Submitted:
12 October 2023
Posted:
13 October 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
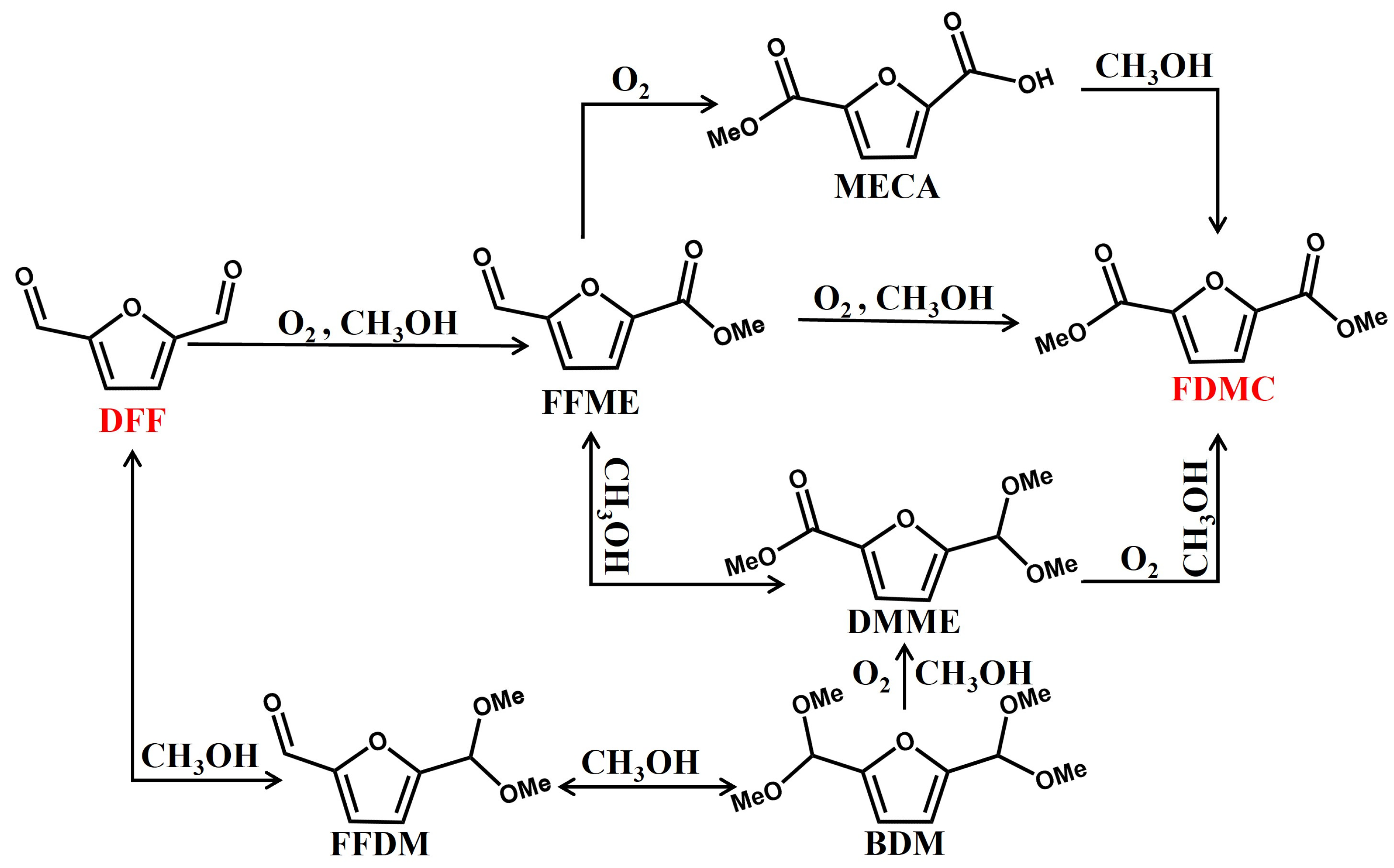
2. Results and Discussion
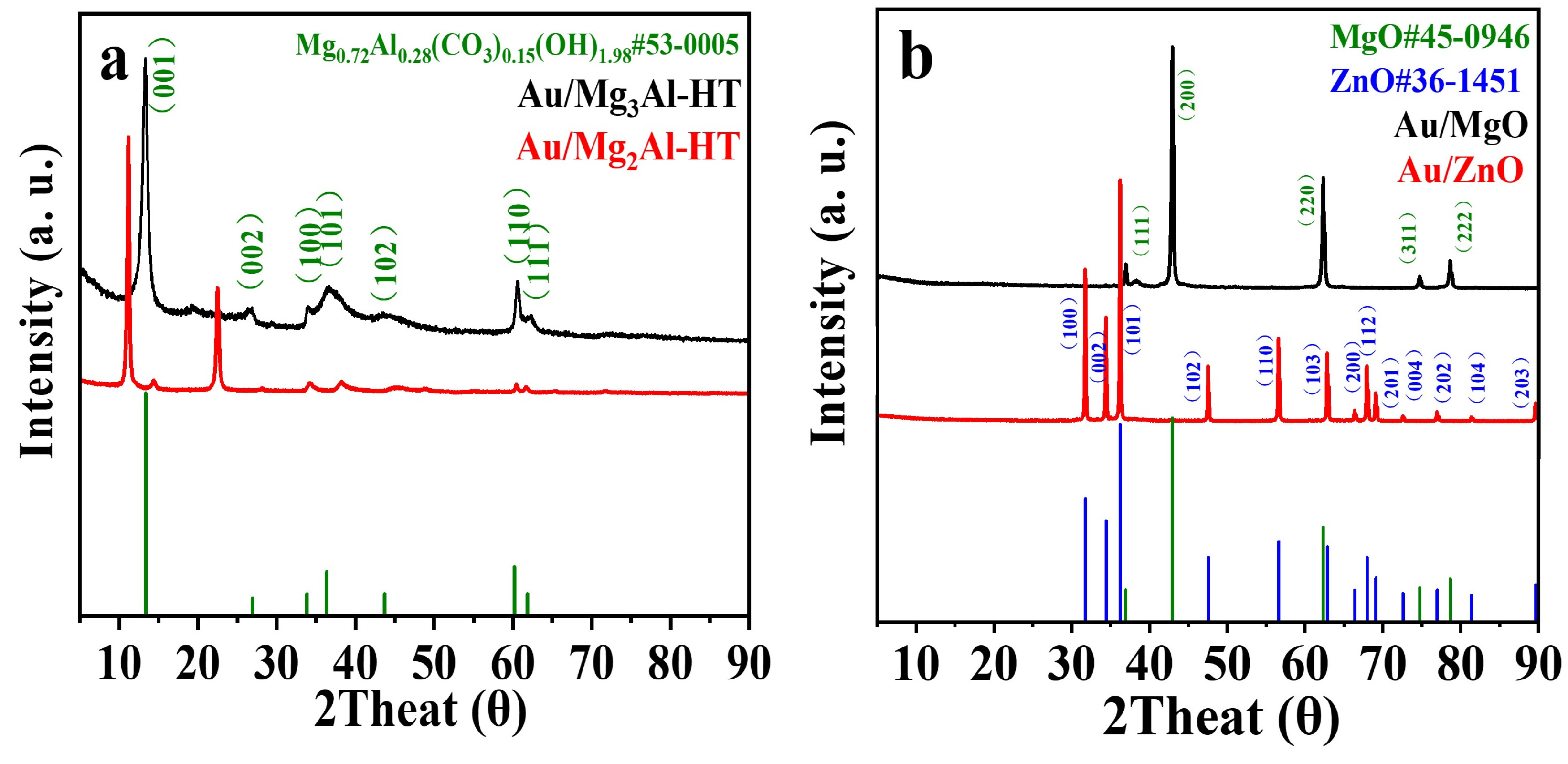
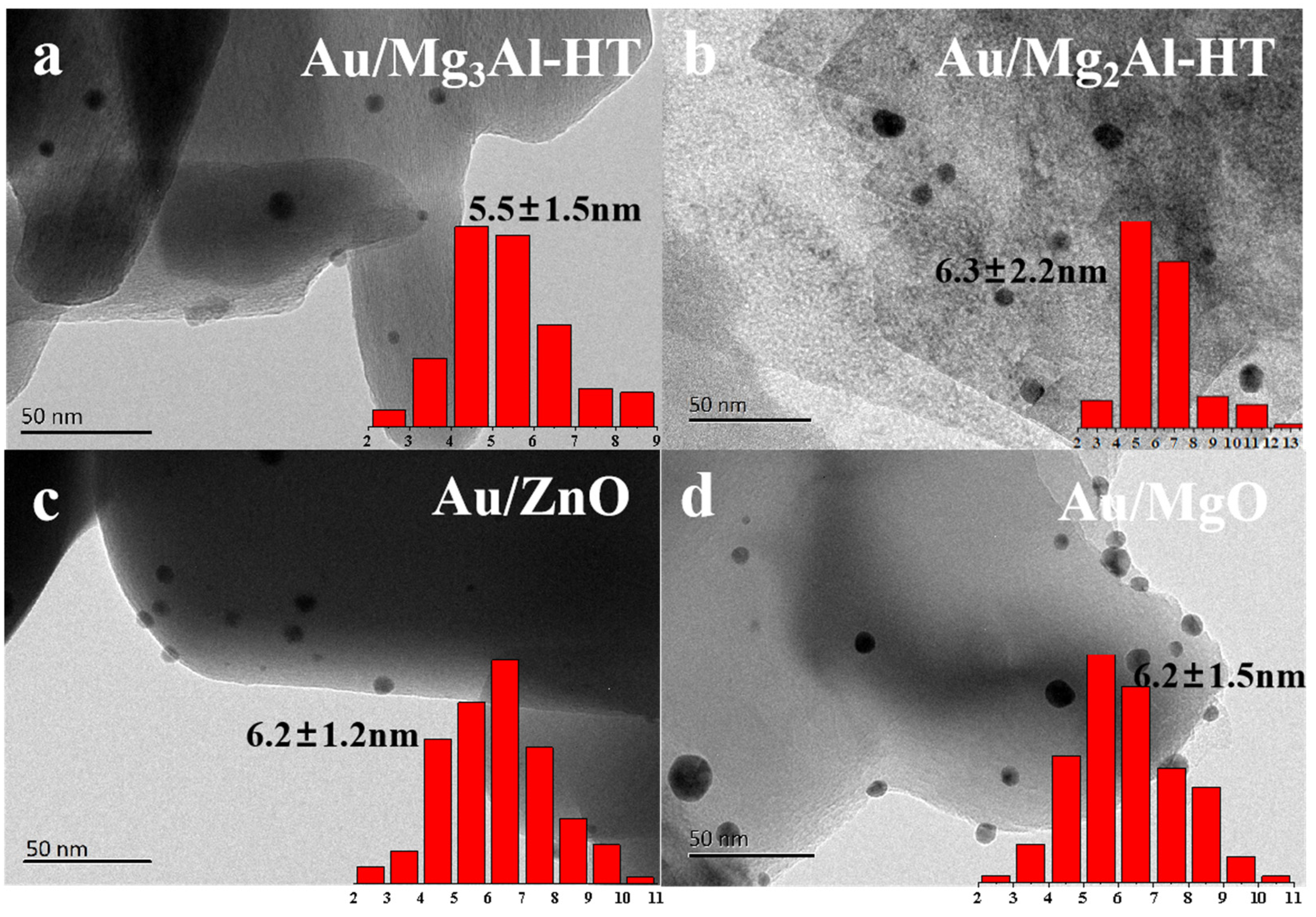
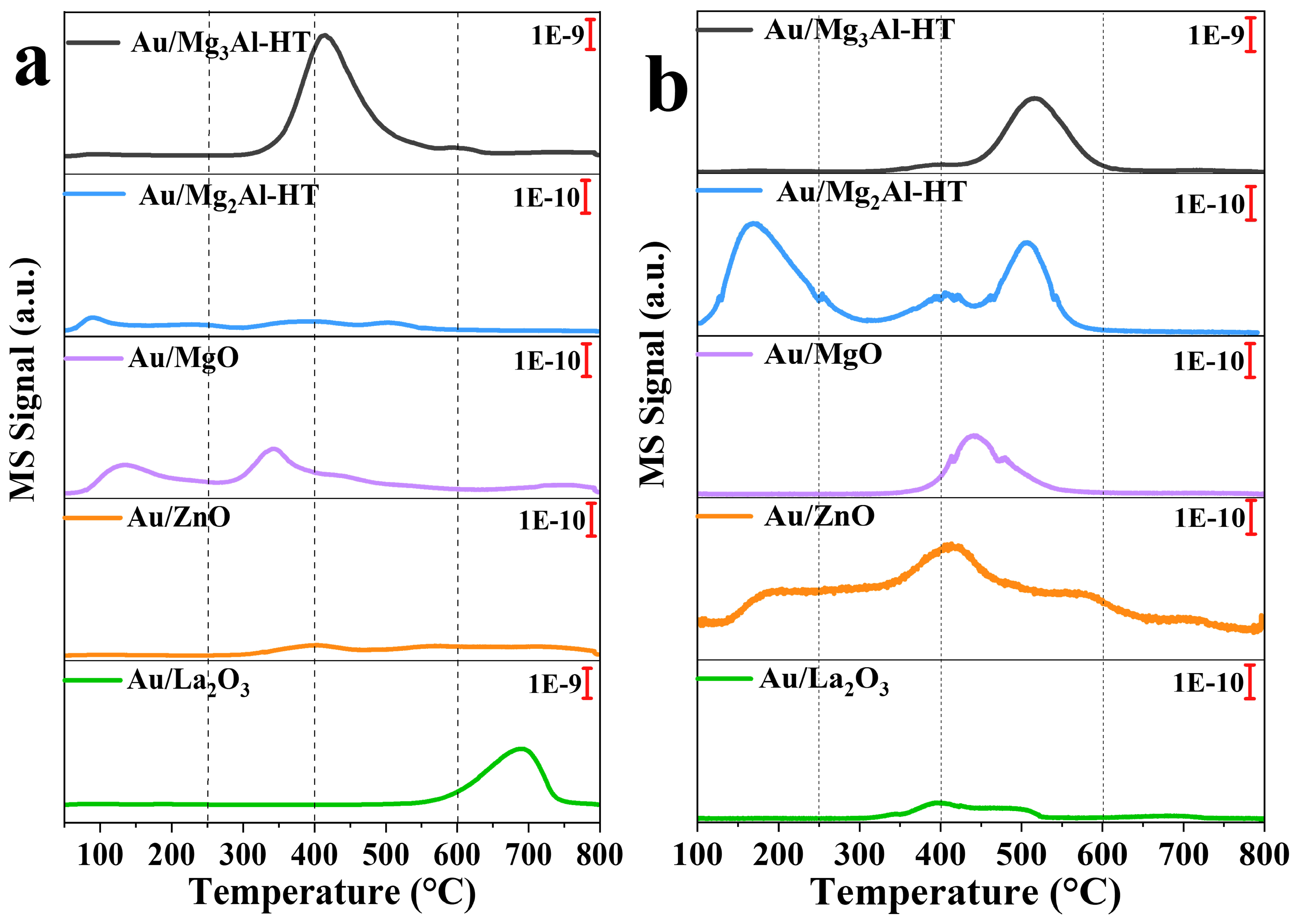
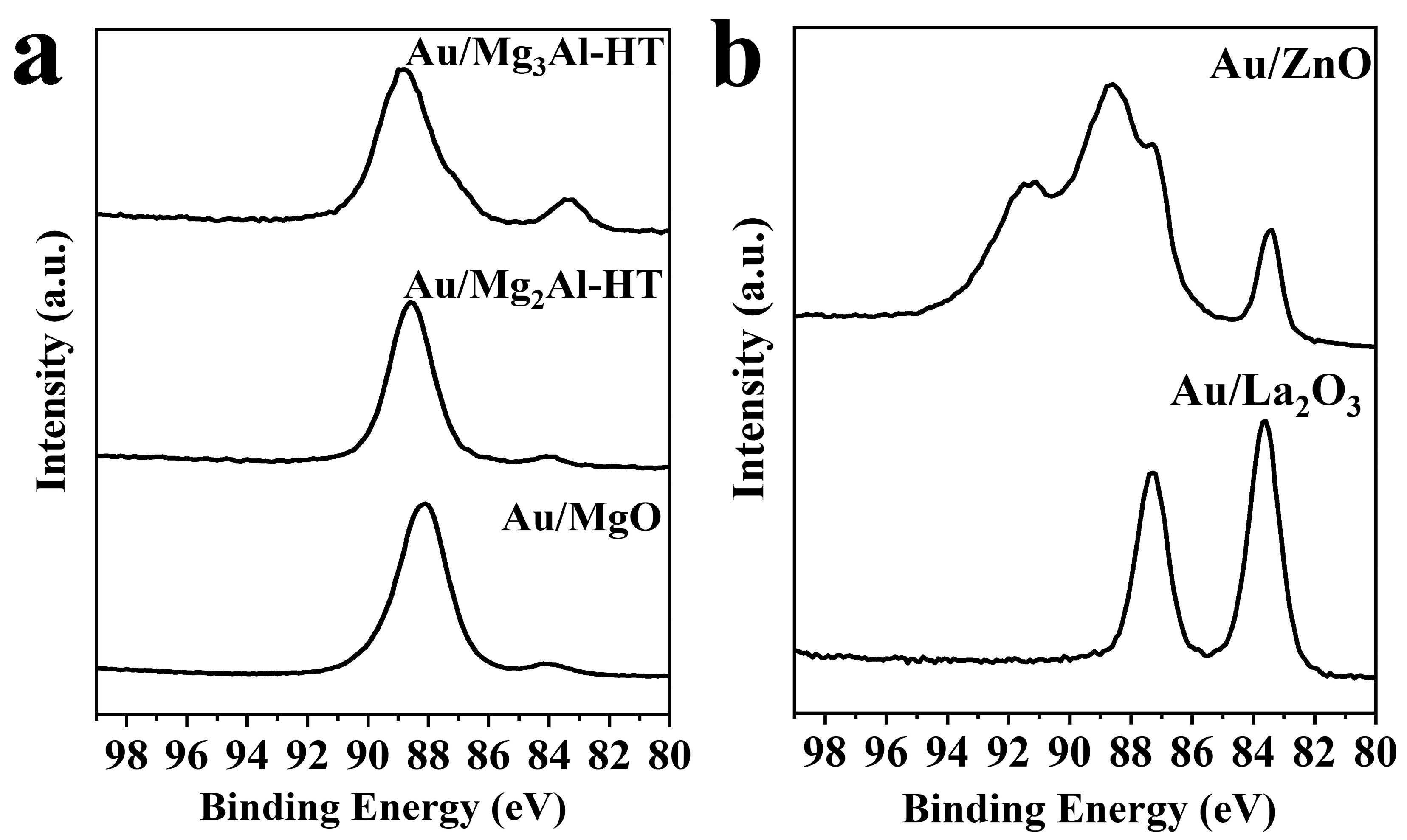
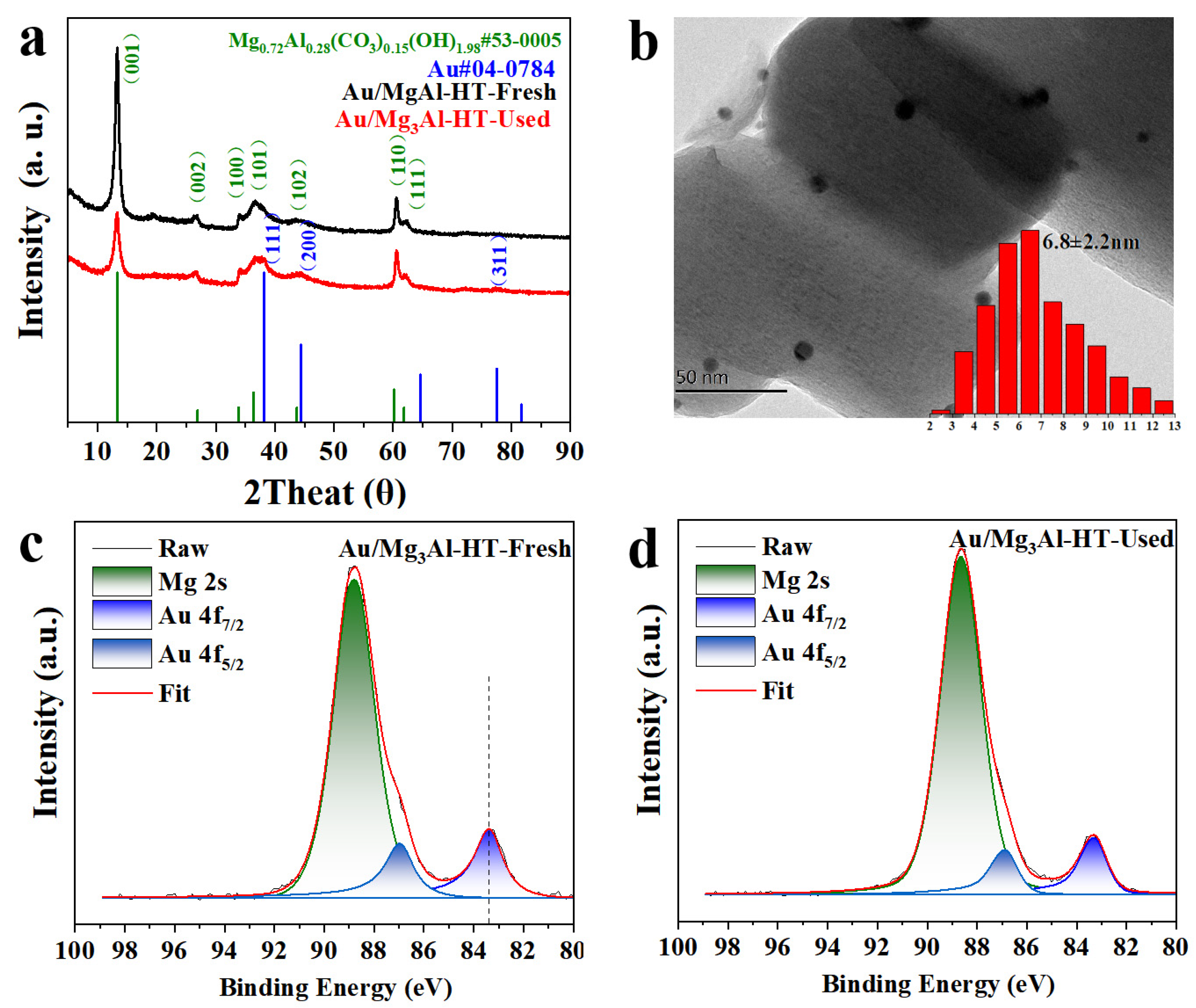
2.1. Catalysts characterization
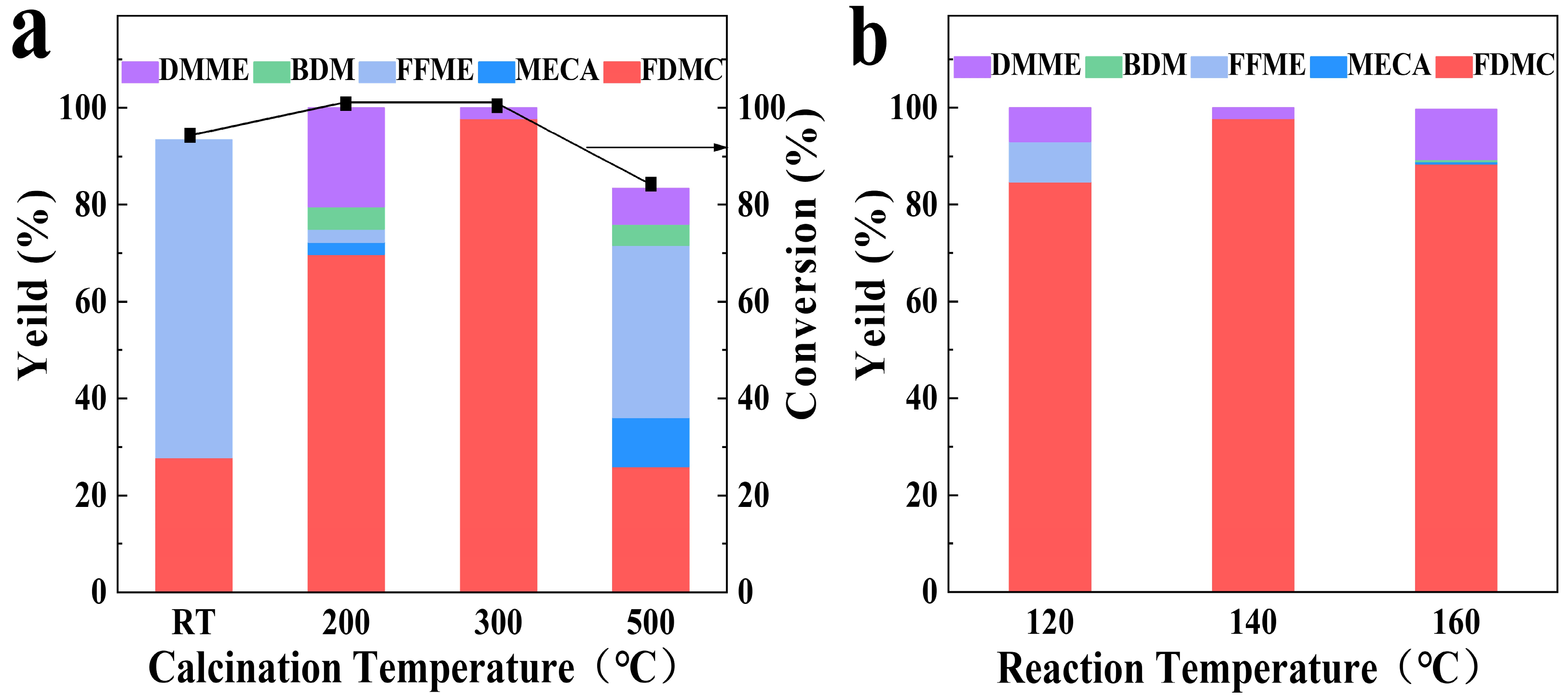
2.2. Effect of calcination temperature of catalyst
2.3. Effect of reaction temperature
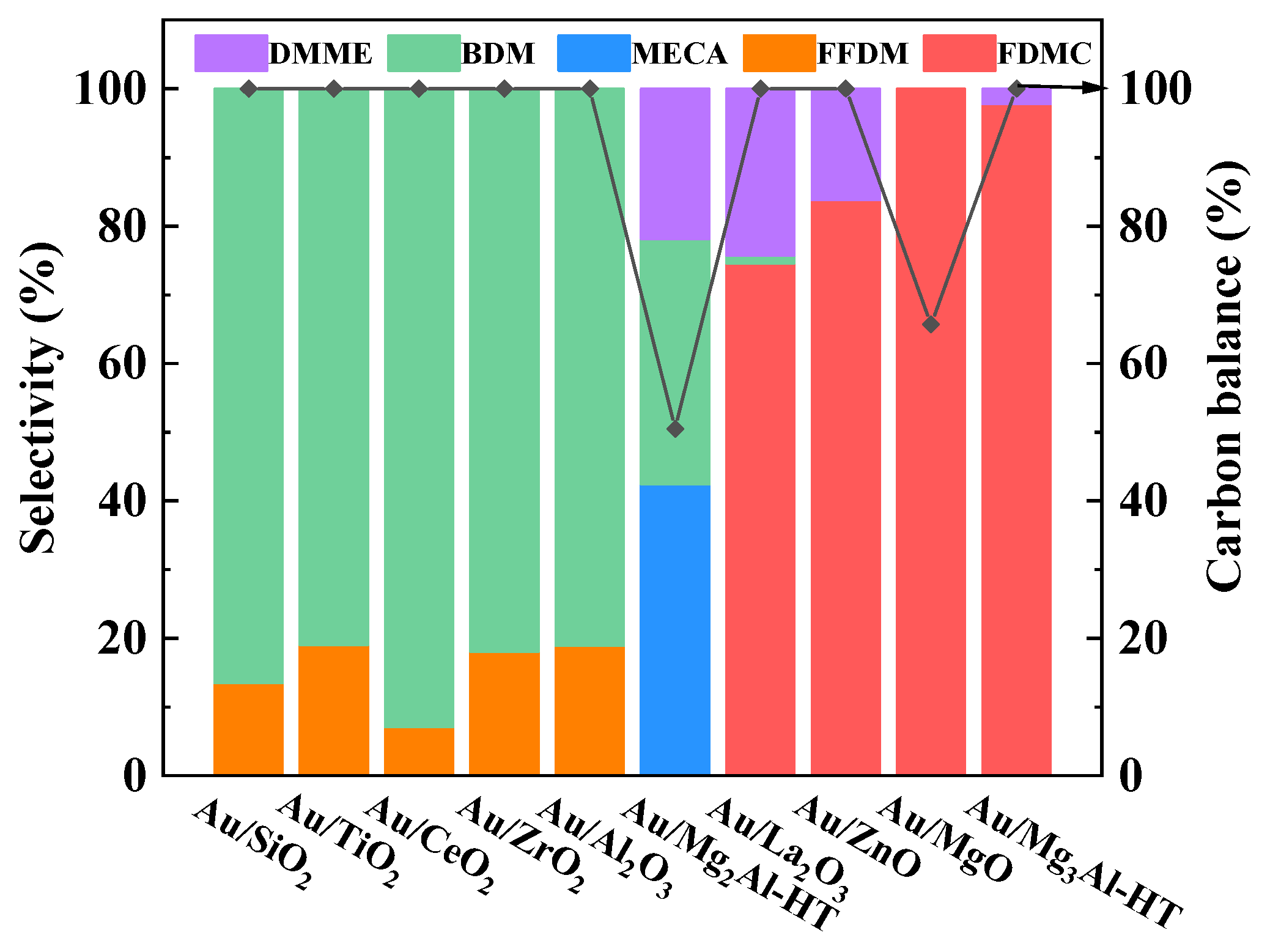
2.4. Catalytic performance of Au/Mg3Al-HT on different substrates
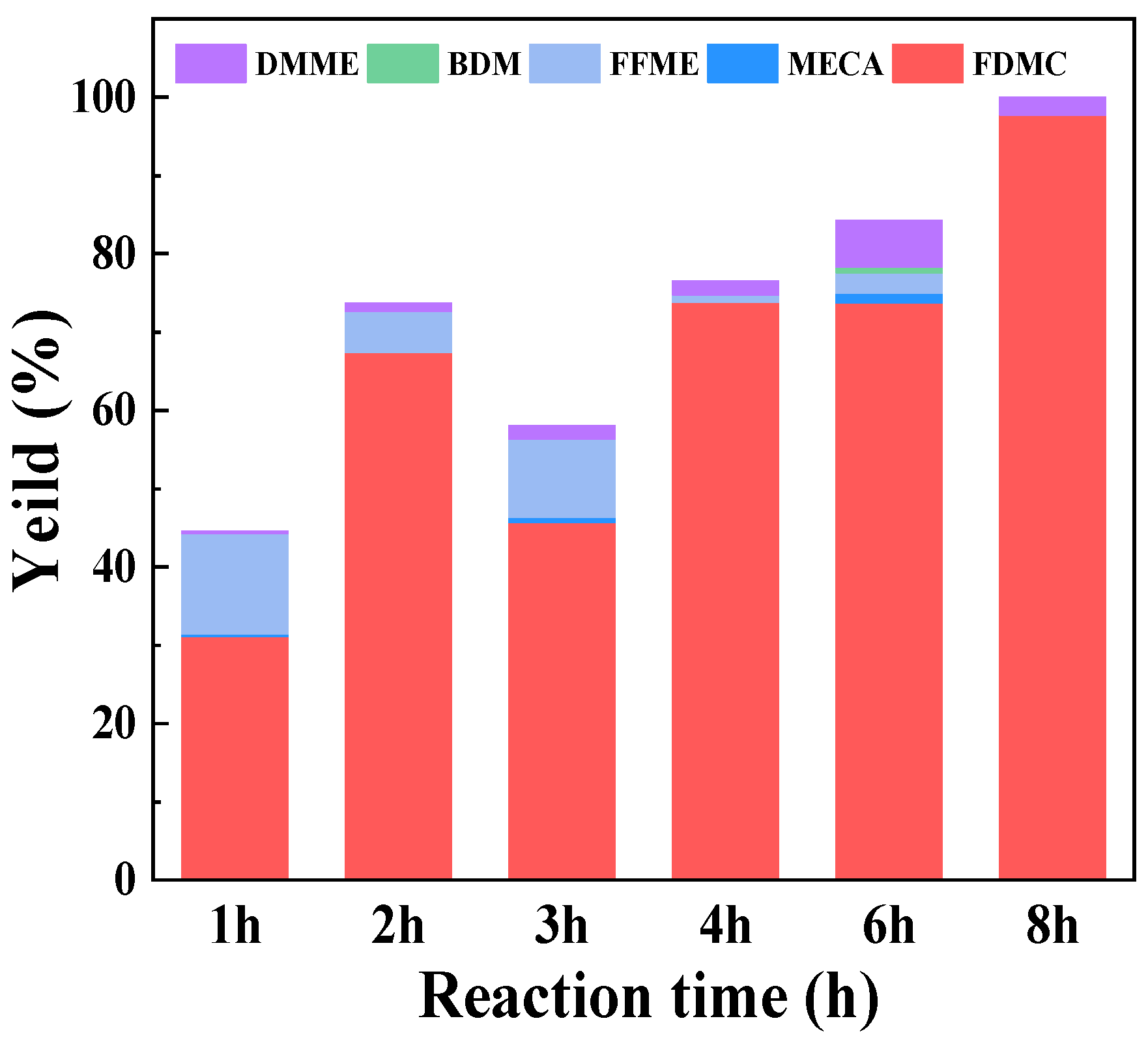
2.5. Stability of Au/Mg3Al-HT catalyst
3. Materials and Methods
3.1. Materials
3.2. Preparation of the catalyst
3.3. Catalytic evaluation
4. Conclusion
Author Contributions
Funding
Conflicts of Interest
References
- Burgess, S.K.; Leisen, J.E.; Kraftschik, B.E.; Mubarak, C.R.; Kriegel, R.M.; Koros, W.J. Chain Mobility, Thermal, and Mechanical Properties of Poly(ethylene furanoate) Compared to Poly(ethylene terephthalate). Macromolecules 2014, 47, 1383–1391. [Google Scholar] [CrossRef]
- Casanova, O.; Iborra, S.; Corma, A. Biomass into chemicals: One pot-base free oxidative esterification of 5-hydroxymethyl-2-furfural into 2, 5-dimethylfuroate with gold on nanoparticulated ceria. J Catal 2009, 265, 109–116. [Google Scholar] [CrossRef]
- Jia, W.; Chen, J.; Yu, X.; Zhao, X.; Feng, Y.; Zuo, M.; Li, Z.; Yang, S.; Sun, Y.; Tang, X.; et al. Toward an integrated conversion of fructose for two-step production of 2, 5-furandicarboxylic acid or furan-2, 5-dimethylcarboxylate with air as oxidant. Chem Eng J 2022, 450, 138172. [Google Scholar] [CrossRef]
- Takei, T.; Akita, T.; Nakamura, I.; Fujitani, T.; Haruta, M. Heterogeneous Catalysis by Gold. Advances in Catalysis 2012, 55, 1–126. [Google Scholar] [CrossRef]
- Davis, S.E.; Houk, L.R.; Tamargo, E.C.; Datye, A.K.; Davis, R.J. Oxidation of 5-hydroxymethylfurfural over supported Pt, Pd and Au catalysts. Catal Today 2011, 160, 55–60. [Google Scholar] [CrossRef]
- Zhang, Y.; Cao, Y.; Yan, C.; Liu, W.; Chen, Y.; Guan, W.; Wang, F.; Liu, Y.; Huo, P. Rationally designed Au-ZrOx interaction for boosting 5-hydroxymethylfurfural oxidation. Chem Eng J 2023, 459, 141644. [Google Scholar] [CrossRef]
- Yang, W.; Fu, M.; Yang, C.; Zhang, Y.; Shen, C. Au−–Ov–Ti3+: Active site of MO -Au/TiO2 catalysts for the aerobic oxidation of 5-hydroxymethylfurfural. Green Energy & Environment 2023, 8, 785–797. [Google Scholar] [CrossRef]
- Taarning, E.; Nielsen, I.S.; Egeblad, K.; Madsen, R.; Christensen, C.H. Chemicals from Renewables: Aerobic Oxidation of Furfural and Hydroxymethylfurfural over Gold Catalysts. ChemSusChem 2008, 1, 75–78. [Google Scholar] [CrossRef] [PubMed]
- Buonerba, A.; Impemba, S.; Litta, A.D.; Capacchione, C.; Milione, S.; Grassi, A. Aerobic Oxidation and Oxidative Esterification of 5-Hydroxymethylfurfural by Gold Nanoparticles Supported on Nanoporous Polymer Host Matrix. ChemSusChem 2018, 11, 3139–3149. [Google Scholar] [CrossRef] [PubMed]
- Dimitratos, N.; Villa, A.; Wang, D.; Porta, F.; Su, D.; Prati, L. Pd and Pt catalysts modified by alloying with Au in the selective oxidation of alcohols. J Catal 2006, 244, 113–121. [Google Scholar] [CrossRef]
- Song, F.; Cen, S.; Wan, C.; Wang, L. Nano-Au Anchored in Organic Base Group-Grafted Silica Aerogel: A Durable and Robust Catalysts for Green Oxidative Esterification of Furfural. ChemCatChem 2022, 14, e202200704. [Google Scholar] [CrossRef]
- P. Ferraz, C.; Braga, A.H.; Ghazzal, M.N.; Zieliński, M.; Pietrowski, M.; Itabaiana, I.; Dumeignil, F.; Rossi, L.M.; Wojcieszak, R. Efficient Oxidative Esterification of Furfural Using Au Nanoparticles Supported on Group 2 Alkaline Earth Metal Oxides. Catalysts 2020, 10, 430. [Google Scholar] [CrossRef]
- Ren, J.; Mebrahtu, C.; Palkovits, R. Ni-based catalysts supported on Mg–Al hydrotalcites with different morphologies for CO2 methanation: exploring the effect of metal–support interaction. Catalysis Science & Technology 2020, 10, 1902–1913. [Google Scholar] [CrossRef]
- Dębek, R.; Radlik, M.; Motak, M.; Galvez, M.E.; Turek, W.; Da Costa, P.; Grzybek, T. Ni-containing Ce-promoted hydrotalcite derived materials as catalysts for methane reforming with carbon dioxide at low temperature – On the effect of basicity. Catal Today 2015, 257, 59–65. [Google Scholar] [CrossRef]
- Liu, H.; Jia, W.; Yu, X.; Tang, X.; Zeng, X.; Sun, Y.; Lei, T.; Fang, H.; Li, T.; Lin, L. Vitamin C-Assisted Synthesized Mn–Co Oxides with Improved Oxygen Vacancy Concentration: Boosting Lattice Oxygen Activity for the Air-Oxidation of 5-(Hydroxymethyl)furfural. ACS Catalysis 2021, 11, 7828–7844. [Google Scholar] [CrossRef]
- Liao, Y.; Yan, H.; Zhou, J.; Yue, Y.; Sun, Y.; Peng, T.; Yuan, X.; Zhou, X.; Liu, Y.; Feng, X.; et al. Interfacial Auδ--OV-Zr3+ structure promoted C H bond activation for oxidative esterification of methacrolein to Methyl methacrylate. Chem Eng J 2023, 454, 140322. [Google Scholar] [CrossRef]
- Sankar, M.; He, Q.; Engel, R.V.; Sainna, M.A.; Logsdail, A.J.; Roldan, A.; Willock, D.J.; Agarwal, N.; Kiely, C.J.; Hutchings, G.J. Role of the Support in Gold-Containing Nanoparticles as Heterogeneous Catalysts. Chem Rev 2020, 120, 3890–3938. [Google Scholar] [CrossRef] [PubMed]
- Wojcieszak, R.; Ferraz, C.; Sha, J.; Houda, S.; Rossi, L.; Paul, S. Advances in Base-Free Oxidation of Bio-Based Compounds on Supported Gold Catalysts. Catalysts 2017, 7, 352. [Google Scholar] [CrossRef]
- Wei, Y.; Zhang, Y.; Chen, Y.; Wang, F.; Cao, Y.; Guan, W.; Li, X. Crystal Faces-Tailored Oxygen Vacancy in Au/CeO2 Catalysts for Efficient Oxidation of HMF to FDCA. ChemSusChem 2021, 15, e202101983. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Sheng, G.; Hu, J.; Chen, C.; Wang, X. The adsorption of Pb(II) on Mg2Al layered double hydroxide. Chem Eng J 2011, 171, 167–174. [Google Scholar] [CrossRef]
| Entry | Substrate | Solvent | Conv. | (di)ester | monoester | monoacetal | monoester- monoacetal | (di)acetal |
|---|---|---|---|---|---|---|---|---|
| entry 1 | FF 1 | methanol | 100.00 | 97.23 | / | / | / | 2.77 |
| entry 2 | BzH 2 | methanol | 100.00 | 99.01 | / | / | / | 0.99 |
| entry 3 | DFF | methanol | 100.00 | 97.78 | / | / | / | 2.22 |
| entry 4 | DFF | ethanol | 100.00 | 54.61 | 12.89 | 6.47 | 24.45 | 1.57 |
| entry 5 | DFF | n-butanol | 94.02 | 6.65 | 3.76 | 82.59 | 6.99 | / |
| entry 6 | DFF | 1-pentanol | 59.24 | / | 2.60 | 34.56 | 62.84 | / |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).




