Submitted:
12 October 2023
Posted:
12 October 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results
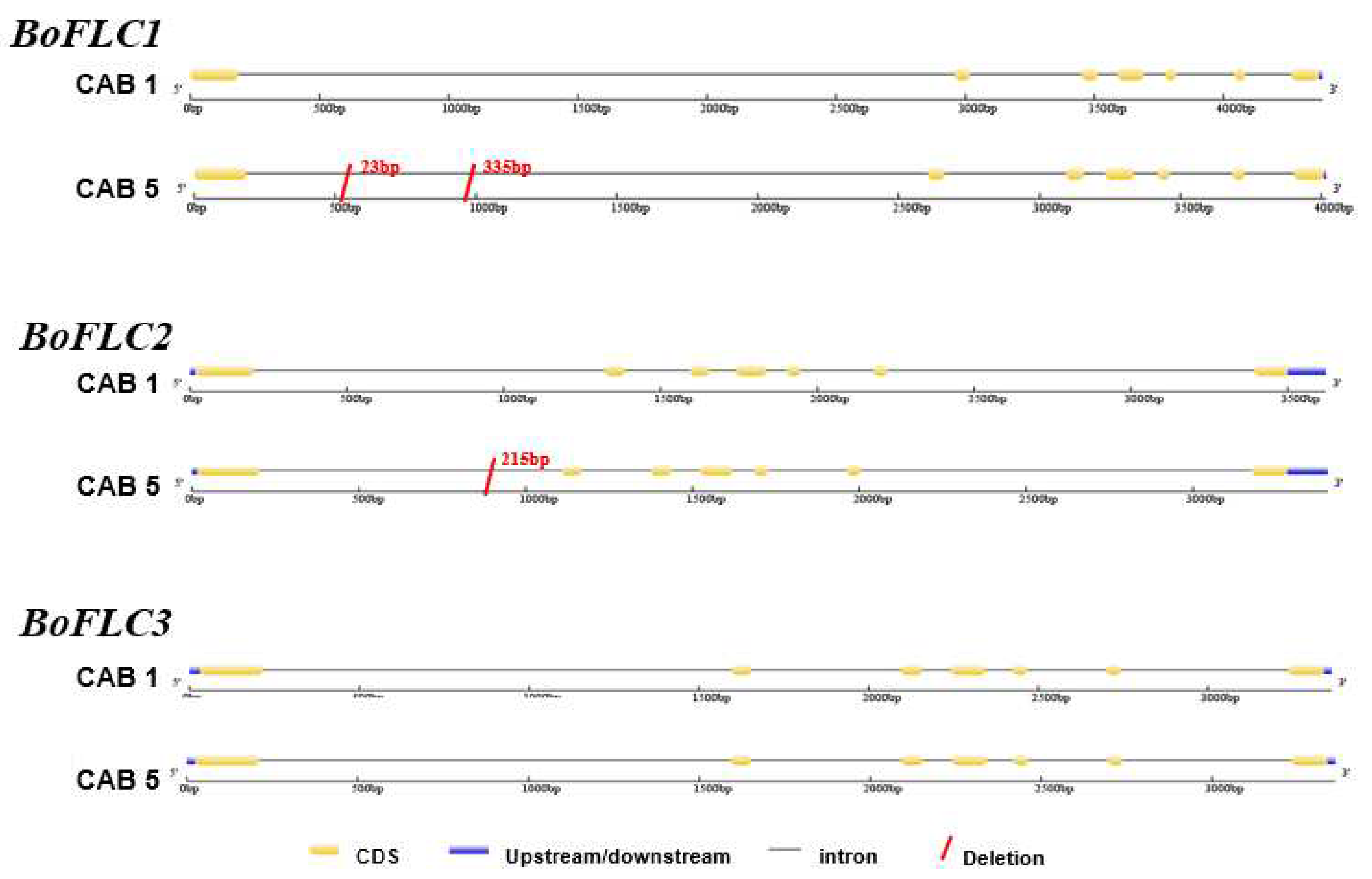
2.1. Molecular characterization of BoFLC-encoding genes from different cabbage bolting and flowering lines (variation in gene structures)
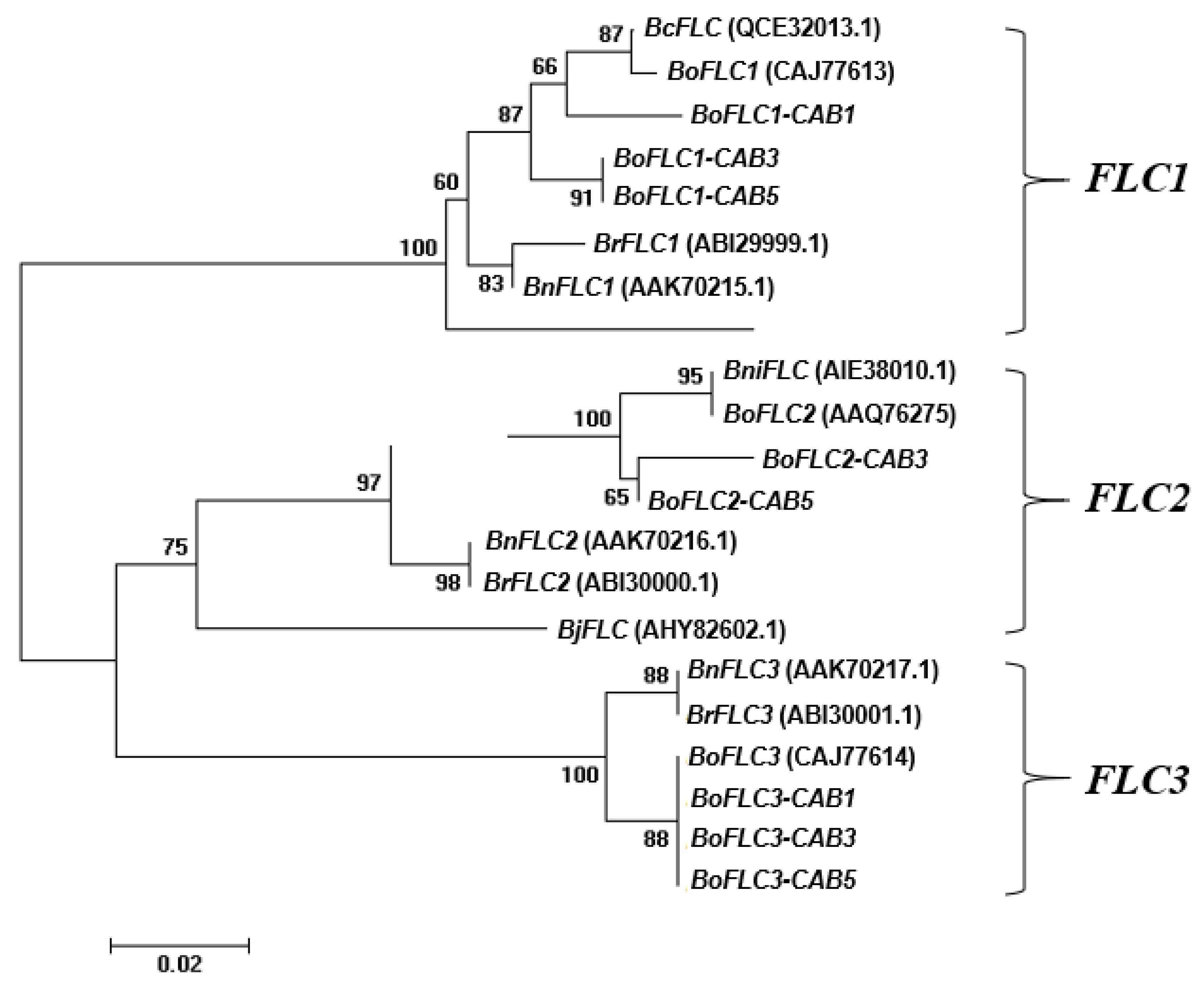
2.2. Phylogenetic relationships among B. oleraceae and other Brassica species
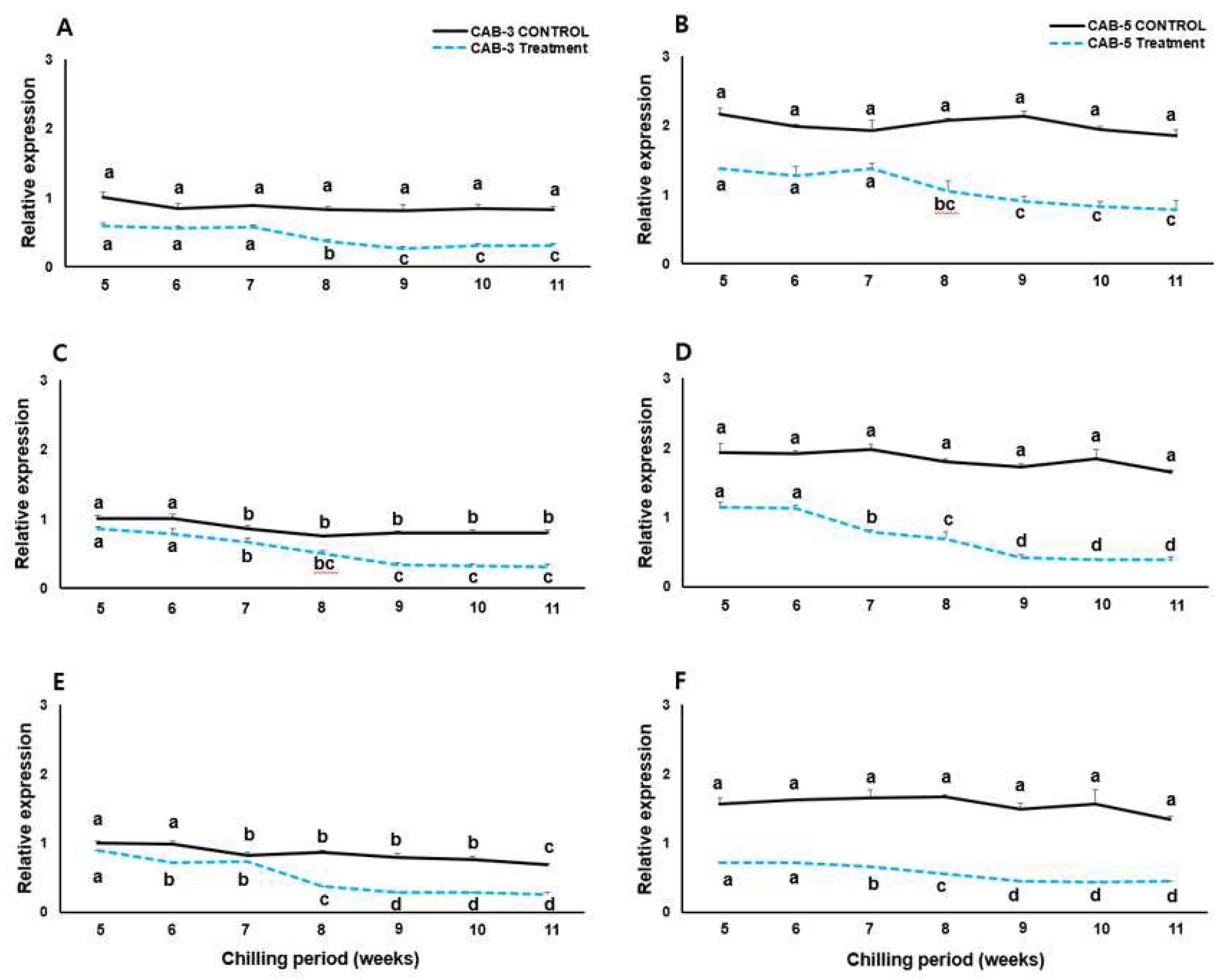
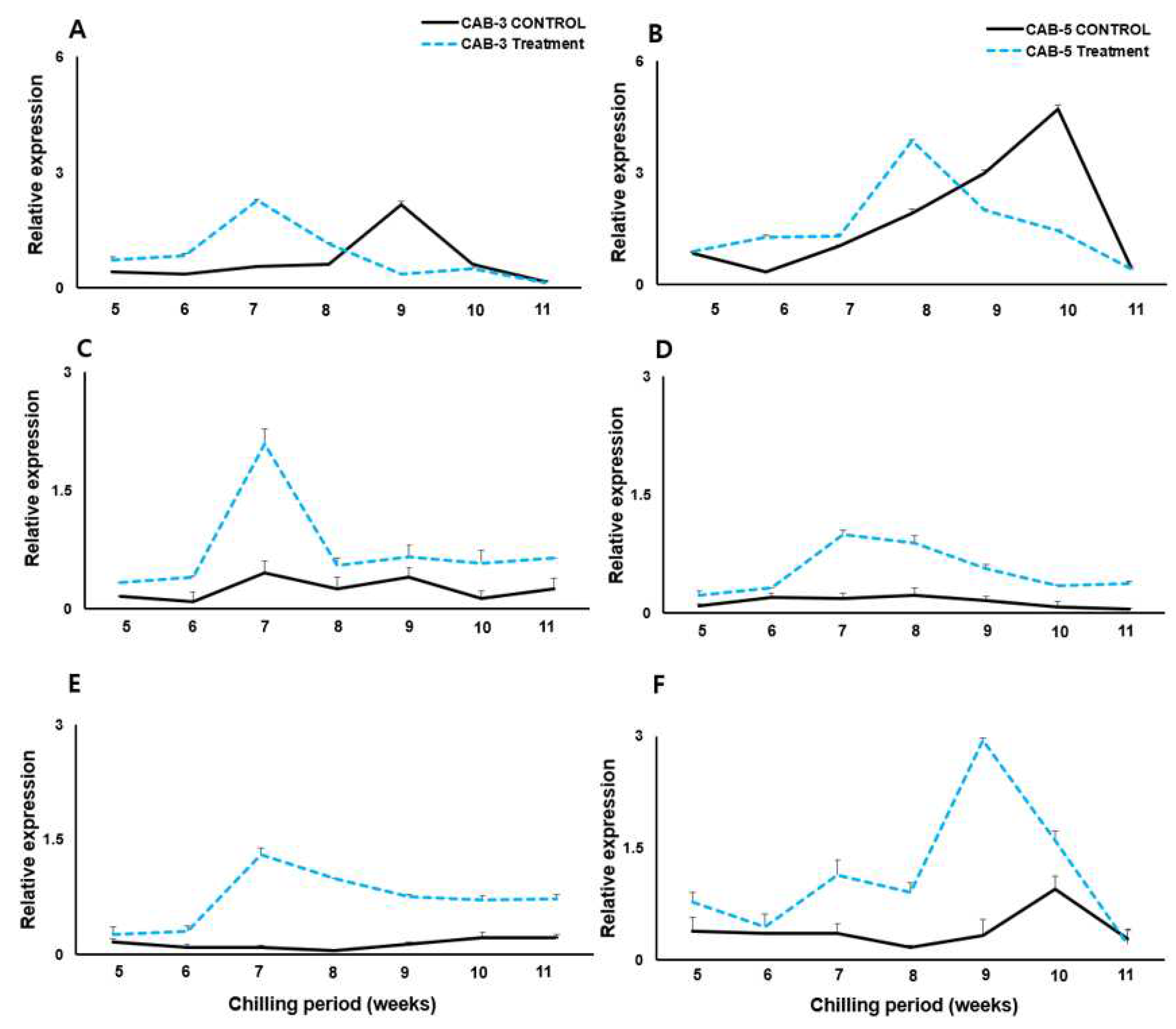
2.3. Transcriptional expression patterns of BoFLC genes by vernalization
2.4. Vernalization response of BoGI, BoCOOLAIR, and BoVIN3 in cabbage
3. Discussion
4. Materials and Methods
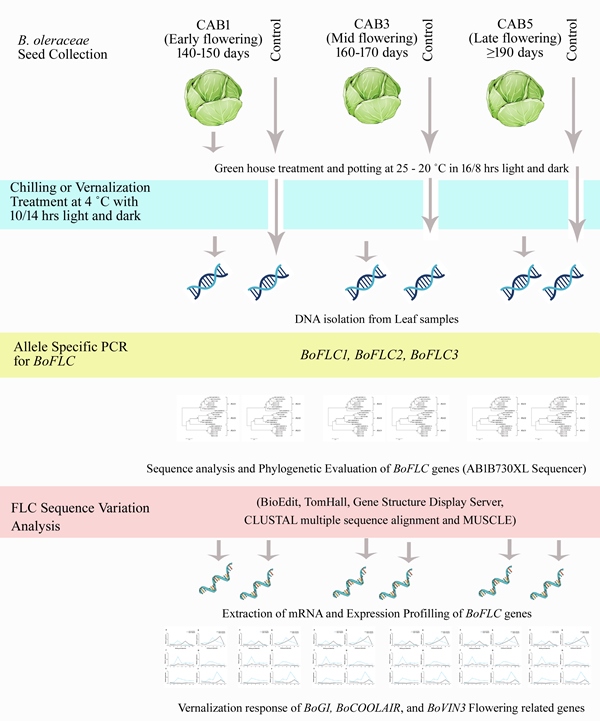
4.1. Plant materials and growth conditions
4.2. Genomic DNA extraction, PCR amplification, and sequencing of BoFLC homologs
4.3. Sequence analyses and Phylogenetic analysis of BoFLC homologs
4.4. Extraction of mRNA and Expression profiling of Flowering genes
4.5. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Andrés, F.; Coupland, G. The Genetic Basis of Flowering Responses to Seasonal Cues. Nat Rev Genet 2012, 13, 627–639.
- Srikanth, A.; Schmid, M. Regulation of Flowering Time: All Roads Lead to Rome. Cellular and Molecular Life Sciences 2011, 68, 2013–2037.
- Fornara, F.; de Montaigu, A.; Coupland, G. SnapShot: Control of Flowering in Arabidopsis. Cell 2010, 141. [CrossRef]
- Ausín, I.; Alonso-Blanco, C.; Martínez-Zapater, J.M. Environmental Regulation of Flowering. International Journal of Developmental Biology 2005, 49, 689–705.
- Motoki, K.; Kinoshita, Y.; Hosokawa, M. Non-Vernalization Flowering and Seed Set of Cabbage Induced by Grafting onto Radish Rootstocks. Front Plant Sci 2019, 9. [CrossRef]
- Mao, Y.; Wu, F.; Yu, X.; Bai, J.; Zhong, W.; He, Y. MicroRNA319a-Targeted Brassica Rapa Ssp. Pekinensis TCP Genes Modulate Head Shape in Chinese Cabbage by Differential Cell Division Arrest in Leaf Regions. Plant Physiol 2014, 164, 710–720. [CrossRef]
- Kitamoto, N.; Nishikawa, K.; Tanimura, Y.; Urushibara, S.; Matsuura, T.; Yokoi, S.; Takahata, Y.; Yui, S. Development of Late-Bolting F1 Hybrids of Chinese Cabbage (Brassica Rapa L.) Allowing Early Spring Cultivation without Heating. Euphytica 2017, 213, 1–13. [CrossRef]
- Dittmar, E.L.; Oakley, C.G.; Ågren, J.; Schemske, D.W. Flowering Time QTL in Natural Populations of Arabidopsis Thaliana and Implications for Their Adaptive Value. Mol Ecol 2014, 23, 4291–4303. [CrossRef]
- Gazzani, S.; Gendall, A.R.; Lister, C.; Dean, C. Analysis of the Molecular Basis of Flowering Time Variation in Arabidopsis Accessions. Plant Physiol 2003, 132, 1107–1114. [CrossRef]
- Okazaki, K.; Sakamoto, K.; Kikuchi, R.; Saito, A.; Togashi, E.; Kuginuki, Y.; Matsumoto, S.; Hirai, M. Mapping and Characterization of FLC Homologs and QTL Analysis of Flowering Time in Brassica Oleracea. Theoretical and Applied Genetics 2007, 114, 595–608. [CrossRef]
- Osborn, T.C.; Kole, C.; Pa~-Kin, I.A.P.; Sharpe, ~ A G; Kuiper, M.; Lydiatet, D.J.; Trickt, M. Comparison of Flowering Time Genes in Brassica Rapa, B. Mpus and Arabidopsis Thulium. Genetics 1997, 146, 1129–1129.
- Camargo, L.E.A.; Osborn, T.C. Mapping Loci Controlling Flowering Time in Brassica Oleracea. Theor Appl Genet 1996, 92, 610–616.
- Ridge, S.; Brown, P.H.; Hecht, V.; Driessen, R.G.; Weller, J.L. The Role of BoFLC2 in Cauliflower (Brassica Oleracea Var. Botrytis L.) Reproductive Development. J Exp Bot 2015, 66, 125–135. [CrossRef]
- Razi, H.; Howell, E.C.; Newbury, H.J.; Kearsey, M.J. Does Sequence Polymorphism of FLC Paralogues Underlie Flowering Time QTL in Brassica Oleracea? Theoretical and Applied Genetics 2008, 116, 179–192. [CrossRef]
- Abuyusuf, M.; Nath, U.K.; Kim, H.T.; Islam, M.R.; Park, J.I.; Nou, I.S. Molecular Markers Based on Sequence Variation in BoFLC1.C9 for Characterizing Early- and Late-Flowering Cabbage Genotypes. BMC Genet 2019, 20. [CrossRef]
- Wu, J.; Wei, K.; Cheng, F.; Li, S.; Wang, Q.; Zhao, J.; Bonnema, G.; Wang, X. A Naturally Occurring InDel Variation in BraA.FLC.b (BrFLC2) Associated with Flowering Time Variation in Brassica Rapa. BMC Plant Biol 2012, 12. [CrossRef]
- Zhao, J.; Kulkarni, V.; Liu, N.; Pino Del Carpio, D.; Bucher, J.; Bonnema, G. BrFLC2 (FLOWERING LOCUS C) as a Candidate Gene for a Vernalization Response QTL in Brassica Rapa. J Exp Bot 2010, 61, 1817–1825. [CrossRef]
- Hou, J.; Long, Y.; Raman, H.; Zou, X.; Wang, J.; Dai, S.; Xiao, Q.; Li, C.; Fan, L.; Liu, B.; et al. A Tourist-like MITE Insertion in the Upstream Region of the BnFLC.A10 Gene Is Associated with Vernalization Requirement in Rapeseed (Brassica Napus L.). BMC Plant Biol 2012, 12. [CrossRef]
- Tadege, M.; Sheldon, C.C.; Helliwell, C.A.; Stoutjesdijk, P.; Dennis, E.S.; Peacock, W.J. Control of Flowering Time by FLC Orthologues in Brassica Napus. Plant Journal 2001, 28, 545–553. [CrossRef]
- Schranz, M.E.; Quijada, P.; Sung, S.-B.; Lukens, L.; Amasino, R.; Osborn, T.C. Characterization and Effects of the Replicated Flowering Time Gene FLC in Brassica Rapa. Genetics 2002, 162, 1457–1468.
- Itabashi, E.; Shea, D.J.; Fukino, N.; Fujimoto, R.; Okazaki, K.; Kakizaki, T.; Ohara, T. Comparison of Cold Responses for Orthologs of Cabbage Vernalizationrelated Genes. Horticulture Journal 2019, 88, 462–470. [CrossRef]
- Irwin, J.A.; Lister, C.; Soumpourou, E.; Zhang, Y.; Howell, E.C.; Teakle, G.; Dean, C. Functional Alleles of the Flowering Time Regulator FRIGIDA in the Brassica Oleracea Genome. BMC Plant Biol 2012, 12. [CrossRef]
- Fadina, O.A.; Pankin, A.A.; Khavkin, E.E. Molecular Characterization of the Flowering Time Gene FRIGIDA in Brassica Genomes A and C. Russian Journal of Plant Physiology 2013, 60, 279–289. [CrossRef]
- Wang, N.; Qian, W.; Suppanz, I.; Wei, L.; Mao, B.; Long, Y.; Meng, J.; Müller, A.E.; Jung, C. Flowering Time Variation in Oilseed Rape (Brassica Napus L.) Is Associated with Allelic Variation in the FRIGIDA Homologue BnaA.FRI.a. J Exp Bot 2011, 62, 5641–5658. [CrossRef]
- Sheldon, C.C.; Rouse, D.T.; Finnegan, E.J.; Peacock, W.J.; Dennis, E.S. The Molecular Basis of Vernalization: The Central Role of FLOWERING LOCUS C (FLC). PNAS 2000, 97, 3753–3758.
- Helliwell, C.A.; Wood, C.C.; Robertson, M.; James Peacock, W.; Dennis, E.S. The Arabidopsis FLC Protein Interacts Directly in Vivo with SOC1 and FT Chromatin and Is Part of a High-Molecular-Weight Protein Complex. Plant Journal 2006, 46, 183–192. [CrossRef]
- Nilsson, O.; Lee, I.; Blázquez, M.A.; Weigel, D. Flowering-Time Genes Modulate the Response to LEAFY Activity. Genetics 1998, 150, 403–410.
- Blázquez, M.A.; Green, R.; Nilsson, O.; Sussman, M.R.; Weigel, D. Gibberellins Promote Flowering of Arabidopsis by Activating the LEAFY Promoter. Plant Cell 1998; 10,791-800.
- Mizoguchi, T.; Wright, L.; Fujiwara, S.; Cremer, F.; Lee, K.; Onouchi, H.; Mouradov, A.; Fowler, S.; Kamada, H.; Putterill, J.; et al. Distinct Roles of GIGANTEA in Promoting Flowering and Regulating Circadian Rhythms in Arabidopsis. Plant Cell 2005, 17, 2255–2270. [CrossRef]
- Kardailsky, I.; Shukla, V.K.; Ahn, J.H.; Dagenais, N.; Christensen, S.K.; Nguyen, J.T.; Chory, J.; Harrison, M.J.; Weigel, D. Activation Tagging of the Floral Inducer FT. Science 1999, 286, 1962–1965.
- Jung, J.H.; Seo, Y.H.; Pil, J.S.; Reyes, J.L.; Yun, J.; Chua, N.H.; Park, C.M. The GIGANTEA-Regulated MicroRNA172 Mediates Photoperiodic Flowering Independent of CONSTANS in Arabidopsis. Plant Cell 2007, 19, 2736–2748. [CrossRef]
- Zhang, J.; Mujahid, H.; Hou, Y.; Nallamilli, B.R.; Peng, Z. Plant Long NcRNAs: A New Frontier for Gene Regulatory Control. Am J Plant Sci 2013, 04, 1038–1045. [CrossRef]
- Swiezewski, S.; Liu, F.; Magusin, A.; Dean, C. Cold-Induced Silencing by Long Antisense Transcripts of an Arabidopsis Polycomb Target. Nature 2009, 462, 799–802. [CrossRef]
- Heo, J.B.; Sung, S. Vernalization-Mediated Epigenetic Silencing by a Long Intronic Noncoding RNA. Science 2011, 331, 76–79. [CrossRef]
- Schiessl, S. V.; Quezada-Martinez, D.; Tebartz, E.; Snowdon, R.J.; Qian, L. The Vernalisation Regulator FLOWERING LOCUS C Is Differentially Expressed in Biennial and Annual Brassica Napus. Sci Rep 2019, 9. [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Tim e Quantitative PCR and the 2 C T Method. METHODS 2001, 2, 402–408.
- Paltiel, J.; Amin, R.; Gover, A.; Ori, N.; Samach, A. Novel Roles for GIGANTEA Revealed under Environmental Conditions That Modify Its Expression in Arabidopsis and Medicago Truncatula. Planta 2006, 224, 1255–1268. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




