21º 20’ 07.91” N 157º 42’ 55.01” W, 23 m |
“ | 0 | 0 | 0 | - |
| Kapaakea Lane, Honolulu | tangerine | 21º 17’ 27.82” N 157º 49’ 25.95” W, 4 m |
“ | 0 | 0 | 0 | - |
| Kapaakea Lane, Honolulu | pummelo | 21º 17’ 25.55” N 157º 49’ 26.61” W, 3.6 m |
“ | 9 | 118 | 1.44 ± 0.49 | 17.8% |
| Pearl City Urban Botanical Garden | Sweet orange | 21º 23’ 38.76” N 157º 58’ 38.08” W, 8.5 m |
27/9/2022 | 71 | 1471 | 1.76 ± 0.78 | 56.6% |
| Diamond Head Community Garden |
tangelo | 21º 16’02.09” N 157º 58’ 59.55” W, 0.9 m |
“ | 0 | 0 | 0 | - |
| Moanalua Gardens | Grapefruit | 21º 20’ 52.68” N 157º 53’ 28.74” W, 6.4 m |
“ | 0 | 0 | 0 | - |
| North Shore. Poamoho Research Station |
Sweet orange | 21º 32’ 38.00” N 158º 05’ 16.28” W, 189 m |
28/9/2022 | 345 | 11981 | 2.17 ± 0.95 | 19.7% |
| Salt Lake Blvd | Sweet lime | 21º 21’ 09.13” N 157º 55’ 30.36” W, 29 m |
“ | 75 | 158 | 1.37 ± 0.48 | 53.8% |
| North Shore, Hawaii Queen bees, Hinshaw Farms |
Sour orange | 21º 32’ 11.10” N 158º 05’ 16.82” W, 226 m |
“ | 0 | 0 | 0 | - |
| North Shore, Hawaii Queen bees, Hinshaw Farms |
lemon | 21º 32’ 17.54” N 158º 05’ 14.90” W, 225 m |
“ | 0 | 0 | 0 | - |
| Hawaii Agriculture Research Center, Kunia | tangelo | 21º 23’ 09.02” N 158º 02’ 14.67” W, 82.6 m |
2/10/2022 | 0 | 0 | 0 | - |
| Hawaii Agriculture Research Center 2 |
lemon | 21º 23’ 07.28” N 158º 02’ 17.16” W, 84.4 m |
“ | 0 | 0 | 0 | - |
| Manoa valley community gardens |
citron | 21º 18’ 53.77” N 157º 48’ 25.49” W, 57 m |
“ | 0 | 0 | 0 | - |
| Nuuanu Mauna ala Royal mausoleum | pummelo | 21º 19’ 30.33” N 157º 50’ 49.81” W, 64 m |
26/10/2022 | 0 | 0 | 0 | - |
| Nuuanu old Pali drive |
lemon | 21º 21’ 14.24” N 157º 48’ 37.68” W, 308 m |
“ | 0 | 0 | 0 | - |
| Tantalus lookout | tangerine | 21º 19’ 06.92” N 157º 49’ 48.23” W, 277 m |
“ | 0 | 0 | 0 | - |
| Kailua | lemon | 21º 23’ 47.09” N 157º 44’ 44.49” W, 0.6 m |
“ | 0 | 0 | 0 | - |
| Mahiole street, Moanaloa | Sour orange | 21º 20’ 49.79” N 157º 53’ 21.81” W, 11 m |
31/10/2022 | 0 | 0 | 0 | - |
| North Shore Poamoho Research Station |
sweet orange, sweet lime | 21º 32’ 38.00” N 158º 05’ 16.28” W, 189 m |
“ | 573 | 23168 | 2.34 ± 0.81 | 24.9% |
| Waikele | tangerine | 21º 24’ 03.18” N 158º 00’ 13.75” W, 60.6 m |
1/11/2022 | 13 | 389 | 2.00 ± 1.03 | 34.4% |
| Aloun farm | pummelo | 21º 22’ 28.47” N 158º 02’ 42.94” W, 56.7 m |
“ | 14 | 212 | 1.78 ± 0.67 | 11.5% |
| Royal Mausoleum of Hawaii |
lime | 21º 19’ 30.33” N 157º 50’ 49.81” W, 64 m |
“ | 0 | 0 | 0 | - |
| Survey area and habitata |
Citrus Plant | GPS and elevation |
Date | Total Number of leaves collected |
Total Number of nymphs |
Level of infestation |
% parasitism |
|---|---|---|---|---|---|---|---|
| Waiakea | Sweet orange | 19º 38’ 30.98” N 155º 04’ 51.67” W, 194 m |
27/10/2022 | 0 | 0 | 0 | - |
| Waiakea Research station | lime | 19º 38’ 41.46” N 155º 04’ 37.82” W, 173 m |
“ | 0 | 0 | 0 | - |
| Hawaii Department of Agriculture, Hilo | lemon | 19º 42’ 23.03” N 155º 04’ 26.25” W, 11 m |
“ | 0 | 0 | 0 | - |
| Kurtistown 1 | Pummelo | 19º 35’ 35.98” N 155º 03’ 30.41” W, 206 m |
“ | 0 | 0 | 0 | - |
| Kurtistown 2 | Pummelo | 19º 35’ 30.36” N 155º 03’ 29.91” W, 199 m |
“ | 0 | 0 | 0 | - |
| Kurtistown 3 | Sweet orange | 19º 35’ 00.29” N 155º 03’ 36.53” W, 242 m |
“ | 0 | 0 | 0 | - |
| Kurtistown 4 | Sour orange | 19º 34’ 38.83” N 155º 03’ 56.19” W, 271 m |
“ | 0 | 0 | 0 | - |
| Hawaiian Paradise Park 1 | Grapefruit | 19º 36’ 12.88” N 154º 56’ 51.46” W, 13 m |
28/10/2022 | 173 | 17848 | 3.14 ± 0.76 | 2.7% |
| Hawaiian Paradise Park 2 | Pummelo | 19º 36’ 12.88” N 154º 56’ 51.46” W, 13 m |
“ | 7 | 423 | 3.00 ± 0.50 | 0.0% |
| Hawaiian Paradise Park 3 | Pummelo | 19º 34’ 17.70” N 154º 57’ 21.50” W, 50 m |
“ | 6 | 212 | 2.50 ± 0.80 | 28.3% |
| Hawaiian Paradise Park 4 | Sweet orange | 19º 34’ 35.47” N 154º 57’ 18.39” W, 9 m |
“ | 0 | 0 | 0 | - |
| Hawaiian Paradise Park 5 | citron | 19º 34’ 52.95” N 154º 57’ 39.69” W, 39 m |
29/10/2022 | 0 | 0 | 0 | - |
| Hawaiian Paradise Park 6 | lime | 19º 34’ 14.46” N 154º 57’ 45.16” W, 40 m |
30/10/2022 | 0 | 0 | 0 | - |
| Hawaiian Paradise Park 7 | lemon | 19º 35’ 39.96” N 154º 57’ 21.77” W, 28 m |
31/10/2022 | 0 | 0 | 0 | - |
| Keeau 1 | Sour orange | 19º 37’ 06.79” N 155º 02’ 37.40” W, 121 m |
29/10/2022 | 0 | 0 | 0 | - |
| Keeau 2 | lemon | 19º 36’ 52.38” N 155º 02’ 48.90” W, 149 m |
“ | 0 | 0 | 0 | - |
| Keeau 3 | tangerine | 19º 36’ 23.02” N 155º 02’ 59.69” W, 177 m |
“ | 0 | 0 | 0 | - |
| Keeau 4 | Sweet orange | 19º 37’ 11.96” N 155º 02’ 32.56” W, 117 m |
“ | 0 | 0 | 0 | - |
| Keeau 5 | Pummelo | 19º 37’ 42.04” N 155º 02’ 19.12” W, 123 m |
“ | 0 | 0 | 0 | - |
| Keeau 6 | tangelo | 19º 38’ 42.04” N 155º 02’ 32.56” W, 79 m |
“ | 0 | 0 | 0 | - |
| Ainaloa 1 | tangerine | 19º 31’ 40.76” N 154º 59’ 35.89” W, 217 m |
“ | 0 | 0 | 0 | - |
| Ainaloa 2 | lime | 19º 31’ 20.12” N 155º 00’ 05.43” W, 226 m |
“ | 0 | 0 | 0 | - |
| Ainaloa 3 | lemon | 19º 31’ 47.83” N 154º 59’ 49.05” W, 201 m |
“ | 0 | 0 | 0 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





