Submitted:
09 October 2023
Posted:
12 October 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Summary of Experimental Sites
2.2. Test Materials and Design
2.3. Index Determination Method
2.3.1. Determination of Plant Growth Index
2.3.2. Leaf Collection and Nutrient Determination
2.3.3. Determination of Fruit Quality Index
2.4 Data Processing and Analysis
3. Results
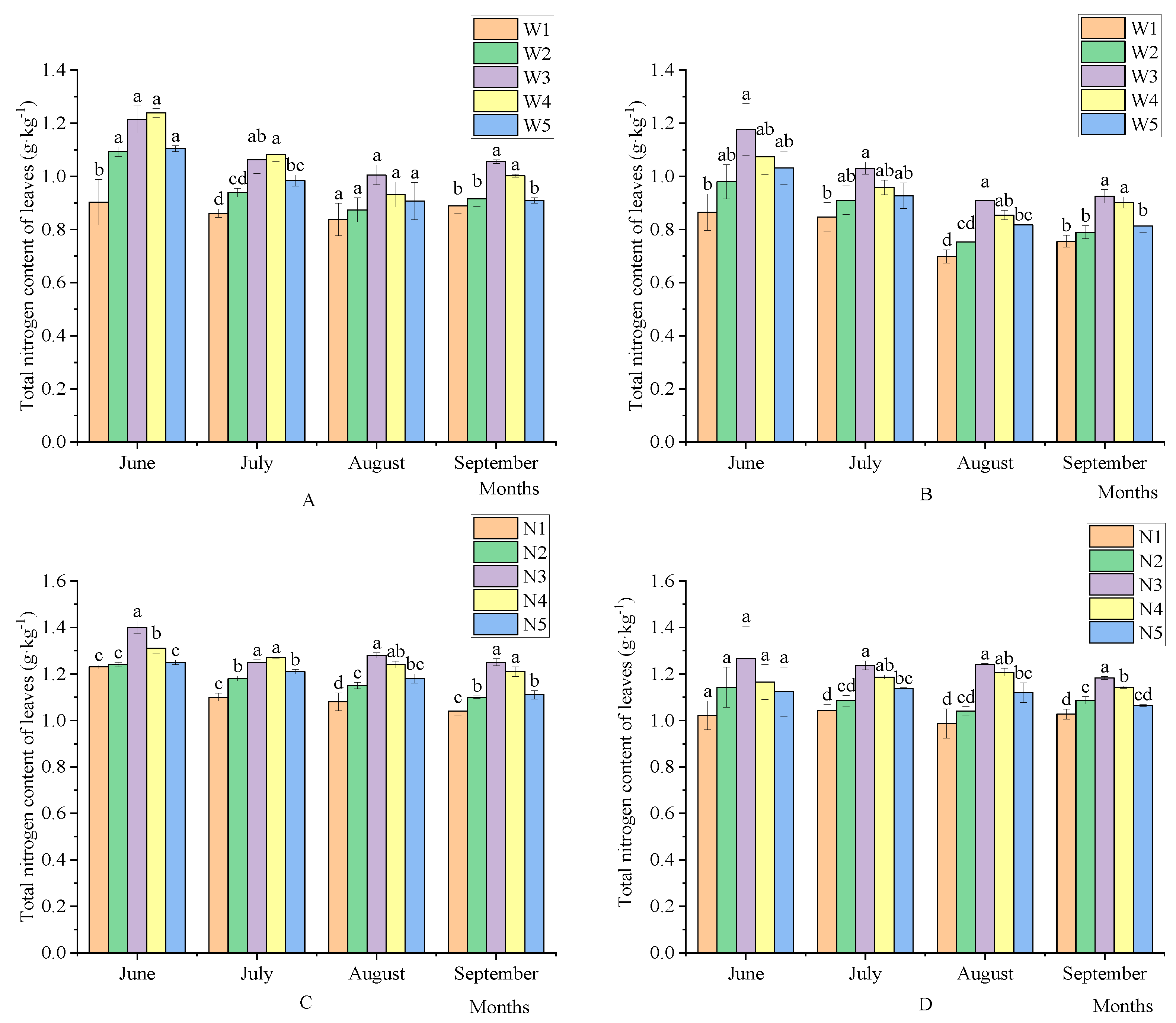
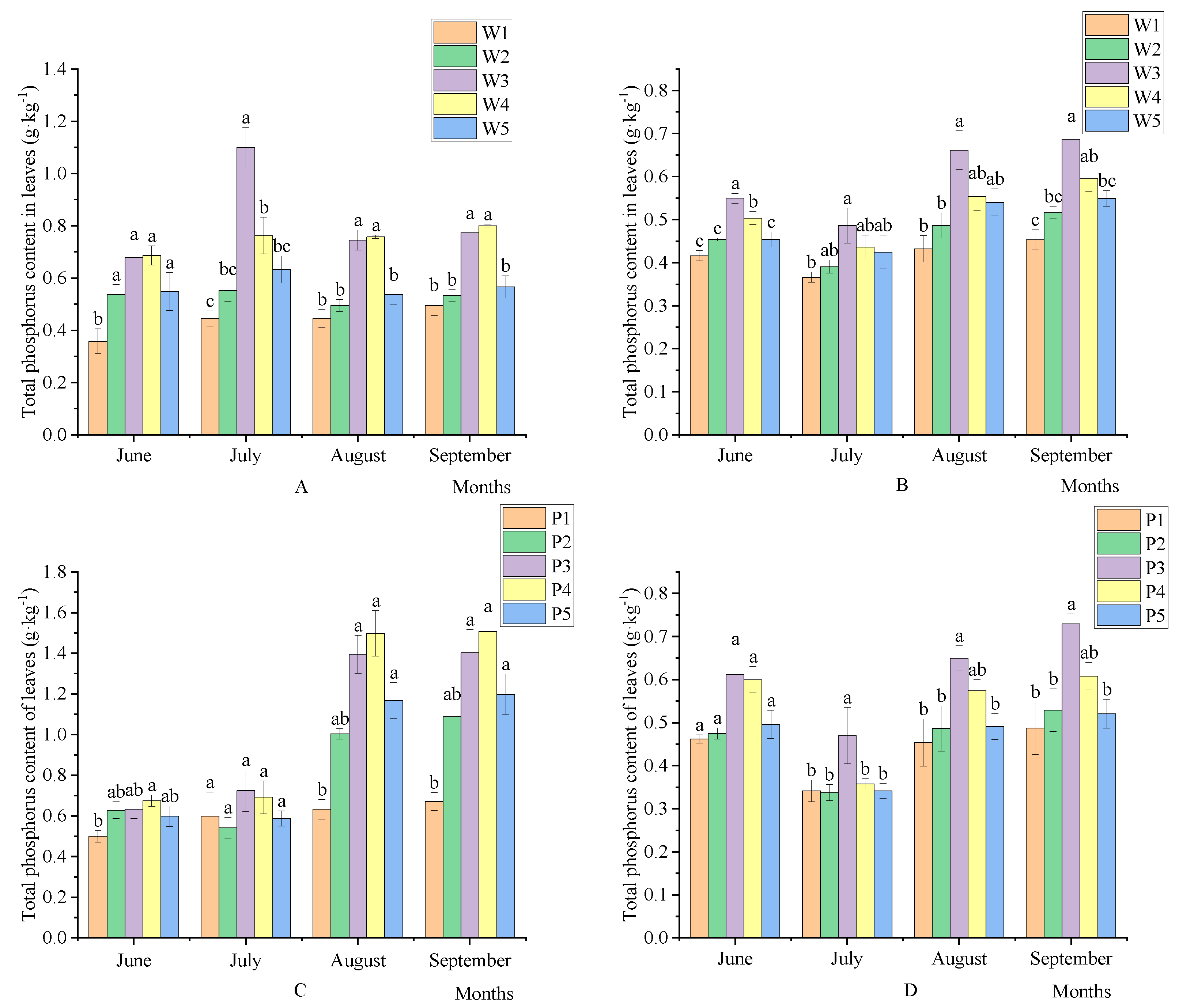
3.1. Effects of the Amount of Water and Fertilizer on Leaf Nutrient Change Change Dynamics in Korla Fragrant Pear
3.1.1. Effects of Water and Fertilizer Dosage on Total Nitrogen in Korla Fragrant Pear Leaves
3.1.2. Influence of Water and Fertilizer Dosage on Total Phosphorus of Korla Fragrant Pear Leaves
3.2. Influence of Water and Fertilizer Dosage on Fruit Quality of Korla Fragrant Pear
3.2.1. Influence of Irrigation Amount on Fruit Quality of Korla Fragrant Pear
3.2.2. Effects of Nitrogen Application Amount on Fruit Quality of Korla Fragrant Pear
3.2.3. Effects of Phosphorus Application Amount on Fruit Quality of Korla Fragrant Pear
3.3. Influence of Water and Fertilizer Coupling on Korla Fragrant Pear Growth
3.3.1. Effects of Water and Fertilizer Coupling on Korla Fragrant Pear Root Density and Root Surface Area
3.3.2. Influence of Water and Fertilizer Coupling on the Change in Leaf Area Index of Korla Fragrant Pear
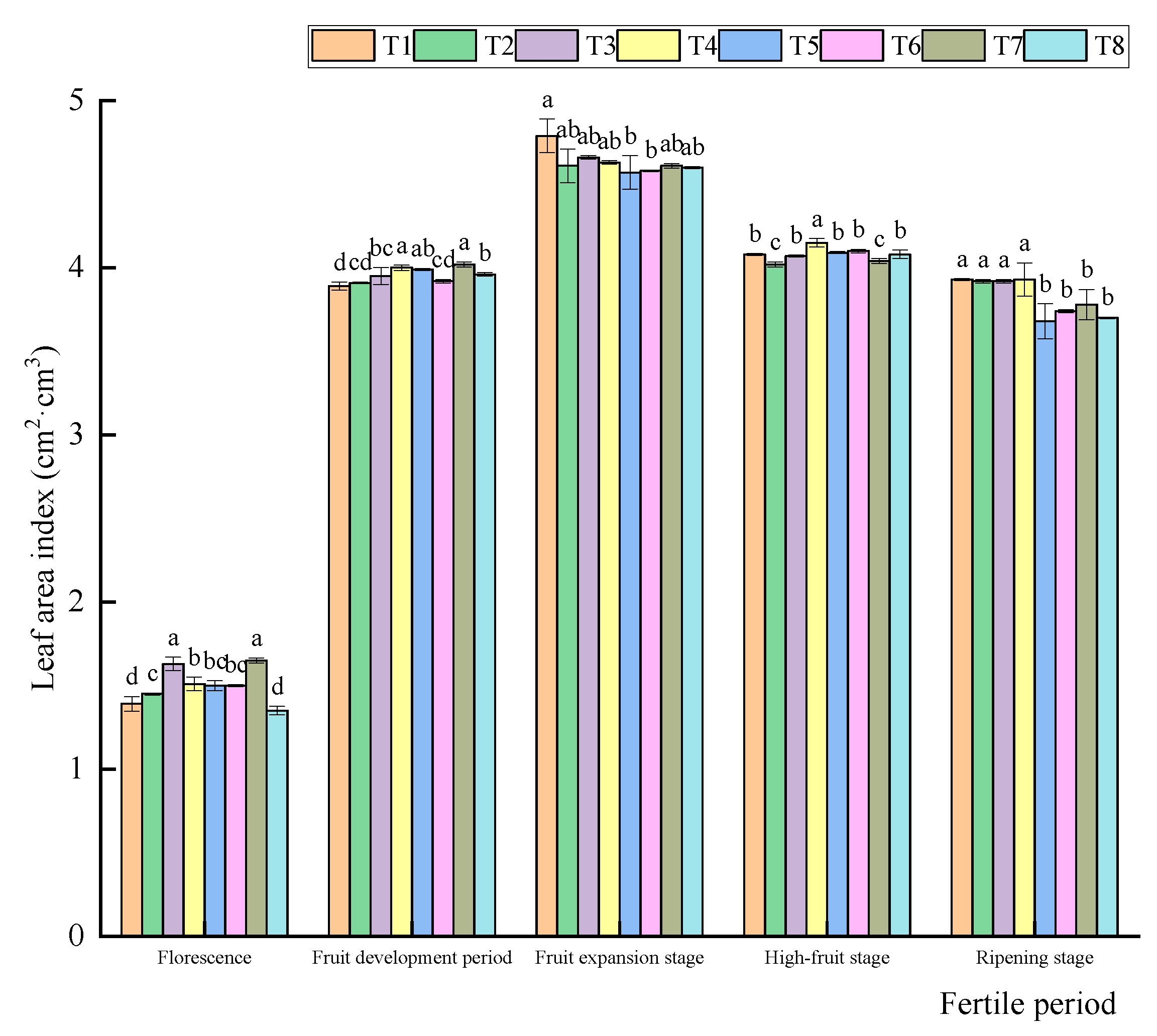
3.3.3. Influence of Water and Fertilizer Coupling on Yield of Korla Fragrant Pear
3.3.4. Influence of Water and Fertilizer Coupling on Fruit Shape Index of Korla Fragrant Pear
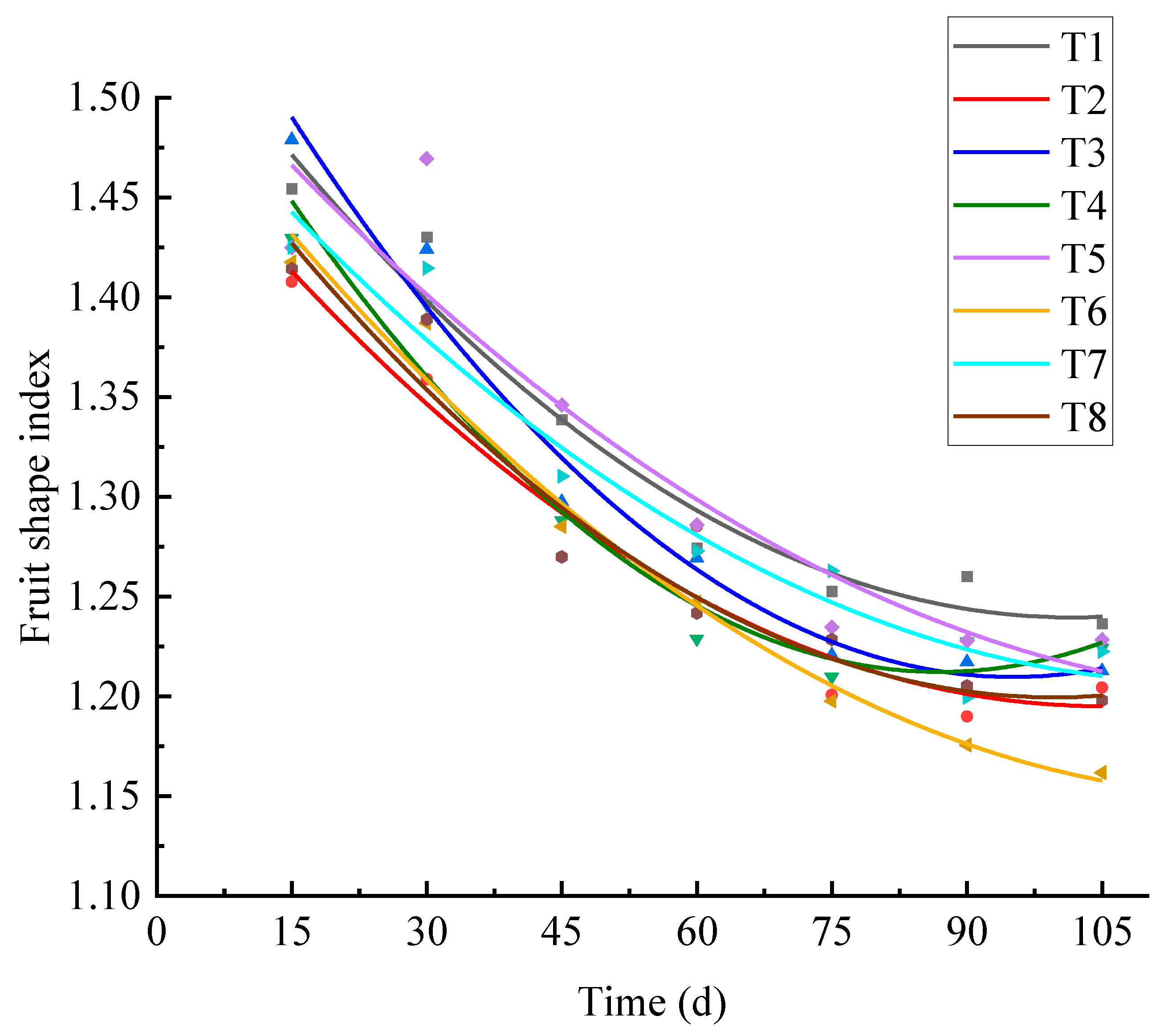
3.4. Effects of Water and Fertilizer Coupling on Fruit Quality of Korla Fragrant Pear
4. Discussions
4.1. Effects of Water, Nitrogen, and Phosphorus on Korla Fragrant Pear Growth and Fruit Quality
4.2. Effects of Water and Fertilizer Coupling on Growth, Fruit Yield, and Quality of Korla Fragrant Pear
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Flora of China. Beijing, Institute of Botany, Chinese Academy of Sciences, 2007-01-01.
- Postman, J. Pear Germplasm Needs and Conservation. 2019, 35–50.
- Zhang, S. (Ed.), Pear Science [M]. China Agriculture Press, 2013.
- Wang, W.H. The development status of pear industry in China under the new situation and some reflections [J]. China Fruit Science, 2019, (04): 4–10.
- Landi, M.; Papadakis, I.E. Editorial: Mineral Nutrition of Fruit Trees[J]. Agronomy, 2021, 11(7), 1315–1315.
- Bould, C.; Jarrett, R.M.; . The Effect of Cover Crops and NPK Fertilizers on Growth, Crop Yield and Leaf Nutrient Status of Young Dessert Apple Trees[J]. J. Hortic. Sci., 2015, 37(1), 58–82.
- Singh, A.; Hidangmayum, A.; Tiwari, P. et al. Chapter 5 - How the soil nitrogen nutrient promotes plant growth—a critical assessment. 2022, 99–118.
- Santos, F.H.D.S.; Almeida, E.F.A.; Frazão, J.E.M.; et al. Nitrogen nutrition of bromeliads[J]. Ornam. Hortic., 2012, 18(1), 39–46.
- Jeet, A.; Sharma, N.C.; Chandel, J.S. et al. Effect of Nitrogen Sources on Yield, Fruit Quality and Nutritional Status of Apple (Malus × Domestica Borkh.)[J]. Int. J. Bio-resour. Stress Manag., 2016, 7(6), 1297–1301.
- Dai, Z.; Fei, L.; Huang, D. et al. Coupling effects of irrigation and nitrogen levels on yield, water and nitrogen use efficiency of surge-root irrigated jujube in a semiarid region[J]. Agric. Water Manag., 2019, 213, 146–154.
- Dawar K.; Khalil, Z.; Mian, I.A., et al. Effects of Farmyard Manure and Different Phosphorus Inorganic Fertilizer Application Rates on Wheat Cultivation in Phosphorus-Deficient Soil[J]. Sustainability, 2022, 14(15), 9030–9030.
- Yang C.; Zhang J.; Zhang G. et al. Potassium deficiency limits water deficit tolerance of rice by reducing leaf water potential and stomatal area[J]. Agric. Water Manag., 2022, 271.
- Porro, D.; Pantezzi, T.; Pedo, S. et al. Interaction of fertigation and water management on apple tree productivity, orchard nutrient status, and fruit quality.[J]. Acta Hortic., 2013, (984), 203–210.
- Ashraf, N. Micro-Irrigation and Fertigation in Fruit Trees[J].Environment and Ecology, 2012, 30(4), 1252–1257.
- Gray, D.M.; Swanson, J.; Dighton, J. The influence of contrasting ground cover vegetation on soil properties in the N J pine barrens [J]. Soil Ecol., 2012, 60, 41–18.
- Chai, Z.; Jiang, P.; Wang, X.; Chen W.; Sun, X. Study on fertilization status of characteristic economic forest in Aksu area [J]. Tianjin Agricultural Sciences, 2008, 14(06), 49–52.
- Liu, H.; Zhang, J.; Baiyun, G.; Zhang, S.; Ding, P. Water consumption characteristics of Korla fragrant pear under drip irrigation [J]. Xinjiang Agricultural Sciences, 2014, 51(12), 2206–2211.
- Liu, X.; Sun G.; Peng, Y.; Yang, Q.; He, H.; Effects of water and fertilizer coupling on photosynthetic characteristics, yield and utilization of mango [J]. Trans. Chin. Soc. Agric. Eng., 2019, 35(16), 125–133. (in Chinese).
- Chen, L.; Wang, H.; Chen, Y.; Zheng, Q.; Wang, Z.; Wang, W.; Bao, J.. Effects of pre-flowering and flowering irrigation on reactive oxygen species metabolism and programmed cell death in calyx tube of Korla Xiangpear [J]. Int. J. Fruit Sci., 2023, 1–20.
- Zhang, Z.; Zhang F.; Zheng C.; Ni F. Effects of soil water and nitrogen nutrition on growth and water conduction of fruit tree seedlings [J]. Trans. Chin. Soc. Agric. Eng., 2009, 25(06), 46–51.
- Ghoname, A.A.; Mona, G.D.; Riad, G.S. et al. Effect of nitrogen forms and biostimulants foliar application on the growth, yield and chemical composition of hot pepper grown under sandy soil conditions[J]. Res. J. Agric. Biol. Sci., 2009, 5(5), 840–852.
- Chen, Q.; Ding, N.; Peng L. et al. Nitrogen fertilizer application techniques for dwarfing apples [J]. Chin. J. Appl. Ecol., 2018, 29(05), 1429–1436. (in Chinese).
- Raese, J.T.; Drake, S.R.; Curry, E.A. Nitrogen fertilizer influences fruit quality, soil nutrients and cover crops, leaf color and nitrogen content, biennial bearing and cold hardiness of ‘Golden Delicious’ [J]. J. Plant Nutr., 2007, 30(10), 1585–1604.
- Wu, P.F.; Ma, X.Q.; Tigabu, M. et al. Root morphological plasticity and biomass production of two Chinese fir clones with high phosphorus efficiency under low phosphorus stress [J]. Can. J. For. Res., 2011, 41(2), 228−234.
- Wu, Y.; Wang, W.; Huang, X.; et al. Effects of deficit irrigation on yield and root growth of mature Korla pear [J]. Trans. Chin. Soc. Agric. Mach., 2012, 43(09), 78–84.
- Wang, J.; Zhang, X.; Gao, B. Effects of water and fertilizer coupling on growth and flowering of dwarfed Fuji Apple saplings [J]. Agric. Res. Arid Areas, 2004, (03), 47–50.
- Hou, Y.; Xue, J.; Zhang, H.; et al. Effects of irrigation and fertilization mode on growth, fruit formation and yield of Longan [J]. Irrig. Drain., 2016, 35(03), 100–104. (in Chinese).
- Sun, X.; Chai, Z.; Jiang, P.; Fang, L. Effects of water and nitrogen coupling on photosynthetic characteristics and fruit quality of apple [J]. Res. Soil Water Conserv., 2010, 17(06), 271–274.
| Soil depth | ||||
|---|---|---|---|---|
| Index | 0–20 cm | 20–40 cm | 40–60 cm | 60–80 cm |
| Soil bulk density (g·cm−3) | 1.60 | 1.56 | 1.43 | 1.38 |
| pH | 8.37 | 8.45 | 8.48 | 8.51 |
| Electric conductivity (μs·cm−1) | 119 | 117 | 92.05 | 90.5 |
| Organic matter (g·kg−1) | 8.16 | 6.44 | 4.36 | 1.43 |
| Salinity (g·kg−1) | 0.61 | 0.68 | 0.71 | 0.74 |
| Alkali-hydrolyzed nitrogen (mg·kg−1) | 12.75 | 8.05 | 3.85 | 1.4 |
| Rapidly available phosphorus (mg·kg−1) | 27.29 | 26.86 | 10.50 | 4.57 |
| Rapidly available potassium (mg·kg−1) | 93 | 81.5 | 70 | 37 |
| ID | Experimental factor | Factor level | Processing code |
|---|---|---|---|
| 1 | Irrigation quantity during growth period (m3·hm−2) | 5460 | W1 |
| 2 | 5880 | W2 | |
| 3 | 6300 | W3 | |
| 4 | 6720 | W4 | |
| 5 | 7140 | W5 | |
| 6 | Nitrogen fertilizer application rate (kg N·hm−2) | 150 | N1 |
| 7 | 225 | N2 | |
| 8 | 300 | N3 | |
| 9 | 375 | N4 | |
| 10 | 450 | N5 | |
| 11 | Amount of phosphate fertilizer applied (kg P2O5·hm−2) | 75 | P1 |
| 12 | 150 | P2 | |
| 13 | 225 | P3 | |
| 14 | 300 | P4 | |
| 15 | 375 | P5 |
| ID | Processing code | Irrigation quantity during growth period (m3·hm−2) | Nitrogen fertilizer application rate (kg N·hm−2) | Amount of phosphate fertilizer applied (kg P2O5·hm−2) |
|---|---|---|---|---|
| 1 | T1 | 6300 | 300 | 225 |
| 2 | T2 | 6300 | 300 | 300 |
| 3 | T3 | 6300 | 375 | 225 |
| 4 | T4 | 6300 | 375 | 300 |
| 5 | T5 | 6720 | 300 | 225 |
| 6 | T6 | 6720 | 300 | 300 |
| 7 | T7 | 6720 | 375 | 225 |
| 8 | T8 | 6720 | 375 | 300 |
| Test treatment | Vc (mg/100g) |
Soluble solid content | Stone cell content (%) | Soluble sugar (%) | Titratable acid (%) |
|---|---|---|---|---|---|
| W1 | 8.71 ± 0.13 d | 10.43 ± 0.25 c | 2.50 ± .033 d | 16.17 ± 1.85 c | 1.24 ± 0.02 a |
| W2 | 9.31 ± 0.07 c | 10.78 ± 0.34 b | 3.20 ± 0.04 c | 16.97 ± 0.76 c | 1.17 ± 0.06 b |
| W3 | 10.10 ± 0.13 a | 11.61 ± 0.26 a | 3.66 ± 0.55 ab | 19.87 ± 0.11 a | 0.95 ± 0.03 d |
| W4 | 9.82 ± 0.02 b | 11.56 ± 0.24 a | 3.82 ± 0.11 a | 18.71 ± 0.88 ab | 1.00 ± 0.03 c |
| W5 | 9.36 ± 0.06 c | 10.92 ± 0.41 b | 3.18 ± 0.38 bc | 17.62 ± 0.64 bc | 1.02 ± 0.03 c |
| Test treatment | Vc (mg/100g) |
Soluble solid content | Stone cell content (%) | Soluble sugar (%) | Titratable acid (%) |
|---|---|---|---|---|---|
| N1 | 9.20 ± 0.15e | 10.94 ± 0.32 c | 2.53 ± 0.46 c | 14.44 ± 2.01 c | 1.14 ± 0.09 a |
| N2 | 9.71 ± 0.11d | 11.38 ± 0.39 b | 2.81 ± 0.26 bc | 15.09 ± 1.05 c | 1.03 ± 0.04 b |
| N3 | 11.11 ± 0.06a | 12.27 ± 0.42 a | 3.49 ± 0.03 a | 20.49 ± 2.84 a | 0.85 ± 0.02 c |
| N4 | 10.31 ± 0.13b | 12.04 ± 0.09 a | 3.25 ± 0.22 a | 20.01 ± 2.86 ab | 0.95 ± 0.02 b |
| N5 | 10.12 ± 0.04c | 11.42 ± 0.50 b | 3.11 ± 0.07 ab | 18.20 ± 2.14 b | 0.97 ± 0.03 b |
| Test treatment | Vc (mg/100g) |
Soluble solid content | Stone cell content (%) | Soluble sugar (%) | Titratable acid (%) |
|---|---|---|---|---|---|
| P1 | 8.25 ± 0.04 d | 10.37 ± 0.28 c | 2.72 ± 0.32 d | 12.01 ± 3.01 c | 1.23 ± 0.05 a |
| P2 | 8.70 ± 0.08 c | 10.88 ± 0.35 b | 2.93 ± 0.34 cd | 13.28 ± 2.47 c | 1.19 ± 0.06 a |
| P3 | 9.98 ± 0.06 a | 11.39 ± 0.32 a | 3.56 ± 0.32 a | 19.36 ± 1.11 a | 0.88 ± 0.06 c |
| P4 | 9.92 ± 0.12 a | 11.02 ± 0.21 b | 3.40 ± 0.07 ab | 16.94 ± 1.51 b | 0.92 ± 0.09 c |
| P5 | 9.33 ± 0.06 b | 10.59 ± 0.30 c | 3.12 ± 0.19 bc | 15.96 ± 1.25 b | 1.08 ± 0.02 b |
| Test treatment | 0~20 root density(cm·cm−3) | 20~40 root density(cm·cm−3) | 40~60 root density (cm·cm−3) | 0~20 root surface area(cm2) | 20~40 root surface area(cm2) | 40~60 root surface area(cm2) |
| T1 | 0.06 ± 0.015a | 0.09 ± 0.006a | 0.16 ± 0.01a | 8.64 ± 1.93ab | 17.78 ± 2.23a | 22.4 ± 1.22a |
| T2 | 0.04 ± 0.012a | 0.11 ± 0.006a | 0.15 ± 0.01a | 5.49 ± 0.51c | 13.78 ± 0.99bc | 17.52 ± 1.32b |
| T3 | 0.05 ± 0.006a | 0.10 ± 0.006a | 0.15 ± 0.01a | 6.22 ± 1.66c | 12.02 ± 2.05b | 18.66 ± 1.11b |
| T4 | 0.04 ± 0.01a | 0.07 ± 0.01a | 0.12 ± 0.01a | 5.35 ± 1.12c | 11.03 ± 0.8bc | 21.92 ± 1.42a |
| T5 | 0.05 ± 0.006a | 0.07 ± 0.006a | 0.13 ± 0.01a | 6.77 ± 0.75bc | 12.01 ± 1.63b | 17.29 ± 1.3b |
| T6 | 0.05 ± 0.006a | 0.10 ± 0.01a | 0.14 ± 0.03a | 9.87 ± 1.09a | 13.37 ± 0.64b | 18.23 ± 1.44b |
| T7 | 0.04 ± 0.006a | 0.09 ± 0.006a | 0.14 ± 0.01a | 6.56 ± 0.94bc | 12.76 ± 2.32b | 21.62 ± 1.06a |
| T8 | 0.04 ± 0.006a | 0.07 ± 0.006a | 0.11 ± 0.01a | 5.28 ± 2.17c | 9.05 ± 1.7c | 17.44 ± 0.78b |
| Test treatment | 0~20 root density(cm·cm−3) | 20~40 root density(cm·cm−3) | 40~60 root density(cm·cm−3) | 0~20 root surface area(cm2) | 20~40root surface area(cm2) | 40~60 root surface area(cm2) |
| T1 | 0.06 ± 0.01a | 0.11 ± 0.01a | 0.21 ± 0.01a | 12.88 ± 0.65a | 19.96 ± 0.62a | 28.38 ± 1.36a |
| T2 | 0.06 ± 0.02a | 0.12 ± 0.012a | 0.17 ± 0.006a | 9.91 ± 0.93bc | 14.40 ± 2.15bc | 23.37 ± 1.8c |
| T3 | 0.05 ± 0.01a | 0.12 ± 0.006a | 0.19 ± 0.01a | 9.66 ± 2.3bc | 15.07 ± 1.09bc | 24.11 ± 1.56bc |
| T4 | 0.06 ± 0.01a | 0.12 ± 0.01a | 0.16 ± 0.01a | 7.79 ± 1.61c | 16.58 ± 3.56b | 26.41 ± 0.9ab |
| T5 | 0.07 ± 0.01a | 0.12 ± 0.012a | 0.18 ± 0.006a | 11.14 ± 1.09ab | 16.83 ± 3.52b | 20.31 ± 0.89d |
| T6 | 0.06 ± 0.01a | 0.11 ± 0.006a | 0.16 ± 0.006a | 11.11 ± 1.69ab | 16.36 ± 1.56b | 19.37 ± 0.33d |
| T7 | 0.07 ± 0.01a | 0.13 ± 0.012a | 0.16 ± 0.006a | 10.38 ± 1.81abc | 16.69 ± 0.95b | 28.61 ± 1.53a |
| T8 | 0.06 ± 0.01a | 0.13 ± 0.006a | 0.15 ± 0.01a | 8.36 ± 2.17bc | 12.31 ± 0.94c | 24.25 ± 1.92bc |
| Test treatment | Weight of single fruit (g) | Yield (kg·hm−2) | Primary fruit rate (%) | Secondary fruit rate (%) |
Tertiary fruit rate (%) |
|---|---|---|---|---|---|
| T1 | 130.28 ± 11.17 ab | 4388.89 c | 67.09% d | 18.38% b | 14.53% a |
| T2 | 122.50 ± 12.01 ab | 4463.89 bc | 73.62% bc | 12.76% d | 13.63% ab |
| T3 | 137.61 ± 6.115 a | 4916.67 a | 78.14% a | 9.72% e | 12.15% bc |
| T4 | 125.40 ± 3.383 ab | 4194.44 d | 64.44% e | 21.85% a | 13.71% ab |
| T5 | 129.90 ± 5.661 ab | 4258.33 d | 64.83% e | 20.73% a | 14.45% a |
| T6 | 115.31 ± 7.566 b | 4533.33 b | 72.43% c | 16.30% c | 11.27% c |
| T7 | 114.39 ± 6.496 b | 4447.22 bc | 74.29% b | 16.21% c | 9.49% d |
| T8 | 126.38 ± 14.97 ab | 4091.67 d | 72.03% c | 18.86% b | 9.10% d |
| Correlation coefficient results | ||||||||
|---|---|---|---|---|---|---|---|---|
| Test treatment | T1 | T2 | T3 | T4 | T5 | T6 | T7 | T8 |
| Stone cell content | 0.9748 | 0.9532 | 0.9941 | 0.9524 | 0.9511 | 0.9349 | 1.0000 | 0.9653 |
| hardness | 0.8212 | 0.8078 | 0.8048 | 0.8043 | 0.8063 | 0.7980 | 0.8049 | 0.8143 |
| Soluble solid | 0.3449 | 0.3622 | 0.3505 | 0.3659 | 0.3593 | 0.3662 | 0.3714 | 0.3504 |
| Soluble sugar | 0.6353 | 0.5874 | 0.6395 | 0.6120 | 0.6132 | 0.5997 | 0.5561 | 0.5871 |
| Titratable acid | 0.5391 | 0.5332 | 0.5414 | 0.5314 | 0.5448 | 0.5260 | 0.5384 | 0.5385 |
| Vc | 0.7382 | 0.7461 | 0.7433 | 0.7408 | 0.7487 | 0.7787 | 0.7449 | 0.7081 |
| Total phenol | 0.5851 | 0.5640 | 0.5714 | 0.5285 | 0.5622 | 0.5428 | 0.5951 | 0.5869 |
| flavonoid | 0.3757 | 0.3752 | 0.3762 | 0.3750 | 0.3755 | 0.3753 | 0.3757 | 0.3759 |
| sort | 2 | 5 | 1 | 8 | 4 | 7 | 3 | 6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





