Submitted:
11 October 2023
Posted:
12 October 2023
You are already at the latest version
Abstract
Keywords:
Introduction
The importance of molybdenum for plants
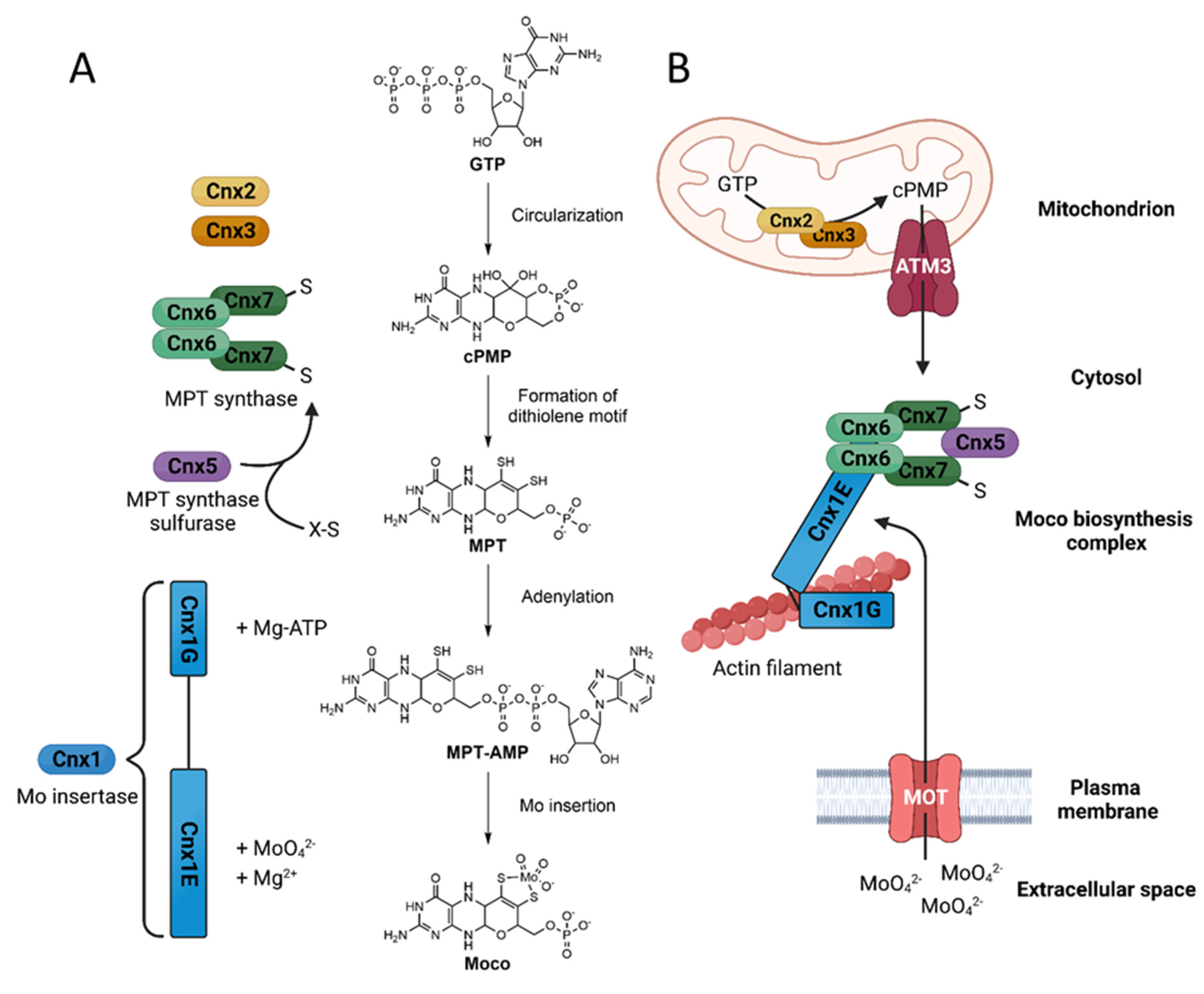
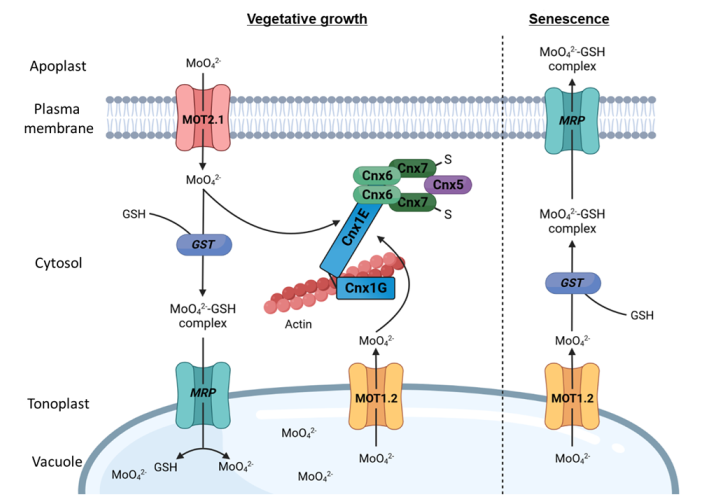
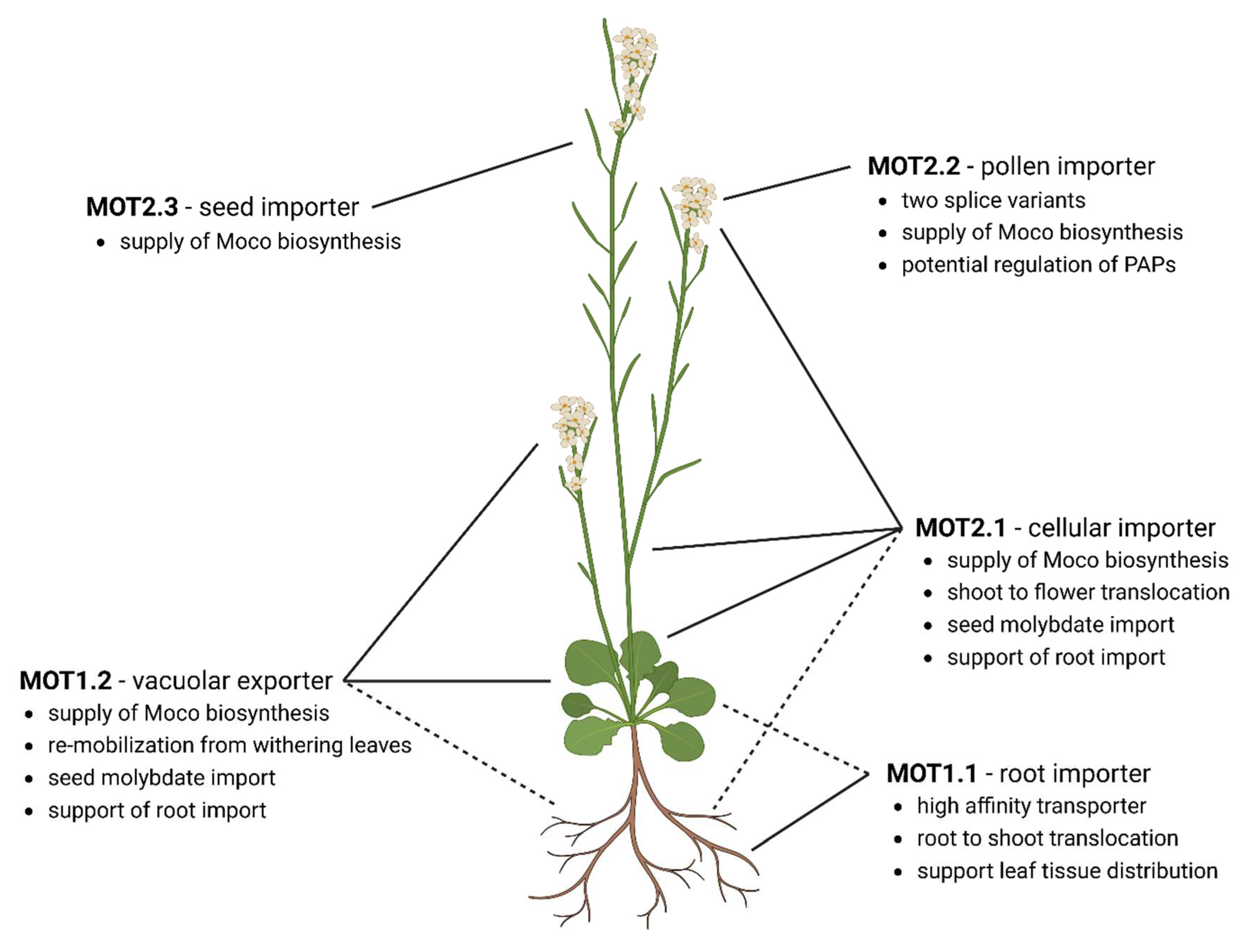
Molybdate transport by specialized membrane transporters
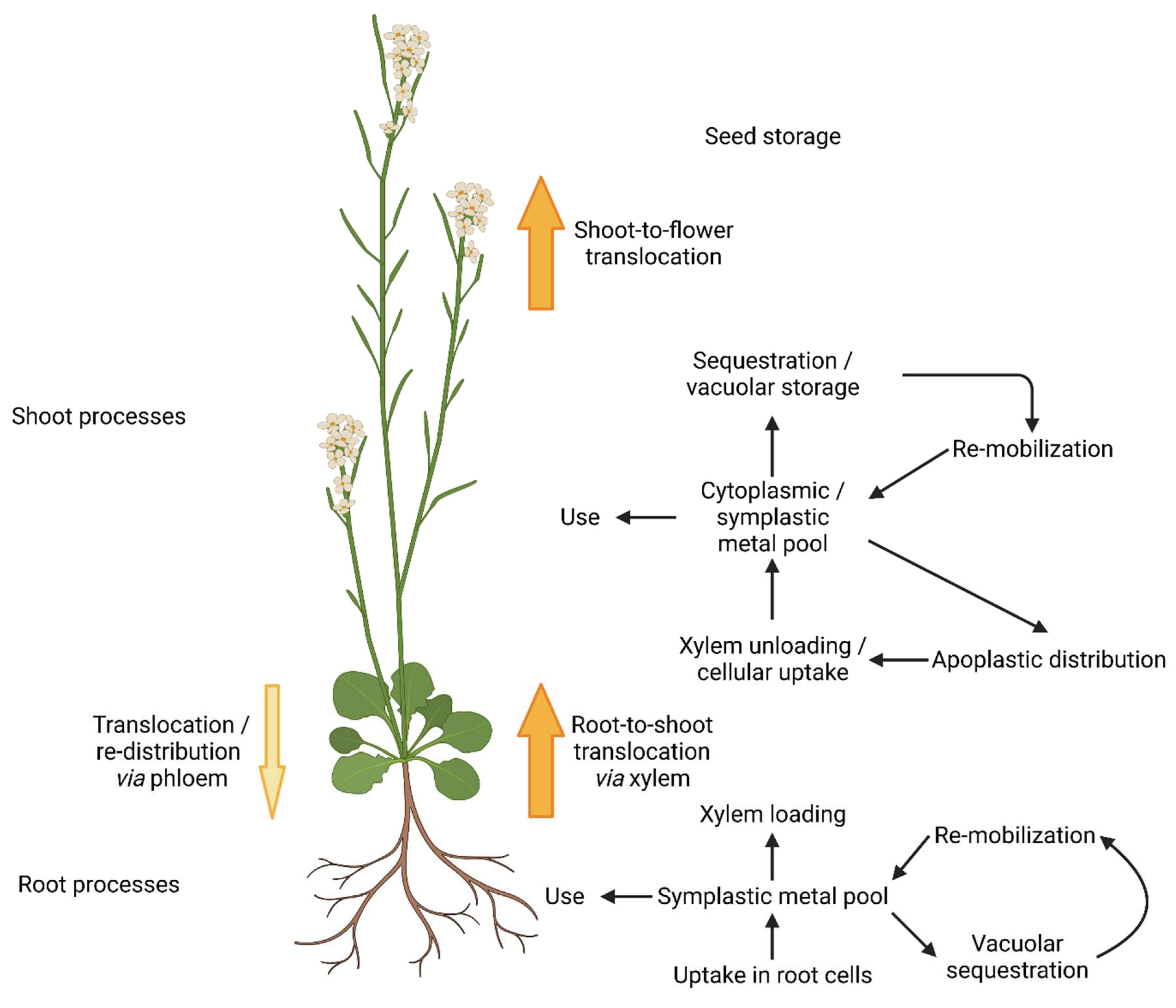
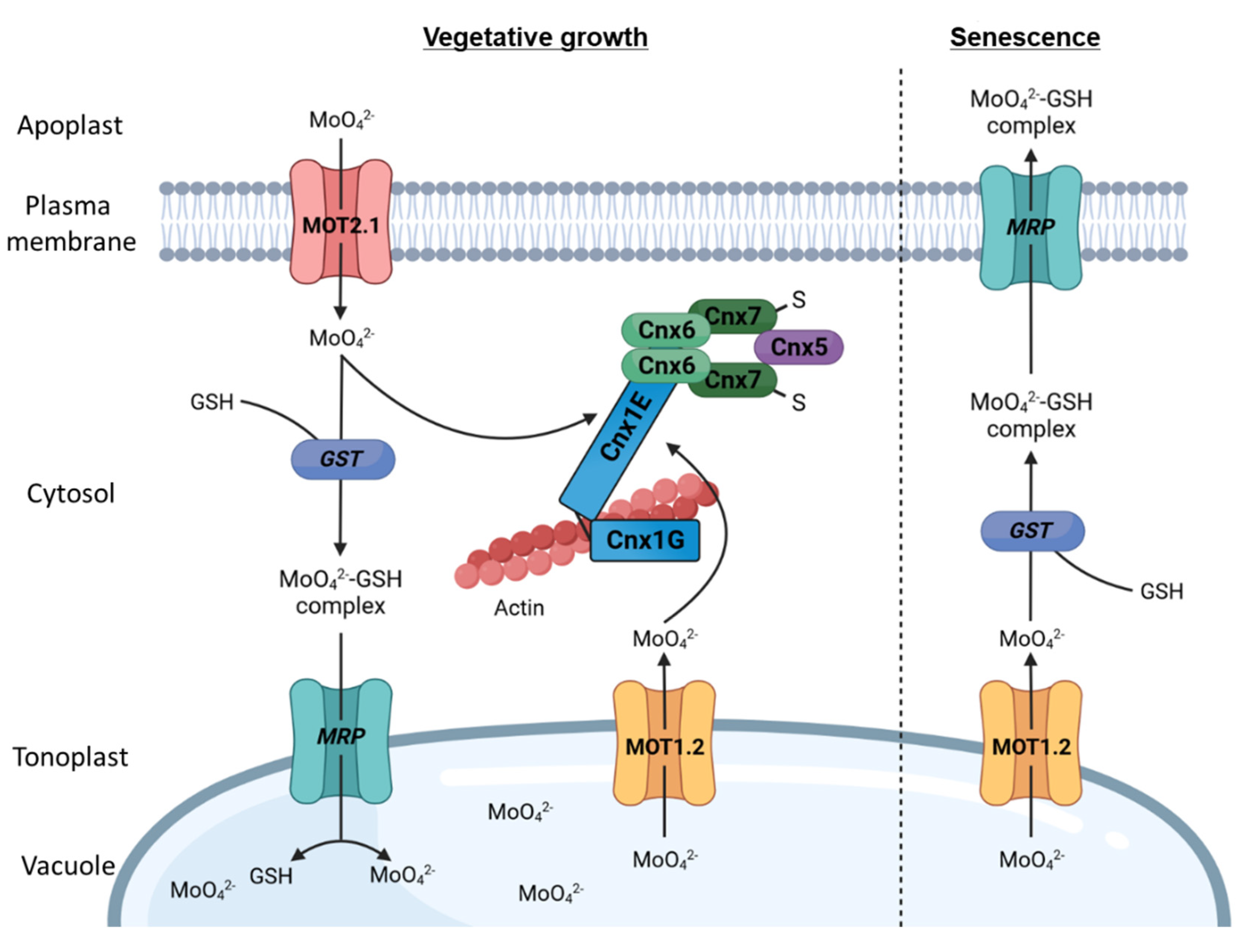
Following molybdate along its way through A. thaliana
Data Availability Statement
Conflicts of Interest
References
- Angulo-Bejarano, P.I.; Puente-Rivera, J.; Cruz-Ortega, R. Metal and Metalloid Toxicity in Plants: An Overview on Molecular Aspects. Plants 2021, 10, 1–28. [Google Scholar] [CrossRef]
- Assunção, A.G.L.; Cakmak, I.; Clemens, S.; González-Guerrero, M.; Nawrocki, A.; Thomine, S. Micronutrient Homeostasis in Plants for More Sustainable Agriculture and Healthier Human Nutrition. J. Exp. Bot. 2022, 73, 1789–1799. [Google Scholar] [CrossRef] [PubMed]
- Colangelo, E.P.; Guerinot, M. Lou Put the Metal to the Petal: Metal Uptake and Transport throughout Plants. Curr. Opin. Plant Biol. 2006, 9, 322–330. [Google Scholar] [CrossRef] [PubMed]
- Krämer, U.; Talke, I.N.; Hanikenne, M. Transition Metal Transport. FEBS Lett. 2007, 581, 2263–2272. [Google Scholar] [CrossRef]
- Clemens, S.; Palmgren, M.G.; Krämer, U. A Long Way Ahead: Understanding and Engineering Plant Metal Accumulation. Trends Plant Sci. 2002, 7, 309–315. [Google Scholar] [CrossRef]
- Andresen, E.; Peiter, E.; Küpper, H. Trace Metal Metabolism in Plants. J. Exp. Bot. 2018, 69, 909–954. [Google Scholar] [CrossRef] [PubMed]
- Küpper, H.; Andresen, E. Mechanisms of Metal Toxicity in Plants. Metallomics 2016, 8, 269–285. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Sitsel, O.; Meloni, G.; Autzen, H.E.; Andersson, M.; Klymchuk, T.; Nielsen, A.M.; Rees, D.C.; Nissen, P.; Gourdon, P. Structure and Mechanism of Zn2+-Transporting P-Type ATPases. Nature 2014, 514, 518–522. [Google Scholar] [CrossRef]
- Murgia, I.; Marzorati, F.; Vigani, G.; Morandini, P. Plant Iron Nutrition: The Long Road from Soil to Seeds. J. Exp. Bot. 2022, 73, 1809–1824. [Google Scholar] [CrossRef]
- Clemens, S. Metal Ligands in Micronutrient Acquisition and Homeostasis. Plant Cell Environ. 2019, 42, 2902–2912. [Google Scholar] [CrossRef]
- Chen, Y.T.; Wang, Y.; Yeh, K.C. Role of Root Exudates in Metal Acquisition and Tolerance. Curr. Opin. Plant Biol. 2017, 39, 66–72. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, D.; Sun, W.; Wang, T. The Adaptive Mechanism of Plants to Iron Deficiency via Iron Uptake, Transport, and Homeostasis. Int. J. Mol. Sci. 2019, 20. [Google Scholar] [CrossRef]
- Merchant, S.S. The Elements of Plant Micronutrients. Plant Physiol. 2010, 154, 512–515. [Google Scholar] [CrossRef] [PubMed]
- Smedley, P.L.; Kinniburgh, D.G. Molybdenum in Natural Waters: A Review of Occurrence, Distributions and Controls. Appl. Geochemistry 2017, 84, 387–432. [Google Scholar] [CrossRef]
- Frascoli, F.; Hudson-Edwards, K.A. Geochemistry, Mineralogy and Microbiology of Molybdenum in Mining-Affected Environments. Minerals 2018, 8, 1–18. [Google Scholar] [CrossRef]
- Tomatsu, H.; Takano, J.; Takahashi, H.; Watanabe-Takahashi, A.; Shibagaki, N.; Fujiwara, T. An Arabidopsis Thaliana High-Affinity Molybdate Transporter Required for Efficient Uptake of Molybdate from Soil. Proc. Natl. Acad. Sci. U. S. A. 2007, 104, 18807–18812. [Google Scholar] [CrossRef]
- Kaiser, B.N.; Gridley, K.L.; Brady, J.N.; Phillips, T.; Tyerman, S.D. The Role of Molybdenum in Agricultural Plant Production. Ann. Bot. 2005, 96, 745–754. [Google Scholar] [CrossRef] [PubMed]
- Mendel, R.R. The Molybdenum Cofactor. J. Biol. Chem. 2013, 288, 13165–13172. [Google Scholar] [CrossRef]
- Mayr, S.J.; Mendel, R.R.; Schwarz, G. Molybdenum Cofactor Biology, Evolution and Deficiency. Biochim. Biophys. Acta - Mol. Cell Res. 2021, 1868, 118883. [Google Scholar] [CrossRef]
- Mendel, R.R.; Schwarz, G. Molybdenum Cofactor Biosynthesis in Plants and Humans. Coord. Chem. Rev. 2011, 255, 1145–1158. [Google Scholar] [CrossRef]
- Teschner, J.; Lachmann, N.; Schulze, J.; Geisler, M.; Selbach, K.; Santamaria-Araujo, J.; Balk, J.; Mendel, R.R.; Bittner, F. A Novel Role for Arabidopsis Mitochondrial ABC Transporter ATM3 in Molybdenum Cofactor Biosynthesis. Plant Cell 2010, 22, 468–480. [Google Scholar] [CrossRef]
- Mendel, R.R.; Leimkühler, S. The Biosynthesis of the Molybdenum Cofactors. J. Biol. Inorg. Chem. 2015, 20, 337–347. [Google Scholar] [CrossRef]
- Krausze, J.; Hercher, T.W.; Zwerschke, D.; Kirk, M.L.; Blankenfeldt, W.; Mendel, R.R.; Kruse, T. The Functional Principle of Eukaryotic Molybdenum Insertases. Biochem. J. 2018, 475, 1739–1753. [Google Scholar] [CrossRef]
- Weber, J.N.; Minner-Meinen, R.; Behnecke, M.; Biedendieck, R.; Hänsch, V.G.; Hercher, T.W.; Hertweck, C.; van den Hout, L.; Knüppel, L.; Sivov, S.; et al. Moonlighting Arabidopsis Molybdate Transporter 2 Family and GSH-Complex Formation Facilitate Molybdenum Homeostasis. Commun. Biol. 2023, 6, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Kaufholdt, D.; Gehl, C.; Geisler, M.; Jeske, O.; Voedisch, S.; Ratke, C.; Bollhöner, B.; Mendel, R.R.; Hänsch, R. Visualization and Quantification of Protein Interactions in the Biosynthetic Pathway of Molybdenum Cofactor in Arabidopsis Thaliana. J. Exp. Bot. 2013, 64, 2005–2016. [Google Scholar] [CrossRef]
- Kaufholdt, D.; Baillie, C.K.; Bikker, R.; Burkart, V.; Dudek, C.A.; Pein, L. von; Rothkegel, M.; Mendel, R.R.; Hänsch, R. The Molybdenum Cofactor Biosynthesis Complex Interacts with Actin Filaments via Molybdenum Insertase Cnx1 as Anchor Protein in Arabidopsis Thaliana. Plant Sci. 2016, 244, 8–18. [Google Scholar] [CrossRef] [PubMed]
- Kaufholdt, D.; Baillie, C.K.; Meyer, M.H.; Schwich, O.D.; Timmerer, U.L.; Tobias, L.; van Thiel, D.; Hänsch, R.; Mendel, R.R. Identification of a Protein-Protein Interaction Network Downstream of Molybdenum Cofactor Biosynthesis in Arabidopsis Thaliana. J. Plant Physiol. 2016, 207, 42–50. [Google Scholar] [CrossRef]
- Lehrke, M.; Rump, S.; Heidenreich, T.; Wissing, J.; Mendel, R.R.; Bittner, F. Identification of Persulfide-Binding and Disulfide-Forming Cysteine Residues in the NifS-like Domain of the Molybdenum Cofactor Sulfurase ABA3 by Cysteine-Scanning Mutagenesis. Biochem. J. 2012, 441, 823–832. [Google Scholar] [CrossRef] [PubMed]
- Kaufholdt, D.; Baillie, C.K.; Meinen, R.; Mendel, R.R.; Hänsch, R. The Molybdenum Cofactor Biosynthesis Network: In Vivo Protein-Protein Interactions of an Actin Associated Multi-Protein Complex. Front. Plant Sci. 2017, 8, 1–8. [Google Scholar] [CrossRef]
- Mendel, R.R. The History of the Molybdenum Cofactor—A Personal View. Molecules 2022, 27. [Google Scholar] [CrossRef]
- Magalon, A.; Mendel, R.R. Biosynthesis and Insertion of the Molybdenum Cofactor. EcoSal Plus 2015, 6. [Google Scholar] [CrossRef]
- Mendel, R.R.; Hänsch, R. Molybdoenzymes and Molybdenum Cofactor in Plants. J. Exp. Bot. 2002, 53, 1689–1698. [Google Scholar] [CrossRef] [PubMed]
- Sano, N.; Marion-Poll, A. Aba Metabolism and Homeostasis in Seed Dormancy and Germination. Int. J. Mol. Sci. 2021, 22. [Google Scholar] [CrossRef]
- Soltabayeva, A.; Srivastava, S.; Kurmanbayeva, A.; Bekturova, A.; Fluhr, R.; Sagi, M. Early Senescence in Older Leaves of Low Nitrate-Grown Atxdh1 Uncovers a Role for Purine Catabolism in n Supply. Plant Physiol. 2018, 178, 1027–1044. [Google Scholar] [CrossRef] [PubMed]
- Yesbergenova, Z.; Yang, G.; Oron, E.; Soffer, D.; Fluhr, R.; Sagi, M. The Plant Mo-Hydroxylases Aldehyde Oxidase and Xanthine Dehydrogenase Have Distinct Reactive Oxygen Species Signatures and Are Induced by Drought and Abscisic Acid. Plant J. 2005, 42, 862–876. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Wang, W.; Bittner, F.; Schmidt, N.; Berkey, R.; Zhang, L.; King, H.; Zhang, Y.; Feng, J.; Wen, Y.; et al. Dual and Opposing Roles of Xanthine Dehydrogenase in Defense-Associated Reactive Oxygen Species Metabolism in Arabidopsis. Plant Cell 2016, 28, 1108–1126. [Google Scholar] [CrossRef] [PubMed]
- Krompholz, N.; Krischkowski, C.; Reichmann, D.; Garbe-Schönberg, D.; Mendel, R.R.; Bittner, F.; Clement, B.; Havemeyer, A. The Mitochondrial Amidoxime Reducing Component (MARC) Is Involved in Detoxification of N-Hydroxylated Base Analogues. Chem. Res. Toxicol. 2012, 25, 2443–2450. [Google Scholar] [CrossRef] [PubMed]
- Maiber, L.; Koprivova, A.; Bender, D.; Kopriva, S.; Fischer-Schrader, K. Characterization of the Amidoxime Reducing Components ARC1 and ARC2 from Arabidopsis Thaliana. FEBS J. 2022, 289, 5656–5669. [Google Scholar] [CrossRef] [PubMed]
- Mohn, M.A.; Thaqi, B.; Fischer-Schrader, K. Isoform-Specific NO Synthesis by Arabidopsis Thaliana Nitrate Reductase. Plants 2019, 8, 1–15. [Google Scholar] [CrossRef]
- Campbell, W.H. Nitrate Reductase Structure, Function and Regulation: Bridging the Gap between Biochemistry and Physiology. Annu. Rev. Plant Biol. 1999, 50, 277–303. [Google Scholar] [CrossRef]
- Nowak, K.; Luniak, N.; Witt, C.; Wüstefeld, Y.; Wachter, A.; Mendel, R.R.; Hänsch, R. Peroxisomal Localization of Sulfite Oxidase Separates It from Chloroplast-Based Sulfur Assimilation. Plant Cell Physiol. 2004, 45, 1889–1894. [Google Scholar] [CrossRef]
- Baillie, C.K.; Kaufholdt, D.; Karpinski, L.H.; Schreiber, V.; Hänsch, S.; Evers, C.; Bloem, E.; Schnug, E.; Kreuzwieser, J.; Herschbach, C.; et al. Detoxification of Volcanic Sulfur Surplus in Planta: Three Different Strategies of Survival. Environ. Exp. Bot. 2016, 126, 44–54. [Google Scholar] [CrossRef]
- Weber, J.N.; Kaufholdt, D.; Minner-Meinen, R.; Bloem, E.; Shahid, A.; Rennenberg, H.; Hänsch, R. Impact of Wildfires on SO2 Detoxification Mechanisms in Leaves of Oak and Beech Trees. Environ. Pollut. 2021, 272. [Google Scholar] [CrossRef]
- Hu, Y.; Ribbe, M.W. Biosynthesis of the Iron-Molybdenum Cofactor of Nitrogenase. J. Biol. Chem. 2013, 288, 13173–13177. [Google Scholar] [CrossRef]
- Bellenger, J.P.; Wichard, T.; Kustka, A.B.; Kraepiel, A.M.L. Uptake of Molybdenum and Vanadium by Nitrogen-Fixing Soil Bacterium Using Siderophores. Nat. Geosci. 2008, 1, 243–246. [Google Scholar] [CrossRef]
- Lindström, K.; Mousavi, S.A. Effectiveness of Nitrogen Fixation in Rhizobia. Microb. Biotechnol. 2020, 13, 1314–1335. [Google Scholar] [CrossRef]
- Haque, N.; Peralta-Videa, J.R.; Jones, G.L.; Gill, T.E.; Gardea-Torresdey, J.L. Screening the Phytoremediation Potential of Desert Broom (Baccharis Sarothroides Gray) Growing on Mine Tailings in Arizona, USA. Environ. Pollut. 2008, 153, 362–368. [Google Scholar] [CrossRef]
- Mendel, R.R.; Bittner, F. Cell Biology of Molybdenum. Biochim. Biophys. Acta - Mol. Cell Res. 2006, 1763, 621–635. [Google Scholar] [CrossRef]
- Schwarz, G.; Mendel, R.R.; Ribbe, M.W. Molybdenum Cofactors, Enzymes and Pathways. Nature 2009, 460, 839–847. [Google Scholar] [CrossRef] [PubMed]
- Wagner, U.G.; Stupperich, E.; Kratky, C. Structure of the Molybdate/Tungstate Binding Protein Mop from Sporomusa Ovata. Structure 2000, 8, 1127–1136. [Google Scholar] [CrossRef] [PubMed]
- Makdessi, K.; Fritsche, K.; Pich, A.; Andreesen, J.R. Identification and Characterization of the Cytoplasmic Tungstate/Molybdate- Binding Protein (Mop) from Eubacterium Acidaminophilum. Arch. Microbiol. 2004, 181, 45–51. [Google Scholar] [CrossRef]
- Demtröder, L.; Narberhaus, F.; Masepohl, B. Coordinated Regulation of Nitrogen Fixation and Molybdate Transport by Molybdenum. Mol. Microbiol. 2019, 111, 17–30. [Google Scholar] [CrossRef] [PubMed]
- Kraepiel, A.M.L.; Bellenger, J.P.; Wichard, T.; Morel, F.M.M. Multiple Roles of Siderophores in Free-Living Nitrogen-Fixing Bacteria. BioMetals 2009, 22, 573–581. [Google Scholar] [CrossRef] [PubMed]
- Thomas, W.; Bellenger, J.P.; Morel, F.M.M.; Kraepiel, A.M.L. Role of the Siderophore Azotobactin in the Bacterial Acquisition of Nitrogenase Metal Cofactors. Environ. Sci. Technol. 2009, 43, 7218–7224. [Google Scholar] [CrossRef]
- Duhme-Klair, A.K. From Siderophores and Self-Assembly to Luminescent Sensors: The Binding of Molybdenum by Catecholamides. Eur. J. Inorg. Chem. 2009, 3689–3701. [Google Scholar] [CrossRef]
- Schalk, I.J.; Hannauer, M.; Braud, A. New Roles for Bacterial Siderophores in Metal Transport and Tolerance. Environ. Microbiol. 2011, 13, 2844–2854. [Google Scholar] [CrossRef] [PubMed]
- Johnstone, T.C.; Nolan, E.M. Beyond Iron: Non-Classical Biological Functions of Bacterial Siderophores. Dalt. Trans. 2015, 44, 6320–6339. [Google Scholar] [CrossRef] [PubMed]
- Vatansever, R.; Filiz, E.; Ozyigit, I.I. In Silico Identification and Comparative Analysis of Molybdenum (Mo) Transporter Genes in Plants. Rev. Bras. Bot. 2016, 39, 87–99. [Google Scholar] [CrossRef]
- Gil-Díez, P.; Tejada-Jiménez, M.; León-Mediavilla, J.; Wen, J.; Mysore, K.S.; Imperial, J.; González-Guerrero, M. MtMOT1.2 Is Responsible for Molybdate Supply to Medicago Truncatula Nodules. Plant Cell Environ. 2019, 42, 310–320. [Google Scholar] [CrossRef]
- Tejada-Jiménez, M.; Gil-Díez, P.; León-Mediavilla, J.; Wen, J.; Mysore, K.S.; Imperial, J.; González-Guerrero, M. Medicago Truncatula Molybdate Transporter Type 1 (MtMOT1.3) Is a Plasma Membrane Molybdenum Transporter Required for Nitrogenase Activity in Root Nodules under Molybdenum Deficiency. New Phytol. 2017, 216, 1223–1235. [Google Scholar] [CrossRef]
- Gao, J.S.; Wu, F.F.; Shen, Z.L.; Meng, Y.; Cai, Y.P.; Lin, Y. A Putative Molybdate Transporter LjMOT1 Is Required for Molybdenum Transport in Lotus Japonicus. Physiol. Plant. 2016, 158, 331–340. [Google Scholar] [CrossRef] [PubMed]
- Minner-Meinen, R.; Weber, J.N.; Kistner, S.; Meyfarth, P.; Saudhof, M.; van den Hout, L.; Schulze, J.; Mendel, R.R.; Hänsch, R.; Kaufholdt, D. Physiological Importance of Molybdate Transporter Family 1 in Feeding the Molybdenum Cofactor Biosynthesis Pathway in Arabidopsis Thaliana. Molecules 2022, 27, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Tejada-Jiménez, M.; Galván, A.; Fernández, E. Algae and Humans Share a Molybdate Transporter. Proc. Natl. Acad. Sci. U. S. A. 2011, 108, 6420–6425. [Google Scholar] [CrossRef] [PubMed]
- Leves, F.P.; Tierney, M.L.; Howitt, S.M. Polar Residues in a Conserved Motif Spanning Helices 1 and 2 Are Functionally Important in the SulP Transporter Family. Int. J. Biochem. Cell Biol. 2008, 40, 2596–2605. [Google Scholar] [CrossRef] [PubMed]
- Shibagaki, N.; Grossman, A.R. The Role of the STAS Domain in the Function and Biogenesis of a Sulfate Transporter as Probed by Random Mutagenesis. J. Biol. Chem. 2006, 281, 22964–22973. [Google Scholar] [CrossRef] [PubMed]
- Baxter, I.; Muthukumar, B.; Hyeong, C.P.; Buchner, P.; Lahner, B.; Danku, J.; Zhao, K.; Lee, J.; Hawkesford, M.J.; Guerinot, M. Lou; et al. Variation in Molybdenum Content across Broadly Distributed Populations of Arabidopsis Thaliana Is Controlled by a Mitochondrial Molybdenum Transporter (MOT1). PLoS Genet. 2008, 4. [Google Scholar] [CrossRef]
- Gasber, A.; Klaumann, S.; Trentmann, O.; Trampczynska, A.; Clemens, S.; Schneider, S.; Sauer, N.; Feifer, I.; Bittner, F.; Mendel, R.R.; et al. Identification of an Arabidopsis Solute Carrier Critical for Intracellular Transport and Inter-Organ Allocation of Molybdate. Plant Biol. 2011, 13, 710–718. [Google Scholar] [CrossRef]
- Temple, H.; Phyo, P.; Yang, W.; Lyczakowski, J.J.; Echevarría-Poza, A.; Yakunin, I.; Parra-Rojas, J.P.; Terrett, O.M.; Saez-Aguayo, S.; Dupree, R.; et al. Discovery of Putative Golgi S-Adenosyl Methionine Transporters Reveals the Importance of Plant Cell Wall Polysaccharide Methylation. bioRxiv 2021. [Google Scholar]
- Yadav, S.K. Heavy Metals Toxicity in Plants: An Overview on the Role of Glutathione and Phytochelatins in Heavy Metal Stress Tolerance of Plants. South African J. Bot. 2010, 76, 167–179. [Google Scholar] [CrossRef]
- Edwards, R.; Dixon, D.P.; Walbot, V. Plant Glutathione S-Transferases: Enzymes with Multiple Functions in Sickness and in Health. Trends Plant Sci. 2000, 5, 193–198. [Google Scholar] [CrossRef]
- Klein, M.; Burla, B.; Martinoia, E. The Multidrug Resistance-Associated Protein (MRP/ABCC) Subfamily of ATP-Binding Cassette Transporters in Plants. FEBS Lett. 2006, 580, 1112–1122. [Google Scholar] [CrossRef] [PubMed]
- Kosakivska, I. V.; Babenko, L.M.; Romanenko, K.O.; Korotka, I.Y.; Potters, G. Molecular Mechanisms of Plant Adaptive Responses to Heavy Metals Stress. Cell Biol. Int. 2021, 45, 258–272. [Google Scholar] [CrossRef] [PubMed]
- Kutrowska, A.; Szelag, M. Low-Molecular Weight Organic Acids and Peptides Involved in the Long-Distance Transport of Trace Metals. Acta Physiol. Plant. 2014, 36, 1957–1968. [Google Scholar] [CrossRef]
- Kuang, R.; Chan, K.H.; Yeung, E.; Lim, B.L. Molecular and Biochemical Characterization of AtPAP15, a Purple Acid Phosphatase with Phytase Activity, in Arabidopsis. Plant Physiol. 2009, 151, 199–209. [Google Scholar] [CrossRef] [PubMed]
- Frascaroli, E.; Tuberosa, R. Effect of Abscisic Acid on Pollen Germination and Tube Growth of Maize Genotypes. Plant Breed. 1993, 110, 250–254. [Google Scholar] [CrossRef]
- Zhao, S.; Wu, Y.; He, Y.; Wang, Y.; Xiao, J.; Li, L.; Wang, Y.; Chen, X.; Xiong, W.; Wu, Y. RopGEF2 Is Involved in ABA-Suppression of Seed Germination and Post-Germination Growth of Arabidopsis. Plant J. 2015, 84, 886–899. [Google Scholar] [CrossRef]
- Frey, A.; Godin, B.; Bonnet, M.; Sotta, B.; Marion-Poll, A. Maternal Synthesis of Abscisic Acid Controls Seed Development and Yield in Nicotiana Plumbaginifolia. Planta 2004, 218, 958–964. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




